Published online Jan 7, 2015. doi: 10.3748/wjg.v21.i1.221
Peer-review started: May 14, 2014
First decision: June 27, 2014
Revised: July 20, 2014
Accepted: August 13, 2014
Article in press: August 28, 2014
Published online: January 7, 2015
Processing time: 237 Days and 20.8 Hours
AIM: To analyze the cellular immune response towards microsatellite-instability (MSI)-induced frameshift-peptides (FSPs) in patients suffering from inflammatory bowel disease (IBD) with and without thiopurine-based immunosuppressive treatment.
METHODS: Frequencies of peripheral blood T cell responses of IBD patients (n = 75) against FSPs derived from 14 microsatellite-containing candidate genes were quantified by interferon-γ enzyme-linked immunospot. T cells derived from 20 healthy individuals served as controls.
RESULTS: Significant T cell reactivities against MSI-induced FSPs were observed in 59 of 75 IBD patients (78.7%). This was significantly more as we could observe in 20 healthy controls (P = 0.001). Overall, the reactivity was significantly influenced by thiopurine treatment (P = 0.032) and duration of disease (P = 0.002) but not by duration or cumulative amount of thiopurine therapy (P = 0.476). Unexpected, 15 of 24 (62.5%) IBD patients without prior thiopurine treatment also showed increased FSP-specific immune responses (P = 0.001).
CONCLUSION: These findings propose FSPs as potential novel class of inflammation-associated antigens and this in turn may have implications for screening, diagnosis as well as clinical management of patients suffering from IBD and other inflammatory conditions.
Core tip: Oxidative stress resulting from chronic inflammation is likely to relax the mismatch repair (MMR) system and to cause DNA damage. On the other hand thiopurine treatment positively selects for cell variants with defective MMR system. inflammatory bowel disease (IBD) patients are often exposed to both factors, hypothetically resulting in an increased number of frameshift mutations. This study shows an increased immune response towards microsatellite-instability-induced frameshift-peptides (FSPs) in patients with IBD. It is the first report which provides functional evidence of FSP expression under non- but possible pre-neoplastic conditions. These findings have potential implications for screening, diagnosis as well as clinical management of IBD patients.
- Citation: Kuehn F, Klar E, Bliemeister A, Linnebacher M. Reactivity against microsatellite instability-induced frameshift mutations in patients with inflammatory bowel disease. World J Gastroenterol 2015; 21(1): 221-228
- URL: https://www.wjgnet.com/1007-9327/full/v21/i1/221.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i1.221
Immunosuppression after organ transplantation is an acknowledged risk factor for skin cancer, lymphoma and acute myeloid leukemia (AML)[1,2]. In a ground-breaking analysis, Offman and coworkers examined whether this might be related to a particular immunosuppressive treatment and they found an association with azathioprine, a thiopurine prodrug[3]. Cellular resistance to thiopurine is associated with DNA mismatch repair (MMR) deficiency and thiopurine treatment of human cells in vitro selects for variants with defective MMR. In addition, the authors demonstrated microsatellite instability (MSI), diagnostic for defective MMR, in bone marrow blasts of 7 out of 7 patients with transplant-related AML/myelodysplastic syndrome[3]. Because MSI occurs infrequently in de novo AML, they concluded that selective proliferation of MMR-defective-and thus azathioprine-resistant - myeloid cells contributes significantly to the development of AML/myelodysplastic syndrome in organ transplant recipients.
Very recently, Schwitalle et al[4] demonstrated that peripheral and tumor infiltrating T cells of patients with MSI+ but not with microsatellite stable colorectal carcinoma specifically react towards carboxy-terminal frameshift-peptides (FSP) originating from short insertion/deletion mutations in coding microsatellites. These MSI-induced FSPs have been proposed as a promising novel class of tumor antigens specific for MSI+ cancers[5-7]. Even more interesting, FSP-specific T cell reactions have been observed in still healthy siblings of Lynch patients but being carriers of germline MMR gene mutations[4]. This implies that FSP- specific T cells as measured by enzyme-linked-immunospot (ELISpot) assay are a sensitive surrogate marker for detection of MSI, even if present at low levels. Nowadays azathioprine is infrequently used as immunosuppressant after organ transplantation but regularly in chronic inflammatory bowel disease (IBD)[8]. We hypothesized that if the findings of Offman and colleagues are true; IBD patients with long-term thiopurine-based immunosuppression are likely to raise MSI+ somatic cells. In combination with results of the Schwitalle and coworkers, FSP-specific T cells may thus be expected in the peripheral blood of these patients.
Ninety-four patients with IBD were recruited from the Departments of Surgery and of Gastroenterology, University of Rostock. Peripheral blood mononuclear cells were isolated from heparinized blood by density gradient centrifugation. Mononuclear cells were cryopreserved prior to analysis in freezing medium (fetal calf serum + 10% DMSO) in cryotubes (10-20 × 106 cells/tube) at -80 °C for one to a maximum of twelve months.
MSI-induced FSPs derived from 14 coding microsatellite containing genes were selected according to the following criteria: high coding microsatellite mutation frequency in MSI+ colorectal cancer, functional relevance of the corresponding wild-type protein, and in vitro immunogenicity data[4-6,9-14] Predicted FSPs (see Supplemental Table 1 for FSP sequences) were synthesized, dissolved to 5 mg/mL in DMSO and further diluted to 500 μg/mL in PBS.
| Patients’ characteristics (n = 75) | Value |
| Diagnosis | |
| CD | 41 (54.7) |
| UC | 34 (45.3) |
| Mean age in years (range) | 44.6 (20-80) |
| Mean duration of disease in years (range) | 11 (1-37) |
| Location of disease (CD) | |
| Small bowel | 11/41 (26.8) |
| Colon | 6/41 (14.6) |
| Small bowel + colon | 24/41 (58.6) |
| Location of disease (UC) | |
| Pancolitis | 17/34 (50.0) |
| Left colon | 14/34 (41.2) |
| Ileal pouch-anal anastomosis | 3/34 (8.8) |
| Co-medication | |
| Prednisolone | 67/75 (89.3) |
| Anti-TNF-α-Antibody | 18/75 (24.0) |
| Tacrolimus | 12/75 (16.0) |
| MTX | 9/75 (12.0) |
| Mean time of azathioprine therapy in months (range) | 50 (4-152) |
Frequencies of FSP-specific T cells were quantified basically as described by determining the number of specific interferon-γ secreting T cells against FSPs by taking advantage of a commercially available ELISpot assay (Mabtech, Nacka, Sweden)[4] Ninety-six-well nitrocellulose plates (Multiscreen; Millipore, Bedford, United States) were coated overnight with mouse anti-human interferon-monoclonal antibodies and blocked with serum containing medium. Cryopreserved mononuclear cells were thawed, washed and counted. Analyses were performed when a minimum amount of 10 × 106 cells could be recovered. Mononuclear cells (1 × 105/well) were plated in 200 μL IMDM with 10% human AB serum. Peptides were added at a final concentration of 10 μg/mL. Interferon-γ ELISpot was conducted with 6-fold determinations. Positive (50 μL anti-human CD3 monoclonal antibody (OKT-3) hybridoma supernatant as polyclonal activator and a recall peptide-mix (WLDARMQAIQNAGLCTLVAMLEETIFWLQE, Epstein-Barr virus, BMLF1 and TRPVLSPLTKGILG-FVFTLTVPGERGLQR, influenza matrix protein) as well as negative controls (no peptides added to the wells) were included. After incubation for 16 h at 37 °C, plates were washed thoroughly, incubated with biotinylated polyclonal rabbit anti-human interferon for 4 h, washed again, and incubated with streptavidin-alkaline phosphatase for 2 h, followed by a final wash. Spots were detected by incubation with nitro-blue tetrazolium and 5-bromo-4-chloro-3’-indolyphosphate for 1 h, reaction was stopped with water, and, after drying, spots were counted with the CTL ImmunoSpot® S5 UV Image Analyzer (Cellular Technology Ltd., OH, United States).
All analyses were conducted according to the Declaration of Helsinki. The study was approved by the local Ethics Committee (identification number: II HV 43/2004). Each patient gave written informed consent prior to scientific evaluation.
T cell reactivity was considered as significant, if antigen-specific spot numbers exceeded background reactivity after adjustment by subtraction of background (no peptide control) and standard deviations of peptide-specific value and no peptide value as described previously[4].
Patients data were evaluated retrospectively and statistically analyzed with software program SPSS (Version 16.0). χ2 and Mann-Whitney-U test were used and P values < 0.05 were considered as statistically significant.
In total 94 IBD patients were recruited for this study. ELISpot-analysis for FSP-specific reactivity was performed when a minimum amount of 10 × 106 peripheral blood lymphocytes could be recovered after transient cryo-conservation. This was possible for 82 patients. Seven ELISpot results were not taken into further consideration because of missing reactivity in the positive controls. Additionally and as a control, FSP-specific T cell reactivity of 20 healthy persons was analyzed.
Thus, 75 patients’ FSP-specific ELISpot results were analyzed in detail (for patient characteristics see Table 1). There were 41 patients (54.7%) with Crohn’s disease (CD) and 34 patients (45.3%) with ulcerative colitis (UC). Mean duration of disease was eleven years (range: 1-37). CD was located in the small bowel in eleven of 41 patients (26.8%), in the colon in six patients (14.6%) and in both compartments in 24 patients (58.6%). Seventeen of 34 patients (50.0%) with UC suffered from a pancolitis, in 14 patients (41.2%) UC was restricted to the left colon and three patients (8.8%) underwent proctocolectomy with an ileal pouch-anal anastomosis prior to this study. T cells isolated from patients with IBD specifically recognized MSI-induced FSPs in 59 of 75 patients (78.7%) (Figure 1A). This was a striking contrast to the healthy controls which did very rarely show significant reactivity (P = 0.001) (Figure 1B). Of note, the reactivity towards a mixture of two viral peptides (derived from EBV BMLF-1 and from the influenza matrix protein) was similar in both groups and it was comparable to the reactivity towards the MSI-induced FSPs in the IBD patient cohort (Figure 1A).
Gender, age, smoking status, disease pattern, co-medication and family anamnesis had no statistically significant influence on the FSP-specific immune responses (Table 2). Also, similar immune response rates against FSPs were detected for UC and CD; 32 of 41 patients (78.0%) with CD compared to 27 of 34 patients (79.4%) with UC showed an increased immune response (P > 0.05).
| Influence of different characteristics on reactivity | P value |
| Duration of disease ( </> 7 yr) | 0.023 (χ2) |
| Duration of disease (IBD) | 0.002 (Mann-Whitney U) |
| Duration of disease (IBD without azathioprine) | 0.008 (Mann-Whitney U) |
| Duration of disease (IBD with weak reactivity) | 0.006 (Mann-Whitney U) |
| Duration of disease (IBD with strong reactivity) | 0.005 (Mann-Whitney U) |
| Duration of azathioprine (among patients with azathioprine) | 0.476 (Mann-Whitney U) |
| Duration of azathioprine (IBD with azathioprine and weak reactivity) | 0.600 (Mann-Whitney U) |
| Duration of azathioprine (IBD with azathioprine and strong reactivity) | 0.350 (Mann-Whitney U) |
| IBD controls vs healthy controls | 0.001 (χ2) |
| IBD with azathioprine vs healthy controls | 0.001 (χ2) |
| IBD with azathioprine vs IBD controls | 0.032 (χ2) |
| IBD with azathioprine vs IBD controls (weak reactivity) | 0.007 (χ2) |
| IBD with azathioprine vs IBD controls (strong reactivity) | 0.342 (χ2) |
| Gender (male vs female) | 0.491 (χ2) |
| Age | 0.522 (Mann-Whitney U) |
| Smoking status | 0.426 (χ2) |
| Disease pattern of CD (small bowel vs large bowel vs combined) | 0.219 (χ2) |
| Disease pattern of UC (left sided colitis vs pancolitis) | 0.412 (χ2) |
| Positive family anamnesis | 0.618 (χ2) |
| Co-medication: | (χ2) |
| Prednisolone | 0.356 |
| TNF-α-antibody | 0.336 |
| Tacrolimus | 0.497 |
| Methotrexate | 0.617 |
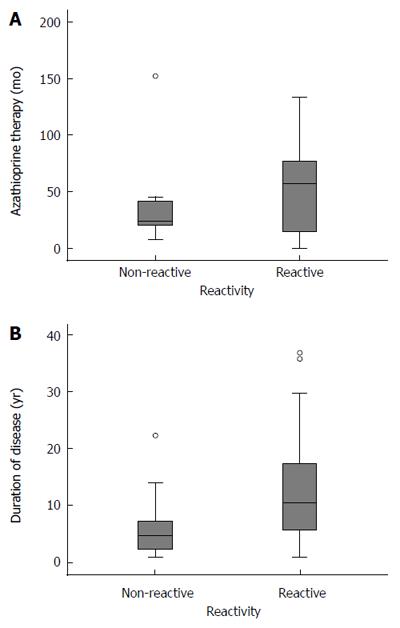
Fifty-one patients with azathioprine treatment for at least three months and with a mean therapy duration of 50 mo (range: 4-152) showed significant higher reactivity rates when compared to 24 patients who never received azathioprine (86.3% vs 62.5%, P = 0.032; Figure 2A, Table 2).
Overall, reactivity was significantly influenced by duration of disease (P = 0.002; Figure 2B, Table 2) but by neither duration nor cumulative amount of thiopurine therapy (P = 0.476). Unexpectedly, IBD patients without prior thiopurine treatment also showed an increased FSP-specific immune response (15 of 24 patients; 62.5%, P = 0.001; Table 2). Detailed analysis revealed that duration of IBD was an independent predictor for FSP-specific immune responses in this patient subgroup (P = 0.008; Table 2). Reactive patients without azathioprine treatment had a significant longer duration of disease than non-reactive patients (14 years vs 6 years, P = 0.008; Table 2).
Referring to the number of interferon-γ-secreting cells, immune responses were further separated into strong and weak reactivity as suggested by Schwitalle et al[4]. In this subgroup analysis, weak responses (36/75 patients; 48.0%) correlated with duration of disease (P = 0.006) and with azathioprine therapy (P = 0.007; Table 2). However, for strong immune responses (23/75 patients; 30.7%), only duration of disease showed a statistically significant influence (P = 0.005), whereas neither azathioprine (P = 0.342) nor duration or cumulative amount of azathioprine therapy had a significant influence (P = 0.350; Table 2).
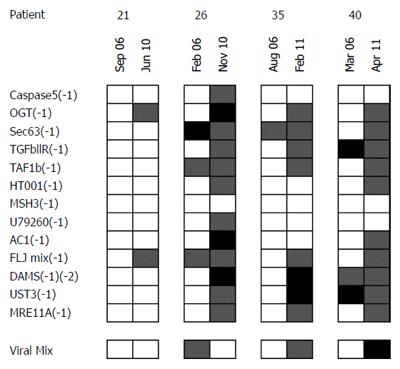
For four patients repetitive measurements at different points of time were possible with a mean time interval between measurements of 5 years. Here, reactivity towards FSPs of the same patients varied at the different dates with a strong trend towards higher reactivity at the later time points (Figure 3).
Starting point of the present study was a report of Offman and colleagues on azathioprine associated MMR-deficiency in patients with post transplantation AML[3]. Nowadays, azathioprine is rarely used as an immunosuppressant for recipients of organ transplants but is a widely used standard for management of chronic inflammatory diseases. And indeed, several very recent cohort studies demonstrated an increased risk for skin cancer and lymphoma in patients with IBD and concomitant thiopurine treatment[15-19].
Azathioprine is a thiopurine prodrug and an incorporation into the DNA leads to mispriming events which are recognized by the cell’s DNA MMR system. When repair is not successful, the MMR system induces apoptosis. This explains the cytotoxicity and thus the therapeutic effect of thiopurines[3,20]. Consequently are cells with functional MMR defects intrinsically resistant towards thiopurine drugs and, as analyzed by Offman and coworkers, will thiopurine treatment positively select for cells with MMR deficiency[3].
The cumulative risk for development of colorectal cancer is increased for patients with UC and CD[21,22]. However, there is controversy about the existence of MSI in IBD, and especially in UC. The scarce literature available about MSI in dysplastic or non-dysplastic mucosa of patients with UC and in UC-related cancer varies in their results. Several groups observed a high incidence of MSI in long standing UC with severe inflammation in histologically non-neoplastic but also in neoplastic mucosa, as well as additionally in adenomas and UC-associated carcinomas[23-25]. It was argued that MSI may reflect the inability of DNA repair mechanisms to compensate for the stress of chronic inflammation, and that it may thus be one mechanism for the heightened neoplastic risk in UC. On the other hand, some studies could not prove an increased incidence for MSI in IBD[26-28].
In the present work, we hypothesized and confirmed a significant influence of azathioprine on the naturally occurring immune response towards MSI-induced FSPs in patients with IBD. However, other findings were surprising. Duration or cumulative amount of azathioprine treatment did not influence the FSP-specific T-cell reactivity. Most unexpected, an elevated immune response towards FSPs was also positively correlated with simple duration of disease, regardless of immunosuppressive treatment. In patients who never received azathioprine, duration of disease was the only predictor for an elevated FSP-response. Exemplarily, we could further confirm these data by repetitive analysis in four cases. As can be depicted from Figure 3, the overall FSP recognition seems to increase with the duration of the disease also on an individual patient level. This implies that a functional link between chronic inflammation and FSP-recognition-as well as presence of some level of MSI - is very likely to exist.
Previous work has demonstrated that oxidative stress not only increases cellular mutation rates but additionally “relaxes“ the MMR system[29,30] Low-frequency MSI is seen in some chronically inflamed but non-neoplastic tissues and additionally in cancer tissues associated with chronic inflammation in the absence of genetic inactivation of the MMR system[30-33]. Boland and Goel explained the phenomenon of MSI in patients with IBD as a biological paradox caused by oxidative stress: at the time of greatest mutational load there is an underactive MMR system at once[34].
More functional evidence may be elucidated from the fact that hMLH1 is located in a susceptibility region for IBD and it has been implicated in the pathogenesis of IBD[35,36]. A germ line alteration of MLH1 (MLH1 I219V) has previously been correlated with a refractory course of UC[37] and it was speculated that “novel immunogenic peptides caused by frameshift mutation events”, i.e., FSPs are the most obvious candidate antigens to promote such a therapy refractory inflammation[38].
Our results on T cell reactivity against MSI-induced FSPs from 75 patients with IBD support the presence of MSI in chronic inflammation. It is the first study that shows an increased immune response towards MSI-induced FSPs in patients with chronic inflammation in general and with IBD in particular. Moreover, it is the first report which provides functional evidence of FSP expression under non- but possible pre-neoplastic conditions. However, our findings have to be verified by other research groups and by direct approaches to validate FSP expression in IBD. The presence of T cells reacting against FSPs is of course only an indirect proof of the antigens’ presence.
To sum up and as an explanation of our findings, we suggest the following functional model: On the one hand, oxidative stress resulting from chronic inflammation is likely to relax the MMR system and to cause DNA damage[29,30,34]. On the other hand, thiopurine (and possibly also other immunosuppressant) treatment positively selects for cell variants with defective MMR system[3]. Cells with such defects will ultimately develop MSI and express frameshift-mutated proteins, which in turn are likely to induce a specific recognition of FSPs by the immune system, since this has been shown for Lynch syndrome mutation carriers[4]
We would like to use the opportunity and hint on the following concerns and questions with possibly strong clinical impact which are raised by our findings: (1) recognition of FSPs is in the light of the above outlined arguments very likely to aggravate and even become a driving force of chronic inflammation. The foreign character of the FSPs may explain why these chronic immune reactions are so strong. However, under conditions of chronic inflammation, FSP-specific immune reactions must be considered as “bad” to the patient’s body; (2) contrary to that, data on MSI and especially on immunological recognition of MSI-induced FSPs clearly show a major prognostic benefit for patients suffering from sporadic MSI+ as well as Lynch-associated cancers[4,39,40]. Thus, under neoplastic conditions, FSP-recognition is obviously “good”; (3) in subsequent studies, we suggest examining the immune response against MSI-induced FSPs in patients suffering from other forms of chronic inflammatory diseases, since FSP expression may well be a more general phenomenon in inflammation; and (4) for potential diagnostic procedures one must consider that FSP-recognition by T cells in the peripheral blood is not necessarily a hint towards the presence of MSI+ malignancies or towards Lynch syndrome but may also hint towards a chronic inflammatory process.
The authors want to thank for the excellent technical assistance of Mathias Krohn.
Long-term immunosuppression is a recognized risk factor for development of neoplasia. Azathioprine treatment positively selects for cell variants with defects in the DNA-mismatch repair (MMR) pathway. Again, MMR-deficiency results in an increased mutation rate typically observed as microsatellite-instability (MSI), i.e., length variations in repetitive DNA sequences. When affecting coding regions, this phenomenon inevitably generates frameshift-peptides (FSPs), which have been repeatedly described as highly immunogenic MSI+ tumor-specific antigens and have also been suggested as drivers for therapy refractory inflammatory bowel disease (IBD).
Despite decades of research, it is still under debate if and when which correlation exists between MSI and IBD. Only few of the actual antigens driving Ulcerative colitis or Crohn`s disease are molecularly defined. FSP-specific T cell reactions are described as specific for MSI+ cancers or Lynch syndrome mutation carriers.
The present work is the first demonstrating an increased immune response towards MSI-induced FSPs in patients with IBD and, more generally speaking, with chronic inflammation. These data imply that a functional link between IBD and MSI likely exists. In addition, these data suggest FSPs as an important group of antigens driving IBD.
FSP-recognition by peripheral blood T cells has been suggested as potential diagnostic tool in the context of MSI+ malignancies and Lynch syndrome. This study widens this towards chronic inflammatory processes. Since FSP-specific T cell responses became stronger with duration of IBD, they may be helpful as diagnostic tool in this context, too.
MSI: defined as variations in length of short, repetitive DNA sequences - typically as a result of cellular MMR deficiency. FSP: carboxyterminal part of a protein differing from the wildtype sequence and resulting from a frameshift mutation.
This is an interesting article that may be helpful for investigation of the mechanism of IBD, which may extend the technology into a potential clinical application in IBD.
P- Reviewer: Zhu ZH S- Editor: Qi Y L- Editor: A E- Editor: Ma S
| 1. | Opelz G, Henderson R. Incidence of non-Hodgkin lymphoma in kidney and heart transplant recipients. Lancet. 1993;342:1514-1516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 488] [Cited by in RCA: 455] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 2. | Gale RP, Opelz G. Commentary: does immune suppression increase risk of developing acute myeloid leukemia? Leukemia. 2012;26:422-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Offman J, Opelz G, Doehler B, Cummins D, Halil O, Banner NR, Burke MM, Sullivan D, Macpherson P, Karran P. Defective DNA mismatch repair in acute myeloid leukemia/myelodysplastic syndrome after organ transplantation. Blood. 2004;104:822-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 119] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 4. | Schwitalle Y, Kloor M, Eiermann S, Linnebacher M, Kienle P, Knaebel HP, Tariverdian M, Benner A, von Knebel Doeberitz M. Immune response against frameshift-induced neopeptides in HNPCC patients and healthy HNPCC mutation carriers. Gastroenterology. 2008;134:988-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 301] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 5. | Maletzki C, Schmidt F, Dirks WG, Schmitt M, Linnebacher M. Frameshift-derived neoantigens constitute immunotherapeutic targets for patients with microsatellite-instable haematological malignancies: frameshift peptides for treating MSI+ blood cancers. Eur J Cancer. 2013;49:2587-2595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Linnebacher M, Gebert J, Rudy W, Woerner S, Yuan YP, Bork P, von Knebel Doeberitz M. Frameshift peptide-derived T-cell epitopes: a source of novel tumor-specific antigens. Int J Cancer. 2001;93:6-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 179] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 7. | Saeterdal I, Bjørheim J, Lislerud K, Gjertsen MK, Bukholm IK, Olsen OC, Nesland JM, Eriksen JA, Møller M, Lindblom A. Frameshift-mutation-derived peptides as tumor-specific antigens in inherited and spontaneous colorectal cancer. Proc Natl Acad Sci USA. 2001;98:13255-13260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 206] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 8. | Prefontaine E, Macdonald JK, Sutherland LR. Azathioprine or 6-mercaptopurine for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2010;CD000545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 9. | Woerner SM, Gebert J, Yuan YP, Sutter C, Ridder R, Bork P, von Knebel Doeberitz M. Systematic identification of genes with coding microsatellites mutated in DNA mismatch repair-deficient cancer cells. Int J Cancer. 2001;93:12-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 75] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Woerner SM, Kloor M, Mueller A, Rueschoff J, Friedrichs N, Buettner R, Buzello M, Kienle P, Knaebel HP, Kunstmann E. Microsatellite instability of selective target genes in HNPCC-associated colon adenomas. Oncogene. 2005;24:2525-2535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 64] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Ripberger E, Linnebacher M, Schwitalle Y, Gebert J, von Knebel Doeberitz M. Identification of an HLA-A0201-restricted CTL epitope generated by a tumor-specific frameshift mutation in a coding microsatellite of the OGT gene. J Clin Immunol. 2003;23:415-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Schwitalle Y, Linnebacher M, Ripberger E, Gebert J, von Knebel Doeberitz M. Immunogenic peptides generated by frameshift mutations in DNA mismatch repair-deficient cancer cells. Cancer Immun. 2004;4:14. [PubMed] |
| 13. | Linnebacher M, Wienck A, Boeck I, Klar E. Identification of an MSI-H tumor-specific cytotoxic T cell epitope generated by the (-1) frame of U79260(FTO). J Biomed Biotechnol. 2010;2010:841451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Garbe Y, Maletzki C, Linnebacher M. An MSI tumor specific frameshift mutation in a coding microsatellite of MSH3 encodes for HLA-A0201-restricted CD8+ cytotoxic T cell epitopes. PLoS One. 2011;6:e26517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Herrinton LJ, Liu L, Weng X, Lewis JD, Hutfless S, Allison JE. Role of thiopurine and anti-TNF therapy in lymphoma in inflammatory bowel disease. Am J Gastroenterol. 2011;106:2146-2153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 151] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 16. | Peyrin-Biroulet L, Khosrotehrani K, Carrat F, Bouvier AM, Chevaux JB, Simon T, Carbonnel F, Colombel JF, Dupas JL, Godeberge P. Increased risk for nonmelanoma skin cancers in patients who receive thiopurines for inflammatory bowel disease. Gastroenterology. 2011;141:1621-28.e1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 349] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 17. | Singh H, Nugent Z, Demers AA, Bernstein CN. Increased risk of nonmelanoma skin cancers among individuals with inflammatory bowel disease. Gastroenterology. 2011;141:1612-1620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 121] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 18. | Long MD, Martin CF, Pipkin CA, Herfarth HH, Sandler RS, Kappelman MD. Risk of melanoma and nonmelanoma skin cancer among patients with inflammatory bowel disease. Gastroenterology. 2012;143:390-399.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 387] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 19. | Setshedi M, Epstein D, Winter TA, Myer L, Watermeyer G, Hift R. Use of thiopurines in the treatment of inflammatory bowel disease is associated with an increased risk of non-melanoma skin cancer in an at-risk population: a cohort study. J Gastroenterol Hepatol. 2012;27:385-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 20. | McLeod HL, Krynetski EY, Relling MV, Evans WE. Genetic polymorphism of thiopurine methyltransferase and its clinical relevance for childhood acute lymphoblastic leukemia. Leukemia. 2000;14:567-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 289] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 21. | Bernstein CN, Blanchard JF, Kliewer E, Wajda A. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer. 2001;91:854-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 22. | Ekbom A, Helmick C, Zack M, Adami HO. Increased risk of large-bowel cancer in Crohn’s disease with colonic involvement. Lancet. 1990;336:357-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 448] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 23. | Ishitsuka T, Kashiwagi H, Konishi F. Microsatellite instability in inflamed and neoplastic epithelium in ulcerative colitis. J Clin Pathol. 2001;54:526-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Brentnall TA, Crispin DA, Bronner MP, Cherian SP, Hueffed M, Rabinovitch PS, Rubin CE, Haggitt RC, Boland CR. Microsatellite instability in nonneoplastic mucosa from patients with chronic ulcerative colitis. Cancer Res. 1996;56:1237-1240. [PubMed] |
| 25. | Tahara T, Inoue N, Hisamatsu T, Kashiwagi K, Takaishi H, Kanai T, Watanabe M, Ishii H, Hibi T. Clinical significance of microsatellite instability in the inflamed mucosa for the prediction of colonic neoplasms in patients with ulcerative colitis. J Gastroenterol Hepatol. 2005;20:710-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Shivakumar BM, Kumar BL, Bhat G, Suvarna D, Rao L, Pai CG, Satyamoorthy K. Molecular alterations in colitis-associated colorectal neoplasia: study from a low prevalence area using magnifying chromo colonoscopy. J Crohns Colitis. 2012;6:647-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Løvig T, Andersen SN, Clausen OP, Rognum TO. Microsatellite instability in long-standing ulcerative colitis. Scand J Gastroenterol. 2007;42:586-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | van Dieren JM, Wink JC, Vissers KJ, van Marion R, Hoogmans MM, Dinjens WN, Schouten WR, Tanke HJ, Szuhai K, Kuipers EJ. Chromosomal and microsatellite instability of adenocarcinomas and dysplastic lesions (DALM) in ulcerative colitis. Diagn Mol Pathol. 2006;15:216-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Gasche C, Chang CL, Rhees J, Goel A, Boland CR. Oxidative stress increases frameshift mutations in human colorectal cancer cells. Cancer Res. 2001;61:7444-7448. [PubMed] |
| 30. | Lee SH, Chang DK, Goel A, Boland CR, Bugbee W, Boyle DL, Firestein GS. Microsatellite instability and suppressed DNA repair enzyme expression in rheumatoid arthritis. J Immunol. 2003;170:2214-2220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 31. | Parsons R, Li GM, Longley M, Modrich P, Liu B, Berk T, Hamilton SR, Kinzler KW, Vogelstein B. Mismatch repair deficiency in phenotypically normal human cells. Science. 1995;268:738-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 209] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 32. | Chang CL, Marra G, Chauhan DP, Ha HT, Chang DK, Ricciardiello L, Randolph A, Carethers JM, Boland CR. Oxidative stress inactivates the human DNA mismatch repair system. Am J Physiol Cell Physiol. 2002;283:C148-C154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 190] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 33. | Brentnall TA, Chen R, Lee JG, Kimmey MB, Bronner MP, Haggitt RC, Kowdley KV, Hecker LM, Byrd DR. Microsatellite instability and K-ras mutations associated with pancreatic adenocarcinoma and pancreatitis. Cancer Res. 1995;55:4264-4267. [PubMed] |
| 34. | Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology. 2010;138:2073-2087.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1677] [Cited by in RCA: 1539] [Article Influence: 102.6] [Reference Citation Analysis (0)] |
| 35. | Annese V, Piepoli A, Andriulli A, Latiano A, Napolitano G, Li HH, Forabosco P, Devoto M. Association of Crohn’s disease and ulcerative colitis with haplotypes of the MLH1 gene in Italian inflammatory bowel disease patients. J Med Genet. 2002;39:332-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 36. | Pokorny RM, Hofmeister A, Galandiuk S, Dietz AB, Cohen ND, Neibergs HL. Crohn’s disease and ulcerative colitis are associated with the DNA repair gene MLH1. Ann Surg. 1997;225:718-723; discussion 723-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 37. | Bagnoli S, Putignano AL, Melean G, Baglioni S, Sestini R, Milla M, d’Albasio G, Genuardi M, Pacini F, Trallori G. Susceptibility to refractory ulcerative colitis is associated with polymorphism in the hMLH1 mismatch repair gene. Inflamm Bowel Dis. 2004;10:705-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 38. | Plotz G, Raedle J, Spina A, Welsch C, Stallmach A, Zeuzem S, Schmidt C. Evaluation of the MLH1 I219V alteration in DNA mismatch repair activity and ulcerative colitis. Inflamm Bowel Dis. 2008;14:605-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 39. | Prall F, Dührkop T, Weirich V, Ostwald C, Lenz P, Nizze H, Barten M. Prognostic role of CD8+ tumor-infiltrating lymphocytes in stage III colorectal cancer with and without microsatellite instability. Hum Pathol. 2004;35:808-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 186] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 40. | de la Chapelle A, Hampel H. Clinical relevance of microsatellite instability in colorectal cancer. J Clin Oncol. 2010;28:3380-3387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 243] [Article Influence: 16.2] [Reference Citation Analysis (0)] |