Published online Feb 28, 2014. doi: 10.3748/wjg.v20.i8.2005
Revised: December 23, 2013
Accepted: January 8, 2014
Published online: February 28, 2014
Processing time: 182 Days and 15.7 Hours
Recurrence of colorectal cancer (CRC) often presents as solitary metastases, oligometastases or oligo-recurrence. Surgical resection became the preferred treatment for patients with CRC lung and hepatic metastases. However, surgical treatment for oligo-recurrence within nodal area is not a widely accepted treatment due to due to their relative rarity and high postoperative morbidity. Stereotactic body radiotherapy (SBRT) is one of the emerging radiation treatment techniques in which a high radiation dose can be delivered to the tumor. High-dose SBRT can ablate the tumor with an efficacy similar to that achieved with surgery, especially for small tumors. However, there have been very few studies on SBRT for oligo-recurrence within nodal area, although several studies have evaluated the role of SBRT in the treatment of liver and lung metastases from CRC. This article reviews the current clinical status of and treatment methods for oligo-recurrence within nodal area from CRC, with particular emphasis on SBRT.
Core tip: Surgical treatment for oligo-recurrence of colorectal cancer (CRC) within nodal area is not a widely accepted treatment due to due to their relative rarity and high postoperative morbidity. High-dose stereotactic body radiotherapy (SBRT) can ablate the tumor with an efficacy similar to that achieved with surgery, especially for small tumors. Recently, several investigators successfully treated oligo-recurrence of CRC within nodal area with SBRT. This article reviews the current clinical status of and treatment methods for oligo-recurrence within nodal area from CRC, with particular emphasis on SBRT.
- Citation: Seo YS, Kim MS, Yoo HJ, Jang WI. Stereotactic body radiotherapy for oligo-recurrence within the nodal area from colorectal cancer. World J Gastroenterol 2014; 20(8): 2005-2013
- URL: https://www.wjgnet.com/1007-9327/full/v20/i8/2005.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i8.2005
Colorectal cancer (CRC) remains a major health problem worldwide and is the third most common cause of cancer-related death globally[1]. It is more common in developed than in developing countries. However, in Asia, the incidence of CRC is rising rapidly, and it is now the third most common malignant disease in both men and women[2-4]. Although surgery, chemotherapy, and radiotherapy (RT) for CRC have all developed rapidly in recent decades, approximately 20%-50% of CRC patients still develop recurrence after definitive treatment[5-7]. This recurrence often presents as solitary metastases or oligometastases, and indeed a study by Tepper et al[6] found that approximately 70% of CRC recurrences were solitary.
The term oligometastases, introduced in 1995[8] and expanded upon more recently[9], describes an intermediate state of cancer spread between localized disease and widespread metastases. The implication of this intermediate state is that metastatic disease might be cured using metastasis-directed therapy. As a further conceptual refinement, Niibe et al[10] suggested the concept of oligo-recurrence as a disease stage in which there are a limited number of metastases and in which the primary tumor has been controlled. Patients with oligo-recurrence have an improved prognosis compared to those with limited metastasis but uncontrolled primary tumors.
Evidence from a number of clinical studies has suggested that surgical resection of lung and hepatic metastases from CRC prolongs survival[11-15]. As a result, surgical resection, became the preferred treatment for patients with CRC lung and hepatic metastases. However, surgical treatment for oligo-recurrence within the nodal area is not a widely accepted treatment, even when lesions are localized, due to their relative rarity, high postoperative morbidity, and unsatisfied surgical margin etc. If patients with oligo-recurrence do not receive treatment, their median survival is typically only 6-15 mo and the disease is frequently accompanied by refractory pain[16-19].
Stereotactic body radiotherapy (SBRT) is one of the emerging radiation treatment techniques in which a high radiation dose can be delivered to the tumor. It allows for high precision with tight planning margins and a sophisticated treatment plan allowing rapid dose fall-off away from the treatment area. Therefore, this technique provides higher tumor dose description with smaller irradiated volumes of normal tissue. And high-dose SBRT in a single or small number of fractions can ablate the tumor with an efficacy similar to that achieved with surgery, especially for small tumors[16,20-24]. However, SBRT can correspondingly cause more damage to normal tissue if it is included in the radiation field because repair mechanism is not expected in high ablative radiation dose. Therefore, it is important to select the optimal indication for SBRT, and one of these may be nodal metastases as they usually have clearly demarcated margins and allow very little movement. However, there have been very few studies on SBRT for oligo-recurrence within the nodal area, although several studies have evaluated the role of SBRT in the treatment of liver and lung metastases from CRC. This article reviews the current clinical status of and treatment methods for oligo-recurrence within the nodal area from CRC, with particular emphasis on SBRT.
Approximately 50% of local recurrences are restricted to the pelvis or associated with operable oligo-recurrence and are thus potentially amenable to curative re-operation[25-27]. Nevertheless, radical surgery is challenging, not commonly performed, and historically associated with high morbidity and mortality. The most important prognostic factor is whether R0 resection can be achieved. Previous studies have reported 5-year overall survival rate for R0 surgical resection ranging from 19% to 53%, whilst the rate is only between 0% and 32% when complete resection cannot be achieved[28-37]. However, in most cases, recurrence is detected as a fixed mass that invades the pelvic wall or sacrum. Pelvic sidewall recurrence in particular is associated with the worst prognosis and the least likelihood of achieving an R0 resection[38]. The disease often involves key structures such as the ureters, iliac vessels, the sciatic nerve, or the bony pelvis itself, and extensive involvement of the sidewall is a relative contraindication for the surgical treatment of recurrent rectal cancer.
Isolated paraaortic lymph node (PALN) recurrences are rarely encountered from CRC, and consequently its treatment is not well established. Recently, Min et al[39] categorized PALN recurrence as a retroperitoneal malignancy, which in turn is a type of locoregional recurrence. Furthermore, several studies[16,40,41] have investigated the therapeutic efficacies of surgery for retroperitoneal, intraabdominal, and PALN recurrences, and several reported outstanding survival rates, which appear to have resulted from the selection of patients with a resectable mass at time of recurrence. In these studies, the reported 5-year survival rates approached a maximum of 56% after complete resection, whereas they ranged from 0% to 7% after incomplete resection. Because radical surgery is rarely feasible for PALN recurrence, they have usually been treated using chemotherapy.
SBRT may differ biologically from conventional RT, which is administered in small doses of 1.8-2 Gy per fraction over 6-8 wk. In addition to the direct cell killing within the high-dose region, vascular and stromal effects also likely contribute to tumor control[42]. Experimental models have demonstrated the importance of sphingomyelinase-mediated endothelial apoptosis of tumor cells when high-dose RT is used[43,44]. Another host factor of potential importance after a high single dose (or a few doses) of RT is the activation of the innate and adaptive immune responses against the tumor[45-47]. Lee et al[47] reported that a single ablative dose of radiation (20 Gy) to the tumor dramatically increased T-cell priming in the draining lymphatic tissues. This CD8(+) T-cell response was essential for the antitumor effects of irradiation and resulted in a reduction in primary tumor size and an abscopal effect[48,49] on distant metastases. The clearance of nonirradiated tumors after localized radiation therapy is known as the abscopal effect. Activation of an antitumor immune response has been proposed as a mechanism for the abscopal effect. The abscopal effect has been reported in several malignancies[50-52]. Stamell et al[52] reported a patient with metastatic melanoma who received palliative radiation to his primary tumor with subsequent clearance of all his nonirradiated in-transit metastases. Anti-MAGEA3 antibodies were found upon serological testing, demonstrating an association between the abscopal effect and a systemic antitumor immune response. While these antitumor effects hardly observed with conventional fractionated RT or with chemotherapy[53,54]. On the basis of these findings, the authors suggested that a new therapeutic strategy may be developed that combines RT with immunotherapy for oligometastasis.
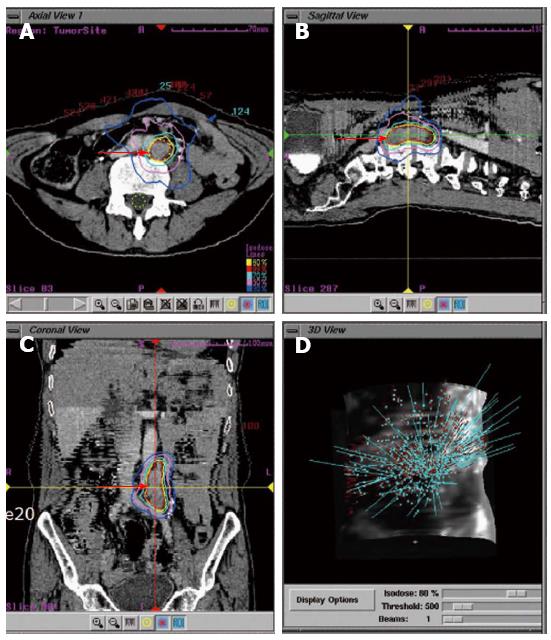
Previously, the delivery of truly ablative doses of radiation has been limited by the risk to normal tissue, and the need for extended fractionation. However, SBRT utilizes stereotactic principles for dose localization and delivers multiple beams to well defined targets in a few fractions. As a result, this technique can deliver higher doses to tumors due to reduced mechanical error margins, and thus cause less normal tissue damage[55] (Figure 1). Regardless of the SBRT treatment delivery unit used, image-guided therapy enables verification of the location of the tumor or target volume before treatment delivery[56]. This image-guided therapy can be performed using three-dimensional volume imaging, using for example cone beam computed tomography (CT). If two-dimensional imaging is used, invasive fiduciary markers positioned in or close to the tumor are required. These image-guidance procedures substantially reduce treatment setup errors, using the tumor itself as a fiducial (frameless SBRT), and will in turn enable the planning target volume to be reduced.
The use of appropriate selection criteria for SBRT in the radical treatment of oligo-recurrence within nodal area remains crucial. In general, indications for SBRT are the same as those for metastasectomy, but without the limits imposed by the need for patients to be fit for surgery. In several reports, the eligibility criteria for SBRT for oligometastatic cancer were described as a limited number of metastases (between 1 and 5), a tumor diameter less than 4 cm, a locally controlled primary tumor, and no additional metastatic sites[57]. Other more specific and recently proposed criteria for the use of SBRT to treat patients with various oligometastatic tumors include a controlled primary tumor, a favorable histology, limited metastatic disease, a metachronous appearance of metastases, young age, and a good performance status[58-60].
As isolated or oligo-recurrence within nodal area is a very rare in CRC cases, clinical trials of SBRT for these recurrences are correspondingly also rare. Kim et al[55] published the results of a study in which SBRT was used to treat isolated PALN recurrence from CRC. The patients criteria for this study included a single conglomerate recurrent node or 2-3 recurrent nodes within 1 cm of each other; and excluded a tumor attached to the stomach or intestine (as determined by CT), or more than 3 separate affected LNs affected. This criteria is focusing to preserve normal tissue surrounding lymph node metastasis.
A further important consideration is the identification of patients with truly oligo-recurrence. Most published surgical oligo-recurrence series describe patients managed in an era before modern imaging techniques such as Positron emission tomography (PET)/computerized tomography (CT) became widely available. Thus, many patients were probably understaged, potentially leading to an underestimation of the effect of aggressive management on truly oligo-recurrence, since some of those patients treated aggressively would have had more extensive disease than was visible on CT or magnetic resonance imaging. Improved imaging will enable better selection of patients. Indeed, these advanced imaging methods (PET/CT scan) and molecular diagnostic techniques were used in some of the most recent studies[14] and are likely to have contributed to better patient selection and improved 5-year survival in this study compared with previous trials[15,61,62].
There is only a little published data on the treatment outcome of using SBRT for CRC oligo-recurrence within nodal area. An overview of published case series and phase 2 trials are presented in Table 1. However, several studies included cohorts that were too heterogeneous to evaluate the effect of SBRTs on these lesions. Greco et al[63] and Milano et al[64,65] studied heterogeneous in terms of the treated site or primary tumor histology. Hoyer et al[66] and Kim et al[55] studied including only a very small number of cases of nodal metastases although all enrolled patients had oligometastases from CRC.
| Ref. | Study year | No. of patients | Proportion of oligo-nodal metastases | SBRTdose (Gy)1; range (median) | Outcomes | ||
| LC | OS | Severe GI toxicity | |||||
| Bae et al[69] | 2012 | 41 | 44% | 45-60 (48) | 57% (5 yr) | 38% (5 yr) | 7% |
| Kang et al[68] | 2010 | 59 | 53% | 36-51 (42) | 24% (5 yr) | 29% (5 yr) | 3% |
| Kim et al[55] | 2009 | 7 | 100% | 36-51 (48) | (-) | 71% (3 yr) | 14% |
| Kim et al[75] | 2008 | 23 | 100% | 30-51 (39) | 74% (4 yr) | 25% (4 yr) | 4% |
| Hoyer et al[66] | 2006 | 64 | 5% | 45 (45) | 63% (2 yr) | 38% (2 yr) | 5% |
In review of SBRT for oligometastases in all primary and all treated sites, Tree et al[67] indicated that generally around 20% of patients remain disease-free 2-4 years after treatment. Kang et al[68] reported the results of a study including 59 CRC patients with LN (31), lung (13), liver (10), and other (5) metastases, which were confined to 1 organ and treated by SBRT (median 42 Gy in 3 fractions). The 3-year overall survival, disease progression free survival, and local control rates were 49%, 25% and 66%, respectively, and the 5-year overall survival, disease progression free survival, and local control rates were to 29% and 19% and 24%, respectively. Focusing to the 31 patients with oligo-recurrence within nodal area, progression-free survival was 25% at 3 years and 19% at 5 years. In further study using high dose SBRT (median 48 Gy in 3 fractions) in same institute, Bae et al[69] reported better survival in the cohort of 41 CRC patients with LN (18), lung (12), and liver (11) metastases confined to a single organ. The 5-year overall survival, disease progression free survival, and local control rates were to 38%, 40% and 57%, respectively. The difference of outcomes between these studies may come from different dose of SBRT. These will be discussed further in the section of “SBRT dose”.
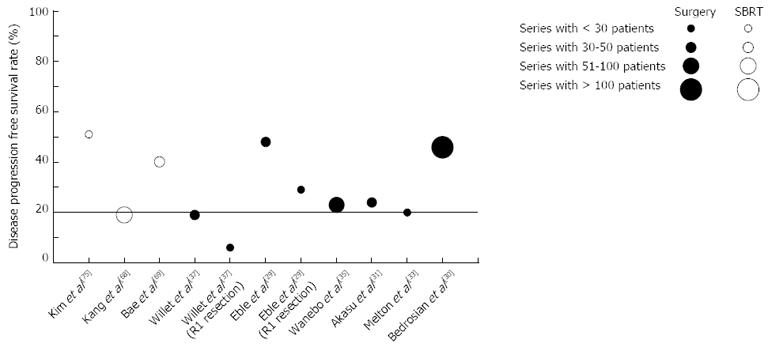
Despite the heterogeneous nature of these studies with respect to the methods used to categorize oligometastatic disease from CRC, the findings indicate that a substantial proportion of patients, generally over 20%, remain disease-free 4-5 years after SBRT (Figure 2). These findings support the idea of an oligometastatic state in which aggressive local therapy could improve cause-specific survival.
The efficacy of SBRT had primarily been investigated in the context of the treatment of early stage non-small cell lung cancer (NSCLC), in which disease a dose-control relationship has been established. Onishi et al[70] reported that the local control and survival rates for patients with stage I NSCLC were significantly better using a biologically effective dose larger than 100 Gy (α/β = 10 Gy). On the basis of this result, dose escalation was performed in a number of primary and metastatic cancer patients, and there were also efforts to escalate the SBRT dose to abdominal LN metastases from CRC. In the study conducted by Kim et al[55], the SBRT dose was escalated in a stepwise manner by 3 Gy from 36 Gy in 3 fractions. During escalation of dose, however, the 2 severe complication resulted in when 48 or 51 Gy was delivered in 3 fractions. They therefore did not escalate the radiation dose over 51 Gy during the treatment of paraaortic LN or pelvic LN. They also found that the radiation dose to tumor was a significant prognostic factor of overall survival. The median survival time was 32 and 72 mo with a SBRT dose of ≤ 42 Gy and > 42 Gy in 3 fractions, respectively. Bae et al[69] also found that SBRT dose was a significant prognostic factor for local control in multivariate analysis and that a dose of ≥ 48 Gy in 3 fractions resulted in a 5-year local control rate of 69%.
In several studies to evaluate SBRT result for oligometastases from heterogeneous primary cancers[71-75], all reports did not suggest that the SBRT dose was a prognostic factor of survival or local control. The SBRT dose ranged from 30 to 51 Gy delivered in 3-6 fractions, and the highest dose was 51 Gy in 3 fractions[76]. Lower doses, such as those used successfully in the study by Bignardi et al[72], might be sufficient to eradicate viable tumor cells. Interestingly, Herfarth et al[77] performed a separate analysis of patients with metastatic disease and found that CRC metastasis had worse local control than metastases from other histological tumor types (45% vs 95%, respectively). In particular, in patients who had previously undergone systemic chemotherapy, tumors may have been radioresistant. Our data[55] support the radiocurative dose for metastases from CRC may be higher than those from other primary tumors as a result of induced cross-resistance from prolonged chemotherapy (discussed above)[77-79]. One hypothesis to explain these phenomenon may be Epidermal growth factor receptor (EGFR), which is reported to be overexpressed in approximately 70%-75% of CRCs[80]. A recent study using CRC-derived cell lines showed that cells with high constitutive EGFR-positive cells within a colorectal adenocarcinoma may have an intrinsic susceptibility to chemotherapy like oxaliplatin and 5-fluorouracil[81] as well as anti-EGFR agents. While, Khalifa et al[78] reported that recurrences following postoperative chemotherapy were approximately 5 times more likely to have lower levels of EGFR expression. In similar pattern, several studies have shown that an absence of EGFR expression is associated with radioresistance[82,83]. Furthermore, in a study of CRC treated with preoperative RT, Zlobec et al[79] reported that a complete pathological response was nearly 6 times more likely in EGFR-positive tumors than in EGFR-negative cases. In this point, the lower EGFR status of recurrent CRC after intensive chemotherapy may induce radio-resistance, requiring higher SBRT dose to achieve local control.
Results from a study of patients with oligo-recurrence within abdominopelvic nodal area suggested that a SBRT dose of more than 42 Gy in 3 fractions is a favorable prognostic factor for overall survival and local control, and dose escalation was recommended. However, there is as yet no consensus on the optimal dose and number of fractions, and further study with larger patient numbers is therefore required[55,69].
When oligo-recurrence within nodal area in the abdominopelvic area is treated with SBRT, the gastrointestinal tract is one of the most important dose-limiting organs. Since Timmerman et al[84] complied unvalidated normal tissue dose constraints for SBRT, most published studies have considered this recommendation or individual empiric data to be the permitted dose constraints for gastrointestinal toxicity. Surely, dosimetric parameters such as maximal point dose (Dmax) and absolute volume of gastrointestine to receive some radiation dose, or fraction number affect complication. Unfortunately, because prospective study to control these variable factors were not available till now, there was no definite conclusion for gastrointestinal tolerance dose. Based on extensive experience to give SBRT to tumor located in abdominopelvic site, using 3 fraction, we suggested the dose constraint for gastroduodenum and intestine[85,86]. For severe gastroduodenal toxicity, Dmax was found to be the best dosimetric predictor. A Dmax of 35 Gy and 38 Gy were respectively associated with a 5% and 10% probability of the development of severe gastroduodenal toxicity. For intestinal toxicity, absolute volume to receive 20 Gy, 25 Gy, 30 Gy, or 35 Gy and Dmax of the intestine were all the valuable predictor of severe toxicity. At Dmax below 37 Gy, no severe intestinal toxicity was not detected. These tolerance dose are higher than expected for SBRT to some extent. Based on limited individualized clinical data, Kavanagh et al[87] and Rusthoven et al[88] suggested Dmax below 30 Gy in 3 fractions for stomach and intestine as the constraint. Timmerman et al[84] suggested Dmax < 27 Gy in 3 fractions for the intestine and < 30 Gy for the colon, which based on the data of the biological effective dose using universal model, not validated by clinical data. One reason to cause discrepancy from these data based on dosimetric uncertainty. Intrafractional and interfractional gastrointestinal movement make it difficult to define accurate radiation dose of gastrointestine. In addition, as the volume of gastrointestine may vary according to the food consumed and respiration, the dose-volume histogram endpoint for pretreatment planning might not accurately reflect the actual dose distribution. In spite of these uncertainties, about Dmax of 30 Gy in 3 fractions in gastroduodenum is supposed to be safe dose constraint
Oligo-recurrence within nodal area from CRC are rarely lethal in themselves. However, aggressive local treatment such as SBRT could prevent further extensive widespread metastatic disease. Several investigators have suggested that higher SBRT doses are associated with a better prognosis with respect to local control and survival. However, there is still no consensus on the optimal dose, number of fractions, or planning constraints. Given the radioresistant nature of CRC oligo-recurrence, increasing the SBRT dose may be a necessity, although because LNs are usually surrounded by radiosensitive normal tissue, the possibility of complications, especially gastrointestinal toxicity, should be carefully considered in treatment planning with SBRT for oligo-recurrence within nodal area in the abdominopelvic area. The constraints for the gastrointestinal tract and colon, a Dmax of 30 Gy could prevent severe gastrointestinal toxicity during SBRT for tumors located in this area.
The outcomes of SBRT for oligo-recurrence within nodal area from CRC appear to be similar to those obtained after surgery despite the fact most studies have only included a small number of patients with a heterogeneous clinical profile. A substantial proportion of patients, generally over 20%, remain disease free 4-5 years after SBRT. This finding supports the idea of an oligo-recurrence state in which aggressive local therapy could improve the cure rate in appropriately selected patients. However, the general aim of oncological interventions for metastatic disease is not cure, but improvement in the quality of life and prolongation of overall survival. To this end, the use of SBRT, which is less invasive, better tolerated, and of a shorter duration than conventional radiation therapy, could have a number of advantages. These include the preservation of the quality of life through delaying further systemic treatment or preventing pain and prolonging survival through reducing subsequent metastatic spread to important organs.
P- Reviewers: Conti A, Hou L, Niibe Y S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN
| 1. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13286] [Cited by in RCA: 13558] [Article Influence: 677.9] [Reference Citation Analysis (1)] |
| 2. | Sung J. Colorectal cancer screening: its time for action in Asia. Cancer Detect Prev. 2007;31:1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Sung JJ, Lau JY, Goh KL, Leung WK. Increasing incidence of colorectal cancer in Asia: implications for screening. Lancet Oncol. 2005;6:871-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 598] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 4. | Pourhoseingholi MA. Increased burden of colorectal cancer in Asia. World J Gastrointest Oncol. 2012;4:68-70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 97] [Cited by in RCA: 106] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 5. | Delpero JR, Pol B, Le Treut P, Bardou VJ, Moutardier V, Hardwigsen J, Granger F, Houvenaeghel G. Surgical resection of locally recurrent colorectal adenocarcinoma. Br J Surg. 1998;85:372-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Tepper JE, O’Connell M, Hollis D, Niedzwiecki D, Cooke E, Mayer RJ. Analysis of surgical salvage after failure of primary therapy in rectal cancer: results from Intergroup Study 0114. J Clin Oncol. 2003;21:3623-3628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Kim MS, Keum KC, Rhee WJ, Kim H, Kim M, Choi S, Nam KC, Koom WS. The location of locoregional recurrence in pathologic T3N0, non-irradiated lower rectal cancer. Radiat Oncol J. 2013;31:97-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. 1995;13:8-10. [PubMed] |
| 9. | Weichselbaum RR, Hellman S. Oligometastases revisited. Nat Rev Clin Oncol. 2011;8:378-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 717] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 10. | Niibe Y, Hayakawa K. Oligometastases and oligo-recurrence: the new era of cancer therapy. Jpn J Clin Oncol. 2010;40:107-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 217] [Cited by in RCA: 273] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 11. | Kanemitsu Y, Kato T, Hirai T, Yasui K. Preoperative probability model for predicting overall survival after resection of pulmonary metastases from colorectal cancer. Br J Surg. 2004;91:112-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 116] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Higashiyama M, Kodama K, Higaki N, Takami K, Murata K, Kameyama M, Yokouchi H. Surgery for pulmonary metastases from colorectal cancer: the importance of prethoracotomy serum carcinoembryonic antigen as an indicator of prognosis. Jpn J Thorac Cardiovasc Surg. 2003;51:289-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Pfannschmidt J, Dienemann H, Hoffmann H. Surgical resection of pulmonary metastases from colorectal cancer: a systematic review of published series. Ann Thorac Surg. 2007;84:324-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 411] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 14. | Pawlik TM, Scoggins CR, Zorzi D, Abdalla EK, Andres A, Eng C, Curley SA, Loyer EM, Muratore A, Mentha G. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241:715-22, discussion 722-4. [PubMed] |
| 15. | Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309-18; discussion 318-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2693] [Cited by in RCA: 2800] [Article Influence: 107.7] [Reference Citation Analysis (1)] |
| 16. | Shibata D, Paty PB, Guillem JG, Wong WD, Cohen AM. Surgical management of isolated retroperitoneal recurrences of colorectal carcinoma. Dis Colon Rectum. 2002;45:795-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Wanebo HJ, Koness RJ, Vezeridis MP, Cohen SI, Wrobleski DE. Pelvic resection of recurrent rectal cancer. Ann Surg. 1994;220:586-95; discussion 595-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 153] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 18. | Gunderson LL, Sosin H. Areas of failure found at reoperation (second or symptomatic look) following “curative surgery” for adenocarcinoma of the rectum. Clinicopathologic correlation and implications for adjuvant therapy. Cancer. 1974;34:1278-1292. [PubMed] |
| 19. | Bakx R, Visser O, Josso J, Meijer S, Slors JF, van Lanschot JJ. Management of recurrent rectal cancer: a population based study in greater Amsterdam. World J Gastroenterol. 2008;14:6018-6023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 58] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Palma D, Visser O, Lagerwaard FJ, Belderbos J, Slotman B, Senan S. Treatment of stage I NSCLC in elderly patients: a population-based matched-pair comparison of stereotactic radiotherapy versus surgery. Radiother Oncol. 2011;101:240-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 130] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 21. | Timmerman R, Paulus R, Galvin J, Michalski J, Straube W, Bradley J, Fakiris A, Bezjak A, Videtic G, Johnstone D. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303:1070-1076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2018] [Cited by in RCA: 1959] [Article Influence: 130.6] [Reference Citation Analysis (0)] |
| 22. | Louie AV, Rodrigues G, Hannouf M, Lagerwaard F, Palma D, Zaric GS, Haasbeek C, Senan S. Withholding stereotactic radiotherapy in elderly patients with stage I non-small cell lung cancer and co-existing COPD is not justified: outcomes of a Markov model analysis. Radiother Oncol. 2011;99:161-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | Mohammed N, Grills IS, Wong CY, Galerani AP, Chao K, Welsh R, Chmielewski G, Yan D, Kestin LL. Radiographic and metabolic response rates following image-guided stereotactic radiotherapy for lung tumors. Radiother Oncol. 2011;99:18-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Andratschke N, Zimmermann F, Boehm E, Schill S, Schoenknecht C, Thamm R, Molls M, Nieder C, Geinitz H. Stereotactic radiotherapy of histologically proven inoperable stage I non-small cell lung cancer: patterns of failure. Radiother Oncol. 2011;101:245-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 25. | McDermott FT, Hughes ES, Pihl E, Johnson WR, Price AB. Local recurrence after potentially curative resection for rectal cancer in a series of 1008 patients. Br J Surg. 1985;72:34-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 240] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 26. | Pilipshen SJ, Heilweil M, Quan SH, Sternberg SS, Enker WE. Patterns of pelvic recurrence following definitive resections of rectal cancer. Cancer. 1984;53:1354-1362. [PubMed] |
| 27. | Rao AR, Kagan AR, Chan PM, Gilbert HA, Nussbaum H, Hintz BL. Patterns of recurrence following curative resection alone for adenocarcinoma of the rectum and sigmoid colon. Cancer. 1981;48:1492-1495. [PubMed] |
| 28. | Bozzetti F, Bertario L, Rossetti C, Gennari L, Andreola S, Baratti D, Gronchi A. Surgical treatment of locally recurrent rectal carcinoma. Dis Colon Rectum. 1997;40:1421-1424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 50] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Eble MJ, Lehnert T, Treiber M, Latz D, Herfarth C, Wannenmacher M. Moderate dose intraoperative and external beam radiotherapy for locally recurrent rectal carcinoma. Radiother Oncol. 1998;49:169-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Bedrosian I, Giacco G, Pederson L, Rodriguez-Bigas MA, Feig B, Hunt KK, Ellis L, Curley SA, Vauthey JN, Delclos M. Outcome after curative resection for locally recurrent rectal cancer. Dis Colon Rectum. 2006;49:175-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Akasu T, Yamaguchi T, Fujimoto Y, Ishiguro S, Yamamoto S, Fujita S, Moriya Y. Abdominal sacral resection for posterior pelvic recurrence of rectal carcinoma: analyses of prognostic factors and recurrence patterns. Ann Surg Oncol. 2007;14:74-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | Hahnloser D, Nelson H, Gunderson LL, Hassan I, Haddock MG, O’Connell MJ, Cha S, Sargent DJ, Horgan A. Curative potential of multimodality therapy for locally recurrent rectal cancer. Ann Surg. 2003;237:502-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 151] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 33. | Melton GB, Paty PB, Boland PJ, Healey JH, Savatta SG, Casas-Ganem JE, Guillem JG, Weiser MR, Cohen AM, Minsky BD. Sacral resection for recurrent rectal cancer: analysis of morbidity and treatment results. Dis Colon Rectum. 2006;49:1099-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 87] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 34. | Palmer G, Martling A, Cedermark B, Holm T. A population-based study on the management and outcome in patients with locally recurrent rectal cancer. Ann Surg Oncol. 2007;14:447-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 151] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 35. | Wanebo HJ, Antoniuk P, Koness RJ, Levy A, Vezeridis M, Cohen SI, Wrobleski DE. Pelvic resection of recurrent rectal cancer: technical considerations and outcomes. Dis Colon Rectum. 1999;42:1438-1448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 179] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 36. | Wiig JN, Poulsen JP, Larsen S, Braendengen M, Waehre H, Giercksky KE. Total pelvic exenteration with preoperative irradiation for advanced primary and recurrent rectal cancer. Eur J Surg. 2002;168:42-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 37. | Willett CG, Shellito PC, Tepper JE, Eliseo R, Convery K, Wood WC. Intraoperative electron beam radiation therapy for recurrent locally advanced rectal or rectosigmoid carcinoma. Cancer. 1991;67:1504-1508. [PubMed] |
| 38. | Moore HG, Shoup M, Riedel E, Minsky BD, Alektiar KM, Ercolani M, Paty PB, Wong WD, Guillem JG. Colorectal cancer pelvic recurrences: determinants of resectability. Dis Colon Rectum. 2004;47:1599-1606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 129] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 39. | Min BS, Kim NK, Sohn SK, Cho CH, Lee KY, Baik SH. Isolated paraaortic lymph-node recurrence after the curative resection of colorectal carcinoma. J Surg Oncol. 2008;97:136-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 40. | Kelly H, Goldberg RM. Systemic therapy for metastatic colorectal cancer: current options, current evidence. J Clin Oncol. 2005;23:4553-4560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 234] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 41. | Gwin JL, Hoffman JP, Eisenberg BL. Surgical management of nonhepatic intra-abdominal recurrence of carcinoma of the colon. Dis Colon Rectum. 1993;36:540-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 42. | Kirkpatrick JP, Meyer JJ, Marks LB. The linear-quadratic model is inappropriate to model high dose per fraction effects in radiosurgery. Semin Radiat Oncol. 2008;18:240-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 348] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 43. | Garcia-Barros M, Paris F, Cordon-Cardo C, Lyden D, Rafii S, Haimovitz-Friedman A, Fuks Z, Kolesnick R. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300:1155-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1192] [Cited by in RCA: 1168] [Article Influence: 53.1] [Reference Citation Analysis (0)] |
| 44. | García-Barros M, Thin TH, Maj J, Cordon-Cardo C, Haimovitz-Friedman A, Fuks Z, Kolesnick R. Impact of stromal sensitivity on radiation response of tumors implanted in SCID hosts revisited. Cancer Res. 2010;70:8179-8186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 45. | Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2084] [Cited by in RCA: 2419] [Article Influence: 134.4] [Reference Citation Analysis (0)] |
| 46. | Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174:7516-7523. [PubMed] |
| 47. | Lee Y, Auh SL, Wang Y, Burnette B, Wang Y, Meng Y, Beckett M, Sharma R, Chin R, Tu T. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114:589-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 917] [Cited by in RCA: 1060] [Article Influence: 66.3] [Reference Citation Analysis (0)] |
| 48. | Takaya M, Niibe Y, Tsunoda S, Jobo T, Imai M, Kotani S, Unno N, Hayakawa K. Abscopal effect of radiation on toruliform para-aortic lymph node metastases of advanced uterine cervical carcinoma--a case report. Anticancer Res. 2007;27:499-503. [PubMed] |
| 49. | Okuma K, Yamashita H, Niibe Y, Hayakawa K, Nakagawa K. Abscopal effect of radiation on lung metastases of hepatocellular carcinoma: a case report. J Med Case Rep. 2011;5:111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 50. | Kingsley DP. An interesting case of possible abscopal effect in malignant melanoma. Br J Radiol. 1975;48:863-866. [PubMed] |
| 51. | Ohba K, Omagari K, Nakamura T, Ikuno N, Saeki S, Matsuo I, Kinoshita H, Masuda J, Hazama H, Sakamoto I. Abscopal regression of hepatocellular carcinoma after radiotherapy for bone metastasis. Gut. 1998;43:575-577. [PubMed] |
| 52. | Stamell EF, Wolchok JD, Gnjatic S, Lee NY, Brownell I. The abscopal effect associated with a systemic anti-melanoma immune response. Int J Radiat Oncol Biol Phys. 2013;85:293-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 306] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 53. | Demaria S, Ng B, Devitt ML, Babb JS, Kawashima N, Liebes L, Formenti SC. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58:862-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 867] [Cited by in RCA: 1051] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 54. | Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, Demaria S. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15:5379-5388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1020] [Cited by in RCA: 1315] [Article Influence: 82.2] [Reference Citation Analysis (0)] |
| 55. | Kim MS, Cho CK, Yang KM, Lee DH, Moon SM, Shin YJ. Stereotactic body radiotherapy for isolated paraaortic lymph node recurrence from colorectal cancer. World J Gastroenterol. 2009;15:6091-6095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 56. | Alongi F, Arcangeli S, Filippi AR, Ricardi U, Scorsetti M. Review and uses of stereotactic body radiation therapy for oligometastases. Oncologist. 2012;17:1100-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 154] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 57. | Onimaru R, Shirato H, Shimizu S, Kitamura K, Xu B, Fukumoto S, Chang TC, Fujita K, Oita M, Miyasaka K. Tolerance of organs at risk in small-volume, hypofractionated, image-guided radiotherapy for primary and metastatic lung cancers. Int J Radiat Oncol Biol Phys. 2003;56:126-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 193] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 58. | Nagata Y, Wulf J, Lax I, Timmerman R, Zimmermann F, Stojkovski I, Jeremic B. Stereotactic radiotherapy of primary lung cancer and other targets: results of consultant meeting of the International Atomic Energy Agency. Int J Radiat Oncol Biol Phys. 2011;79:660-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 59. | Norihisa Y, Nagata Y, Takayama K, Matsuo Y, Sakamoto T, Sakamoto M, Mizowaki T, Yano S, Hiraoka M. Stereotactic body radiotherapy for oligometastatic lung tumors. Int J Radiat Oncol Biol Phys. 2008;72:398-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 156] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 60. | Rubin P, Brasacchio R, Katz A. Solitary metastases: illusion versus reality. Semin Radiat Oncol. 2006;16:120-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 61. | Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P, Jaeck D. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Française de Chirurgie. Cancer. 1996;77:1254-1262. [PubMed] |
| 62. | Hughes KS, Simon R, Songhorabodi S, Adson MA, Ilstrup DM, Fortner JG, Maclean BJ, Foster JH, Daly JM, Fitzherbert D. Resection of the liver for colorectal carcinoma metastases: a multi-institutional study of patterns of recurrence. Surgery. 1986;100:278-284. [PubMed] |
| 63. | Greco C, Zelefsky MJ, Lovelock M, Fuks Z, Hunt M, Rosenzweig K, Zatcky J, Kim B, Yamada Y. Predictors of local control after single-dose stereotactic image-guided intensity-modulated radiotherapy for extracranial metastases. Int J Radiat Oncol Biol Phys. 2011;79:1151-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 64. | Milano MT, Katz AW, Muhs AG, Philip A, Buchholz DJ, Schell MC, Okunieff P. A prospective pilot study of curative-intent stereotactic body radiation therapy in patients with 5 or fewer oligometastatic lesions. Cancer. 2008;112:650-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 197] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 65. | Milano MT, Katz AW, Schell MC, Philip A, Okunieff P. Descriptive analysis of oligometastatic lesions treated with curative-intent stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72:1516-1522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 109] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 66. | Hoyer M, Roed H, Traberg Hansen A, Ohlhuis L, Petersen J, Nellemann H, Kiil Berthelsen A, Grau C, Aage Engelholm S, Von der Maase H. Phase II study on stereotactic body radiotherapy of colorectal metastases. Acta Oncol. 2006;45:823-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 295] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 67. | Tree AC, Khoo VS, Eeles RA, Ahmed M, Dearnaley DP, Hawkins MA, Huddart RA, Nutting CM, Ostler PJ, van As NJ. Stereotactic body radiotherapy for oligometastases. Lancet Oncol. 2013;14:e28-e37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 379] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 68. | Kang JK, Kim MS, Kim JH, Yoo SY, Cho CK, Yang KM, Yoo HJ, Seo YS, Lee DH, Kang HJ, Kim YH, Shin US. Oligometastases confined one organ from colorectal cancer treated by SBRT. Clin Exp Metastasis. 2010;27:273-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 69. | Bae SH, Kim MS, Cho CK, Kang JK, Kang HJ, Kim YH, Shin US, Moon SM, Lee DH. High dose stereotactic body radiotherapy using three fractions for colorectal oligometastases. J Surg Oncol. 2012;106:138-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 70. | Onishi H, Araki T, Shirato H, Nagata Y, Hiraoka M, Gomi K, Yamashita T, Niibe Y, Karasawa K, Hayakawa K. Stereotactic hypofractionated high-dose irradiation for stage I nonsmall cell lung carcinoma: clinical outcomes in 245 subjects in a Japanese multiinstitutional study. Cancer. 2004;101:1623-1631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 687] [Cited by in RCA: 664] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 71. | Choi CW, Cho CK, Yoo SY, Kim MS, Yang KM, Yoo HJ, Seo YS, Kang JK, Lee DH, Lee KH. Image-guided stereotactic body radiation therapy in patients with isolated para-aortic lymph node metastases from uterine cervical and corpus cancer. Int J Radiat Oncol Biol Phys. 2009;74:147-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 105] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 72. | Bignardi M, Navarria P, Mancosu P, Cozzi L, Fogliata A, Tozzi A, Castiglioni S, Carnaghi C, Tronconi MC, Santoro A. Clinical outcome of hypofractionated stereotactic radiotherapy for abdominal lymph node metastases. Int J Radiat Oncol Biol Phys. 2011;81:831-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 73. | Jereczek-Fossa BA, Fariselli L, Beltramo G, Catalano G, Serafini F, Garibaldi C, Cambria R, Brait L, Possanzini M, Bianchi LC. Linac-based or robotic image-guided stereotactic radiotherapy for isolated lymph node recurrent prostate cancer. Radiother Oncol. 2009;93:14-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 74. | Jereczek-Fossa BA, Piperno G, Ronchi S, Catalano G, Fodor C, Cambria R, Fossati Ing P, Gherardi F, Alterio D, Zerini D. Linac-based Stereotactic Body Radiotherapy for Oligometastatic Patients With Single Abdominal Lymph Node Recurrent Cancer. Am J Clin Oncol. 2012;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 75. | Kim MS, Choi C, Yoo S, Cho C, Seo Y, Ji Y, Lee D, Hwang D, Moon S, Kim MS. Stereotactic body radiation therapy in patients with pelvic recurrence from rectal carcinoma. Jpn J Clin Oncol. 2008;38:695-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 76. | Kim MS, Yoo SY, Cho CK, Yoo HJ, Yang KM, Kang JK, Lee DH, Lee JI, Bang HY, Kim MS. Stereotactic body radiotherapy for isolated para-aortic lymph node recurrence after curative resection in gastric cancer. J Korean Med Sci. 2009;24:488-492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 77. | Herfarth KK, Debus J, Lohr F, Bahner ML, Rhein B, Fritz P, Höss A, Schlegel W, Wannenmacher MF. Stereotactic single-dose radiation therapy of liver tumors: results of a phase I/II trial. J Clin Oncol. 2001;19:164-170. [PubMed] |
| 78. | Khalifa MA, Rowsell CH, Gladdy R, Ko YJ, Hanna S, Smith A, Law C. Is EGFR expression altered following postoperative chemotherapy for colorectal adenocarcinoma? World J Surg Oncol. 2006;4:92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 79. | Zlobec I, Vuong T, Compton CC, Lugli A, Michel RP, Hayashi S, Jass JR. Combined analysis of VEGF and EGFR predicts complete tumour response in rectal cancer treated with preoperative radiotherapy. Br J Cancer. 2008;98:450-456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 80. | Salomon DS, Brandt R, Ciardiello F, Normanno N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol. 1995;19:183-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1911] [Cited by in RCA: 1921] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 81. | Van Schaeybroeck S, Karaiskou-McCaul A, Kelly D, Longley D, Galligan L, Van Cutsem E, Johnston P. Epidermal growth factor receptor activity determines response of colorectal cancer cells to gefitinib alone and in combination with chemotherapy. Clin Cancer Res. 2005;11:7480-7489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 82. | Bentzen SM, Atasoy BM, Daley FM, Dische S, Richman PI, Saunders MI, Trott KR, Wilson GD. Epidermal growth factor receptor expression in pretreatment biopsies from head and neck squamous cell carcinoma as a predictive factor for a benefit from accelerated radiation therapy in a randomized controlled trial. J Clin Oncol. 2005;23:5560-5567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 185] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 83. | Eriksen JG, Steiniche T, Overgaard J. The influence of epidermal growth factor receptor and tumor differentiation on the response to accelerated radiotherapy of squamous cell carcinomas of the head and neck in the randomized DAHANCA 6 and 7 study. Radiother Oncol. 2005;74:93-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 72] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 84. | Timmerman RD. An overview of hypofractionation and introduction to this issue of seminars in radiation oncology. Semin Radiat Oncol. 2008;18:215-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 348] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 85. | Bae SH, Kim MS, Kim SY, Jang WI, Cho CK, Yoo HJ, Kim KB, Lee DH, Han CJ, Yang KY. Severe intestinal toxicity after stereotactic ablative radiotherapy for abdominopelvic malignancies. Int J Colorectal Dis. 2013;28:1707-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 86. | Bae SH, Kim MS, Cho CK, Kang JK, Lee SY, Lee KN, Lee DH, Han CJ, Yang KY, Kim SB. Predictor of severe gastroduodenal toxicity after stereotactic body radiotherapy for abdominopelvic malignancies. Int J Radiat Oncol Biol Phys. 2012;84:e469-e474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 87. | Kavanagh BD, Schefter TE, Cardenes HR, Stieber VW, Raben D, Timmerman RD, McCarter MD, Burri S, Nedzi LA, Sawyer TE. Interim analysis of a prospective phase I/II trial of SBRT for liver metastases. Acta Oncol. 2006;45:848-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 142] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 88. | Rusthoven KE, Kavanagh BD, Cardenes H, Stieber VW, Burri SH, Feigenberg SJ, Chidel MA, Pugh TJ, Franklin W, Kane M. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol. 2009;27:1572-1578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 617] [Article Influence: 38.6] [Reference Citation Analysis (0)] |