Published online Feb 21, 2014. doi: 10.3748/wjg.v20.i7.1701
Revised: November 18, 2013
Accepted: December 5, 2013
Published online: February 21, 2014
Processing time: 141 Days and 23.2 Hours
This article reviews the epidemiological evidence linking diabetes and gastric cancer and discusses some of the potential mechanisms, confounders and biases in the evaluation of such an association. Findings from four meta-analyses published from 2011 to 2013 suggest a positive link, which may be more remarkable in females and in the Asian populations. Putative mechanisms may involve shared risk factors, hyperglycemia, Helicobacter pylori (H. pylori) infection, high salt intake, medications and comorbidities. Diabetes may increase the risk of gastric cancer through shared risk factors including obesity, insulin resistance, hyperinsulinemia and smoking. Hyperglycemia, even before the clinical diagnosis of diabetes, may predict gastric cancer in some epidemiological studies, which is supported by in vitro, and in vivo studies. Patients with diabetes may also have a higher risk of gastric cancer through the higher infection rate, lower eradication rate and higher reinfection rate of H. pylori. High salt intake can act synergistically with H. pylori infection in the induction of gastric cancer. Whether a higher risk of gastric cancer in patients with diabetes may be ascribed to a higher intake of salt due to the loss of taste sensation awaits further investigation. The use of medications such as insulin, metformin, sulfonylureas, aspirin, statins and antibiotics may also influence the risk of gastric cancer, but most of them have not been extensively studied. Comorbidities may affect the development of gastric cancer through the use of medications and changes in lifestyle, dietary intake, and the metabolism of drugs. Finally, a potential detection bias related to gastrointestinal symptoms more commonly seen in patients with diabetes and with multiple comorbidities should be pointed out. Taking into account the inconsistent findings and the potential confounders and detection bias in previous epidemiological studies, it is expected that there are still more to be explored for the clarification of the association between diabetes and gastric cancer.
Core tip: Epidemiological studies suggested a possible higher risk of gastric cancer in patients with diabetes. This article summarizes the findings in four meta-analyses and proposes some mechanisms explaining the association. Findings in the meta-analyses suggested that the association between diabetes and gastric cancer is more remarkable in females and in the Asian populations. Although the mechanisms for such a link remain to be explored, these may involve shared risk factors between diabetes and gastric cancer (such as obesity, insulin resistance, hyperinsulinemia and smoking), hyperglycemia, Helicobacter pylori infection, high salt intake, medications and comorbidities.
- Citation: Tseng CH, Tseng FH. Diabetes and gastric cancer: The potential links. World J Gastroenterol 2014; 20(7): 1701-1711
- URL: https://www.wjgnet.com/1007-9327/full/v20/i7/1701.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i7.1701
Diabetes mellitus may increase the risk of several cancers involving the breast[1,2], liver[3,4], pancreas[5-7], colorectum[8-10], endometrium[11,12], kidney[13], non-Hodgkin lymphoma[14,15] and urinary bladder[16-20]. The underlying mechanisms for a higher risk of cancer in patients with diabetes may be due to insulin resistance, poor glycemic control, oxidative stress and pro-inflammatory status[21,22]. In addition, the use of anti-diabetic drugs, diabetes duration and the severity of diabetes status accompanied by various comorbidities may play some roles[22-24].
Gastric cancer is more common in men and in people aged 50 years or older[25-27]. Obesity, smoking, salt intake and Helicobacter pylori (H. pylori) infection are important risk factors[28,29]. Gastric cancer is very common in developing countries in East Asia, East Europe and South America; while the incidence is low in North America and most parts of Africa[26]. The prognosis of gastric cancer is very poor, with a 5-year survival < 20% for advanced disease[27]. The incidence of gastric cancer has decreased in most parts of the world in recent years, probably due to the increasing use of refrigerators and less dependence on salt for food preservation, increasing availability of fresh fruits and vegetables, and the control of chronic infection with H. pylori. However, it remains as a major cancer affecting human health, and in 2008 it may account for 8% of the total cancer incidence and 10% of the total cancer death worldwide[26].
Recent observational studies suggested that diabetes or hyperglycemia may increase the risk of gastric cancer incidence or mortality[30-36]. In this article, we review the current evidence, and discuss the potential mechanisms, confounders and biases in the evaluation of such an association.
Whether diabetes may increase the risk of gastric cancer has become a focus of attention in recent years. On October 1, 2013, we used the keywords of “diabetes, gastric cancer, meta-analysis” to search the Pubmed, eight papers were available. After further scrutiny, four of them were excluded because they are not related to the topic under review. Finally, there are four meta-analyses[33-36] published within a 3-year period from 2011 to 2013. The main findings of these four meta-analyses are summarized in Table 1 and briefly described below.
| Ref. | Studies included | Summary RR (95%CI) | Notes and comments for specific studies | Limitations common to the meta-analysis studies | |
| Overall | Subgroup analysis | ||||
| Ge et al[33], 2011 | 4 case-control and 17 cohort | 1.09 (0.98-1.22) | Women: 1.18 (1.01-1.39)Men: 1.04 (0.94-1.15)Duration of follow-up < 10 yr: 0.95 (0.72-1.26)Duration of follow-up ≥ 10 yr: 1.14 (1.01-1.29) | Evaluating incidence and mortality togetherA mixture of incidence and mortality studies may not be appropriateEthnicity differences not considered | Heterogeneity in terms of study design, population demographics, diabetes ascertainment, duration of follow-up, and confoundersType 1 and type 2 diabetes not distinguished in most studiesCardia and non-cardia gastric cancer not discerned in most studiesConfounding effects of H. pylori, smoking and diet are not considered in most studiesNumbers of studies in subgroup analyses varied and may be too small for some analysesMost studies included in meta-analyses were conducted in developed western countries and not primarily designed for evaluating the association between diabetes and gastric cancerPublication bias is possible |
| Marimuthu et al[34], 2011 | 20 population-based cohort | Incidence: 1.01 (0.90-1.11)Mortality: 1.62 (1.36-1.89) | Type 1 diabetes (incidence): 1.60 (0.56-2.64)Asians (mortality): 1.98 (1.57-2.39) | Evaluating incidence and mortality separately in overall analysisConsidering type 1 diabetes and ethnicity differences in subgroup analyses | |
| Tian et al[35], 2012 | 7 case-control and 18 cohort | Incidence: 1.11 (1.00-1.24) Mortality: 1.29 (1.04-1.59) | Asians: 1.19 (1.07-1.32)Cohort design: 1.14 (1.01-1.30) Type 2 diabetes: 1.16 (1.01-1.33) Studies adjusted for more confounders: 1.16 (1.03-1.30) | Evaluating incidence and mortality separately in overall analysisSubgroup analysis was conducted with a mixture of incidence and mortality | |
| Yoon et al[36], 2013 | 6 case-control and 11 cohort | 1.19 (1.08-1.31) | Cohort design: 1.20 (1.08-1.34)Case-control design: 1.12 (0.87-1.45)East Asian countries: 1.19 (1.02-1.38)Western countries: 1.18 (1.03-1.36)Men: 1.10 (0.97-1.24)Women: 1.24 (1.01-1.52)Studies adjusted for smoking: 1.17 (1.01-1.34)Studies adjusted for infection of H. pylori: 2.35 (1.24-4.46)Cardia cancer: 1.39 (0.72-2.69)Noncardia cancer: 1.19 (0.80-1.77) | Evaluating only incidenceStrengths include considering subgroup analyses in studies with adjustment for smoking and H. pylori infectionSubgroup analyses on cardia and noncardia cancer are available, but only 2 studies are included | |
In the first meta-analysis by Ge et al[33], which included 21 (4 case-control and 17 cohort) studies evaluating either incidence or mortality of gastric cancer, patients with diabetes did not show an overall higher risk of gastric cancer when sex was not analyzed separately. The summary relative risk (SRR) was 1.09, 95%CI: 0.98-1.22. However, when men and women were analyzed separately, diabetes was associated with a significantly increased risk of gastric cancer in women (SRR = 1.18, 95%CI: 1.01-1.39) but not in men (SRR = 1.04, 95%CI: 0.94-1.15)[33]. In other subgroup analyses including both sexes, studies with a follow-up duration < 10 years showed a null association, but those with a follow-up duration ≥ 10 years showed a significant SRR (1.14, 95%CI: 1.01-1.29)[33].
The second meta-analysis by Marimuthu et al[34] included 20 population-based cohort studies evaluating gastric cancer incidence and mortality separately. The overall SRR for gastric cancer incidence was 1.01 (95%CI: 0.90-1.11). The null association was similarly observed in studies conducted in Europe, Asia and United States. It is interesting that the link with gastric cancer incidence was more remarkable, though not significant, in patients with type 1 diabetes (< 30 years of age at diagnosis), with SRR of 1.60 (95%CI: 0.56-2.64) derived from two studies[34]. When gastric cancer mortality was evaluated, patients with diabetes had a significantly higher risk in overall analysis (SRR = 1.62, 95%CI: 1.36-1.89) and in studies from Asian populations (SRR = 1.98, 95%CI: 1.57-2.39), but not in studies from Europe or the United States[34].
The third meta-analysis by Tian et al[35] included 25 (7 case-control and 18 cohort) studies involving incidence and mortality of gastric cancer. The overall analysis showed a significant link between diabetes and gastric cancer incidence and mortality with respective SRR of 1.11 (95%CI: 1.00-1.24) (P = 0.045) and 1.29 (95%CI: 1.04-1.59)[35]. Subgroup analyses from various numbers of studies with a mixture of incidence and mortality of gastric cancer showed a positive association in studies conducted in Asian countries, in cohort study design, in patients with type 2 diabetes and in studies adjusted for more confounders, with respective SRR of 1.19 (95%CI: 1.07-1.32), 1.14 (95%CI: 1.01-1.30), 1.16 (95%CI: 1.01-1.33) and 1.16 (95%CI: 1.03-1.30)[35].
The latest meta-analysis by Yoon et al[36] included 17 (6 case-control and 11 cohort) studies comparing gastric cancer incidence between patients with diabetes and control subjects. This meta-analysis excluded studies investigating only mortality but not incidence or studies reporting only standardized incidence ratios without control groups. The overall SRR was 1.19 (95%CI: 1.08-1.31), and was consistently significant in subgroup analyses conducted in cohort studies, in studies done in populations of either Western or Eastern countries, in females, and in studies with high quality[36]. The significantly higher risk was also demonstrated in analyses confined to studies controlling well-known risk factors such as smoking or H. pylori infection, with respective SRR of 1.17 (95%CI: 1.01-1.34) and 2.35 (95%CI: 1.24-4.46). Another strength is the subgroup analysis for cardia and noncardia gastric cancer, with respective SRR of 1.39 (95%CI: 0.72-2.69) and 1.19 (95%CI: 0.80-1.77). But only two studies are available for these site-specific analyses.
Because the findings are inconsistent from observational studies[30-36], a consensus report does not support diabetes as a risk factor for gastric cancer[22]. However, some common limitations in the above meta-analysis studies should be pointed out (Table 1).
First, heterogeneity exists in study design, diabetes diagnosis, cancer ascertainment, use of incidence, prevalence or mortality, consideration of confounders (e.g., age, sex, obesity, smoking, salt intake and H. pylori infection), follow-up duration, and population demography.
Second, most studies did not differentiate type 1 and type 2 diabetes, and did not discern between different histopathology (adenocarcinoma, lymphoma or other types) or anatomical sites (cardia or noncardia) of gastric cancer. It is worth to point out that patients with diabetes may increase the risk of adenocarcinoma located specifically at the gastric cardia by 89% in one United States population-based study[37]. On the other hand, H. pylori infection-related gastric cancer may be primarily located at the noncardiac portion[38].
Third, because most studies were conducted in the developed western countries where gastric cancer is less common, and these studies were mainly designed to evaluate the risk of all or multiple cancer sites and not specifically of gastric cancer, they might not have sufficient power for investigating the association between diabetes and gastric cancer. If the effect of diabetes on gastric cancer is smaller than its effect on the other types of cancer, then a much larger sample size will be required.
Fourth, dietary factors may also modify the development of gastric cancer induced by carcinogens[27], but most of these factors have not been considered in previous studies.
As described in the above meta-analyses, there are signals indicating a positive link between diabetes and gastric cancer, especially in females[33,36] and in Asian populations[34,35]. Estrogen has been shown to interact with insulin/insulin-like growth factor 1 (IGF-1) in the development of breast cancer[39], and gastrointestinal tissues may express estrogen receptor[40,41]. Therefore, estrogen may play a role in the differential effect between men and women on the link between diabetes and gastric cancer. The stronger link found in studies conducted in the Asian populations may either indicate a higher H. pylori infection rate in the patients with diabetes in these populations, or it may suggest an effect of different ethnic/genetic backgrounds, dietary habits, lifestyle, or disease prevalence.
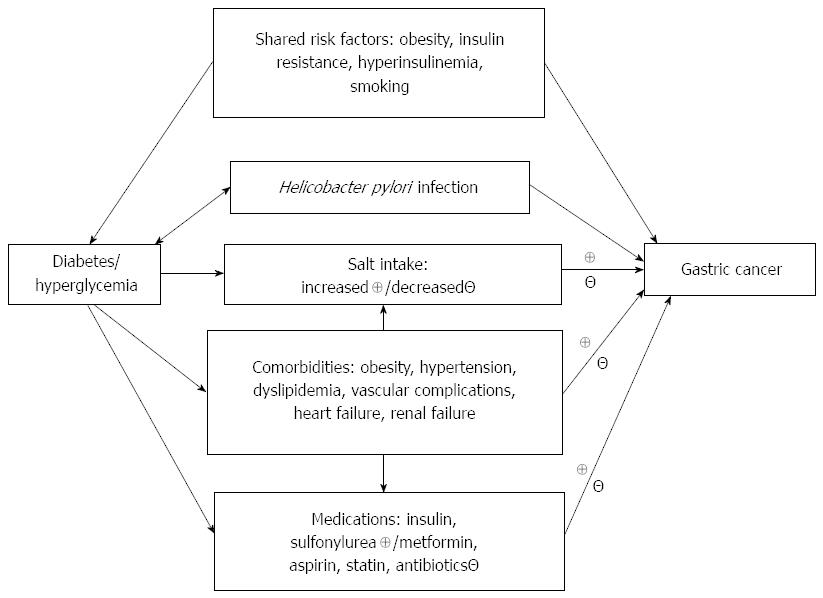
The putative mechanisms linking diabetes and gastric cancer are shown in Figure 1. Table 2 summarizes the explanations for such links and discusses some limitations for each possible link. These potential links will be discussed below under the subtitles of: (1) shared risk factors; (2) hyperglycemia; (3) H. pylori infection; (4) salt intake; (5) medications; and (6) comorbidities.
| Factors | Explanations/limitations |
| Shared risk factors | Explanations: Diabetes and gastric cancer may share common risk factors such as obesity, insulin resistance, hyperinsulinemia and smoking Limitations: These shared risk factors are known to cause cancer. Therefore, if these shared risk factors are in play, they may also increase the risk of other types of cancer, like colorectal cancer and lung cancer. As demonstrated in some studies, the link between diabetes and gastric cancer may be independent of smoking. Evidence for such an effect in humans needs to be fortified by further studies |
| Hyperglycemia | Explanations: Hyperglycemia is associated with pro-inflammatory status, oxidative stress, impaired immune function and increased insulin secretion. All of these may contribute to the development of gastric cancer. Epidemiological studies conducted in Japan support hyperglycemia as a risk factor for gastric cancer, and an interaction between hyperglycemia and H. pylori infection. Such a link may also be supported by findings from in vitro studies Limitations: Confirmation of such a link in other ethnicities is necessary |
| H. pylori infection | Explanations: Diabetes and H. pylori infection may be mutually causative. Patients with diabetes may have a higher infection rate, a lower eradication rate, and/or a higher reinfection rate of H. pylori. On the other hand, the inflammatory process induced by H. pylori infection may also increase the risk of diabetes Limitations: Findings in epidemiological studies are controversial with regards to the higher infection rate of H. pylori in patients with diabetes. Detection bias can not be excluded because patients with diabetes may suffer from more gastrointestinal symptoms leading to the diagnosis of H. pylori infection and gastric cancer |
| Salt intake | Explanations: A synergistic effect between H. pylori infection and salt intake on gastric cancer is supported by recent human studies and by in vivo and in vitro studies. Patients with diabetes may consume more salt because of loss of sensitivity to taste Limitations: Patients with diabetes may also be advised to take less salt especially in those with hypertension, kidney disease or congestive heart failure. Epidemiological studies evaluating the link between salt intake and gastric cancer in patients with diabetes are lacking |
| Medications | Explanations: Insulin and sulfonylureas may increase the risk of cancer. On the other hand, metformin, aspirin and statin may potentially reduce the risk of gastric cancer. Patients who repeatedly use antibiotics may have a lower risk of infection with H. pylori Limitations: Research of well quality on the use of medications and gastric cancer risk is lacking |
| Comorbidities | Explanations: Patients with diabetes may have multiple comorbidities including obesity, hypertension, dyslipidemia, vascular complications and heart failure. All of these may affect the development of gastric cancer, either positively or negatively, through the use of medications and changes in lifestyle, salt intake, dietary components, and the metabolism of drugs Limitations: A detection bias on H. pylori infection or gastric cancer is possible in patients with multiple comorbidities. Studies clarifying such links are still lacking |
Diabetes and gastric cancer may share common risk factors such as obesity, insulin resistance, hyperinsulinemia and smoking.
Obesity is associated with inflammation, oxidative stress, insulin resistance and hyperinsulinemia. All of these may contribute to a higher risk of gastric cancer[42]. Because some patients with diabetes may be obese[43-46], it is possible that this shared risk factor may partly explain the higher risk of gastric cancer in patients with diabetes.
Insulin has both metabolic and mitogenic properties[47,48]. Hyperinsulinemia, especially in the presence of insulin resistance, may promote cancer cell growth either through the mitogenic pathways triggered by insulin receptor or IGF-1 receptor, or via increased bioavailability of free IGF-1 by inhibiting the expression of IGF binding proteins[47-49]. These effects have also been well demonstrated in gastric cancer cell lines and in in vivo studies[50-54].
Smoking is another common risk factor for diabetes[55] and gastric cancer[27]. Therefore, smoking may also confound the association between diabetes and gastric cancer. However, because the higher risk of gastric cancer associated with diabetes remains significant after adjustment for multiple risk factors including smoking[35,36], the link between diabetes and gastric cancer can also be independent of smoking.
It should also be pointed out that if shared risk factors are in play, their effects may not be site-specific and the risk of other types of cancer like colorectal cancer and lung cancer may also be increased. Furthermore, evidence for such a link through shared risk factors is not sufficient in humans and needs to be fortified by further studies.
Patients with diabetes are characterized by an increased serum level of glucose. Similar observation of an increased risk of gastric cancer in patients with type 1 diabetes[34,56,57] and type 2 diabetes[35,36] may imply a mechanism involving hypgerglycemia, which is independent of insulin effect because type 1 diabetes is characterized by insulin deficiency. This is supported by human studies conducted in Japan showing an association between hyperglycemia even before the diagnosis of diabetes with a higher risk of gastric cancer[31,58]. Furthermore an interaction between hyperglycemia and H. pylori infection was reported to markedly increase the risk[31,58]. However, such a link with hyperglycemia needs to be confirmed in other ethnicities.
It is worth mentioning that a higher risk of gastric cancer in patients with type 1 diabetes may not completely exclude a mechanism involving insulin resistance or hyperinsulinemia because of the following facts: (1) recent studies strongly support the presence of insulin resistance in patients with type 1 diabetes[59]; (2) insulin injected subcutaneously bypasses the first-pass clearance by the liver; and (3) therapeutic insulin dose can not always be adjusted exactly to the physiological demands and hyperinsulinemia should be the usual phenomenon if glycemic control is aimed close to the normal range.
Some in vitro and in vivo studies may support a link between hyperglycemia and gastric cancer. An in vitro study indicated that glucose per se may affect the development of cancer viaβ-catenin acetylation with increased Wnt signaling[60], which is also a characteristic of gastric cancer[61]. Patients with diabetes may have an increased expression of pro-inflammatory cytokines such as interleukin-1, interleukin-6 and tumor necrosis factor-α[62]. It is also shown that these factors may upregulate and activate the Wnt/β-catenin pathway[63]. An animal study supported that gastric cancer induced by N-methyl-N-nitrosourea is enhanced in diabetic (db/db) mice through the effects of hyperglycemia and/or hyperinsulinemia[64].
Hyperglycemia may also promote carcinogenesis via increasing reactive oxygen species resulting in DNA damage[65] or increasing the expression of vascular endothelial growth factor, which is correlated with tumor vascularity and metastasis[66]. Furthermore, hyperglycemia may impair immune function rendering susceptibility to H. pylori infection and delaying wound healing in gastric ulcer following H. pylori infection. Hyperglycemia may also trigger insulin secretion, leading to hyperinsulinemia, especially in the presence of insulin resistance, which may increase the risk of cancer through insulin signaling. Because cancer cells are less efficient in using glucose for energy expenditure and they may consume more glucose than normal cells (the Warburg effect)[67], hyperglycemia provides a more suitable condition for tumor cells to grow.
H. pylori infection is well known as a risk factor for gastric ulcer and cancer[27,68,69], possibly through DNA damage induced by reactive oxygen species in the infected gastric epithelial cells[70]. A research conducted in Taiwan suggested that early H. pylori eradication decreases the risk of gastric cancer in patients with peptic ulcer disease[71]. However, the role of diabetes on the relation between H. pylori infection and gastric cancer is still under investigation.
The relation between H. pylori infection and diabetes can be mutually causative. The increased risk of gastric cancer in patients with diabetes may be explained by either one of the following conditions related to H. pylori infection[72-76]: (1) higher infection rate; (2) lower eradication rate; or (3) higher reinfection rate. Patients with diabetes may be more susceptible to H. pylori infection because of impaired immune function associated with hyperglycemia[77]. However, whether patients with diabetes may really have a higher rate of H. pylori infection is controversial in epidemiological studies. Although studies from Qatar[78] and Egypt[79] suggested an increased infection rate in the patients with diabetes, this could not be similarly observed in studies conducted in Turkey[80] and Japan[81]. Because diabetes or poor glycemic control may be associated with an increased prevalence of gastrointestinal symptoms[82,83], it is not known whether the higher rate of H. pylori infection in some of the studies may be due to detection bias related to the symptoms[84]. Furthermore, it should be pointed out that an evaluation of the prevalence rate of H. pylori infection may not necessarily indicate an increased risk in terms of incidence. Patients with diabetes may have a lower eradication rate[72,76,85-88] and a higher reinfection rate after H. pylori infection[73,75,76]. Therefore, even in the condition that the incidence of H. pylori infection may not be increased in patients with diabetes, the prevalence rate may be significantly higher.
On the other hand, H. pylori infection can lead to diabetes because the active chronic inflammation may affect the normal secretion and function of insulin leading to glucose dysregulation[89-91]. For example, in a human study measuring the HOMA-IR (homeostasis model assessment of insulin resistance) levels in patients with and without H. pylori infection, insulin resistance is well demonstrated in those having H. pylori infection[89]. H. pylori infection may also affect the secretion of gastrointestinal hormones, such that basal and stimulated levels of serum gastrin are elevated but somatostatin level is decreased[92,93]. Gastrin increases food- or glucose-stimulated insulin secretion; but somatostatin inhibits the release of insulin. As a result, hyperinsulinemia may be seen following H. pylori infection. Whether H. pylori infection may directly affect insulin secretion from pancreas is not known. If the inflammatory process and oxidative stress induced by H. pylori infection[91] could also be demonstrated in the pancreas, it is expected that insulin secretion may be impaired. Insulin resistance, as induced by H. pylori infection, may also accelerate β-cell loss and leads finally to the clinical onset of diabetes[94]. Therefore, insulin deficiency as well as insulin resistance might be seen in chronic H. pylori infection.
High salt intake has long been recognized as an important risk factor for gastric cancer[95-101], which can be independent of H. pylori infection[101]. However, some recent human studies showed a synergistic effect between salt intake and H. pylori infection[96,100]. Evidence from an in vivo study using Mongolian gerbils confirmed that high salt intake may exacerbate the risk of gastric cancer induced by H. pylori infection[102], which could probably be due to the upregulation of CagA synthesis in the bacteria in response to increased concentration of salt. The CagA protein is a bacterial oncoprotein related to the H. pylori-induced gastric cancer[102].
Whether high salt intake could be responsible for the increased risk of gastric cancer in patients with diabetes remains to be answered. It has been speculated that people with easy access to sugary, salty and fatty foods, which are calorie-rich but micronutrient-poor, may cause diseases such as obesity and diabetes[103]. On the other hand, patients with diabetes may consume more salt than people without diabetes because of the loss of sensitivity to taste, especially in those with a late stage of the disease complicated with neuropathy[104,105]. However, it is also possible that patients with diabetes may be advised to consume less salt than people without diabetes by their physicians, especially when the patients also suffer from hypertension, renal disease or congestive heart failure.
Exogenous insulin use has also been shown to increase the risk of several cancer types[106,107]. Whether this could also be applied to gastric cancer has not been extensively studied. In studies conducted in Taiwan, patients with diabetes who used insulin had a significantly higher risk of H. pylori eradication, but none of the other anti-diabetic drugs including sulfonylurea, metformin, acarbose, pioglitazone or rosiglitazone was associated with H. pylori eradication[84]. However, insulin use was not associated with an increased risk of gastric cancer[108]. It has been explained that the use of insulin might indicate poor glycemic control with more severe disease conditions in the H. pylori eradication study[84], suggesting a deteriorating metabolic control following H. pylori infection.
Insulin glargine, a long-acting insulin analog, may increase the risk of certain cancers involving colon, pancreas and breast[107,109,110]. This has always been ascribed to the very high affinity of insulin glargine to the IGF-1 receptor in in vitro studies[111]. However, this may not be the case when insulin glargine is injected subcutaneously because it is converted at the injection site to less mitogenic metabolites[112]. It remains unknown whether clinical use of exogenous human insulin or insulin analogs may affect the risk of gastric cancer.
Metformin may protect against a number of cancers[10,107,113], but sulfonylureas may be associated with an increased risk[106,114]. Whether these medications may affect the risk of gastric cancer in humans has rarely been studied. An inhibitory effect of metformin on gastric cancer cell proliferation can be demonstrated in in vitro and in vivo studies[115]. Similarly, an early in vitro study suggested that glibenclamide (a sulfonylurea) may exert antitumor activity in a human gastric cancer cell line[116]. However, a preliminary human study conducted in Taiwan showed a slightly higher but not significant risk ratio while comparing users of sulfonylureas only to users of metformin only in patients with type 2 diabetes (age-sex-adjusted OR = 1.855, 95%CI: 0.779-4.419)[106]. Thiazolidinediones may also demonstrate some antitumor effects on gastric cancer cells in in vitro and in vivo studies[117,118]. However, whether this can be translated into a preventive effect on gastric cancer growth in humans remains unknown.
From meta-analyses, use of statins is associated with a significantly 32% lower risk of gastric cancer[119], and aspirin may significantly reduce the risk with a SRR of 0.71 (95%CI: 0.60-0.82)[120]. Although without evidence, patients who repeatedly use antibiotics may happen to have a reduced risk of H. pylori infection. The confounding effects of these commonly used medications have rarely been controlled in previous studies investigating the association between diabetes and gastric cancer. Some studies suggested a sex difference in the use of insulin (more common in women)[121] and statins (more common in men)[122] in patients with type 2 diabetes. Whether this may contribute to a sex difference in the association between diabetes and gastric cancer awaits further investigation.
Obesity, hypertension and dyslipidemia are common comorbidities observed in patients with diabetes[43-46,123]. All of these may be associated with insulin resistance. Patients with ischemic heart disease, other vascular complications, congestive heart failure or chronic kidney disease/end-stage renal disease may have changed their lifestyle, daily activity, salt intake and dietary components or may have taken some other medications, supplements or alternative treatment. Hepatic or renal insufficiency may also affect the metabolism of medications. The confounding effects of comorbidities in the association between diabetes and gastric cancer have rarely been addressed in previous studies.
A detection bias related to multiple comorbidities is also possible. Patients with more comorbidities may have a higher probability of receiving laboratory examinations leading to the diagnosis of gastric cancer. This detection bias should be seriously taken into account in future studies.
Epidemiological evidence signals a higher risk of gastric cancer in patients with diabetes, which is more remarkable in females and in the Asian populations. Potential mechanisms may include shared risk factors, hyperglycemia, H. pylori infection, high salt intake, medications and comorbidities. It should be recognized that epidemiological findings are inconsistent, the estimated relative risk is moderate, and most studies have inherent limitations related to study design, sample size, confounders and biases. Therefore, more well-designed epidemiological studies are required to confirm the association between diabetes and gastric cancer in humans, and in-depth mechanistic studies are necessary to explain the possible links.
The authors thank the following institutes in Taiwan for their continuous support on epidemiological studies of diabetes and arsenic-related health hazards: the Department of Health (DOH97-TD-D-113-97009); and the National Science Council (NSC 101-2314-B-002-117, NSC-102-2314-B-002-067).
P- Reviewers: Buchler C, Marchesini G, Naoaki S S- Editor: Ma YJ L- Editor: A E- Editor: Liu XM
| 1. | Larsson SC, Mantzoros CS, Wolk A. Diabetes mellitus and risk of breast cancer: a meta-analysis. Int J Cancer. 2007;121:856-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 628] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 2. | Tseng CH, Chong CK, Tai TY. Secular trend for mortality from breast cancer and the association between diabetes and breast cancer in Taiwan between 1995 and 2006. Diabetologia. 2009;52:240-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 3. | El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol. 2006;4:369-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 600] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 4. | Tseng CH. Type 2 diabetes, smoking, insulin use and mortality from hepatocellular carcinoma: a 12-year follow-up of a national cohort in Taiwan. Hepatol Int. 2013;7:693-702 [DOI 10.1007/s12072-012-9405-0]. |
| 5. | Ben Q, Cai Q, Li Z, Yuan Y, Ning X, Deng S, Wang K. The relationship between new-onset diabetes mellitus and pancreatic cancer risk: a case-control study. Eur J Cancer. 2011;47:248-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 6. | Tseng CH. New-onset diabetes with a history of dyslipidemia predicts pancreatic cancer. Pancreas. 2013;42:42-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Tseng CH. Diabetes, insulin use, smoking, and pancreatic cancer mortality in Taiwan. Acta Diabetol. 2013;50:879-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst. 2005;97:1679-1687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 719] [Cited by in RCA: 753] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 9. | Tseng CH. Diabetes but not insulin is associated with higher colon cancer mortality. World J Gastroenterol. 2012;18:4182-4190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Tseng CH. Diabetes, metformin use, and colon cancer: a population-based cohort study in Taiwan. Eur J Endocrinol. 2012;167:409-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 11. | Friberg E, Orsini N, Mantzoros CS, Wolk A. Diabetes mellitus and risk of endometrial cancer: a meta-analysis. Diabetologia. 2007;50:1365-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 372] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 12. | Saltzman BS, Doherty JA, Hill DA, Beresford SA, Voigt LF, Chen C, Weiss NS. Diabetes and endometrial cancer: an evaluation of the modifying effects of other known risk factors. Am J Epidemiol. 2008;167:607-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 13. | Larsson SC, Wolk A. Diabetes mellitus and incidence of kidney cancer: a meta-analysis of cohort studies. Diabetologia. 2011;54:1013-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 137] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 14. | Tseng CH. Diabetes and non-Hodgkin’s lymphoma: analyses of prevalence and annual incidence in 2005 using the National Health Insurance database in Taiwan. Ann Oncol. 2012;23:153-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Tseng CH. Diabetes, insulin use, and non-Hodgkin lymphoma mortality in Taiwan. Metabolism. 2012;61:1003-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Tseng CH. Insulin use and smoking jointly increase the risk of bladder cancer mortality in patients with type 2 diabetes. Clin Genitourin Cancer. 2013;11:508-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Tseng CH, Chong CK, Tseng CP, Chan TT. Age-related risk of mortality from bladder cancer in diabetic patients: a 12-year follow-up of a national cohort in Taiwan. Ann Med. 2009;41:371-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Tseng CH. Diabetes and risk of bladder cancer: a study using the National Health Insurance database in Taiwan. Diabetologia. 2011;54:2009-2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 19. | Tseng CH. Pioglitazone and bladder cancer: a population-based study of Taiwanese. Diabetes Care. 2012;35:278-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 20. | Tseng CH. Benign prostatic hyperplasia is a significant risk factor for bladder cancer in diabetic patients: a population-based cohort study using the National Health Insurance in Taiwan. BMC Cancer. 2013;13:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Arcidiacono B, Iiritano S, Nocera A, Possidente K, Nevolo MT, Ventura V, Foti D, Chiefari E, Brunetti A. Insulin resistance and cancer risk: an overview of the pathogenetic mechanisms. Exp Diabetes Res. 2012;2012:789174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 294] [Cited by in RCA: 396] [Article Influence: 30.5] [Reference Citation Analysis (1)] |
| 22. | Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, Pollak M, Regensteiner JG, Yee D. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33:1674-1685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1291] [Cited by in RCA: 1460] [Article Influence: 97.3] [Reference Citation Analysis (1)] |
| 23. | Tseng CH, Tseng FH. Peroxisome proliferator-activated receptor agonists and bladder cancer: lessons from animal studies. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2012;30:368-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 24. | Tseng CH. Pioglitazone and bladder cancer in human studies: is it diabetes itself, diabetes drugs, flawed analyses or different ethnicities? J Formos Med Assoc. 2012;111:123-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10002] [Cited by in RCA: 10451] [Article Influence: 696.7] [Reference Citation Analysis (0)] |
| 26. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25535] [Article Influence: 1823.9] [Reference Citation Analysis (7)] |
| 27. | Nagini S. Carcinoma of the stomach: A review of epidemiology, pathogenesis, molecular genetics and chemoprevention. World J Gastrointest Oncol. 2012;4:156-169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 324] [Cited by in RCA: 330] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 28. | Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030-3044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1946] [Cited by in RCA: 1971] [Article Influence: 103.7] [Reference Citation Analysis (0)] |
| 29. | Correa P, Piazuelo MB. Helicobacter pylori Infection and Gastric Adenocarcinoma. US Gastroenterol Hepatol Rev. 2011;7:59-64. [PubMed] |
| 30. | Tseng CH. Diabetes conveys a higher risk of gastric cancer mortality despite an age-standardised decreasing trend in the general population in Taiwan. Gut. 2011;60:774-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 31. | Ikeda F, Doi Y, Yonemoto K, Ninomiya T, Kubo M, Shikata K, Hata J, Tanizaki Y, Matsumoto T, Iida M. Hyperglycemia increases risk of gastric cancer posed by Helicobacter pylori infection: a population-based cohort study. Gastroenterology. 2009;136:1234-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 32. | Gong Y, Yang YS, Zhang XM, Su M, Wang J, Han JD, Guo MZ. ABO blood type, diabetes and risk of gastrointestinal cancer in northern China. World J Gastroenterol. 2012;18:563-569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 33. | Ge Z, Ben Q, Qian J, Wang Y, Li Y. Diabetes mellitus and risk of gastric cancer: a systematic review and meta-analysis of observational studies. Eur J Gastroenterol Hepatol. 2011;23:1127-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 34. | Marimuthu SP, Vijayaragavan P, Moysich KB, Jayaprakash V. Diabetes mellitus and gastric carcinoma: Is there an association? J Carcinog. 2011;10:30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Tian T, Zhang LQ, Ma XH, Zhou JN, Shen J. Diabetes mellitus and incidence and mortality of gastric cancer: a meta-analysis. Exp Clin Endocrinol Diabetes. 2012;120:217-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 36. | Yoon JM, Son KY, Eom CS, Durrance D, Park SM. Pre-existing diabetes mellitus increases the risk of gastric cancer: a meta-analysis. World J Gastroenterol. 2013;19:936-945. [PubMed] |
| 37. | Lin SW, Freedman ND, Hollenbeck AR, Schatzkin A, Abnet CC. Prospective study of self-reported diabetes and risk of upper gastrointestinal cancers. Cancer Epidemiol Biomarkers Prev. 2011;20:954-961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 38. | Cavaleiro-Pinto M, Peleteiro B, Lunet N, Barros H. Helicobacter pylori infection and gastric cardia cancer: systematic review and meta-analysis. Cancer Causes Control. 2011;22:375-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 136] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 39. | Lanzino M, Morelli C, Garofalo C, Panno ML, Mauro L, Andò S, Sisci D. Interaction between estrogen receptor alpha and insulin/IGF signaling in breast cancer. Curr Cancer Drug Targets. 2008;8:597-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 40. | Matsuyama S, Ohkura Y, Eguchi H, Kobayashi Y, Akagi K, Uchida K, Nakachi K, Gustafsson JA, Hayashi S. Estrogen receptor beta is expressed in human stomach adenocarcinoma. J Cancer Res Clin Oncol. 2002;128:319-324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 90] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 41. | Enmark E, Pelto-Huikko M, Grandien K, Lagercrantz S, Lagercrantz J, Fried G, Nordenskjöld M, Gustafsson JA. Human estrogen receptor beta-gene structure, chromosomal localization, and expression pattern. J Clin Endocrinol Metab. 1997;82:4258-4265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 279] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 42. | Yang P, Zhou Y, Chen B, Wan HW, Jia GQ, Bai HL, Wu XT. Overweight, obesity and gastric cancer risk: results from a meta-analysis of cohort studies. Eur J Cancer. 2009;45:2867-2873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 246] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 43. | Tseng CH. Body composition as a risk factor for coronary artery disease in Chinese type 2 diabetic patients in Taiwan. Circ J. 2003;67:479-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 44. | Tseng CH. Body mass index and blood pressure in adult type 2 diabetic patients in Taiwan. Circ J. 2007;71:1749-1754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 45. | Tseng CH, Chong CK, Tseng CP, Shau WY, Tai TY. Hypertension is the most important component of metabolic syndrome in the association with ischemic heart disease in Taiwanese type 2 diabetic patients. Circ J. 2008;72:1419-1424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 46. | Tseng CH. Obesity paradox: differential effects on cancer and noncancer mortality in patients with type 2 diabetes mellitus. Atherosclerosis. 2013;226:186-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 47. | Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8:915-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1484] [Cited by in RCA: 1565] [Article Influence: 92.1] [Reference Citation Analysis (0)] |
| 48. | Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Cancer. 2012;12:159-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 771] [Cited by in RCA: 838] [Article Influence: 64.5] [Reference Citation Analysis (0)] |
| 49. | Giovannucci E. Insulin, insulin-like growth factors and colon cancer: a review of the evidence. J Nutr. 2001;131:3109S-3120S. [PubMed] |
| 50. | Yi HK, Hwang PH, Yang DH, Kang CW, Lee DY. Expression of the insulin-like growth factors (IGFs) and the IGF-binding proteins (IGFBPs) in human gastric cancer cells. Eur J Cancer. 2001;37:2257-2263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 73] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 51. | Lee DY, Yi HK, Hwang PH, Oh Y. Enhanced expression of insulin-like growth factor binding protein-3 sensitizes the growth inhibitory effect of anticancer drugs in gastric cancer cells. Biochem Biophys Res Commun. 2002;294:480-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 52. | Adachi Y, Li R, Yamamoto H, Min Y, Piao W, Wang Y, Imsumran A, Li H, Arimura Y, Lee CT. Insulin-like growth factor-I receptor blockade reduces the invasiveness of gastrointestinal cancers via blocking production of matrilysin. Carcinogenesis. 2009;30:1305-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 53. | Pavelić K, Kolak T, Kapitanović S, Radosević S, Spaventi S, Kruslin B, Pavelić J. Gastric cancer: the role of insulin-like growth factor 2 (IGF 2) and its receptors (IGF 1R and M6-P/IGF 2R). J Pathol. 2003;201:430-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 54. | Thompson MA, Cox AJ, Whitehead RH, Jonas HA. Autocrine regulation of human tumor cell proliferation by insulin-like growth factor II: an in-vitro model. Endocrinology. 1990;126:3033-3042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 55. | Bi Y, Wang T, Xu M, Xu Y, Li M, Lu J, Zhu X, Ning G. Advanced research on risk factors of type 2 diabetes. Diabetes Metab Res Rev. 2012;28 Suppl 2:32-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 56. | Zendehdel K, Nyrén O, Ostenson CG, Adami HO, Ekbom A, Ye W. Cancer incidence in patients with type 1 diabetes mellitus: a population-based cohort study in Sweden. J Natl Cancer Inst. 2003;95:1797-1800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 193] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 57. | Swerdlow AJ, Laing SP, Qiao Z, Slater SD, Burden AC, Botha JL, Waugh NR, Morris AD, Gatling W, Gale EA. Cancer incidence and mortality in patients with insulin-treated diabetes: a UK cohort study. Br J Cancer. 2005;92:2070-2075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 148] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 58. | Yamagata H, Kiyohara Y, Nakamura S, Kubo M, Tanizaki Y, Matsumoto T, Tanaka K, Kato I, Shirota T, Iida M. Impact of fasting plasma glucose levels on gastric cancer incidence in a general Japanese population: the Hisayama study. Diabetes Care. 2005;28:789-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 59. | Fourlanos S, Narendran P, Byrnes GB, Colman PG, Harrison LC. Insulin resistance is a risk factor for progression to type 1 diabetes. Diabetologia. 2004;47:1661-1667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 168] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 60. | Chocarro-Calvo A, García-Martínez JM, Ardila-González S, De la Vieja A, García-Jiménez C. Glucose-induced β-catenin acetylation enhances Wnt signaling in cancer. Mol Cell. 2013;49:474-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 132] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 61. | Wu WK, Cho CH, Lee CW, Fan D, Wu K, Yu J, Sung JJ. Dysregulation of cellular signaling in gastric cancer. Cancer Lett. 2010;295:144-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 130] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 62. | Wieser V, Moschen AR, Tilg H. Inflammation, cytokines and insulin resistance: a clinical perspective. Arch Immunol Ther Exp (Warsz). 2013;61:119-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 165] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 63. | Katoh M. Dysregulation of stem cell signaling network due to germline mutation, SNP, Helicobacter pylori infection, epigenetic change and genetic alteration in gastric cancer. Cancer Biol Ther. 2007;6:832-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 64. | Yoshizawa N, Yamaguchi H, Yamamoto M, Shimizu N, Furihata C, Tatematsu M, Seto Y, Kaminishi M. Gastric carcinogenesis by N-Methyl-N-nitrosourea is enhanced in db/db diabetic mice. Cancer Sci. 2009;100:1180-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 65. | Lorenzi M, Montisano DF, Toledo S, Barrieux A. High glucose induces DNA damage in cultured human endothelial cells. J Clin Invest. 1986;77:322-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 191] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 66. | Mahdy RA, Nada WM. Evaluation of the role of vascular endothelial growth factor in diabetic retinopathy. Ophthalmic Res. 2011;45:87-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 67. | Warburg O. On the origin of cancer cells. Science. 1956;123:309-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9117] [Cited by in RCA: 9895] [Article Influence: 143.4] [Reference Citation Analysis (0)] |
| 68. | Shimizu H, Monden T, Matsumura M, Domeki N, Kasai K. Effects of twice-daily injections of premixed insulin analog on glycemic control in type 2 diabetic patients. Yonsei Med J. 2010;51:845-849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 69. | Yamagata H, Kiyohara Y, Aoyagi K, Kato I, Iwamoto H, Nakayama K, Shimizu H, Tanizaki Y, Arima H, Shinohara N. Impact of Helicobacter pylori infection on gastric cancer incidence in a general Japanese population: the Hisayama study. Arch Intern Med. 2000;160:1962-1968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 78] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 70. | Obst B, Wagner S, Sewing KF, Beil W. Helicobacter pylori causes DNA damage in gastric epithelial cells. Carcinogenesis. 2000;21:1111-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 114] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 71. | Wu CY, Kuo KN, Wu MS, Chen YJ, Wang CB, Lin JT. Early Helicobacter pylori eradication decreases risk of gastric cancer in patients with peptic ulcer disease. Gastroenterology. 2009;137:1641-8.e1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 209] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 72. | Gasbarrini A, Ojetti V, Pitocco D, Franceschi F, Candelli M, Torre ES, Gabrielli M, Cammarota G, Armuzzi A, Pola R. Insulin-dependent diabetes mellitus affects eradication rate of Helicobacter pylori infection. Eur J Gastroenterol Hepatol. 1999;11:713-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 73. | Ojetti V, Pitocco D, Bartolozzi F, Danese S, Migneco A, Lupascu A, Pola P, Ghirlanda G, Gasbarrini G, Gasbarrini A. High rate of helicobacter pylori re-infection in patients affected by type 1 diabetes. Diabetes Care. 2002;25:1485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 74. | Ojetti V, Migneco A, Silveri NG, Ghirlanda G, Gasbarrini G, Gasbarrini A. The role of H. pylori infection in diabetes. Curr Diabetes Rev. 2005;1:343-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 75. | Ojetti V, Migneco A, Nista EC, Gasbarrini G, Gasbarrini A, Pitocco D, Ghirlanda G. H pylori re-infection in type 1 diabetes: a 5 years follow-up. Dig Liver Dis. 2007;39:286-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 76. | Ojetti V, Pellicano R, Fagoonee S, Migneco A, Berrutti M, Gasbarrini A. Helicobacter pylori infection and diabetes. Minerva Med. 2010;101:115-119. [PubMed] |
| 77. | Daoud AK, Tayyar MA, Fouda IM, Harfeil NA. Effects of diabetes mellitus vs. in vitro hyperglycemia on select immune cell functions. J Immunotoxicol. 2009;6:36-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 78. | Bener A, Micallef R, Afifi M, Derbala M, Al-Mulla HM, Usmani MA. Association between type 2 diabetes mellitus and Helicobacter pylori infection. Turk J Gastroenterol. 2007;18:225-229. [PubMed] |
| 79. | Hamed SA, Amine NF, Galal GM, Helal SR, Tag El-Din LM, Shawky OA, Ahmed EA, Abdel Rahman MS. Vascular risks and complications in diabetes mellitus: the role of helicobacter pylori infection. J Stroke Cerebrovasc Dis. 2008;17:86-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 39] [Reference Citation Analysis (0)] |
| 80. | Demir M, Gokturk HS, Ozturk NA, Kulaksizoglu M, Serin E, Yilmaz U. Helicobacter pylori prevalence in diabetes mellitus patients with dyspeptic symptoms and its relationship to glycemic control and late complications. Dig Dis Sci. 2008;53:2646-2649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 81. | Ariizumi K, Koike T, Ohara S, Inomata Y, Abe Y, Iijima K, Imatani A, Oka T, Shimosegawa T. Incidence of reflux esophagitis and H pylori infection in diabetic patients. World J Gastroenterol. 2008;14:3212-3217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 82. | Bytzer P, Talley NJ, Hammer J, Young LJ, Jones MP, Horowitz M. GI symptoms in diabetes mellitus are associated with both poor glycemic control and diabetic complications. Am J Gastroenterol. 2002;97:604-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 139] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 83. | Kim JH, Park HS, Ko SY, Hong SN, Sung IK, Shim CS, Song KH, Kim DL, Kim SK, Oh J. Diabetic factors associated with gastrointestinal symptoms in patients with type 2 diabetes. World J Gastroenterol. 2010;16:1782-1787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (2)] |
| 84. | Tseng CH. Diabetes, insulin use and Helicobacter pylori eradication: a retrospective cohort study. BMC Gastroenterol. 2012;12:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 85. | Selinger C, Robinson A. Helicobacter pylori eradication in diabetic patients: still far off the treatment targets. South Med J. 2010;103:975-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 86. | Demir M, Gokturk HS, Ozturk NA, Serin E, Yilmaz U. Efficacy of two different Helicobacter pylori eradication regimens in patients with type 2 diabetes and the effect of Helicobacter pylori eradication on dyspeptic symptoms in patients with diabetes: a randomized controlled study. Am J Med Sci. 2009;338:459-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 87. | Demir M, Gokturk HS, Ozturk NA, Arslan H, Serin E, Yilmaz U. Clarithromycin resistance and efficacy of clarithromycin-containing triple eradication therapy for Helicobacter pylori infection in type 2 diabetes mellitus patients. South Med J. 2009;102:1116-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 88. | Sargýn M, Uygur-Bayramicli O, Sargýn H, Orbay E, Yavuzer D, Yayla A. Type 2 diabetes mellitus affects eradication rate of Helicobacter pylori. World J Gastroenterol. 2003;9:1126-1128. [PubMed] |
| 89. | Aydemir S, Bayraktaroglu T, Sert M, Sokmen C, Atmaca H, Mungan G, Gun BD, Borazan A, Ustundag Y. The effect of Helicobacter pylori on insulin resistance. Dig Dis Sci. 2005;50:2090-2093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 66] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 90. | Gunji T, Matsuhashi N, Sato H, Fujibayashi K, Okumura M, Sasabe N, Urabe A. Helicobacter pylori infection significantly increases insulin resistance in the asymptomatic Japanese population. Helicobacter. 2009;14:144-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 91. | Aslan M, Horoz M, Nazligul Y, Bolukbas C, Bolukbas FF, Selek S, Celik H, Erel O. Insulin resistance in H pylori infection and its association with oxidative stress. World J Gastroenterol. 2006;12:6865-6868. [PubMed] |
| 92. | Kaneko H, Konagaya T, Kusugami K. Helicobacter pylori and gut hormones. J Gastroenterol. 2002;37:77-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 93. | Calam J. Helicobacter pylori modulation of gastric acid. Yale J Biol Med. 1999;72:195-202. [PubMed] |
| 94. | Wilkin TJ. Is autoimmunity or insulin resistance the primary driver of type 1 diabetes? Curr Diab Rep. 2013;13:651-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 95. | Ge S, Feng X, Shen L, Wei Z, Zhu Q, Sun J. Association between Habitual Dietary Salt Intake and Risk of Gastric Cancer: A Systematic Review of Observational Studies. Gastroenterol Res Pract. 2012;2012:808120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 96. | Shikata K, Kiyohara Y, Kubo M, Yonemoto K, Ninomiya T, Shirota T, Tanizaki Y, Doi Y, Tanaka K, Oishi Y. A prospective study of dietary salt intake and gastric cancer incidence in a defined Japanese population: the Hisayama study. Int J Cancer. 2006;119:196-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 179] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 97. | Wang XQ, Terry PD, Yan H. Review of salt consumption and stomach cancer risk: epidemiological and biological evidence. World J Gastroenterol. 2009;15:2204-2213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 114] [Cited by in RCA: 122] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 98. | Tsugane S, Sasazuki S. Diet and the risk of gastric cancer: review of epidemiological evidence. Gastric Cancer. 2007;10:75-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 325] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 99. | Tsugane S. Salt, salted food intake, and risk of gastric cancer: epidemiologic evidence. Cancer Sci. 2005;96:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 203] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 100. | Zhong C, Li KN, Bi JW, Wang BC. Sodium intake, salt taste and gastric cancer risk according to Helicobacter pylori infection, smoking, histological type and tumor site in China. Asian Pac J Cancer Prev. 2012;13:2481-2484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 101. | Peleteiro B, Lopes C, Figueiredo C, Lunet N. Salt intake and gastric cancer risk according to Helicobacter pylori infection, smoking, tumour site and histological type. Br J Cancer. 2011;104:198-207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 102. | Gaddy JA, Radin JN, Loh JT, Zhang F, Washington MK, Peek RM, Algood HM, Cover TL. High dietary salt intake exacerbates Helicobacter pylori-induced gastric carcinogenesis. Infect Immun. 2013;81:2258-2267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 152] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 103. | Breslin PA. An evolutionary perspective on food and human taste. Curr Biol. 2013;23:R409-R418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 254] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 104. | Bajaj S, Prasad S, Gupta A, Singh VB. Oral manifestations in type-2 diabetes and related complications. Indian J Endocrinol Metab. 2012;16:777-779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 105. | Gondivkar SM, Indurkar A, Degwekar S, Bhowate R. Evaluation of gustatory function in patients with diabetes mellitus type 2. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:876-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 106. | Hsieh MC, Lee TC, Cheng SM, Tu ST, Yen MH, Tseng CH. The influence of type 2 diabetes and glucose-lowering therapies on cancer risk in the Taiwanese. Exp Diabetes Res. 2012;2012:413782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 128] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 107. | Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009;52:1766-1777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 859] [Cited by in RCA: 848] [Article Influence: 53.0] [Reference Citation Analysis (1)] |
| 108. | Tseng CH. Diabetes, insulin use, and gastric cancer: a population-based analysis of the Taiwanese. J Clin Gastroenterol. 2013;47:e60-e64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 109. | Jonasson JM, Ljung R, Talbäck M, Haglund B, Gudbjörnsdòttir S, Steineck G. Insulin glargine use and short-term incidence of malignancies-a population-based follow-up study in Sweden. Diabetologia. 2009;52:1745-1754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 312] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 110. | Hemkens LG, Grouven U, Bender R, Günster C, Gutschmidt S, Selke GW, Sawicki PT. Risk of malignancies in patients with diabetes treated with human insulin or insulin analogues: a cohort study. Diabetologia. 2009;52:1732-1744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 463] [Cited by in RCA: 446] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 111. | Smith U, Gale EA. Does diabetes therapy influence the risk of cancer? Diabetologia. 2009;52:1699-1708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 229] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 112. | Sommerfeld MR, Müller G, Tschank G, Seipke G, Habermann P, Kurrle R, Tennagels N. In vitro metabolic and mitogenic signaling of insulin glargine and its metabolites. PLoS One. 2010;5:e9540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 113. | Lee MS, Hsu CC, Wahlqvist ML, Tsai HN, Chang YH, Huang YC. Type 2 diabetes increases and metformin reduces total, colorectal, liver and pancreatic cancer incidences in Taiwanese: a representative population prospective cohort study of 800,000 individuals. BMC Cancer. 2011;11:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 314] [Cited by in RCA: 365] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 114. | Tseng CH. Thyroid cancer risk is not increased in diabetic patients. PLoS One. 2012;7:e53096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 115. | Kato K, Gong J, Iwama H, Kitanaka A, Tani J, Miyoshi H, Nomura K, Mimura S, Kobayashi M, Aritomo Y. The antidiabetic drug metformin inhibits gastric cancer cell proliferation in vitro and in vivo. Mol Cancer Ther. 2012;11:549-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 186] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 116. | Qian X, Li J, Ding J, Wang Z, Duan L, Hu G. Glibenclamide exerts an antitumor activity through reactive oxygen species-c-jun NH2-terminal kinase pathway in human gastric cancer cell line MGC-803. Biochem Pharmacol. 2008;76:1705-1715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 117. | Zhang L, Hu JF, Li GQ, Xiao X, Su Q. Rosiglitazone reverses mitomycin C resistance in human gastric cancer cells. Am J Med Sci. 2012;343:382-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 118. | Leung WK, Bai AH, Chan VY, Yu J, Chan MW, To KF, Wu JR, Chan KK, Fu YG, Chan FK. Effect of peroxisome proliferator activated receptor gamma ligands on growth and gene expression profiles of gastric cancer cells. Gut. 2004;53:331-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 119. | Singh PP, Singh S. Statins are associated with reduced risk of gastric cancer: a systematic review and meta-analysis. Ann Oncol. 2013;24:1721-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 124] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 120. | Ye X, Fu J, Yang Y, Gao Y, Liu L, Chen S. Frequency-risk and duration-risk relationships between aspirin use and gastric cancer: a systematic review and meta-analysis. PLoS One. 2013;8:e71522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 121. | Franzini L, Ardigò D, Cavalot F, Miccoli R, Rivellese AA, Trovati M, Zavaroni I, Vaccaro O. Women show worse control of type 2 diabetes and cardiovascular disease risk factors than men: results from the MIND.IT Study Group of the Italian Society of Diabetology. Nutr Metab Cardiovasc Dis. 2013;23:235-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 122. | Fu AZ, Zhang Q, Davies MJ, Pentakota SR, Radican L, Seck T. Underutilization of statins in patients with type 2 diabetes in US clinical practice: a retrospective cohort study. Curr Med Res Opin. 2011;27:1035-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 123. | Tseng CH, Chong CK, Chan TT, Bai CH, You SL, Chiou HY, Su TC, Chen CJ. Optimal anthropometric factor cutoffs for hyperglycemia, hypertension and dyslipidemia for the Taiwanese population. Atherosclerosis. 2010;210:585-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |