Published online Dec 28, 2014. doi: 10.3748/wjg.v20.i48.18330
Revised: August 6, 2014
Accepted: September 5, 2014
Published online: December 28, 2014
Processing time: 246 Days and 10.2 Hours
AIM: To evaluate the protective effects of Aloe vera on gastric injury in rats with indomethacin (IMN)-induced gastropathy.
METHODS: Male Sprague-Dawley rats were randomly divided into three groups. Group 1 (control, n = 6) was given distilled water (DW) orally. Group 2 (IMN, n = 6) was given oral IMN (150 mg/kg) dissolved in 5% sodium bicarbonate (NaHCO3-) at time 0 and 4 h. Group 3 (Aloe vera-treated, n = 6) was given oral Aloe vera (150 mg/kg) dissolved in DW and IMN at time 0 and 4 h. Eight hours later, the stomach was removed to determine gastric malondialdehyde (MDA), the number of interleukin (IL)-18 positive stained cells (%) by immunohistochemistry, and for histopathological examination. Then, the serum was collected to determine tumor necrosis factor (TNF)-α and cytokine-induced neutrophil chemoattractant (CINC)-1 by sandwich enzyme linked immunosorbent assay method.
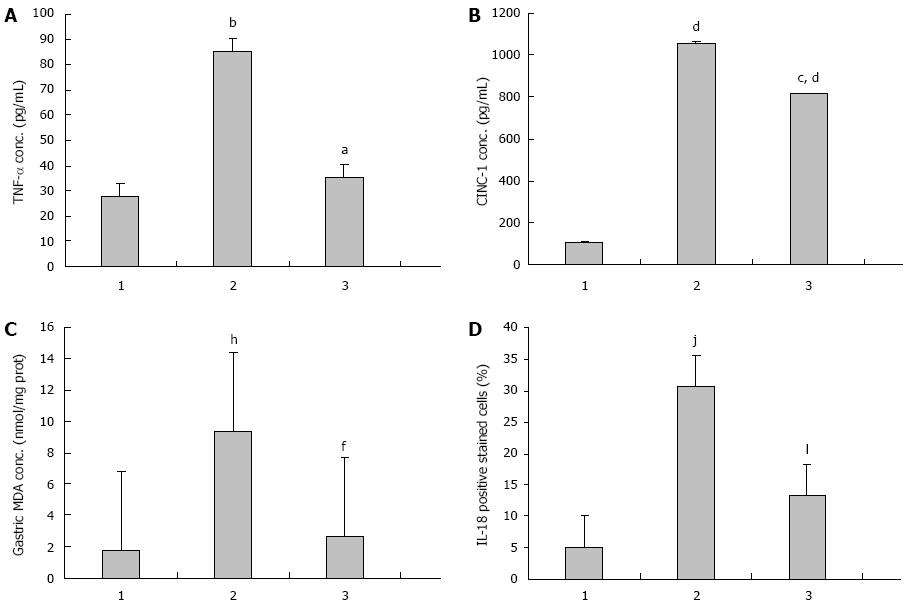
RESULTS: In the IMN group, serum TNF-α, CINC-1 and gastric MDA were significantly increased when compared to the control group (27.78 ± 1.52 pg/mL vs 85.07 ± 49.11 pg/mL, P = 0.009; 104.55 ± 45.80 pg/mL vs 1054.70 ± 20.38 pg/mL, and 1.74 ± 0.21 nmol/mg vs 9.36 ± 1.07 nmol/mg protein, P = 0.000, respectively). The mean level of TNF-α, CINC-1 and gastric MDA in the Aloe vera-treated group were improved as compared with the IMN group (85.07 ± 49.11 pg/mL vs 35.19 ± 1.61 pg/mL, P = 0.021; 1054.70 ± 20.38 pg/mL vs 813.56 ± 239.04 pg/mL, P = 0.025; and 9.36 ± 1.07 nmol/mg vs 2.67 ± 0.64 nmol/mg protein, P = 0.000, respectively). The number of IL-18 positive stained cells (%) in the gastric epithelial cells of the IMN group was significantly higher than the control group (5.01% ± 3.73% vs 30.67% ± 2.03%, P = 0.000, respectively). In contrast, Aloe vera treatment decreased the number of IL-18 positive stained cells (%) significantly when compared with the IMN group (30.67% ± 2.03% vs 13.21% ± 1.10%, P = 0.000, respectively). Most rats in the IMN group developed moderate to severe gastric inflammation and erosions. The gastric erosions and neutrophil infiltration scores were significantly reduced in the Aloe vera-treated group.
CONCLUSION: Aloe vera attenuated IMN-induced gastropathy in rats by the reduction of oxidative stress, inflammation, and improvement of gastric histopathology.
Core tip: Nonsteroidal anti-inflammatory drugs (NSAIDs)-induced gastric damage is the major side effect of this kind of medication. Although the underlying pathogenesis of NSAIDs-induced gastric damage is unclear, neutrophils are believed to play an important role in the development of gastric inflammation and injury following NSAIDs administration. Aloe vera (Aloe barbadensis Mill) possesses several biological activities, including an anti-inflammatory and ulcer healing effects. The authors postulated that Aloe vera, acting through inflammatory inhibition, could reduce the inflammatory cytokine, neutrophil chemoattractant, and oxidative stress thus resulting in attenuation of gastric injury in indomethacin-induced gastropathy in rats.
-
Citation: Werawatganon D, Rakananurak N, Sallapant S, Prueksapanich P, Somanawat K, Klaikeaw N, Rerknimitr R.
Aloe vera attenuated gastric injury on indomethacin-induced gastropathy in rats. World J Gastroenterol 2014; 20(48): 18330-18337 - URL: https://www.wjgnet.com/1007-9327/full/v20/i48/18330.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i48.18330
Nonsteroidal anti-inflammatory drugs (NSAIDs) are among the most commonly prescribed medications worldwide. However, NSAIDs have adverse effects on the gastric mucosa, resulting in various clinical presentations, ranging from nonspecific dyspepsia to ulceration, upper gastrointestinal bleeding, and death, summarized by the term “NSAIDs gastropathy”[1]. NSAIDs-induced gastric damage is the major side effect of this kind of drug[2].
The main action of NSAIDs is to inhibit prostaglandin synthesis. There is substantial evidence supporting the view that the ulcerogenic effect of this medication correlates with its ability to suppress prostaglandin synthesis[3-5]. Endogenous prostaglandins normally regulate mucosal blood flow, epithelial cell proliferation, epithelial restitution, mucosal immunocyte function, mucus and bicarbonate secretion, and basal acid secretion[6]. Therefore, decreases in prostaglandins, protective factors for ulcer formation, lead to gastric mucosal injury.
Animal studies have shown that neutrophil adherence to the endothelium of gastric microcirculation is critical in NSAIDs injury[7]. Neutrophil adherence damages the mucosa by producing oxygen-free radicals, releasing proteases, and obstructing capillary blood flow. NSAIDs might induce the synthesis of tumor necrosis factor (TNF)-α and leukotrienes[8,9]. These inflammatory mediators subsequently stimulate neutrophil adherence by the upregulation of adhesion molecules[10].
NSAIDs administration in rats caused a rapid and significant increase in adhesion between neutrophils and vascular endothelial cells in both gastric and mesenteric venules[11-13]. This was dependent on intercellular adhesion molecule (ICAM)-1 expression on the endothelium and CD11/CD18 expression on the leukocyte[14,15]. Interestingly, Thong-Ngam et al[16] recently reported that administration of indomethacin (IMN) to rats resulted in a significant increase in ICAM-1 and TNF-α expression in the gastric mucosa. Andrews et al[10] reported that administration of aspirin or IMN to rats resulted in a significant increase in ICAM-1 expression in the gastric microcirculation.
Aloe vera (Aloe barbadensis Mill) is classified in family of Aloaceae, originated in the dry areas of Africa, Asia, and Southern Europe, especially in the Mediterranean regions. Aloe vera and other species of Aloe are succulent and xerophytic plants that are adapted to living in areas with little water. These plants possess extensive water storage tissue in their leaves, the part of the plant which is used for its therapeutic properties[17]. The largest components (60%) in the dry matter, are the carbohydrates (soluble sugar and complex polysaccharides). Aloe is known well for its topical use as an anti-inflammatory and for curing wounds and burns[18]. Anti-oxidative, anti-inflammatory, and immunosuppressive activities of Aloe vera have been indicated[19,20]. Acemannan is a key compound that stimulates the immunity mediated by the cells[21].
Several studied showed that Aloe vera is also an anti-inflammatory and ulcer healing substance. It can protect gastric mucosa by its anti-inflammatory[22], cytoprotective, healing, and mucus stimulatory effects[23].
Preliminary data obtained in animal models of NSAIDs-induced gastropathy showed that treatment with Aloe vera could be effective in limiting gastric damage, but the exact mechanism of these effects is still largely undefined.
There are currently limited studies investigating the effects of Aloe vera on NSAIDs-induced gastropathy. To date, there are no experimental data to prove its beneficial effects on inflammatory mediators, principally interleukin (IL)-18 level changes.
IL-18, originally known as interferon-γ (IFN-γ)-inducing factor (IGIF), is a cytokine that shares structural and functional properties with IL-1[24,25]. This cytokine is mainly produced by activated macrophages, but may also be expressed by Kupffer cells, T cells, B cells, keratinocytes, astrocytes, and osteoblasts[26]. Like IL-1, IL-18 is synthesized as an inactive precursor (pro-IL-18, 24 kDa), which is cleaved by interleukin-1 β-converting enzyme (ICE or caspase-1) into an active 18 kDa mature form[26-28].
Therefore, the aim of the present study was to test the effects of Aloe vera on oxidative stress, inflammation, and gastric histopathology in rats with IMN-induced gastropathy and also determine IL-18 levels, which is a proinflammatory cytokine.
Male Sprague-Dawley rats weighing 180-220 g, purchased from the National Laboratory Animal Center, Mahidol University, Salaya (n = 18), Nakorn pathom, were used in an experimental study. All rats were kept in a controlled temperature room at 25 ± 1 °C under standard conditions (12 h day-night rhythm). They were cared for in accordance with the Ethical Committee, Faculty of Medicine, Chulalongkorn University, Thailand. NSAIDs or indomethacin(IMN) was dissolved in 5% sodium bicarbonate (NaHCO3-). Aloe vera extract in powder form (Sigma®) was dissolved in distilled water (DW).
All rats were randomly divided into three experimental groups. Group 1 (control, n = 6): rats were given DW 1 mL/rat orally via an intragastric tube. Group 2 (IMN, n = 6): rats were given IMN 150 mg/kg body weight (BW) dissolved in 5% NaHCO3- 1 mL/rat orally via an intragastric tube at time 0 and 4 hour. Group 3 (Aloe vera-treated, n = 6): rats were given Aloe vera 200 mg/kg BW dissolved in DW and IMN 150 mg/kg BW dissolved in 5% NaHCO3- 1 mL/rat orally via an intragastric tube at 0 and 4 h.
Eight hours later, all rats were anesthetized with an intraperitoneal injection of thiopental 60 mg/kg (Jagsonpal Pharmaceuticals Ltd Haryana, India). The stomach was removed and dissected along the greater curvature and washed twice with ice-cold phosphate-buffered saline, frozen in liquid nitrogen, and stored at -80 °C to examine tissue malondialdehyde (MDA) using thiobarbituric acid reactive substances (TBARS) assay kit (Cayman Chemical Company, United States) and was expressed as nanomole per milligram (nmol/mg) protein. The remaining stomach was cut into multiple 5-micron-thick sections and fixed in 10% formalin solution, which were later stained with hematoxylin and eosin (H and E) for histopathologic examination. Then, a blood sample was collected by cardiac puncture. The blood was allowed to clot at room temperature for 2 h before being centrifuged at 1000 g for 20 min. Serum was separated and stored at -80 °C to measure the TNF-α and CINC-1 levels using sandwich enzyme linked immunosorbent assay (ELISA) technique (R and D Systems, Inc., United States) and was expressed as picogram per milliliter (pg/mL).
Tissue samples of stomach were excised and fixed with 10% formalin and later processed by routine technique before paraffin embedding. Sections were cut at 5 μm thickness and stained with H and E. One experienced gastrointestinal pathologist examined all blinded samples by using light microscopy with magnification × 20. All histopathological findings were recorded and graded by using gastric erosion and polymorphonuclear leukocyte infiltration scores as follows; Score 0: no erosion; Score 1: erosion 1/3 of epithelium depth; Score 2: erosion 2/3 of epithelium depth, or development of ulcer. For PMN infiltration score, Score 0: no infiltration; Score 1: PMN infiltrate 1/3 of epithelium; Score 2: PMN infiltrate 2/3 of epithelium; Score 3: PMN infiltrate all depths of epithelium which represent normal, mild, moderate and severe gastric mucosal injury respectively[29].
The stomach sections were deparaffinized with xylene and ethanol for 10 min. After water washing, the antigen was retrieved from sections with citrate buffer pH 6.0 in microwave for 13 min. Next, 3% H2O2 and 3% normal horse serum were added to the slides to block endogenous peroxidase activity for 5 min and block nonspecific binding for 20 min, respectively. Then, the primary antibody used for IL-18, a monoclonal antibody against the Rat IL-18, was applied at a dilution of 1:100 (R and D Systems, Inc., United States) and 1:200 (Gene Tex, United States) respectively for 1 h at room temperature and incubated with the secondary antibody for IL-18 (ready-to-use; Dako, Denmark) for 30 min. When the development of the color with DAB was detected, the slides were counterstained with hematoxylin.
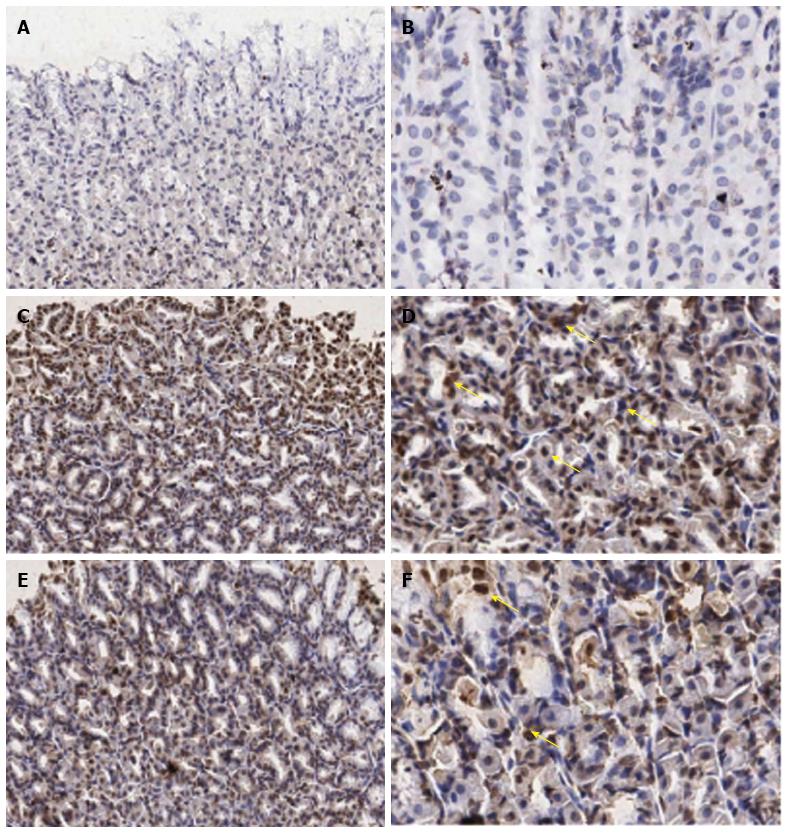
Under light microscopy (Nikon E50i, Nikon Corporation, Japan), the number of IL-18 positive cells presented as dark brown stained nuclei of gastric epithelial cells. Images were obtained at × 20 and × 40 magnification field from each sample. Ten images from two sections per animals were analyzed. The numbers of IL-18 positive stained cells were counted manually using Point tool in the IMAGE-PRO PLUS software program (version 6.1). A thousand gastric epithelial cells were counted for each sample. The results were expressed as the number of IL-18 gastric epithelial positive stained cells (%) calculating from this equation:
The number of positive stained cells (%) = (number of nuclei stained cells/number of examined cells) × 100%.
All data are presented as mean ± SD. We evaluated group differences with one-way analysis of variance (one-way ANOVA) followed by Tukey PostHoc comparision by using SPSS for windows version 17.0 (SPSS Inc., Chicago, IL, United States). A P-value of < 0.05 was considered significant.
The mean serum TNF-α level in the IMN group was significantly higher than in the control group (27.78 ± 1.52 pg/mL vs 85.07 ± 49.11 pg/mL, P = 0.009). In the Aloe vera-treated group, the serum TNF-α was significantly reduced when compared to the IMN group (35.19 ± 1.61 pg/mL vs 85.07 ± 49.11 pg/mL, P = 0.021). The averages of serum TNF-α level of all groups were shown in Figure 1A and Table 1.
The average concentrations of serum CINC-1 were 104.55 ± 45.80 pg/mL, 1054.70 ± 20.38 pg/mL, and 813.56 ± 239.04 pg/mL in the control group, the IMN group, and the Aloe vera-treated group respectively. The mean serum CINC-1 level in the IMN group was significantly higher than in the control group (P = 0.000) and significantly decreased in the Aloe vera-treated group when compared with the IMN group (P = 0.025). The averages of serum TNF-α level of all groups were shown in Figure 1B and Table 1.
The level of gastric MDA was significantly higher in the IMN group compared with the control group (1.74 ± 0.21 nmol/mg vs 9.36 ± 1.07 nmol/mg protein, P = 0.000). In the Aloe vera-treated group, there was a significant decrease in the elevated gastric MDA level compared with the IMN group (9.36 ± 1.07 nmol/mg vs 2.67 ± 0.64 nmol/mg protein, P = 0.000). The averages of serum TNF-α level of all groups were shown in Figure 1C and Table 1.
The expression of IL-18 in gastric epithelial cells was determined by an immunohistochemistry method and showed as dark brown stained nuclei of this cell.
The data of IL-18 expression in all experimental groups were given in Figure 1D, Figure 2 and Table 1. From the results, the number of IL-18 positive stained cells in the gastric epithelial cells of the IMN group was significantly higher than the control group (30.67 ± 3.73 vs 5.01 ± 3.73, P = 0.000, respectively). In contrast, the Aloe vera-treated group had a significantly decreased number of IL-18 positive stained cells when compared with the IMN group (13.21% ± 1.10% vs 30.67% ± 2.03%, P = 0.000, respectively).
In the IMN group, 5 rats developed gastric erosion (score 2+) and one rat developed 0.5 cm gastric ulcer. The PMN infiltration scores in the IMN group were 1+ (n = 4) and 0 (n = 2). In the Aloe vera-treated group, stomach histopathology was improved when compared to the IMN group, especially in the reduction of PMN leukocyte infiltration (score 0: n = 4 and score 1+: n = 2). The severity of gastric erosion was reduced from score 2+ to score 1+. Additionally, no gastric ulcer was found, as shown in Table 2.
In this study, we investigated the effect of Aloe vera on gastric injury in rats with IMN-induced gastropathy. The results clearly demonstrated that Aloe vera administration prevented the ulcerogenic effect of IMN, possibly through its anti-inflammatory action. Evidence suggests that NSAIDs-induced gastric ulceration is a neutrophil-dependent process. NSAIDs administration to rats caused a rapid and significant increase in adhesion between neutrophils and vascular endothelial cells in both the gastric and mesenteric venules[11,12]. Indeed, monoclonal antibodies that blocked NSAIDs-induced neutrophil adherence to vascular endothelium could significantly alleviate NSAIDs-induced gastric mucosal injury[7,15,29]. Neutrophils play an important role in the development of inflammation and tissue injury by releasing a variety of inflammatory mediators[30,31]. These inflammatory mediators are capable of producing tissue injury; therefore, they may be involved in the pathogenesis of NSAIDs-induced gastric mucosal injury[32]. Furthermore, adhesion molecules expressed on activated neutrophils, such as CD11b and CD18 have been shown to play an important role in neutrophil-induced tissue injury[14].
Moreover, NSAIDs are believed to have an effect on nuclear translocation of nuclear factor (NF)-κB, which modulates the expression of several adhesion molecules, including ICAM-1[33]. ICAM-1, one of the major adhesion molecules, plays a pivotal role in the inflammatory reaction by increasing leukocyte adhesion to endothelium and promoting transendothelial migration of leukocytes to inflammatory sites[34]. Another important mechanism that induces ICAM-1 expression is the increment of TNF-α level[34,35]. The inhibitory effect of NSAIDs on COX-2 leads to reduced prostaglandin E2 (PGE2) levels. Thus, TNF-α production, which is normally inhibited by PGE2, increases[35]. TNF-α is an important mediator causing NSAIDs-induced gastropathy. Apart from its effect on adhesion molecules, TNF-α may have the ability to activate pro-apoptosis caspases, which regulate gastric epithelial cells apoptosis in NSAIDs-treated rats[34].
In the present study, after administration of 150 mg/kg of IMN, erosions and ulcer developed along with an increase in PMN infiltration around the gastric lesions. With Aloe vera treatment, gastric histopathology was improved compared with the IMN group, especially in the reduction of PMN leukocyte infiltration score and reduction in gastric erosion severity score. Serum TNF-α, IL-18, serum CINC-1 and gastric MDA, which is a proinflammatory cytokine, a neutrophil chemoattractant and a metabolite of intracellular lipid peroxidation reaction respectively, levels in the IMN group were significantly higher than the control group and were significantly reduced in the Aloe vera-treated group.
TNF-α, the major proinflammatory cytokine released from the migrated macrophages, plays an important role in the pathogenesis of gastric ulcers through stimulation of ICAM-1 expression on vascular endothelial cells which increases leukocyte adhesion to the endothelial surface on post-capillary venules and promotes transendothelial migration of leukocytes to inflammatory sites[36]. TNF-α also increases intracellular oxidative stress and up-regulation of CINC-1 mRNA and protein in gastric epithelial cells in rats[35]. The Aloe vera-treated group had significantly reduced TNF-α levels, in concordance with the previous studies, resulting in a decrease of leukocyte adherence and promotion of gastric ulcer healing[23,37].
The significantly higher level of serum CINC-1 in the IMN group compared with the control group demonstrates the pathophysiologic mechanism of CINC-1 as a functional chemoattractant for neutrophils into the gastric ulcer area leading to a decrease in blood flow by their adhesion to microvessels. This results in the production of reactive oxygen species, MPO, and protease, which exert a noxious effect on the gastric mucosa. The CINC-1 level gradually decreases as the ulcer heals[38]. With Aloe vera treatment, there was significant reduction in serum CINC-1 compared with the IMN group, which may be responsible for the reduced PMN infiltration score.
Gastric MDA, which is the main metabolite in the intracellular lipid peroxidation reaction, represents the extent of the reactive oxygen species injuring the membrane structures of the cells which was reported to play a major role in the pathomechanism of IMN-induced gastric lesion[39] and also to play a key role in the mechanism of the gastric carcinogenesis[40,41].
IL-18, originally known as IFN-γ-inducing factor (IGIF), is a cytokine that shares structural and functional properties with IL-1[24,25]. This cytokine is mainly produced by activated macrophages, but may also be expressed by Kupffer cells, T cells, B cells, keratinocytes, astrocytes, and osteoblasts[26]. Like IL-1, IL-18 is synthesized as an inactive precursor (pro-IL-18, 24 kDa), which is cleaved by interleukin-1 β-converting enzyme (ICE or caspase-1) into an active 18 kDa mature form[26-28].
Aloe vera treatment significant reduced the gastric MDA and proinflammatory cytokine IL-18 levels compared with the IMN group, which may reflect the reduced amount of the reactive oxygen species-mediated gastric injury and also reflect the inhibition of inflammatory cells expression.
In conclusion, Aloe vera attenuated IMN-induced gastropathy in rats by the reduction of inflammatory cytokines, neutrophil chemoattractant, oxidative stress, and improvement of gastric histopathology.
Nonsteroidal anti-inflammatory drugs (NSAIDs)-induced gastric damage is the major side effect of this kind of medication. Although the underlying pathogenesis of NSAIDs-induced gastric damage is unclear, neutrophils are believed to play an important role in the development of gastric inflammation and injury following NSAIDs administration. Aloe vera (Aloe barbadensis Mill) possesses several biological activities, including an anti-inflammatory and ulcer healing effects. The authors postulated that Aloe vera, acting through inflammatory inhibition, could reduce inflammatory cytokines, neutrophil chemoattractants, and oxidative stress thus resulting in attenuation of gastric injury in indomethacin (IMN)-induced gastropathy in rats.
Aloe vera (Aloe barbadensis Mill) is classified in the family of Aloaceae. These plants possess extensive water storage tissue in their leaves, the part of the plant which is used for its therapeutic properties and exerts many biological activities by inhibition of inflammatory responses. NSAIDs-induced gastropathy has various pathogenesis mechanisms, including directed inhibition of the prostaglandins, directed toxic effect of NSAIDs, and indirect effects through stimulation of inflammatory mediators leading to gastric damage. The hallmark of this study was that we showed an attenuation of gastric injury through decrease serum tumor necrosis factor-α level, interleukin-18 level, serum cytokine-induced neutrophil chemoattractant-1 level, and gastric malondialdehyde, which is a proinflammatory cytokine, a neutrophil chemoattractant and a metabolite of intracellular lipid peroxidation reaction respectively.
Preliminary data obtained in animal models of NSAIDs-induced gastropathy showed that treatment with Aloe vera could be effective in limiting gastric damage, but the exact mechanism of these effects is still largely undefined. In this study, the authors investigated the protective effect of Aloe vera on gastric injury in rats with IMN-induced gastropathy. The authors found that Aloe vera could attenuate gastric injury by the reduction of proinflammatory cytokines, neutrophil chemoattractants, and oxidative stress, and by improvement of gastric histopathology.
Aloe vera might be used as a novel therapeutic strategy against NSAIDs-induced gastric damage in clinical practice and may be an interesting option for the treatment of cellular or tissue injury in the future.
NSAIDs gastropathy: NSAIDs are one of the most commonly prescribed drugs worldwide and are known to induce gastric injury from multiple mechanisms, ranging from nonspecific dyspepsia to ulceration, upper gastrointestinal bleeding and death. Aloe vera is a species of succulent plant. Aloe vera is known to effectively decrease inflammation and promote ulcer healing.
This is an interesting study of the effect of Aloe vera on NSAIDs-induced gastropathy in rats. The results clearly demonstrated that Aloe vera administration prevented the ulcerogenic effects of IMN, possibly through its anti-inflammatory action.
P- Reviewer: Lo GH S- Editor: Ma YJ L- Editor: O’Neill M E- Editor: Ma S
| 1. | Becker JC, Domschke W, Pohle T. Current approaches to prevent NSAID-induced gastropathy--COX selectivity and beyond. Br J Clin Pharmacol. 2004;58:587-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 79] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 2. | Wolfe MM, Lichtenstein DR, Singh G. Gastrointestinal toxicity of nonsteroidal antiinflammatory drugs. N Engl J Med. 1999;340:1888-1899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1465] [Cited by in RCA: 1316] [Article Influence: 50.6] [Reference Citation Analysis (2)] |
| 3. | Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971;231:232-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5183] [Cited by in RCA: 4957] [Article Influence: 91.8] [Reference Citation Analysis (0)] |
| 4. | Whittle BJ. Temporal relationship between cyclooxygenase inhibition, as measured by prostacyclin biosynthesis, and the gastrointestinal damage induced by indomethacin in the rat. Gastroenterology. 1981;80:94-98. [PubMed] |
| 5. | Rainsford KD, Willis C. Relationship of gastric mucosal damage induced in pigs by antiinflammatory drugs to their effects on prostaglandin production. Dig Dis Sci. 1982;27:624-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 110] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Wallace JL. Nonsteroidal anti-inflammatory drugs and gastroenteropathy: the second hundred years. Gastroenterology. 1997;112:1000-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 384] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 7. | Wallace JL, Keenan CM, Granger DN. Gastric ulceration induced by nonsteroidal anti-inflammatory drugs is a neutrophil-dependent process. Am J Physiol. 1990;259:G462-G467. [PubMed] |
| 8. | Appleyard CB, McCafferty DM, Tigley AW, Swain MG, Wallace JL. Tumor necrosis factor mediation of NSAID-induced gastric damage: role of leukocyte adherence. Am J Physiol. 1996;270:G42-G48. [PubMed] |
| 9. | Rainsford KD. Microvascular injury during gastric mucosal damage by anti-inflammatory drugs in pigs and rats. Agents Actions. 1983;13:457-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 38] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Andrews FJ, Malcontenti-Wilson C, O’Brien PE. Effect of nonsteroidal anti-inflammatory drugs on LFA-1 and ICAM-1 expression in gastric mucosa. Am J Physiol. 1994;266:G657-G664. [PubMed] |
| 11. | Wallace JL, McKnight W, Miyasaka M, Tamatani T, Paulson J, Anderson DC, Granger DN, Kubes P. Role of endothelial adhesion molecules in NSAID-induced gastric mucosal injury. Am J Physiol. 1993;265:G993-G998. [PubMed] |
| 12. | Asako H, Kubes P, Wallace J, Gaginella T, Wolf RE, Granger DN. Indomethacin-induced leukocyte adhesion in mesenteric venules: role of lipoxygenase products. Am J Physiol. 1992;262:G903-G908. [PubMed] |
| 13. | Asako H, Kubes P, Wallace J, Wolf RE, Granger DN. Modulation of leukocyte adhesion in rat mesenteric venules by aspirin and salicylate. Gastroenterology. 1992;103:146-152. [PubMed] |
| 14. | Wallace JL, Arfors KE, McKnight GW. A monoclonal antibody against the CD18 leukocyte adhesion molecule prevents indomethacin-induced gastric damage in the rabbit. Gastroenterology. 1991;100:878-883. [PubMed] |
| 15. | Wallace JL, McCafferty DM, Carter L, McKnight W, Argentieri D. Tissue-selective inhibition of prostaglandin synthesis in rat by tepoxalin: anti-inflammatory without gastropathy? Gastroenterology. 1993;105:1630-1636. [PubMed] |
| 16. | Thong-Ngam D, Choochuai S, Patumraj S, Chayanupatkul M, Klaikeaw N. Curcumin prevents indomethacin-induced gastropathy in rats. World J Gastroenterol. 2012;18:1479-1484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Rodríguez Rodríguez E, Darias Martín J, Díaz Romero C. Aloe vera as a functional ingredient in foods. Crit Rev Food Sci Nutr. 2010;50:305-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 18. | Shelton RM. Aloe vera. Its chemical and therapeutic properties. Int J Dermatol. 1991;30:679-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 141] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | Ozsoy N, Candoken E, Akev N. Implications for degenerative disorders: antioxidative activity, total phenols, flavonoids, ascorbic acid, beta-carotene and beta-tocopherol in Aloe vera. Oxid Med Cell Longev. 2009;2:99-106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Hegazy SK, El-Bedewy M, Yagi A. Antifibrotic effect of aloe vera in viral infection-induced hepatic periportal fibrosis. World J Gastroenterol. 2012;18:2026-2034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Manna S, McAnalley BH. Determination of the position of the O-acetyl group in a beta-(1-->4)-mannan (acemannan) from Aloe barbardensis Miller. Carbohydr Res. 1993;241:317-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 45] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Park CH, Nam DY, Son HU, Lee SR, Lee HJ, Heo JC, Cha TY, Baek JH, Lee SH. Polymer fraction of Aloe vera exhibits a protective activity on ethanol-induced gastric lesions. Int J Mol Med. 2011;27:511-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Eamlamnam K, Patumraj S, Visedopas N, Thong-Ngam D. Effects of Aloe vera and sucralfate on gastric microcirculatory changes, cytokine levels and gastric ulcer healing in rats. World J Gastroenterol. 2006;12:2034-2039. [PubMed] |
| 24. | Gracie JA, Robertson SE, McInnes IB. Interleukin-18. J Leukoc Biol. 2003;73:213-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 528] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 25. | Mühl H, Pfeilschifter J. Interleukin-18 bioactivity: a novel target for immunopharmacological anti-inflammatory intervention. Eur J Pharmacol. 2004;500:63-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin- 18 regulates both Th1 and Th2 responses. Annu Rev Immunol. 2001;19:423-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 958] [Cited by in RCA: 1022] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 27. | Fantuzzi G, Dinarello CA. Interleukin-18 and interleukin-1 beta: two cytokine substrates for ICE (caspase-1). J Clin Immunol. 1999;19:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 372] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 28. | Dinarello CA. Interleukin-18. Methods. 1999;19:121-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 337] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 29. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3221] [Cited by in RCA: 3553] [Article Influence: 122.5] [Reference Citation Analysis (3)] |
| 30. | Alican I, Coşkun T, Corak A, Yeğen BC, Oktay S, Kurtel H. Role of neutrophils in indomethacin-induced gastric mucosal lesions in rats. Inflamm Res. 1995;44:164-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Campbell EJ, Senior RM, McDonald JA, Cox DL. Proteolysis by neutrophils. Relative importance of cell-substrate contact and oxidative inactivation of proteinase inhibitors in vitro. J Clin Invest. 1982;70:845-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 167] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 32. | Weiss SJ, LoBuglio AF. An oxygen-dependent mechanism of neutrophil-mediated cytotoxicity. Blood. 1980;55:1020-1024. [PubMed] |
| 33. | Zimmerman BJ, Granger DN. Reperfusion-induced leukocyte infiltration: role of elastase. Am J Physiol. 1990;259:H390-H394. [PubMed] |
| 34. | Lazzaroni M, Bianchi Porro G. Gastrointestinal side-effects of traditional non-steroidal anti-inflammatory drugs and new formulations. Aliment Pharmacol Ther. 2004;20 Suppl 2:48-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 104] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 35. | Konturek PC, Duda A, Brzozowski T, Konturek SJ, Kwiecien S, Drozdowicz D, Pajdo R, Meixner H, Hahn EG. Activation of genes for superoxide dismutase, interleukin-1beta, tumor necrosis factor-alpha, and intercellular adhesion molecule-1 during healing of ischemia-reperfusion-induced gastric injury. Scand J Gastroenterol. 2000;35:452-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 59] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 36. | Kast RE. Tumor necrosis factor has positive and negative self regulatory feed back cycles centered around cAMP. Int J Immunopharmacol. 2000;22:1001-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 37. | Handa O, Naito Y, Takagi T, Shimozawa M, Kokura S, Yoshida N, Matsui H, Cepinskas G, Kvietys PR, Yoshikawa T. Tumor necrosis factor-alpha-induced cytokine-induced neutrophil chemoattractant-1 (CINC-1) production by rat gastric epithelial cells: role of reactive oxygen species and nuclear factor-kappaB. J Pharmacol Exp Ther. 2004;309:670-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 38. | Duansak D, Somboonwong J, Patumraj S. Effects of Aloe vera on leukocyte adhesion and TNF-alpha and IL-6 levels in burn wounded rats. Clin Hemorheol Microcirc. 2003;29:239-246. [PubMed] |
| 39. | Yamada H, Takahashi S, Fujita H, Kobayashi N, Okabe S. Cytokine-induced neutrophil chemoattractants in healing of gastric ulcers in rats: expression of >40-kDa chemoattractant in delayed ulcer healing by indomethacin. Dig Dis Sci. 1999;44:889-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 40. | Kwiecień S, Brzozowski T, Konturek PCh, Konturek SJ. The role of reactive oxygen species in action of nitric oxide-donors on stress-induced gastric mucosal lesions. J Physiol Pharmacol. 2002;53:761-773. [PubMed] |
| 41. | Wang SH, Wang YZ, Zhang KY, Shen JH, Zhou HQ, Qiu XY. Effect of superoxide dismutase and malondialdehyde metabolic changes on carcinogenesis of gastric carcinoma. World J Gastroenterol. 2005;11:4305-4310. [PubMed] |