Published online Dec 28, 2014. doi: 10.3748/wjg.v20.i48.18216
Revised: June 8, 2014
Accepted: July 11, 2014
Published online: December 28, 2014
Processing time: 262 Days and 16.3 Hours
AIM: To evaluate and characterize motility patterns from small intestinal gut segments depending on different perfusion media and pressures.
METHODS: Experiments were carried out in a custom designed perfusion chamber system to validate and standardise the perfusion technique used. The perfusion chamber was built with a transparent front wall allowing for optical motility recordings and a custom made fastener to hold the intestinal segments. Experiments with different perfusion and storage media combined with different luminal pressures were carried out to evaluate the effects on rat small intestine motility. Software tools which enable the visualization and characterization of intestinal motility in response to different stimuli were used to evaluate the videotaped experiments. The data collected was presented in so called heatmaps thus providing a concise overview of form and strength of contractility patterns. Furthermore, the effect of different storage media on tissue quality was evaluated. Haematoxylin-Eosin stainings were used to compare tissue quality depending on storage and perfusion mode.
RESULTS: Intestinal motility is characterized by different repetitive motility patterns, depending on the actual situation of the gut. Different motility patterns could be recorded and characterized depending on the perfusion pressure and media used. We were able to describe at least three different repetitive patterns of intestinal motility in vitro. Patterns with an oral, anal and oro-anal propagation direction could be recorded. Each type of pattern finalized its movement with or without a subsequent distension of the wavefront. Motility patterns could clearly be distinguished in heatmap diagrams. Furthermore undirected motility could be observed. The quantity of the different patterns varies and is highly dependent on the perfusion medium used. Tissue preservation varies depending on the perfusion medium utilized, therefore media with a simple composition as Tyrode solution can only be recommended for short time experiments. The more complex media, MEM-HEPES medium and especially AQIX® RS-I tissue preservation reagent preserved the tissue much better during perfusion.
CONCLUSION: Perfusion media have to be carefully chosen considering type and duration of the experiments. If excellent tissue quality is required, complex media are favorable. Perfusion pressure is also of great importance due to the fact that a minimum amount of luminal pressure seems to be necessary to trigger intestinal contractions.
Core tip: Perfused intestinal segments can be used as a pharmacological model in order to test drugs acting on motility and gastrointestinal physiology. Different motility patterns can be distinguished using visualization and appropriate software tools. In order to use intestinal segments for pharmacological testing it is important to know which factors affect intestinal motility. Motility measurement and characterization of motility pattern in the ex vivo system is much easier compared to in vivo measurements. The main disadvantage of the ex vivo system is its limited stability. Therefore longer ex vivo times are desirable. In this study we could demonstrate effects of different perfusion media on tissue viability and visualize motility in response to perfusion conditions.
-
Citation: Schreiber D, Jost V, Bischof M, Seebach K, Lammers WJ, Douglas R, Schäfer KH. Motility patterns of
ex vivo intestine segments depend on perfusion mode. World J Gastroenterol 2014; 20(48): 18216-18227 - URL: https://www.wjgnet.com/1007-9327/full/v20/i48/18216.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i48.18216
The ability of the gastrointestinal tract (GIT) to digest food and to ensure energy supply to the body depends largely on its motility. As a result of external and internal stimuli, GIT contractions can be assessed. Especially the enteric nervous system (ENS) in cooperation with pacemaker cells, mechano- and chemo-sensory cells is crucial for the coordination of movements[1-3]. Motor control systems in the gut are redundant. If one mechanism fails, others can quickly replace it. This is a very well adapted endogenous strategy in response to the fact that intestine paralysis is a life-threatening condition[4]. Due to the complexity of factors that influence in vivo motility, intestinal in vitro perfusion is a promising alternative for the investigation of pharmacological stimuli on motility.
In the history of biomedical sciences, perfused organs have been widely used as model systems. They are superior to cell culture approaches considering physiological reactions due to their higher grade of complexity. In vivo organ reactions can be simulated under standardised conditions in vitro without live animal testing. Therefore in vitro evaluation of organ reactions to specific stimuli and quantification can be accomplished more reproducibly.
Various in vitro and in vivo GIT perfusion and motility studies have been carried out by Lammers and others[4-14], partly in combination with modern bioinformatics tools to evaluate different pharmacological stimuli or to characterize physiological reactions of the intestine.
So far, the individual studies can hardly be compared with each other due to the different approaches. The comparability of the data provided by the individual groups strongly depends on compliance with a standardised protocol. If the perfusion mode or pressure changes the data output, conclusions made from the single experiments will be different.
In the actual study, we used a modified small intestinal in vitro model, similar to the one used by Lammers[5]. Motility of the small intestine in response to perfusion can be video-recorded and interpreted by using optical analysis software. We used different pressures and perfusion media to investigate the influence of both upon the read out of the experiment.
Eighteen 19-to-22-d-old Wistar rats of either gender were used for the experiments. The rats were sacrificed by decapitation prior to median laparotomy. Animal experiments were approved by German legislation and the responsible authorities.
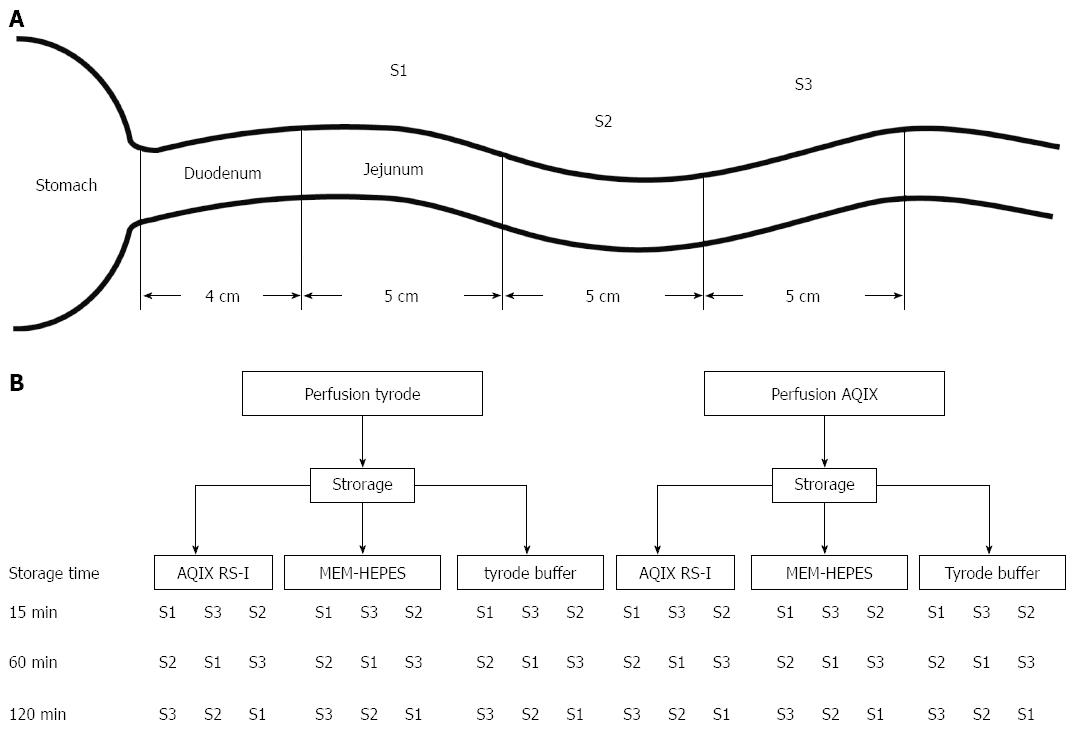
In each animal, three identical subsequent 5 cm long gut segments were excised (S1-S3, Figure 1A), beginning at the ligament of Treitz. Different storage solutions depending on the experimental setting were used until the individual segment was used for the experiment. Segments were stored in 5 mL of the ice cold solution. To investigate the influence of storage upon the tissues, different storage media were used: commercially available storage medium AQIX® RS-I (Aqix ltd, London), MEM-HEPES medium with EBSS (stabilized Glutamine, 25 mmol/L HEPES and 2.2 g/L NaHCO3, PAN-Biotech P04-08250) or Tyrode solution were utilized. Storage was always done on crushed ice.
Tyrode solution contains 130 mmol/L NaCl, 24.2 mmol/L NaHCO3, 11 mmol/L Glucose, 4.5 mmol/L KCl, 2.2 mmol/L CaCl2, 1.2 mmol/L NaH2PO4, 0.6 mmol/L MgCl2. MEM medium (P04-08250) as well as AQIX solution both contain inorganic salts, organic buffer [HEPES for MEM respectively BES (N,N-Bis-(2-hydroxyethyl)-2-aminoethane sulfonic acid) for AQIX] and Glucose. Glucose concentration varys from 5.5 mmol/L for MEM medium to 11 mmol/L for Tyrode solution, respectively 10 mmol/L for AQIX RS-1. In comparison to MEM medium, AQIX contains a smaller variety of amino acids and does not contain any vitamins except thiamine. In contrast to MEM medium AQIX solution contains the hormone insulin, glycerol and carnitine. AQIX RS-I does in contrast to both other media not contain any phosphate.
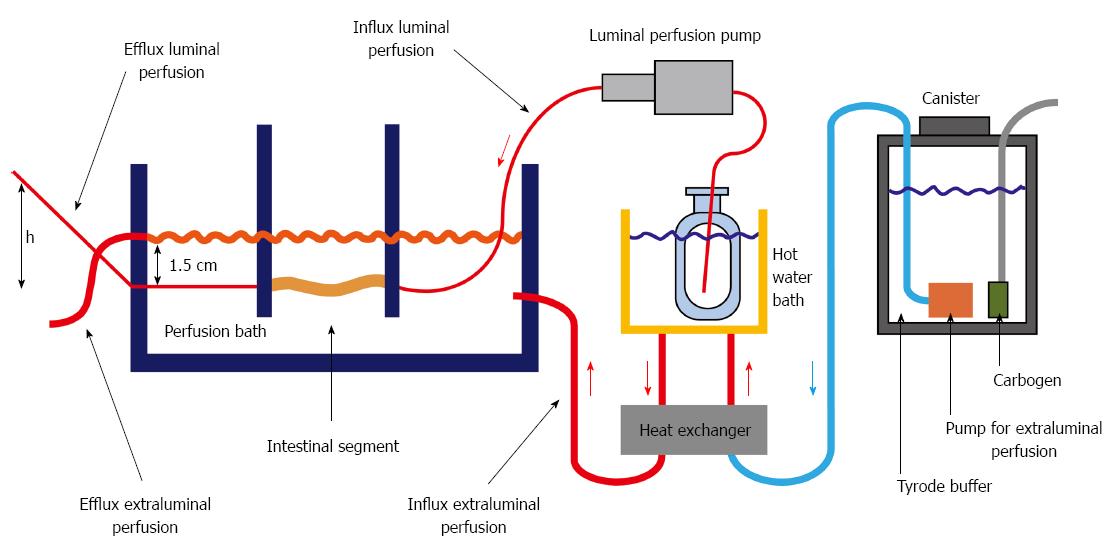
To avoid a bias based on storing time and location, the order of the individual segments was changed from animal to animal. The resected segments were perfused one after the other in different orders (Figure 1B). Luminal pressure was increased from 0 to 10 cm water column (height h see Figure 2) by increasing the height of the luminal efflux tubing. Each perfusion pressure setting was applied for three min, after a three min stabilizing period, which gives a total perfusion time of 33 min. Taken into account the time needed for mounting the segment in the apparatus a time window of 60 min was calculated for each segment.
Perfusion was performed both supraserosally and luminally.
Tyrode solution was chosen as an external perfusion medium for serosal superfusion[5]. Due to the more sensitive mucous layer Tyrode solution or AQIX® RS-I was used for the luminal perfusion.
Three different solutions (Tyrode, MEM-Hepes, AQIX® RS-I) were evaluated for their influence on tissue quality and intestinal motility. Using different liquid perfusion media, the influence of mechanic stimuli by solid nutritional contents can be excluded. Therefore nutritional contents, osmolarity and pH value as well as perfusion pressure are the main factors to influence motility during perfusion. Perfusion was carried out in a custom designed organ bath (6.7 cm × 5 cm × 30 cm). The isolated intestinal segment was superfused with Tyrode at a constant flow rate of about 100 mL/min (Heissner submerged pump Typ P6), which superfused the isolated intestinal segment. Thus a continuous video recording could be performed. The Tyrode solution was gassed with carbogen (95% O2, 5% CO2). During the perfusion the pH was kept constant at 7.35 ± 0.05, the temperature at 37 °C. The intestinal lumen was flushed with different media: AQIX RS-1 solution and Tyrode solution. For luminal perfusion, a mzr-7205 gear pump (HPN Mikrosysteme GmbH) was used to generate a constant flow of 2.5 mL/min. A model of the perfusion apparatus is shown in Figure 2.
Motility patterns of nine intestinal segments per approach were evaluated depending on storage and perfusion medium. Motility patterns were counted and mapped for different luminal pressures. Perfusion pressure was increased step by step.
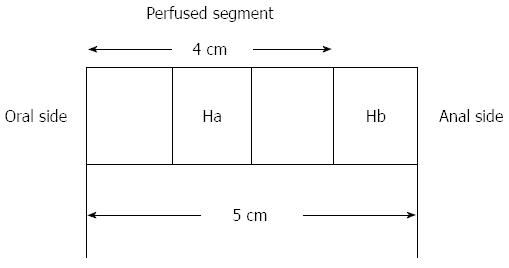
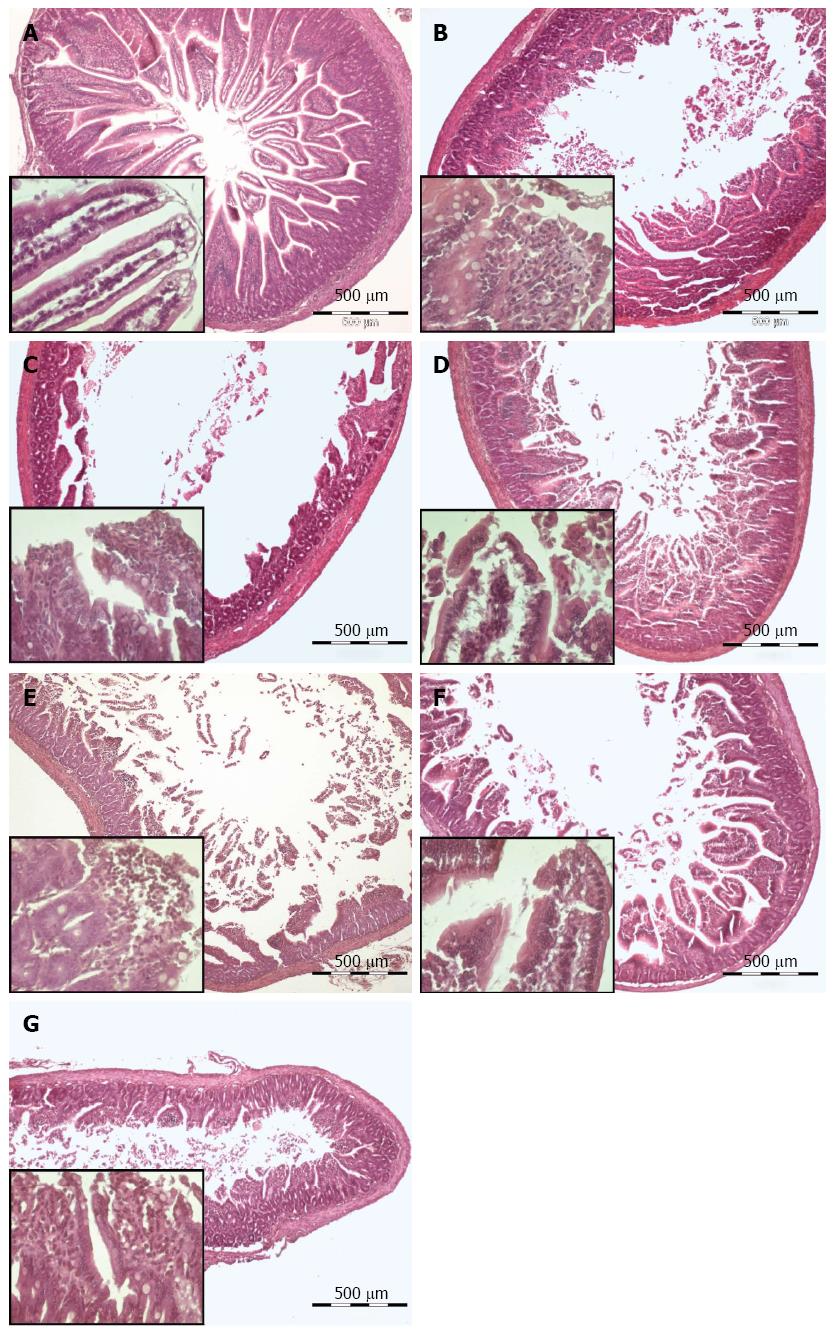
Prior to perfusion a 1 cm-sample of the distal part of each individual segment (Hb) was removed and fixed in 4% formaldehyde in Phosphate Buffered Saline (AppliChem) for histological evaluation. After the perfusion an equivalent piece of tissue (Ha) was resected from the middle of the perfused segment and processed likewise (Figure 3). The comparison of the two samples allowed to analyze the influence of the perfusion on the tissue.
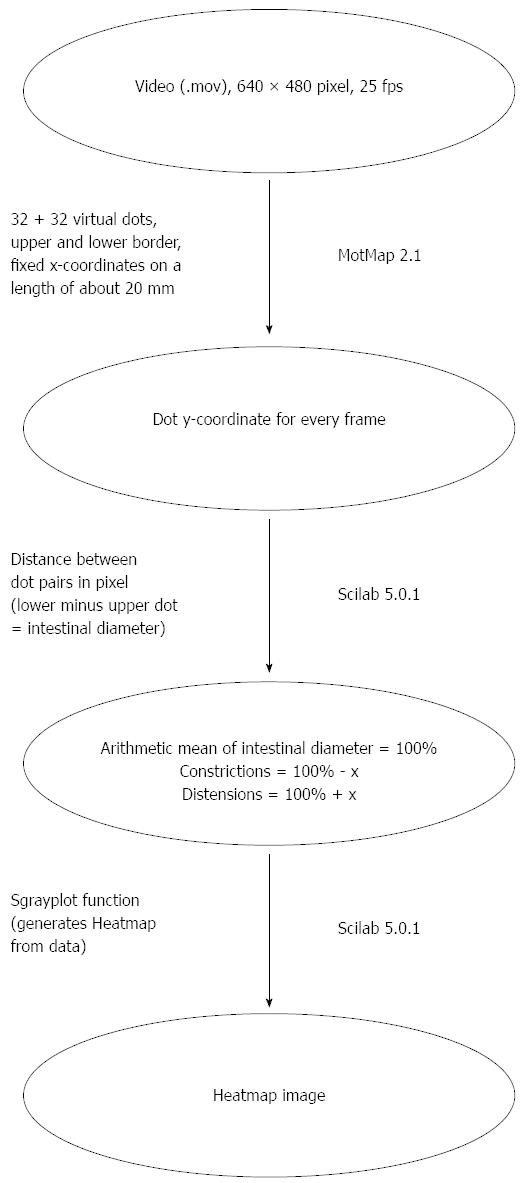
Gut motility in the perfusion chamber was recorded with a Panasonic NV-GS500 video camera at 25 fps (frames per second). In order to generate heatmap images, the program Motmap 2.1 (http://www.smoothmap.org) was used to quantify motility. Thirty-two tracking dots were virtually assigned to a 2 cm long intestinal borderline for both upper and lower side of the gut. The individual dots could be followed and the movements of each pair of dots (upper and lower) were individually calculated for every frame of the video. The difference between the lower and upper value is a measure for the diameter of the gut at a given position and time. Small values mean strong contractions and vice versa. One minute of every pressure setting was evaluated for each gut segment. The diameter of each dot pair on the intestinal boarder for every frame of the video was documented and saved in a table. The data was standardised and outlier values erased. The motility tables were graphically depicted in heatmap diagrams (Figure 4). For every given dot pair (y-axis) at every given time (x-axis) a color coded diameter value was plotted. Then Scilab 5.0.1 (http://www.scilab.org) was used for generating the heatmap images that revealed different motility. For a detailed description of the heatmap generation see Figure 5.
For statistical analysis one way ANOVA (OriginPro 9.1) was used. Data was considered statistically significant if P was smaller than 0.05.
Three distinguishable motility patterns were found: Motility pattern 1 (MP1) are contractions in the anal direction in connection with (Figure 4B) or without a distension of the wavefront (Figure 4A). Distensions can be recognized by a broadening of the wave front on the heatmap (see markings in Figure 4B, D and F). MP2 are contractions in the oral direction in connection with (Figure 4D) or without a distension of the intestine (Figure 4C). MP3 are contractions in the anal and in the oral direction in connection with (Figure 4F) or without a distension of the intestine (Figure 4E). Irregular contractions that cannot be allocated to any other type of contraction (Figure 4G) were recorded in between the distinguishable patterns. They are mainly characterized by short distance anal and oral waves as well as pendular movements and tonic contractions (Figure 4).
Intestinal motility was characterized depending on the direction of the propagating contractions. The direction of contractions in the Heatmap image can be determined by the direction of the waves (see arrows in Figure 4). The stronger the luminal diameter of the intestine decreases at a certain measuring point and a certain time, the darker the heatmap. The units of scale are percentage of the average intestinal diameter.
Three individual patterns can be seen: contractions starting at the oral side going to the anal side (Figure 4A, B) with (Figure 4B) or without (Figure 4A) distensions, contractions starting at the anal side going to the oral side (Figure 4C, D), also with (Figure 4D) or without (Figure 4C) distensions, as well as contractions in both directions (Figure 4E, F), also with (Figure 4F) or without (Figure 4E) distensions of the wavefront. The most common forms were irregular contractions of different shapes (Figure 4G). Distensions of the wavefront where seldom observed.
The amount of contractions in different intraluminal pressure situations was recorded. Contractions were evaluated and assigned to the categories “increasing amount of contractions”, “stable amount of contractions” and “decreasing amount of contractions”. With increasing luminal pressure, an increasing amount of contractions could be recorded, a phenomena that could be observed up to a pressure of about 3 cm of water column. Between 4 and 9 cm the amount and quality of contractions was stable. At 10 cm water column the amount of contractions slightly decreased. Therefore the pressure area from 4 to 9 cm water column was chosen for the evaluation of motility patterns dependent on storage and perfusion media.
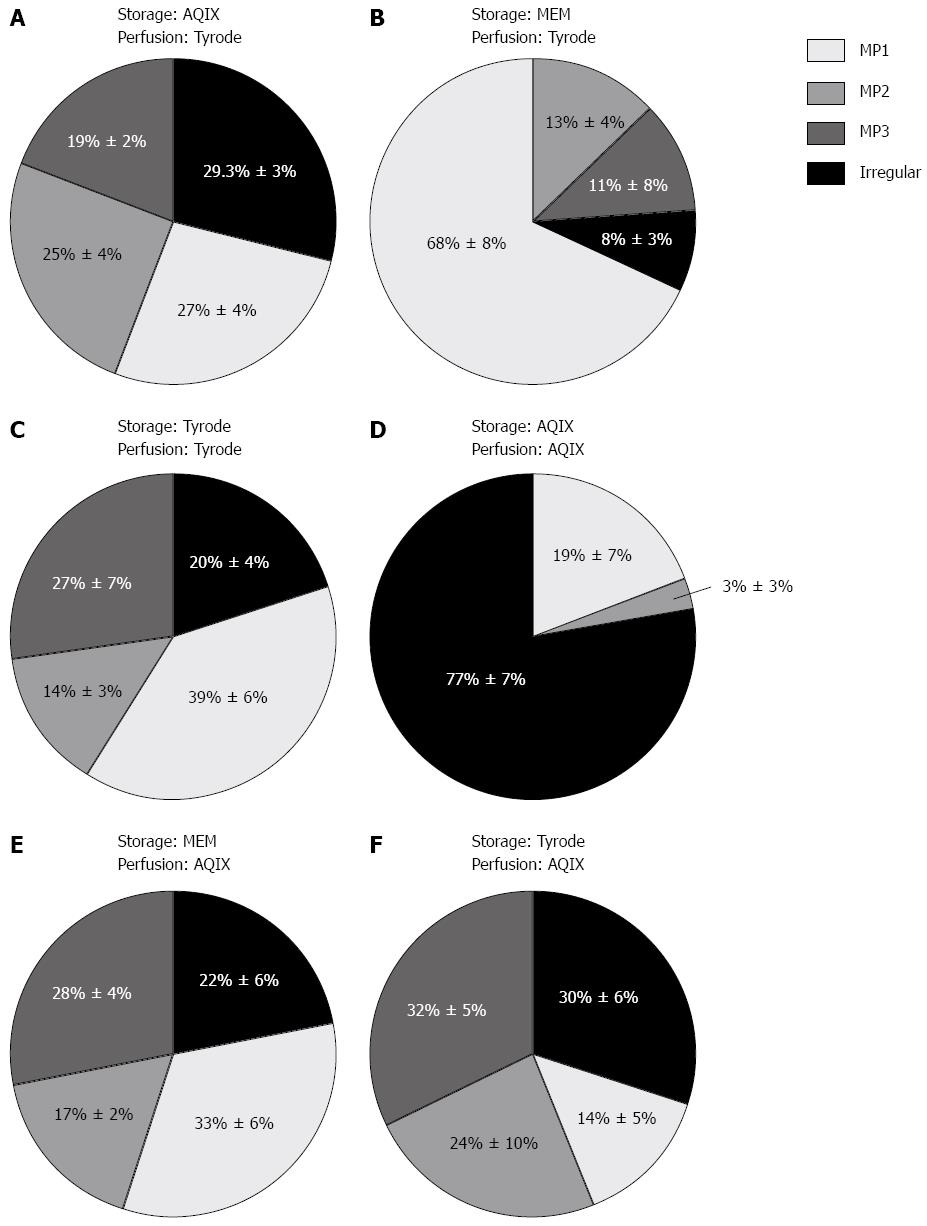
The perfusion medium influenced quantity and quality of motility patterns observed (Figure 6). Storage and perfusion medium are indicated in the figures. Perfusion with AQIX RS-I solution results in a bias towards irregular motility. Especially if AQIX solution is used for storage and perfusion, irregular motility is quite dominant. The percentage of irregular motility on the different pressure levels shown in Figure 6 is significantly higher in segments stored and perfused with AQIX RS-I solution compared to the other media combinations. If Tyrode solution is used for perfusion MP1 is observed as the dominant contraction type. To sum up the effects of the selected perfusion media, a combination of MEM Medium for storage and Tyrode solution for perfusion as well as Tyrode solution for storage and perfusion led to a consistent recording of MP1 in the whole pressure range. In MEM stored/Tyrode perfused segments the percentage of MP1 is significantly higher on the different pressure levels compared to all other media combinations. The other combinations of storage and perfusion medium investigated led to a relatively homogenous distribution of the different motility patterns with a slight bias towards irregular motility. Regarding the percentage of the different motility pattern there are no statistically significant differences between AQIX/Tyrode, Tyrode/Tyrode and MEM/AQIX for storage/perfusion.
With increasing pressure, the amount and type of contractions changed (Figure 7). In case that a minimum pressure of about 3 cm of water column is reached, the increasing luminal pressure does not seem to affect movements. The quantity and quality of intestinal peristaltics are quite stable in a pressure range between 4 and 9 cm of water column. Low perfusion pressure leads to a low frequency of contractions. Irregular contractions make up most contractions in the 0-1 cm pressure range independent of the perfusion medium used (Figure 7). The total amount of contractions in the medium pressure range between 4 and 9 cm is significantly lower compared to the other media combinations if AQIX is used for storage and perfusion.
Storage duration (15, 60 or 120 min) did not decrease the intestine’s ability to contract. For histological comparison intestinal segments were stored for 60 min in different media on ice and subsequently perfused for 33 min with the same medium. Storage and perfusion with AQIX led to a superior tissue quality in contrast to storage and perfusion with Tyrode solution. In addition, the effect of MEM medium for storage and perfusion was evaluated, which led to an intermediate tissue quality. Mucosa damage was strongly decreased using AQIX as storage and perfusion medium (Figure 8). Light microscopically there were no differences seen concerning the muscle layer between the different perfusion media. The muscular layer is morphologically intact after perfusion with all three perfusion media (Figure 8C, E, G). Mild destruction of the intestinal villi tips can be observed after perfusion with MEM medium and AQIX solution. After Tyrode perfusion large mucosal areas are completely depleted.
A variety of perfused intestine models currently available for pharmacological testing use intestinal muscle strips which are mounted on an isometric apparatus to test muscle contraction strength in response to different stimuli. Strips or sections of intestinal tissue which are connected to a force transducer can be used to characterize substances that affect smooth muscle function[15,16]. Depending on the conditions of perfusion or applied substances, an increase or decrease in contraction strength can be detected. The advantage of this system lies in a high reproducibility and a simple readout. Nevertheless the experimental setting is quite artificial, due to the fact that the natural division of the intestine in the body into a luminal and a peritoneal side is completely ignored. Moreover it happens very often that only longitudinal or circular muscle is used.
A valid and reproducible model for the recording and evaluation of intestinal peristalsis in vitro was established by Benard et al[17]. Benard used vertically fixed intestinal segments which are spanned into a perfusion bath under isometric conditions. In contrast to our model he used Krebs solution and segments were about 10 cm in length. He did not characterize different motility patterns into detail, although propulsive movements were described in Benard’s study. Similar to our model it allows a much more sophisticated evaluation of intestinal motility compared to the isometric testing based on muscle strips. Vertical and horizontal contractions can be distinguished. Therefore specific effects of pharmacological substances or perfusion media can be assessed much more into detail. Although the model by Benard is quite useful for pharmacological testing it has certain limitations. Pharmacological substances which are supposed to be tested have to be added to a large volume of perfusion medium. In contrast to the model we used pharmacological active compounds were only added to the luminal and not to the supraserosal perfusion medium. Luminal and supraserosal perfusion are not separated. This does not allow luminal perfusion with a different perfusion medium and pressure. In our study, however, especially these parameters turned out to be crucial for triggering different types of peristaltic patterns.
Similar to the media effects, perfusion pressure also influences the types of contractions observed. In the context of our pressure dependent perfusion experiment it is important to know that contractions of the intestine can be mechanically triggered by solid nutritional components. Low perfusion pressure leads to a low frequency of contractions. Food contents perform mechanical pressure on the intestine and are thereby involved in the triggering of contractions. The peristaltic reflex, which leads to a transportation of food contents in distal direction in the guinea pig small intestine, can be triggered by distension of the intestinal lumen[18]. In our setting mechanical pressure and therefore distension of the intestinal wall was induced by hydrostatic pressure of the perfusion fluid on the complete inner surface of the intestine. It is important to remove food residues from the lumen before starting perfusion in order to avoid mechanical pressure triggered by solid contents.
In our setting about 4 cm of water column were sufficient to generate stable contractions. Pressures over 9 cm of water column decreased slightly the ability of the intestine to contract. A reason for this could be the unphysiological expansion of the intestinal lumen. After reaching a minimum pressure of about 2 cm of water column the intestinal diameter did not distend any further.
In vivo intestinal motility in mammals is characterized by the interdigestive migrating motor complex between digestive periods[19]. So called housekeeping contractions clean the intestinal lumen in interdigestive periods from bacteria and food residues. During digestion mixing movements as well as peristaltic movements in anal direction so called “fed pattern” occur. It is also known that different food compositions produce different myoelectric patterns and hence different types of intestinal motility[20,21]. We found that different media compositions similar to food composition in vitro affected the quantity of the motility patterns observed. Quantitative and qualitative effects of the three different perfusion media on intestinal motility could be documented. The small intestine changes its motility patterns depending on the perfusion medium used. As soon as a certain minimum pressure level is reached the effect of different perfusion media and their different ingredients seems to be more important than intraluminal pressure.
MP1 is the dominant contraction type in the small intestine with most perfusion media and pressure settings used. This contraction pattern is characterized by waves which spread in anal direction. The physiological function of this sort of contraction is probably transportation from the oral to the anal side of the GIT in combination with mixing of the food pulp as a form of propulsive peristaltics. MP2 is the second most abundant motility pattern consisting of waves with a oral direction. MP3, a more seldom type of intestinal motility, describes contractions which move in oral as well as in anal direction. Apart from that irregular contractions, which cannot be compared to other types of contraction non-propulsive peristaltics, rhythmic segmentation, pendular movements as well as tonic contractions could be observed and quantified. Irregular contractions are often locally restricted and do not make up rhythmic, repetitive patterns. The amount of contractions with a specific direction is reduced. In vivo this pendular peristalsis is necessary for mixing up the food pulp. The in vitro situation which does not reflect the autonomic nervous system and hormonal mediated influences could be a further important reason for the generation of irregular contractions without specific directions.
Perfusion with AQIX RS-I solution results in a bias towards irregular contractions. If Tyrode solution is used for perfusion, MP1 was observed as the dominant contraction type. A combination of MEM Medium for storage and Tyrode solution for perfusion as well as Tyrode solution for storage and perfusion led to a consistent recording of MP1 in the whole pressure range. All other combinations of storage and perfusion medium investigated led to a relatively homogenous distribution of the three different motility patterns.
Due to the fact that the complete pattern of different intestinal movements can be observed using Tyrode solution for luminal perfusion, Tyrode solution can be recommended for short time experiments. Long-term perfusion with Tyrode usually causes tissue damage. If intestinal tissue has to be stored, AQIX RSI solution or MEM medium are superior to Tyrode solution concerning tissue preservation. AQIX RS-I can be recommended for tissue preservation and perfusion, if the focus is on a superior tissue quality. This includes testing for the uptake of substances from the luminal perfusion medium as well as the transplantation or transportation of intact tissue samples. It is important to standardise the perfusion medium, in case a series of experiments is performed. Pharmacological test results cannot be compared, if different perfusion media were used during the experiments. Different physiological effects can be triggered when using different perfusion media.
Experiments with different perfusion media in adult rat and mouse intestine have shown that histological features of the mucus layer are usually destroyed after 30-120 min of luminal perfusion depending on the perfusion medium used. The submucosal layer, muscle layers and the ENS maintain their functional activity over longer periods of time, ranging from several hours to days. Therefore we can recommend Tyrode solution only for short time experiments lasting 30 min or less, especially if superior tissue quality is crucial. Although Tyrode solution contains different salts in physiological concentrations and glucose, it cannot render the mucosa undamaged over longer periods of time probably due to the lack of amino acids and vitamines.
First steps have already been taken to set up a luminal/vasal combined perfusion model that allows motility recordings of short intestinal segments with an adhering mesenteric root[14]. This approach does not only allow for motility evaluation but also maintains tissue quality over longer periods of time similar to the perfusion system created by Lautenschläger et al[22]. As shown by this group, it is possible to maintain tissue integrity and nearly normal physiological functions over some hours in vitro using luminal/vasal perfusion. The combined perfusion allows for the induction of inflammation or other pathophysiological conditions that can be treated in vitro. Slow physiological processes that cannot be investigated in a luminal only perfused model could be simulated especially in order to test pharmacological treatments. Our luminal/vasal perfused model also facilitates real-time motility monitoring and characterization. Conditions that influence motility can be identified. Quantitative as well as qualitative changes in motility patterns can be evaluated. In all approaches which were performed, different types of repetitive patterns could be identified. Equivalent gastrointestinal motility patterns can also be found in vivo. The quantity of the single patterns depends strongly on the perfusion medium used and the perfusion pressure used.
Perfused intestinal segments show a broad spectrum of motility in vitro which is controlled by the enteric nervous system. The enteric nervous system is located in the gut wall. Intestinal segments can be perfused through the intestinal lumen. Vascular perfusion is also possible. The segments can be used as an ex vivo model to expand our knowledge on gastrointestinal physiology and pharmacology. Nevertheless perfusion conditions have to be carefully evaluated and standardised to ensure comparability between different experimental settings.
Intestinal ex vivo models can expand the knowledge on how digestion and intestinal physiology in general works. Visualization and recording of intestinal activity as well as evaluation of influential factors are important hotspots in the area of gastrointestinal physiology. They can help us understand how the autonomous nervous system of the gut, these “second brain” works.
Numerous studies have shown the feasibility of the in vitro perfused intestine in short time experiments. The effects of pharmacological stimulation increasing or decreasing motility could be shown. In more recent studies visualization and heatmap images were used, which allow the reader to obtain a more distinct picture of how motility works. Due to different perfusion media and conditions used in those studies their contribution to general knowledge on gastrointestinal physiology is limited.
By understanding how different perfusion conditions and media affect motility in the small intestine different studies can be compared more easily. It is especially important to stress the effect of perfusion pressure on the amount of contractions and the effects of different media on tissue preservation. Under carefully chosen perfusion conditions, intestinal segments can be kept vital ex vivo over longer time periods.
Intestinal motility is made up of contractions of the intestine governed by the so called enteric nervous system (ENS). The ENS is located in the intestinal wall. It is a part of the autonomous nervous system and independent from the central nervous system. The ENS provokes and coordinates contractions which often have a specific direction, for example an anal direction in order to transport gastrointestinal contents. The intestine can generate different motility patterns for example propulsive peristaltics with an anal or an oral direction, pendular movements, constrictions and combinations of different patterns.
This study describes a perfused small intestine model using rat intestines. Different perfusion media and pressure settings were characterized in order to show their effects on motility. Tissue quality dependent on the perfusion media was also assessed.
P- Reviewer: Capasso R, Edward C, Plaza MA S- Editor: Nan J L- Editor: A E- Editor: Ma S
| 1. | Kunze WA, Furness JB. The enteric nervous system and regulation of intestinal motility. Annu Rev Physiol. 1999;61:117-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 265] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 2. | Nakagawa T, Misawa H, Nakajima Y, Takaki M. Absence of peristalsis in the ileum of W/W(V) mutant mice that are selectively deficient in myenteric interstitial cells of Cajal. J Smooth Muscle Res. 2005;41:141-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Sanders KM. Regulation of smooth muscle excitation and contraction. Neurogastroenterol Motil. 2008;20 Suppl 1:39-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 122] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 4. | Costa M, Furness JB. The peristaltic reflex: an analysis of the nerve pathways and their pharmacology. Naunyn Schmiedebergs Arch Pharmacol. 1976;294:47-60. [PubMed] |
| 5. | Lammers WJ. Spatial and temporal coupling between slow waves and pendular contractions. Am J Physiol Gastrointest Liver Physiol. 2005;289:G898-G903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Lentle RG, Janssen PW, Asvarujanon P, Chambers P, Stafford KJ, Hemar Y. High definition mapping of circular and longitudinal motility in the terminal ileum of the brushtail possum Trichosurus vulpecula with watery and viscous perfusates. J Comp Physiol B. 2007;177:543-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Lin X, Hayes J, Peters LJ, Chen JD. Entrainment of intestinal slow waves with electrical stimulation using intraluminal electrodes. Ann Biomed Eng. 2000;28:582-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Seerden TC, Lammers WJ, De Winter BY, De Man JG, Pelckmans PA. Spatiotemporal electrical and motility mapping of distension-induced propagating oscillations in the murine small intestine. Am J Physiol Gastrointest Liver Physiol. 2005;289:G1043-G1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Storkholm JH, Zhao J, Villadsen GE, Gregersen H. Spontaneous and bolus-induced motility in the chronically obstructed guinea-pig small intestine in vitro. Dig Dis Sci. 2008;53:413-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Thor PJ, Konturek JW, Konturek SJ. Pancreatic polypeptide and intestinal motility in dogs. Dig Dis Sci. 1987;32:513-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Trendelenburg P. Physiological and pharmacological investigations of small intestinal peristalsis. Translation of the article “Physiologische und pharmakologische Versuche über die Dünndarmperistaltik”, Arch. Exp. Pathol. Pharmakol. 81, 55-129, 1917. Naunyn Schmiedebergs Arch Pharmacol. 2006;373:101-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Vantrappen G, Hellemans J, Vandenbroucke J. A method for the analysis of intestinal motility records. Dig Dis Sci. 1965;10:449-454. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Costa M, Wiklendt L, Arkwright JW, Spencer NJ, Omari T, Brookes SJ, Dinning PG. An experimental method to identify neurogenic and myogenic active mechanical states of intestinal motility. Front Syst Neurosci. 2013;7:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Schreiber D, Klotz M, Laures K, Clasohm J, Bischof M, Schäfer KH. The mesenterially perfused rat small intestine: A versatile approach for pharmacological testings. Ann Anat. 2014;196:158-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Gagon DJ. Intestinal smooth muscles: demonstration of catecholamines-induced contraction mediated through alpha-adrenergic receptors. Eur J Pharmacol. 1970;10:297-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Triggle CR, Grant WF, Triggle DJ. Intestinal smooth muscle contraction and the effects of cadmium and A23187. J Pharmacol Exp Ther. 1975;194:182-190. [PubMed] |
| 17. | Benard T, Bouchoucha M, Dupres M, Cugnenc PH. In vitro analysis of rat intestinal wall movements at rest and during propagated contraction: a new method. Am J Physiol. 1997;273:G776-G784. [PubMed] |
| 18. | Kosterlitz HW, Pirie VW, Robinson JA. The mechanism of the peristaltic reflex in the isolated guinea-pig ileum. J Physiol. 1956;133:681-694. [PubMed] |
| 19. | Romański KW. Migrating motor complex in biological sciences: characterization, animal models and disturbances. Indian J Exp Biol. 2009;47:229-244. [PubMed] |
| 20. | Eeckhout C, Vantrappen G, Peeters TL, Janssens J, De Wever I. Different meals produce different digestive motility patterns. Dig Dis Sci. 1984;29:219-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Soffer EE, Adrian TE. Effect of meal composition and sham feeding on duodenojejunal motility in humans. Dig Dis Sci. 1992;37:1009-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Lautenschläger I, Dombrowsky H, Frerichs I, Kuchenbecker SC, Bade S, Schultz H, Zabel P, Scholz J, Weiler N, Uhlig S. A model of the isolated perfused rat small intestine. Am J Physiol Gastrointest Liver Physiol. 2010;298:G304-G313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |