Published online Dec 28, 2014. doi: 10.3748/wjg.v20.i48.18121
Revised: August 10, 2014
Accepted: September 29, 2014
Published online: December 28, 2014
Processing time: 213 Days and 14.3 Hours
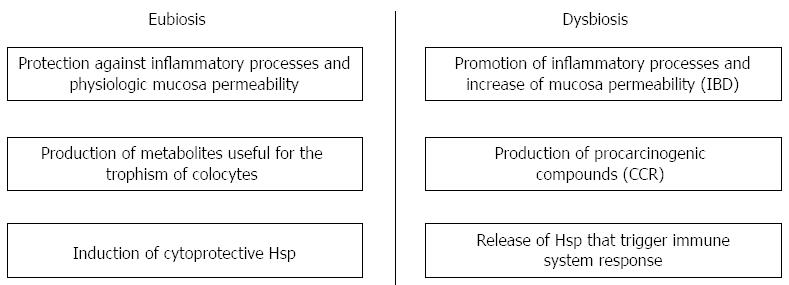
Patients with inflammatory bowel disease (IBD) have an increased risk of 10%-15% developing colorectal cancer (CRC) that is a common disease of high economic costs in developed countries. The CRC has been increasing in recent years and its mortality rates are very high. Multiple biological and biochemical factors are responsible for the onset and progression of this pathology. Moreover, it appears absolutely necessary to investigate the environmental factors favoring the onset of CRC and the promotion of colonic health. The gut microflora, or microbiota, has an extensive diversity both quantitatively and qualitatively. In utero, the intestine of the mammalian fetus is sterile. At birth, the intestinal microbiota is acquired by ingesting maternal anal or vaginal organisms, ultimately developing into a stable community, with marked variations in microbial composition between individuals. The development of IBD is often associated with qualitative and quantitative disorders of the intestinal microbial flora (dysbiosis). The healthy human gut harbours about 10 different bacterial species distributed in colony forming units which colonize the gastrointestinal tract. The intestinal microbiota plays a fundamental role in health and in the progression of diseases such as IBD and CRC. In healthy subjects, the main control of intestinal bacterial colonization occurs through gastric acidity but other factors such as endoluminal temperature, competition between different bacterial strains, peristalsis and drugs can influence the intestinal microenvironment. The microbiota exerts diverse physiological functions to include: growth inhibition of pathogenic microorganisms, synthesis of compounds useful for the trophism of colonic mucosa, regulation of intestinal lymphoid tissue and synthesis of amino acids. Furthermore, mucus seems to play an important role in protecting the intestinal mucosa and maintaining its integrity. Changes in the microbiota composition are mainly influenced by diet and age, as well as genetic factors. Increasing evidence indicates that dysbiosis favors the production of genotoxins and metabolites associated with carcinogenesis and induces dysregulation of the immune response which promotes and sustains inflammation in IBD leading to carcinogenesis. A disequilibrium in gut microflora composition leads to the specific activation of gut associated lymphoid tissue. The associated chronic inflammatory process associated increases the risk of developing CRC. Ulcerative colitis and Crohn’s disease are the two major IBDs characterized by an early onset and extraintestinal manifestations, such as rheumatoid arthritis. The pathogenesis of both diseases is complex and not yet fully known. However, it is widely accepted that an inappropriate immune response to microbial flora can play a pivotal role in IBD pathogenesis.
Core tip: Dysbiosis could be the common denominator of inflammatory bowel disease (IBD) and colorectal cancer. A well balanced gut microbiota promotes the health of colocytes through the production of important compounds and the correct modulation of immune system. Qualitative and quantitative modifications in the bacterial composition are responsible of changes in the biochemistry and in the cell cycle of colocytes. The aim of this work is to focus on the molecular mechanisms that connect dysbiosis, IBD and colorectal cancer. Experimental studies are oriented towards the discovery of new probiotic-based therapies for the treatment and prevention of inflammatory and carcinogenetic processes.
- Citation: Tomasello G, Tralongo P, Damiani P, Sinagra E, Trapani BD, Zeenny MN, Hussein IH, Jurjus A, Leone A. Dismicrobism in inflammatory bowel disease and colorectal cancer: Changes in response of colocytes. World J Gastroenterol 2014; 20(48): 18121-18130
- URL: https://www.wjgnet.com/1007-9327/full/v20/i48/18121.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i48.18121
Inflammatory bowel diseases (IBD), such as Crohn’s disease (CD) and Ulcerative colitis (UC), are pathologies characterized by a chronic inflammation of the gastrointestinal tract. In UC, rectal and descending colon involvement is a typical feature whereas CD can affect any part of the gastrointestinal tract from the mouth to the anus. Their aetiopathogenesis is not yet fully understood[1,2]. It is now well established that close interactions exist between the luminal bacterial flora and the intestinal immune cells and tissues. Such interactions are involved in the onset and progression of several diseases including IBDs and colorectal cancer (CRCs)[3-7]. Dysbiosis of gut microflora may represent the “primum movens” of the chronic inflammation characterizing IBD and triggering the stimulation of the immune system[8-13]. Dismicrobism can cause alterations in the immune response and can, in particular, lead to gut associated lymphoid tissue (GALT) activation[14]. The terms dysbiosis and dismicrobism are being used interchangeably, however, dismicrobism is sometimes more used in reference to the condition evolving consequent to the administration of antibiotics. Specifically, the microbiological imbalance (dysbiosis) causes to a modification of intercellular tight junctions[15] leading to an effective penetration of antigens responsible of the activation of GALT with consequent tissue damage[16]. Olsen and Co-workers tested the reactivity of intestinal T cells derived from patients with CD, UC and undetermined colitis against some bacteria. The results showed that T lymphocytes isolated from CD patients had a high reactivity against Mycobacterium avium compared to patients affected by UC and undetermined colitis. Moreover, the isolated T cells produced inflammatory cytokines like interferon (IFN)-γ and IL-17[17]. These data suggest a possible involvement of Mycobacteria in CD immunopathology. Other bacteria belonging to Clostridia group, Fusobacterium, Mycobacterium, adherent invasive Escherichia coli (E. coli), Proteus mirabilis, Klebsiella pneumonia and Proteobacteria such as Helicobacter spp were found over-represented in the IBD condition[18,19]. Nowadays several studies focus on the possibility to control IBD through the modulation of the immune system by re-establishment of eubiosis through specific probiotic mixtures. The homeostasis of the immune system is very important and involves the equilibrium between pro-inflammatory and anti-inflammatory cytokines such as IL-10 and others. Mgl1-/- mice with a DSS induced colitis were studied before and after probiotic treatment. The induction of colitis in wild type mice showed a less severe phenotype compared to Mgl1-/- mice and a lowered expression of IL-10 in the mutant mice, leading to the evidence that Mgl1 is involved in the regulation of IL-10 production. The results showed that Lactobacillus and Streptococcus were able to bind to Mgl1 receptor, leading to the hypothesis that these bacterial strains may be able to induce an anti-inflammatory response[20]. These findings highlight the importance of gut microbiota both in the triggering and in the modulation of immune system in the chronic inflammatory diseases of the gastrointestinal tract.
There is a relatively wide spectrum of diagnostic tests used for the diagnosis of dysbiosis. They cover all four patterns of dysbiosis by analysis of both stool and urine for respective markers. Reference laboratories specializing in the evaluation of dysbiosis offer a comprehensive testing of various aspects of digestion, absorption, microbiology, metabolic markers and immunology including fetal secretory IgA and sometimes calprotectin. In addition, in some cases of medical necessity, fecal analysis may also include other standard components such as stool culture, stool parasitology and fecal occult blood[21,22].
The etiology of IBD is linked to three basic parameters: (1) genetic predisposition; (2) a dysregulated immune response; and (3) an altered reaction to gut microorganisms. It is also well established that the mechanism of intestinal inflammation, acute or chronic, involves a complex myriad of pathogens (bacteria, viruses or fungi), chemicals, radiation, dietary components and hormones among others[1,11,23]. It also involves another complex series interactions between immune cells (T cells B cells, NK cells, mast cells, macrophages), interleukins, bradykinins, complement system factors, prostaglandins, tumour necrosis factors, histamines, hormones and activated neutrophils products like myeloperxidase, radicals and oxydants[1,23]. In some cases, the body’s reaction is not enough to rid itself from etiological agents and infecting organisms. The reaction persists as a chronic inflammation which proliferates into multiple complications, escalating the damage, especially in genetically susceptible individuals. The big question is how could this chronic inflammation progress and express itself, and how could its complications lead to the formation of cancerous cells? It is well documented that patients with this condition are more prone to the development of malignancy and that the microbiota could sustain the chronicity of the inflammatory state in the presence of a dysregulated immune response[3,6]. Such a persistent inflammation is believed to play a significant role in CRC development[24-26]. Studies in this field reported underlying genetic, proteomic, and epigenetic changes in IBD’s and CRC’s. Individuals with IBD have mostly permissive rather than causative genes[23].
In CD, an early discovery on chromosome 16 (IBD 1 gene) led to the identification of 3 single nucleotide polymorphisms (2 missense, 1 frameshift) in the NOD2 gene (now called CARD 15 is a polymorphic gene involved in the innate immune system and has more than 60 variations, of which 3 play a role in 27% of patients with CD, primarily in patients with ileal disease. Another early genome-wide association study looked at Jewish and non-Jewish case-control cohorts and identified 2 single nucleotide polymorphisms in the lL23R gene, which encode 1 subunit of the interleukin-23 receptor protein. Actually, 21 new loci were identified with an increased risk of developing CD such as the genes CCR6, IL 12B, STAT3, JAK2, LRRK2, CDKAL 1, and PTPN22. Most of these genes are involved in signal transduction in immune function. There is also a strong support for IBD-susceptibility genes on chromosome 5p131.1 which is a gene desert but does modulate expression of the PTGER4 gene. A murine PTGER4 knockout model has also a significant susceptibility to severe colitis[26,27].
In UC, however, the genetic predisposition is less than that of CD. Actually, there is a set of genetic susceptibilities that show significant overlap with CD. A recent genome-wide association study found a previously unknown susceptibility locus at ECM1 and showed several common risk loci between UC and CD. Genes that are a risk of both diseases appear to have an influence on the immune environment of the intestine[28].
Further susceptibility loci for UC have been found on 1p36 and 12q15. The lp36 single nucleotide polymorphism is located near the PLA2G2E gene; involved in releasing arachidonic acid from membrane phospholipids, which leads to other pro-inflammatory lipids. The first 12q15 signal is located near the interferon (IFN)-γ, interleukin (IL)-26, and IL-22 genes, whereas the second 12q15 signal is located in IL-26. These genes have roles in the immune response to pathogens as well as the tissue inflammation processes. It was also shown that genetic predisposition increases the risk for one form of IBD while decreasing that of the other[27].
Chlorinated adducts of DNA and RNA, which are generated from reaction with MPO products, are associated with repetitive infiltration of neutrophils and macrophages to the site of inflammation. Furthermore, these chlorination products increased in amount as the inflammatory state lengthened. Importantly, infiltration by these innate immune cells was accompanied by cancer initiation and progression. Actually, the contributing factors to the onset of colon carcinoma were infiltration of phagocytes and macromolecular damage to DNA and RNA (chlorination) mediated by neutrophil activity. In cases of UC, IL-8, Cl-Tyr, SAA, CRP, procalcitonin, G-CSF, and tissue plasminogen activator have been identified, whereas for CD cases, procalcitonin, SAA, Nitro-Tyr, and IP-10 were identified as the most influential factors[23].
Albumin is the most abundant chlorinated species found in serum; it is synthesized and released in the liver, indicating the liver as a possible site for innate cell activation in IBD patients, and therefore a possible source of the chlorination and nitration products observed in serum. This finding suggests that 5-CI-dC may be a contributing factor in the initiation of colon carcinogenesis through either mutational or gene silencing mechanisms. In addition, two interesting proteins were identified from a proteomics study: Orosomucoid 2 (“-1-acid glycoprotein 2) and Peptidylglycan recognition protein 2 (PGRP2, also named N-acetylmuramoyl-L-alanine amidase). Association of orosmucoid 2 with UC disease activity was not established, but it was associated with development of colorectal cancer[23].
Concerning colorectal cancer, it has been reported for several decades that carcinomas mostly develop from adenomas and chronic inflammations. In 1988, Vogelstein described four specific mutations that accumulate during the progression of adenomas to carcinomas. These mutations seem to involve gatekeeper genes, which enable genetic or epigenetic instability and support tumor growth[29].
Various factors have been shown to be responsible for the accumulation of mutations in CRC including inheritance and environmental factors (e.g., composition of diet, obesity, diabetes mellitus, smoking, alcohol consumption) as well, besides the fact that chronic inflammation sustained by microbial flora is regarded as an important risk factor for the development of cancer[30].
On the other hand, IL-6 is regarded as an important tumor promoting factor in many types of human cancer including glioma, lymphoma, melanoma as well as breast, ovarian, pancreatic, prostate, renal and, colorectal cancer[29,31].
Various studies have found an increased expression of lL-6 in patients with CRC, where lL-6 levels are elevated in the serum of patients and in tumor tissue itself. According to a review article by Knupfer and Preiss, lL-6 expression can be associated with tumor stage, size, metastasis and survival of patients with CRC[32].
Many studies have revealed that lL-6 is an important regulator of IBD pathogenesis, mainly through its effect on immune cell function in the gut. lL-6 trans-signalling has been shown to activate T cells in the lamina propria of patients with IBD and induces resistance of these cells against apoptosis through upregulation of anti-apoptotic factors such as Bcl-2 and Bcl-xl. IL-6 is deemed to act as a link between chronic inflammation and tumor development due to its increased expression in IBD patients[29].
There is a myriad of factors, in addition to intestinal microbiota, that play a crucial role in the development of IBD and CRC. An altered microbiota, termed dysbiosis, could lead to altered immune functions and increased risk of disease[33]. The influence of diet on the composition of the microbiota has been well documented during the initial colonization phase, postnatally and beyond, even in adult life[34]. Several studies demonstrated that dietary factors have a dominating role in altering the microbial community resulting in biological changes to the host. Actually dietary changes could explain 57% of the total structural variation whereas genetic factors accounted for less than 12%[35]. For example, the “Western” diet, which is high in sugar and fat, causes dysbiosis affecting both gastrointestinal (GI) metabolism and immune homeostasis[30]. Vegetarianism, which is high in fibbers, results in increased short chain fatty acid production by microbes leading to a decrease in intestinal pH and consequently preventing the growth of potentially pathogenic bacteria such as E.coli and members of the Enterobacteriaceae[36]. In brief, it is becoming well documented that the microbiota could be modified through dietary factors to enrich beneficial microbes and prevent diseases associated with dysbiosis or promote such diseases like; IBDs metabolic diseases like diabetes, obesity as well as irritable bowel syndrome, celiac disease and also CRC. Diet-induced dysbiosis affects disease susceptibility[37].
Understanding how extrinsic factors such as diet alter disease susceptibility through modification in the microbiota could shed light onto the function of microbes in healthy and diseased individuals. A strong and well documented correlation exists between the composition of the gut microbiota and diet. In general, dietary change could explain most of the total structural variation in gut microbiota in metabolic syndrome repeats[35].
Such microbial changes in the GI tract have profound effects on host inflammatory and metabolic responses. So far, it is not known for sure whether diet-induced dysbiosis is a transient or long-term event[38]. This is a very dynamic area of research since dietary choices are proven to modify the ecology of intestinal microbiota which could increase susceptibility to disease like, IBD and CRC.
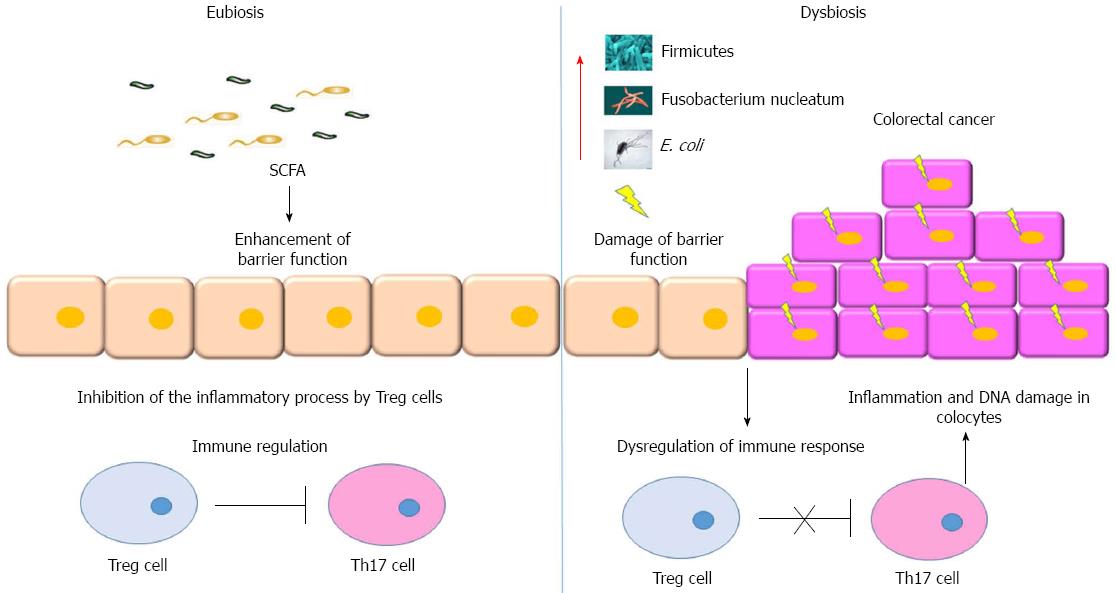
The next-generation sequencing and taxonomic studies, based on ribosomal 16S bacterial genes, give a clearer picture of the microorganisms inhabiting our intestine in physiological and pathological conditions, although a healthy core microbiota has not been clearly identified. The microbiota analysis on tissues and fecal materials has identified various microbial groups associated with CRC. Stool samples derived from CRC patients harbour a higher population of bacteria belonging to the group Bacteroides-Prevotella compared with normal controls[24]. Another study showed an enrichment in the luminal compartment of CRC patients compared to controls of bacteria belonging to Enterococcus, Escherichia, Shigella, Klebsiella, Streptococcus, and Peptostreptococcus, and a depletion of bacteria belonging to Lachnospiraceae family (butyrate-producing bacteria)[25,39]. CRC is a multi-step process that includes adenoma formation. Studies of microbiota on intermediate stages revealed an increased abundance of Firmicutes, Bacteroidetes, and Proteobacteria at the intestinal mucosal surface in patients with adenoma compared with non-adenoma subjects[34]. Moreover, resected tissues from adenocarcinoma patients and adjacent non-malignant sites showed expansion of the phyla Bacteroidetes and reduction of Firmicutes in tumors compared with healthy subjects[4]. Interestingly, through whole-genome and RNA-sequencing approaches two independent groups found a high prevalence of Fusobacterium (over-represented also in IBD) in colonic tissues of CRC patients compared with normal controls[40,41].
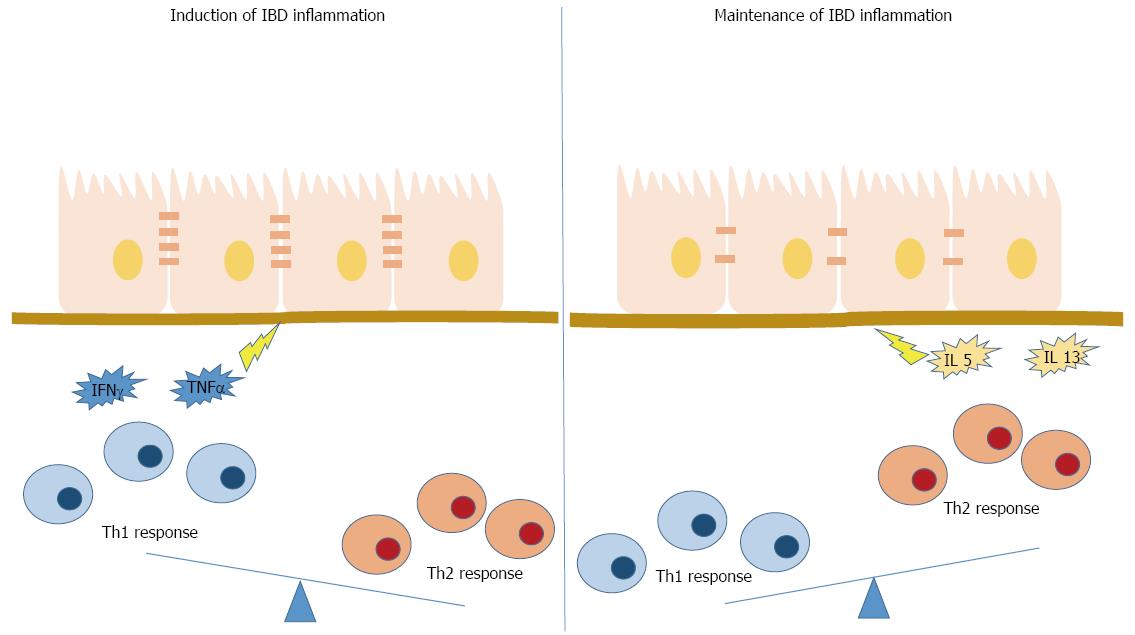
The intestinal microbiota is involved in aetiopathogenesis of autoimmune and allergic diseases and can also play a role in the development of CRC[6]. Many cancers arise from sites of infection and chronic inflammation. The strongest association of chronic inflammation with malignant diseases is found in inflammatory bowel diseases of the colon with a lifetime incidence of 10% or more. Thus, patients with IBD have a higher risk of CRC occurrence[1,42]. Both innate and adaptive immune responses have been involved in the pathogenesis of IBD. The first one involves particular receptors called Toll like receptors or PRR (Pattern recognition receptors). It has been considered as an impairment of the innate immune response rather than an excessive activation, which may be involved in the development of CD. In fact some genes such as NOD2 (an intracellular PRR involved in the recognition of the bacterial muramyl dipeptide) showed mutations consisting in a loss rather than a gain of function[43]. Regarding the adaptive immune response, IBD is characterized by dysregulated T lymphocyte effector cells, and shows a Th1 and Th17 activation to the endogenous microbiota, and a lack of immune suppression typically afforded by Tregs[44]. CD is considered a disease with an increased Th1 response while UC is a Th2-mediated disease. This paradigm relies on evidence showing that intestinal tissues derived from patients with active CD contain elevated levels of IL-12 and IL-18 (Th1-polarizing cytokines), compared with UC patients[45,46]. Moreover, in CD patients, an increased amount of Th1 lymphocytes has been reported compared to control subjects[45]. Evidence from studies conducted on humans and animal models suggest that the different subsets of lymphocytes play their role in different stages of IBDs[43]. Studies of CD pathogenesis in SAMP1/YitFc mice indicate an involvement of Th1 cells in the initiation process of the disease. The establishment of chronic inflammation is supported by IL-5 and IL-13 cytokines, indicating a role of Th2 subset in this stage of CD[47]. These data indicate that adaptive immune responses involved in IBD pathogenesis are more complex than the traditionally dichotomous Th1/Th2 paradigm (Figure 1).
About 1%-2% of CRC patients have a pathological background consisting of intestinal mucosa inflammation[48]. This “inflammatory background” of colonic mucosa can evolve to a less (low-grade) or more severe (high-grade) dysplasia, which through neoplastic transformation gives rise to carcinoma “in situ” and finally “invasive” carcinoma. Interestingly, many lines of evidence highlighted the importance of intestinal microbiota in the development of CRC[42] and that the type of immune response generated by the gut commensal bacteria could potentially influence tumor immunity[49]. Mice colonized with enterotoxigenic B. Fragilis exhibit colonic Th17 inflammatory infiltrates that are involved in induction of colon tumors, as indicated by inhibition of colon tumor formation following treatment with anti-IL-17 antibody[50]. It is, therefore, possible that a microbiota favouring commensal bacteria, that induces a Th17 response, could have differential effects on tumors depending on the type of tumor or the stage of tumor development[49]. The amount of lymphocytes infiltrated in the tumoral tissue is correlated to the prognosis of the patients affected by CRC[51]. Among the different subsets of T lymphocytes studied in the immune system is the γδ population. This lymphocytic subset is characterized by the presence of γδ chains in their TCR and their representation in peripheral blood is < 10%. Since γδ T cells express both natural killer receptors (NKG2D) and γδ T cell receptors, they are considered as a link between innate and adaptive immunity[52]. CRC comprises a small population of cancer stem cells (CSC) responsible for tumor resistance to therapies. It has been observed that the treatment with the bisphosphonate zoledronate sensitizes colon CSCs to γ9δ2 T cell cytotoxicity through the production of pro-inflammatory cytokines [IFN γ and tumor necrosis factor (TNF)-α] and granule exocytosis pathway. The γδ T cells response is highly dependent on isoprenoid production by tumor cells[53]. Isoprenoids are a class of natural compounds derived from five-carbon isoprene units assembled and modified in different ways. Most of them have multicyclic structures found in all classes of living organisms such as bacteria. These compounds are under investigation for their involvement in bacterial metabolism and immunomodulation of γ9δ2 T lymphocytes. Isoprenoids are synthesized through the mevalonate pathway or the alternative 2C-methyl-D-erythritol 4-phosphate (MEP) pathway. It has been observed that the MEP intermediate pathway 1-hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate (HMB-PP) can activate human γ9δ2 T cells. Some pathogen bacteria such as Campylobacter jejuni and Salmonella enterica have genes involved in the MEP pathway. Since HMB-PP and other isoprenoids are able to induce activation of γ9δ2 T cells, this metabolic feature shared by bacteria and host cells should be used to fight together dysbiosis and the tumorigenesis process of CRC.
The “chaperoning system” is a concept that was proposed in 2008[54]. Heat shock proteins (Hsps) are cellular complexes involved in processes like folding, refolding, translocation and degradation of intracellular proteins under normal and stress conditions[6,55]. Hsp chaperones are also involved in many cellular processes and interact with the immune system. They start to be considered as potential biomarkers of carcinogenesis, correlating their expression to the degree of differentiation and aggressiveness of certain tumors. Pathological conditions in which chaperones become etiological factors are called “chaperonopathies” and are classified into three cathegories: by defect, by excess, and by mistake[56]. Commensal microbiota may affect the immune system influencing the expression of specific Hsps. In particular, Hsp60 and Hsp70, both constitute a group of auto-antigens able to trigger immune-regulatory pathways, involved also in other human inflammatory processes such as rheumatoid arthritis[57], Type 1 diabetes mellitus[58], atherosclerosis[59] and allergy[60], through the stimulation of both innate and adaptive immune responses[61]. These proteins were found increased in both tissue and serum of patients with UC[7]. The microbiota is able to regulate Hsp expression. Studies conducted on patients affected by IBD showed a decrease of Hsp60, Hsp10 and Hsp70 in epithelium and lamina propria after a combined therapy of 5- amino salicylic acid plus probiotics (Lactobacillus Salivarius, Lactobacillus Acidophilus, and Bifidobacterium Bifidum)[62]. Treatment of gut epithelial cells with Lactobacillus GG showed an increase in Hsp25 and Hsp72 (considered as cytoprotective Hsps) in a time and concentration dependent manner. These data suggest that the microbiota is able to influence Hsps expression in colocytes.
Gut microflora has a great importance; it influences the colonocyte’s life under multiple aspects. In certain groups of individuals, the onset of CRC is strongly related to diet and composition of the microbiota[42]. The aforementioned correlation could be explained through the anticancer effects of bioproducts of microflora metabolism[63]. Small-chain fatty acids (SCFAs), particularly butyrate, formed from the bacterial fermentation of indigestible carbohydrates, are nutrients and growth signals for the intestinal epithelium and may play a role in CRC prevention[64]. In normal colonocytes, butyrate prevents apoptosis and subsequent mucosal atrophy[10,65]. In contrast, in colon carcinoma cells, butyrate exerts a protective role against the onset of colonic cancer stimulating differentiation, inhibiting cell proliferation, inducing apoptosis and inhibiting angiogenesis[66]. The role of butyrate resides in its effects on enzymatic functions such as the inhibition of the histone deacetylase. This enzyme is responsible of the maintenance of the acetylated state of the histones. Acetylation is important for the access to the chromatin by the enzymes responsible of the transcription of important genes such as those involved in the inhibition of tumorigenesis (p21/Waf1)[67]. Inhibition of histone deacethyltransferases enzymes (HDAC) is one of the therapeutic approaches used in anticancer therapy. Microbial metabolism of cruciferous vegetables or garlic leads to the production of compounds such as sulforaphane N-acetyl-cysteine, allyl mercaptan and butyrate. Thus, microbe-derived metabolites can counteract the carcinogenetic process by triggering cell cycle arrest and apoptosis of tumoral cells through interference with HDAC activity[10,68]. Endoluminal accumulation of toxic compounds can exert a mutagenic action on intestinal mucosa. The microbial fermentation of charred meat leads to the production of poli-heterocyclyc amines (PAH). These compounds are able to damage the DNA of colonocytes. Bacteria belonging to the Clostridium species are able to convert the bile acids into secondary products like deoxycholic acid (DCA). This compound is capable to induce the carcinogenesis process[69]. Tumorigenesis is a multi-step process in which the damage in the DNA is the essential pre-condition for tumoral induction. E. coli has a genic cluster called Pks that encodes for a toxin called calibactin. This was illustrated in studies conducted in mice which are not able to produce IL-10. They were treated either with one strain of E. coli in which Pks was deleted and the others with the wild type strain. The results of this experiment showed that the genic cluster Pks was only able to induce damage on the DNA (assessed trough the presence of H2AX marker) but not sufficient to induce rectal carcinogenesis[65,70]. Experimental data show that consumption of omega-3 polyunsaturated fatty acids is able to decrease the incidence of sporadic colorectal cancer. Eicosapentaenoic free fatty acid reduces polyp formation and growth in models of familial adenomatous polyposis[71]. The intake of docosahexaenoic acid can modify the gut microflora. Breast milk is rich in omega-3 fatty acids. Some studies showed that Bifidobacteria are more represented in breastfed infants rather than in formula-fed infants. In another study, it was shown that the supplementation with fish oil is able to determine changes in Bacteriodetes among children 9-18 mo old[72]. An understanding of the comprehension of the correct equilibrium in the prevalence of the different bacterial strains composing human microbiota may represent one of the keys for the better understanding of CRC etiopathogenesis. In fact, bacterial metabolism has a high impact on the biochemistry of colocytes (Figure 2). This field of study may open the road to therapies oriented not only towards specific probiotic-based therapies but also to targeted “prebiotic-based” approaches for the prevention or treatment of CRC.
The role of environmental factors involved in CRC aetiopathogenesis remains to be fully elucidated. The qualitative and quantitative modifications of the microbiota can make people more susceptible to develop IBD. In fact, these changes can trigger the production of inflammatory mediators in the mucosa that, in a large temporal window, can also exert a pro-cancerogenic role. The immunological and biochemical evidences related to dysbiosis allow the global comprehension of the background on which relies the development of CRC (Figure 3). These findings suggest a close connection between dysbiosis, IBD and CRC. Up till now, the direct or indirect role of bacteria in the induction of genomic damages in colocytes is not fully comfirmed. Several clinical trials have focused the preventive and therapeutic action of probiotics in the treatment of digestive diseases. Probiotics could inhibit the inflammatory and the carcinogenetic processes through several pathways including the re-equilibration of the host immune responses and through the anti-proliferative activity on tumor cells. However, further clinical studies seem necessary to clarify the complex interplay between dysbiosis, inflammation and CRC.
P- Reviewer: Reggiani-Bonetti L, Teramoto-Matsubara OT, Tsai JF S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN
| 1. | Hajj Hussein IA, Tohme R, Barada K, Mostafa MH, Freund JN, Jurjus RA, Karam W, Jurjus A. Inflammatory bowel disease in rats: bacterial and chemical interaction. World J Gastroenterol. 2008;14:4028-4039. [PubMed] |
| 2. | Margiotta G, Sanfilippo A, Accardo FM, Damiani P, Geraci A, Tomasello G. Chronic Inflammatory Bowel Disease in patients with orthopedic manifestation. Comparison with the data reported in international literauture. Euromediterranean Biomedical J. 2012;7:33-38. |
| 3. | Sobhani I, Amiot A, Le Baleur Y, Levy M, Auriault ML, Van Nhieu JT, Delchier JC. Microbial dysbiosis and colon carcinogenesis: could colon cancer be considered a bacteria-related disease? Therap Adv Gastroenterol. 2013;6:215-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 4. | Marchesi JR, Dutilh BE, Hall N, Peters WH, Roelofs R, Boleij A, Tjalsma H. Towards the human colorectal cancer microbiome. PLoS One. 2011;6:e20447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 442] [Cited by in RCA: 429] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 5. | Turner ND, Ritchie LE, Bresalier RS, Chapkin RS. The microbiome and colorectal neoplasia: environmental modifiers of dysbiosis. Curr Gastroenterol Rep. 2013;15:346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Tomasello G, Bellavia M, Damiano G, Gioviale MG, Lo Monte AI. Possible relation between gut microflora composition and oncogenic risk: is stimulation of inflammation the one ring of connection? Rev Med Microbiol. 2012;23:52-57. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Bettelheim KA, Breadon A, Faiers MC, O’Farrell SM, Shooter RA. The origin of O serotypes of Escherichia coli in babies after normal delivery. J Hyg (Lond). 1974;72:67-70. [PubMed] |
| 8. | Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9101] [Cited by in RCA: 7831] [Article Influence: 522.1] [Reference Citation Analysis (4)] |
| 9. | Fujimura KE, Slusher NA, Cabana MD, Lynch SV. Role of the gut microbiota in defining human health. Expert Rev Anti Infect Ther. 2010;8:435-454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 289] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 10. | Tralongo P, Tomasello G, Sinagra E; Damiani P, Leone A, Palumbo VD, Giammanco M, Di Majo D, Damiani F, Abruzzo A, Bruno A. The role of butyric acid as a protective agent against Inflammatory Bowel Diseases. Euro Biomed J. 2014;9:24-35. |
| 11. | Manichanh C, Borruel N, Casellas F, Guarner F. The gut microbiota in IBD. Nat Rev Gastroenterol Hepatol. 2012;9:599-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 771] [Cited by in RCA: 895] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 12. | Hamer HM, Jonkers DM, Loof A, Vanhoutvin SA, Troost FJ, Venema K, Kodde A, Koek GH, Schipper RG, van Heerde WL. Analyses of human colonic mucus obtained by an in vivo sampling technique. Dig Liver Dis. 2009;41:559-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Trubitsyna IE, Drozdov VN, Baryshnikov EN, Chikunova BZ, Petrakov AV. [The damaging role of endogenous nitric oxide in the development of the experimental DSS-induced colitis in rats]. Eksp Klin Gastroenterol. 2010;74-77. [PubMed] |
| 14. | Yu JE, De Ravin SS, Uzel G, Landers C, Targan S, Malech HL, Holland SM, Cao W, Harpaz N, Mayer L. High levels of Crohn’s disease-associated anti-microbial antibodies are present and independent of colitis in chronic granulomatous disease. Clin Immunol. 2011;138:14-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Llopis M, Antolin M, Carol M, Borruel N, Casellas F, Martinez C, Espín-Basany E, Guarner F, Malagelada JR. Lactobacillus casei downregulates commensals’ inflammatory signals in Crohn’s disease mucosa. Inflamm Bowel Dis. 2009;15:275-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 118] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 16. | Arseneau KO, Cominelli F. Leukocytapheresis in ulcerative colitis: a possible alternative to biological therapy? Dig Liver Dis. 2009;41:551-552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Olsen I, Tollefsen S, Aagaard C, Reitan LJ, Bannantine JP, Andersen P, Sollid LM, Lundin KE. Isolation of Mycobacterium avium subspecies paratuberculosis reactive CD4 T cells from intestinal biopsies of Crohn’s disease patients. PLoS One. 2009;4:e5641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Grivennikov SI. Inflammation and colorectal cancer: colitis-associated neoplasia. Semin Immunopathol. 2013;35:229-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 422] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 19. | Zhu Q, Gao R, Wu W, Qin H. The role of gut microbiota in the pathogenesis of colorectal cancer. Tumour Biol. 2013;34:1285-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 160] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 20. | Saba K, Denda-Nagai K, Irimura T. A C-type lectin MGL1/CD301a plays an anti-inflammatory role in murine experimental colitis. Am J Pathol. 2009;174:144-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Gallan L. Dysbiotic relationships in the bowel. American College of Advancement in Medicine Conference; Spring. 1992;. |
| 23. | Knutson CG, Mangerich A, Zeng Y, Raczynski AR, Liberman RG, Kang P, Ye W, Prestwich EG, Lu K, Wishnok JS. Chemical and cytokine features of innate immunity characterize serum and tissue profiles in inflammatory bowel disease. Proc Natl Acad Sci USA. 2013;110:E2332-E2341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 24. | Sobhani I, Tap J, Roudot-Thoraval F, Roperch JP, Letulle S, Langella P, Corthier G, Tran Van Nhieu J, Furet JP. Microbial dysbiosis in colorectal cancer (CRC) patients. PLoS One. 2011;6:e16393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 630] [Cited by in RCA: 620] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 25. | Wang T, Cai G, Qiu Y, Fei N, Zhang M, Pang X, Jia W, Cai S, Zhao L. Structural segregation of gut microbiota between colorectal cancer patients and healthy volunteers. ISME J. 2012;6:320-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 738] [Cited by in RCA: 948] [Article Influence: 67.7] [Reference Citation Analysis (0)] |
| 26. | Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883-899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8437] [Cited by in RCA: 8170] [Article Influence: 544.7] [Reference Citation Analysis (0)] |
| 27. | Rowe WA, Lichtenstein GR, Katz J. Inflammatory Bowel Disease. Medscape Drugs, Diseases and Procedures. 2014; Available from: http://emedicine.medscape.com/article/179037-overview. |
| 28. | Fisher SA, Tremelling M, Anderson CA, Gwilliam R, Bumpstead S, Prescott NJ, Nimmo ER, Massey D, Berzuini C, Johnson C. Genetic determinants of ulcerative colitis include the ECM1 locus and five loci implicated in Crohn’s disease. Nat Genet. 2008;40:710-712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 333] [Cited by in RCA: 339] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 29. | Waldner MJ, Foersch S, Neurath MF. Interleukin-6--a key regulator of colorectal cancer development. Int J Biol Sci. 2012;8:1248-1253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 233] [Cited by in RCA: 305] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 30. | Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90:859-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 31. | Gong J, Zhang JP, Li B, Zeng C, You K, Chen MX, Yuan Y, Zhuang SM. MicroRNA-125b promotes apoptosis by regulating the expression of Mcl-1, Bcl-w and IL-6R. Oncogene. 2013;32:3071-3079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 163] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 32. | Maggio M, Guralnik JM, Longo DL, Ferrucci L. Interleukin-6 in aging and chronic disease: a magnificent pathway. J Gerontol A Biol Sci Med Sci. 2006;61:575-584. [PubMed] |
| 33. | Brown K, DeCoffe D, Molcan E, Gibson DL. Diet-induced dysbiosis of the intestinal microbiota and the effects on immunity and disease. Nutrients. 2012;4:1095-1119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 402] [Cited by in RCA: 466] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 34. | Fallani M, Young D, Scott J, Norin E, Amarri S, Adam R, Aguilera M, Khanna S, Gil A, Edwards CA. Intestinal microbiota of 6-week-old infants across Europe: geographic influence beyond delivery mode, breast-feeding, and antibiotics. J Pediatr Gastroenterol Nutr. 2010;51:77-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 35. | Zhang C, Zhang M, Wang S, Han R, Cao Y, Hua W, Mao Y, Zhang X, Pang X, Wei C. Interactions between gut microbiota, host genetics and diet relevant to development of metabolic syndromes in mice. ISME J. 2010;4:232-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 36. | Zimmer J, Lange B, Frick JS, Sauer H, Zimmermann K, Schwiertz A, Rusch K, Klosterhalfen S, Enck P. A vegan or vegetarian diet substantially alters the human colonic faecal microbiota. Eur J Clin Nutr. 2012;66:53-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 37. | Turnbaugh PJ, Bäckhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008;3:213-223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2421] [Cited by in RCA: 2189] [Article Influence: 128.8] [Reference Citation Analysis (0)] |
| 38. | Nielsen S, Nielsen DS, Lauritzen L, Jakobsen M, Michaelsen KF. Impact of diet on the intestinal microbiota in 10-month-old infants. J Pediatr Gastroenterol Nutr. 2007;44:613-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 39. | Sanapareddy N, Legge RM, Jovov B, McCoy A, Burcal L, Araujo-Perez F, Randall TA, Galanko J, Benson A, Sandler RS. Increased rectal microbial richness is associated with the presence of colorectal adenomas in humans. ISME J. 2012;6:1858-1868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 169] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 40. | Castellarin M, Warren RL, Freeman JD, Dreolini L, Krzywinski M, Strauss J, Barnes R, Watson P, Allen-Vercoe E, Moore RA. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012;22:299-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1164] [Cited by in RCA: 1494] [Article Influence: 106.7] [Reference Citation Analysis (0)] |
| 41. | Kostic AD, Gevers D, Pedamallu CS, Michaud M, Duke F, Earl AM, Ojesina AI, Jung J, Bass AJ, Tabernero J. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012;22:292-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1199] [Cited by in RCA: 1495] [Article Influence: 106.8] [Reference Citation Analysis (0)] |
| 42. | O’Keefe SJ, Ou J, Aufreiter S, O’Connor D, Sharma S, Sepulveda J, Fukuwatari T, Shibata K, Mawhinney T. Products of the colonic microbiota mediate the effects of diet on colon cancer risk. J Nutr. 2009;139:2044-2048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 115] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 44. | Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2937] [Cited by in RCA: 3944] [Article Influence: 328.7] [Reference Citation Analysis (1)] |
| 45. | Monteleone G, Biancone L, Marasco R, Morrone G, Marasco O, Luzza F, Pallone F. Interleukin 12 is expressed and actively released by Crohn’s disease intestinal lamina propria mononuclear cells. Gastroenterology. 1997;112:1169-1178. [PubMed] |
| 46. | Pizarro TT, Michie MH, Bentz M, Woraratanadharm J, Smith MF, Foley E, Moskaluk CA, Bickston SJ, Cominelli F. IL-18, a novel immunoregulatory cytokine, is up-regulated in Crohn’s disease: expression and localization in intestinal mucosal cells. J Immunol. 1999;162:6829-6835. [PubMed] |
| 47. | Bamias G, Martin C, Mishina M, Ross WG, Rivera-Nieves J, Marini M, Cominelli F. Proinflammatory effects of TH2 cytokines in a murine model of chronic small intestinal inflammation. Gastroenterology. 2005;128:654-666. [PubMed] |
| 48. | Davis CD, Milner JA. Gastrointestinal microflora, food components and colon cancer prevention. J Nutr Biochem. 2009;20:743-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 226] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 49. | Kosiewicz MM, Zirnheld AL, Alard P. Gut microbiota, immunity, and disease: a complex relationship. Front Microbiol. 2011;2:180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 133] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 50. | Wu S, Rhee KJ, Albesiano E, Rabizadeh S, Wu X, Yen HR, Huso DL, Brancati FL, Wick E, McAllister F. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15:1016-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1113] [Cited by in RCA: 1303] [Article Influence: 81.4] [Reference Citation Analysis (0)] |
| 51. | Huh JW, Lee JH, Kim HR. Prognostic significance of tumor-infiltrating lymphocytes for patients with colorectal cancer. Arch Surg. 2012;147:366-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 131] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 52. | Wu YL, Ding YP, Tanaka Y, Shen LW, Wei CH, Minato N, Zhang W. γδ T cells and their potential for immunotherapy. Int J Biol Sci. 2014;10:119-135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 118] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 53. | Todaro M, D’Asaro M, Caccamo N, Iovino F, Francipane MG, Meraviglia S, Orlando V, La Mendola C, Gulotta G, Salerno A. Efficient killing of human colon cancer stem cells by gammadelta T lymphocytes. J Immunol. 2009;182:7287-7296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 241] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 54. | Macario AJL, Conway de Macario E. The chaperoning system: physiology and pathology. Experimental Medicine Reviews. Anguilla: Plumelia, 2008/ 2009; 9-21 (ISBN 978-88-89876-15-2). |
| 55. | Macario AJ, Lange M, Ahring BK, Conway de Macario E. Stress genes and proteins in the archaea. Microbiol Mol Biol Rev. 1999;63:923-67, table of contents. [PubMed] |
| 56. | Rappa F, Farina F, Zummo G, David S, Campanella C, Carini F, Tomasello G, Damiani P, Cappello F, DE Macario EC. HSP-molecular chaperones in cancer biogenesis and tumor therapy: an overview. Anticancer Res. 2012;32:5139-5150. [PubMed] |
| 57. | van Eden W, Thole JE, van der Zee R, Noordzij A, van Embden JD, Hensen EJ, Cohen IR. Cloning of the mycobacterial epitope recognized by T lymphocytes in adjuvant arthritis. Nature. 1988;331:171-173. [PubMed] |
| 58. | Raz I, Elias D, Avron A, Tamir M, Metzger M, Cohen IR. Beta-cell function in new-onset type 1 diabetes and immunomodulation with a heat-shock protein peptide (DiaPep277): a randomised, double-blind, phase II trial. Lancet. 2001;358:1749-1753. [PubMed] |
| 59. | Maron R, Sukhova G, Faria AM, Hoffmann E, Mach F, Libby P, Weiner HL. Mucosal administration of heat shock protein-65 decreases atherosclerosis and inflammation in aortic arch of low-density lipoprotein receptor-deficient mice. Circulation. 2002;106:1708-1715. [PubMed] |
| 60. | Rha YH, Taube C, Haczku A, Joetham A, Takeda K, Duez C, Siegel M, Aydintug MK, Born WK, Dakhama A. Effect of microbial heat shock proteins on airway inflammation and hyperresponsiveness. J Immunol. 2002;169:5300-5307. [PubMed] |
| 61. | Cappello F, Conway de Macario E, Di Felice V, Zummo G, Macario AJ. Chlamydia trachomatis infection and anti-Hsp60 immunity: the two sides of the coin. PLoS Pathog. 2009;5:e1000552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 62. | Tomasello G, Sciumé C, Rappa F, Rodolico V, Zerilli M, Martorana A, Cicero G, De Luca R, Damiani P, Accardo FM. Hsp10, Hsp70, and Hsp90 immunohistochemical levels change in ulcerative colitis after therapy. Eur J Histochem. 2011;55:e38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 63. | Wächtershäuser A, Stein J. Rationale for the luminal provision of butyrate in intestinal diseases. Eur J Nutr. 2000;39:164-171. [PubMed] |
| 64. | Zoran DL, Barhoumi R, Burghardt RC, Chapkin RS, Lupton JR. Diet and carcinogen alter luminal butyrate concentration and intracellular pH in isolated rat colonocytes. Nutr Cancer. 1997;27:222-230. [PubMed] |
| 65. | Basson MD, Liu YW, Hanly AM, Emenaker NJ, Shenoy SG, Gould Rothberg BE. Identification and comparative analysis of human colonocyte short-chain fatty acid response genes. J Gastrointest Surg. 2000;4:501-512. [PubMed] |
| 66. | Myzak MC, Dashwood RH. Histone deacetylases as targets for dietary cancer preventive agents: lessons learned with butyrate, diallyl disulfide, and sulforaphane. Curr Drug Targets. 2006;7:443-452. [PubMed] |
| 67. | Davie JR. Inhibition of histone deacetylase activity by butyrate. J Nutr. 2003;133:2485S-2493S. [PubMed] |
| 68. | Shenderov BA. Gut indigenous microbiota and epigenetics. Microb Ecol Health Dis. 2012;23:17195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 69. | Huycke MM, Gaskins HR. Commensal bacteria, redox stress, and colorectal cancer: mechanisms and models. Exp Biol Med (Maywood). 2004;229:586-597. [PubMed] |
| 70. | Arthur JC, Perez-Chanona E, Mühlbauer M, Tomkovich S, Uronis JM, Fan TJ, Campbell BJ, Abujamel T, Dogan B, Rogers AB. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012;338:120-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1374] [Cited by in RCA: 1661] [Article Influence: 127.8] [Reference Citation Analysis (1)] |
| 71. | Piazzi G, D’Argenio G, Prossomariti A, Lembo V, Mazzone G, Candela M, Biagi E, Brigidi P, Vitaglione P, Fogliano V. Eicosapentaenoic acid free fatty acid prevents and suppresses colonic neoplasia in colitis-associated colorectal cancer acting on Notch signaling and gut microbiota. Int J Cancer. 2014;135:2004-2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 72. | Andersen AD, Mølbak L, Michaelsen KF, Lauritzen L. Molecular fingerprints of the human fecal microbiota from 9 to 18 months old and the effect of fish oil supplementation. J Pediatr Gastroenterol Nutr. 2011;53:303-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |