Published online Dec 21, 2014. doi: 10.3748/wjg.v20.i47.18044
Revised: June 30, 2014
Accepted: July 29, 2014
Published online: December 21, 2014
Processing time: 243 Days and 4.9 Hours
Fundic gland polyps (FGPs) are currently the most common type of gastric polyps and are usually benign. However, although rare, gastric adenocarcinoma of FGP has been recently proposed as a new variant of gastric adenocarcinoma. Here we report the first case of a 49-year-old woman with focal signet ring cell carcinoma that arose from an FGP of the stomach. The tumor was completely excised by endoscopic snare polypectomy. FGPs should therefore be evaluated for malignant changes although they occur rarely, if the FGP has an erosive or irregular surface.
Core tip: We report the first case of a 49-year-old woman diagnosed with focal signet ring cell carcinoma that arose from a fundic gland polyp in the stomach. The tumor was detected and completely excised by endoscopic snare polypectomy. Although malignant transformation of fundic gland polyps (FGPs) is extremely rare, endoscopists should consider the association of gastric polyps with gastric cancer for both hyperplastic polyps and FGPs.
- Citation: Jeong YS, Kim SE, Kwon MJ, Seo JY, Lim H, Park JW, Kang HS, Moon SH, Kim JH, Park CK. Signet-ring cell carcinoma arising from a fundic gland polyp in the stomach. World J Gastroenterol 2014; 20(47): 18044-18047
- URL: https://www.wjgnet.com/1007-9327/full/v20/i47/18044.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i47.18044
Gastric fundic gland polyps (FGPs) are the most common lesions, and they have been regarded as benign. These polyps can be sporadic or associated with an inherited polyposis syndrome. Sporadic FGPs are typically regarded as benign lesions with no risk of malignant transformation. Some reports have described sporadic FGPs containing low-grade or high-grade dysplasia[1,2]. Adenocarcinoma of the fundic gland was recently proposed as a new variant of gastric adenocarcinoma[3,4]. However, there are no reports of signet ring cell carcinoma arising from a sporadic FGP. Herein, we report the case of a woman diagnosed with focal signet ring cell carcinoma originating from an FGP. The FGP with focal signet ring cell carcinoma was completely endoscopically excised by snare polypectomy.
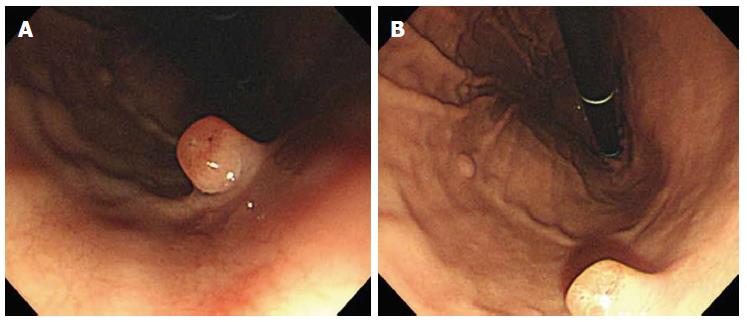
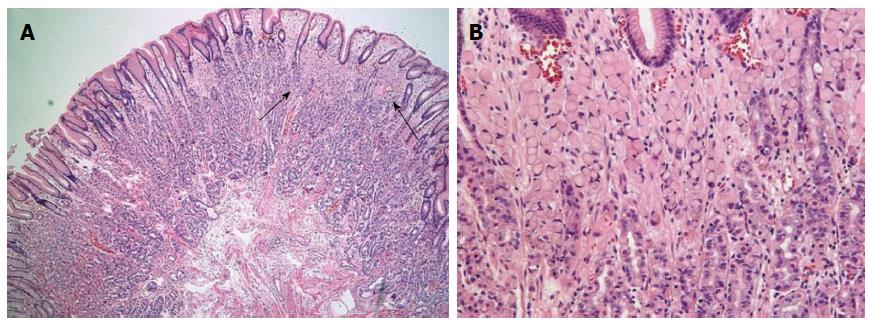
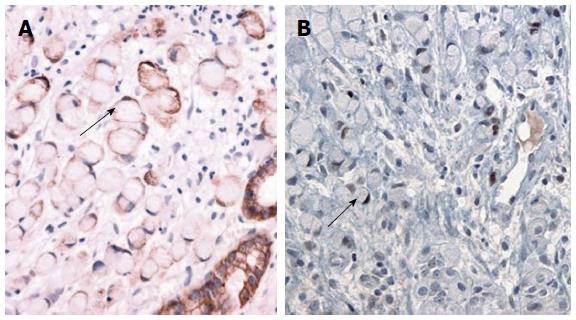
A 49-year-old woman was referred for gastric polyps in a mass-screening endoscopic examination at a local clinic. The patient did not describe any important past history data or complaints at admission. No specific familial history was identified. The physical examination and laboratory data were also unremarkable. Endoscopic examination of the upper digestive tract revealed four gastric polyps. The first polyp (0.8 cm in diameter, pedunculated, reddish, and with an erosive surface) was located at the mid body on the anterior wall (Figure 1A). The second and third polyps (0.5 cm in diameter, pedunculated, and with a smooth surface) were located at the mid body on the greater curvature. The fourth polyp (0.4 cm in diameter, pedunculated, and smooth) was at the high body on the greater curvature (Figure 1B). The first polyp was removed by snare polypectomy after epinephrine injection due to the erosive surface, even though it was small and suspected to be an FGP. The second, third and fourth polyps were removed using hot biopsy forceps. Histologically, the lesion consisted mainly of polypoid proliferation of fundic oxyntic glands, which were partially dilated and distorted, which is consistent with FGP. We also found a focus of loosely cohesive round cells that had a foamy eosinophilic cytoplasm and peripherally placed nuclei infiltrated lamina propria, measuring 0.2 cm × 0.1 cm, in the superficial portion of the polyp. The tumor cells were consistent with signet ring cell carcinoma, and they appeared to originate from fundic oxyntic cells of the FGP. The cancerous focus was confined within the mucosa (Figure 2). The tumor cells were positive for cytokeratin using immunohistochemistry. Approximately 80% of the tumor cells were positive for p53 protein (1:2000, clone DO-7, Novacastra, Newcastle upon Tyne, United Kingdom), while proliferative oxyntic cells of the FGP were negative (Figure 3). Helicobacter pylori (H. pylori) infection was negative. The second, third and fourth polyps were FGPs.
The first polyp was resected completely by snare polypectomy, and the resection margins were negative for carcinoma.
This novel case describes a signet ring cell carcinoma that originated from an FGP. The relationship between adenocarcinoma and gastric adenoma is well established[5]. However, gastric FGPs, which are the most common lesions, have typically been considered benign lesions with no risk of malignant transformation.
Sporadic FGPs are the most common type of gastric polyps that are found during upper endoscopy[6]. The prevalence of FGPs appears to be increasing due to the increased use of upper endoscopy, increased use of acid suppressive medications, and decreased prevalence of H. pylori infection[2].
The pathogenesis and malignant potential of FGPs are unknown. FGPs rarely occur before adulthood and 40%-50% spontaneously regress; both features are inconsistent with the traditional assumptions about hamartomas[7]. A case of dysplasia and adenocarcinoma has been documented in the FGPs of patients with attenuated adenomatous polyposis coli[8]. A female predominance in nonfamilial FGPs suggests that hormones play a role in the development of FGPs[9]. Some reports have described sporadic FGPs with low-grade or high-grade dysplasia[10,11] and adenocarcinoma[3,4,6]. There are no reports of gastric signet ring cell carcinoma arising from sporadic FGPs.
In the present case, the FGP (0.8 cm in diameter) was associated with a minute signet ring cell carcinoma and removed. The histologic differential diagnoses of signet ring cell carcinoma in gastric polyp included mucosal xanthelasma, clusters of mucin-laden macrophages in the lamina propria, and metastatic carcinomas composed of loosely cohesive cells, such as lobular carcinoma of the breast. In the present case, the tumor cells were positive for cytokeratin, which differentiates histiocytes from signet ring cell carcinoma. The present tumor cells were negative for the estrogen and progesterone receptors, and the systemic evaluation of the patient did not reveal any other malignancies.
Although the malignant transformation of FGPs is extremely rare, endoscopists should consider the association of gastric polyps and gastric cancer for both hyperplastic polyps and FGPs.
The polyp had a minute signet ring cell carcinoma that was removed using snare polypectomy due to the erosive surface, even if it was a suspected FGP. Histologically, the tumor cells were in the superficial portion of the polyp, but the relationship between the cancerous foci and the erosive surface was not clear. Little is known about the pathogenesis of and risk factors for the malignant transformation of FGPs. Inflammation or molecular alterations related to an erosive surface may be associated with the malignant transformation of FGPs.
Therefore, endoscopists should perform a thorough visual inspection of gastric FGPs. Any lesions, no matter how small, that appear significantly different from others (e.g., erosive or irregular surface) should be biopsied or removed. When the surface of an FGP is eroded, the regenerative appearance can be interpreted as dysplasia; however, true dysplasia or malignant transformation of sporadic FGPs should be considered. Biopsies or endoscopically resected stomach specimens should be carefully examined microscopically for the possibility of malignancy.
In conclusion, we report the first case of a woman who was diagnosed with focal signet ring cell carcinoma originating from an FGP. This case shows that sporadic FGPs may also be related to gastric cancer. Although the association of FGPs and gastric cancer is not clearly established, biopsies or resection of FGPs, no matter how small, should be considered when the FGP has an erosive surface.
A 49-year-old woman with incidentally found gastric polyps.
Fundic gland polyp.
Familial polyposis syndrome, malignancy.
The laboratory datas were unremarkable.
Computed tomography scans of abdomen and pelvis were unremarkable.
Snare polypectomy revealed a focal signet ring cell carcinoma originating from a fundic gland polyp.
The patient was not treated any further after snare polypectomy because the polyp was resected completely and the resection margins were negative for carcinoma.
Some reports have described sporadic fundic gland polyps (FGPs) containing low-grade or high-grade dysplasia. A gastric adenocarcinoma of the fundic gland was recently proposed as a new variant of gastric adenocarcinoma.
FGP means fundic gland polyp.
This case report not only represents focal signet ring cell carcinoma in a FGP that have not been reported, but also provides a lesson that sporadic FGPs with different appearance from others should undergo a biopsy examination or, if possible, be removed.
The direct histologic relationship between the cancerous foci and the erosive surface of FGP is not clear. But this case shows the possibility of malignant transformation in a sporadic FGP. This case can give endoscopists a lesson in the management of a sporadic FGP.
P- Reviewer: Dumitrascu DL, Freund JN, Iizuka T S- Editor: Qi Y L- Editor: A E- Editor: Liu XM
| 1. | Wu TT, Kornacki S, Rashid A, Yardley JH, Hamilton SR. Dysplasia and dysregulation of proliferation in foveolar and surface epithelia of fundic gland polyps from patients with familial adenomatous polyposis. Am J Surg Pathol. 1998;22:293-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 96] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 2. | Carmack SW, Genta RM, Schuler CM, Saboorian MH. The current spectrum of gastric polyps: a 1-year national study of over 120,000 patients. Am J Gastroenterol. 2009;104:1524-1532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 199] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 3. | Ueyama H, Yao T, Nakashima Y, Hirakawa K, Oshiro Y, Hirahashi M, Iwashita A, Watanabe S. Gastric adenocarcinoma of fundic gland type (chief cell predominant type): proposal for a new entity of gastric adenocarcinoma. Am J Surg Pathol. 2010;34:609-619. [PubMed] |
| 4. | Tsukamoto T, Yokoi T, Maruta S, Kitamura M, Yamamoto T, Ban H, Tatematsu M. Gastric adenocarcinoma with chief cell differentiation. Pathol Int. 2007;57:517-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 67] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 5. | Leung WK, Ng EKW, Sung JJY. Tumors of the stomach. Textbook of gastroenterology. 5th ed. Oxford: Blackwell Publishing Ltd 2009; . |
| 6. | Ueyama H, Matsumoto K, Nagahara A, Hayashi T, Yao T, Watanabe S. Gastric adenocarcinoma of the fundic gland type (chief cell predominant type). Endoscopy. 2014;46:153-157. [PubMed] |
| 7. | Eidt S, Stolte M. Gastric glandular cysts--investigations into their genesis and relationship to colorectal epithelial tumors. Z Gastroenterol. 1989;27:212-217. [PubMed] |
| 8. | Zwick A, Munir M, Ryan CK, Gian J, Burt RW, Leppert M, Spirio L, Chey WY. Gastric adenocarcinoma and dysplasia in fundic gland polyps of a patient with attenuated adenomatous polyposis coli. Gastroenterology. 1997;113:659-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 107] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Marcial MA, Villafaña M, Hernandez-Denton J, Colon-Pagan JR. Fundic gland polyps: prevalence and clinicopathologic features. Am J Gastroenterol. 1993;88:1711-1713. [PubMed] |
| 10. | Abraham SC, Nobukawa B, Giardiello FM, Hamilton SR, Wu TT. Sporadic fundic gland polyps: common gastric polyps arising through activating mutations in the beta-catenin gene. Am J Pathol. 2001;158:1005-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 112] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 11. | Jalving M, Koornstra JJ, Götz JM, van der Waaij LA, de Jong S, Zwart N, Karrenbeld A, Kleibeuker JH. High-grade dysplasia in sporadic fundic gland polyps: a case report and review of the literature. Eur J Gastroenterol Hepatol. 2003;15:1229-1233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |