Published online Dec 21, 2014. doi: 10.3748/wjg.v20.i47.18001
Revised: June 12, 2014
Accepted: July 11, 2014
Published online: December 21, 2014
Processing time: 245 Days and 9.7 Hours
AIM: To investigate the efficacy and safety of gemcitabine (Gem)-based combination chemotherapies for the treatment of advanced biliary tract cancer.
METHODS: Clinical trials were identified by searching scientific literature databases (PubMed, EMBASE and the Cochrane Library) for studies published between 1975 and 2013. Two reviewers independently evaluated the relevant studies and manually searched references from these reports to locate additional eligible studies. The disease response and control rates, progression-free and overall survivals, and the grade 3-4 toxicities were evaluated by a meta-analysis. Odds-ratios (ORs) of the disease response and control rates and grade 3-4 toxicities, and the mean difference (MD) of both progression-free and overall survivals were calculated and used for statistical analysis.
RESULTS: Seven randomized trials with a total of 858 patients were selected and included in the final analysis. The studies were divided into subgroups based on the chemotherapy regimens, including Gem-based and non-Gem-based chemotherapies. The overall analyses revealed that the patients treated with Gem-based combination chemotherapy had significantly higher disease response rates [OR = 1.69, 95% confidence interval (CI): 1.17-2.43; P = 0.01], a longer progression-free survival (MD = 1.95, 95%CI: 0.90-3.00; P = 0.00) and a longer overall survival (MD = 1.85, 95%CI: 0.26-3.44; P = 0.02). A higher incidence of grade 3-4 hematological toxicities, including leukopenia (OR = 2.98, 95%CI: 1.44-6.20; P = 0.00), anemia (OR = 2.96, 95%CI: 1.79-4.92; P = 0.00) and neutropenia (OR = 2.80, 95%CI: 1.39-5.64; P = 0.00) was found in the Gem-based combination chemotherapy group compared with the Gem monotherapy and non-Gem-based chemotherapy groups.
CONCLUSION: Gem-based combination chemotherapy is a potential first-line treatment for advanced biliary tract cancer as a result of improved survival, though with additional toxicity.
Core tip: To investigate the efficacy and safety of gemcitabine (Gem)-based combination chemotherapy for the treatment of advanced biliary tract cancer, the authors analyzed the potential impact of Gem-based combination chemotherapy and other regimens on the outcomes and toxicities of the patients using meta-analysis methodologies. Meta-analysis showed that compared with Gem monotherapy and non-Gem-based chemotherapy, Gem-based combination chemotherapy provided a modest improvement in survival but was associated with more toxicity.
- Citation: Liu H, Zhang QD, Li ZH, Zhang QQ, Lu LG. Efficacy and safety of gemcitabine-based chemotherapies in biliary tract cancer: A meta-analysis. World J Gastroenterol 2014; 20(47): 18001-18012
- URL: https://www.wjgnet.com/1007-9327/full/v20/i47/18001.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i47.18001
Biliary tract cancer (BTC) refers to tumors that develop in the bile ducts and gall bladder and includes cholangiocarcinomas and gallbladder carcinomas[1]. BTC is a heterogeneous group of relatively rare tumors that account for about 3% of all gastrointestinal malignancies[1] and is the second most common cause of primary hepatic tumors[2]. Globally, hepatobiliary malignancies account for 13% of cancer-related deaths; 10%-20% of these deaths are attributable to BTC[3]. Epidemiologic studies have indicated that the incidence of BTC has increased rapidly worldwide in previous decades, particularly in Asian countries[1,4,5]. Despite advances in the diagnosis, staging and surgical management of BTC during the past decade, patients with BTC have a reported five-year survival rate that approaches only 15%[4] and an overall median survival of only 6.3 mo[6]. Surgical resection may be the only potentially curative therapeutic option. Unfortunately, due to the late clinical presentation, most BTC patients are diagnosed at an advanced stage when surgical resection is not feasible and treatment options are limited[5]. Hence, chemotherapeutic treatment is usually recommended for patients with unresectable advanced BTC or for patients who relapse subsequent to surgery[7].
Gemcitabine (Gem) emerged as a treatment for pancreatic cancer and has been explored as a treatment for advanced biliary cancer since 1998[8]. To improve the clinical efficacy, systemically administered Gem is often combined with a second cytotoxic agent, such as platinum analogs, fluoropyrimidine, or a targeted cytotoxic agent. The results from several phase II studies suggest that Gem, alone or in combination with other agents, has been relatively effective for treating BTC[5,9]. However, most of these studies were small, single-arm and nonrandomized trials. Therefore, the role of Gem-based chemotherapy for patients with advanced BTC has not been clearly established. Until 2010, data from the largest randomized biliary tract trial to date indicated that the overall survival was significantly higher in the Gem and cisplatin arms vs the Gem single-agent treatment (11.7 mo vs 8.1 mo)[10]. Based on these results, Gem combined with cisplatin was established as the new standard of therapy for advanced, unresectable BTC[7]. Since that time, several randomized trials[11-14] comparing Gem-based combination chemotherapy with other regimens have been published. However, the results of these trials were conflicting, which has made the role of Gem-based combination chemotherapy controversial. Using these data, we conducted a meta-analysis to evaluate the efficacy and safety of Gem-based combination chemotherapy in advanced BTC treatment. The aim of this study was to assess whether Gem-based combination chemotherapy improves BTC prognosis compared with other treatment regimens.
PubMed, EMBASE and the Cochrane Library were systematically searched using the following combination of search terms: “biliary tract cancer”, “gallbladder carcinoma”, “cholangiocarcinoma” and “gemcitabine”. The search was performed in August 2013 and updated in November 2013 to identify relevant publications between 1975 and 2013; there were no language restrictions. All potentially relevant studies were retrieved, and their references were evaluated to identify additional eligible studies.
Studies were eligible for inclusion in the meta-analysis if they met all of the following criteria: (1) advanced BTC patients (with unresectable or metastatic cancer); (2) Gem-based combination chemotherapy at any line; (3) reported disease response rate (DRR), disease control rate (DCR), progression-free survival (PFS), overall survival (OS) and toxicities; and (4) structured as randomized controlled trials. Non-randomized trials and studies that repeated existing research were excluded to avoid clinical heterogeneity between studies.
Two independent reviewers (Liu H and Zhang QD) extracted the data from the eligible studies. A third reviewer (Lu LG) was consulted to resolve any disagreements. The following data were extracted from the included studies: first author’s name, year of publication, number of patients enrolled in each treatment group, patient age (median and range), proportion of male participants, treatment regimens, numbers and rates of DRR, DCR, PFS, and OS and numbers and rates of each type of grade 3-4 toxicity.
Study quality was assessed with Jadad scores[15] using the following criteria: quality of randomization, quality of allocation concealment, quality of double-blinding, and quality of withdrawals and dropouts in the study description. The studies scored one point for each criterion met. Additional points were given for each of the following conditions that were met: randomization sequence method was described by computer or randomized number, method of allocation concealment was described and was appropriate, or a detailed description of proper double blinding methods was provided. Based on these criteria, high-quality studies scored a total of at least four points.
Pooled odds ratios (ORs) with 95% confidence intervals (CIs) were calculated for the DRR and the DCR using Review Manger 5.2 software (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012). The mean differences (MDs) with 95%CIs were calculated for the DRR and the DCR. Statistical heterogeneity among studies was assessed using Cochran’s Q-test and the I2 statistic[16]. A P value < 0.10 for the Q-test or an I2 > 50% indicated study heterogeneity; in either case, a DerSimonian-Laird random effects model was used. Otherwise, a Mantel-Haenszel fixed effects method was used. If heterogeneity was found, a sensitivity analysis was performed to identify the potential sources of heterogeneity. Egger’s and Begg’s tests were used to measure potential publication bias. Statistical significance from two-sided analyses was indicated by a P < 0.05. Subgroup analyses were used to compare the efficacy and safety of gemcitabine-based combination chemotherapy with Gem monotherapy and non-Gem-based chemotherapy.
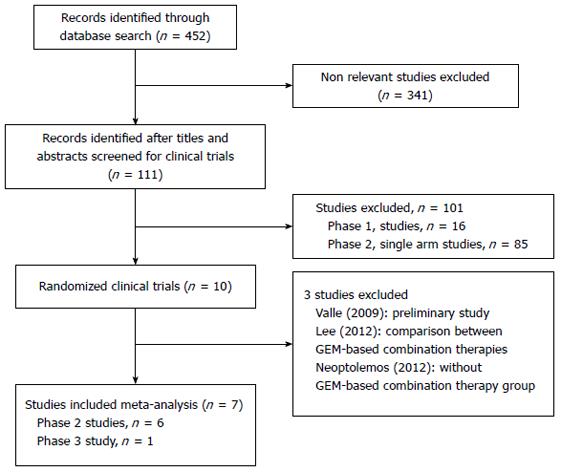
The study selection flow is depicted in Figure 1. Initially, 452 records were identified from PubMed, EMBASE and the Cochrane Library. After the initial review of the titles and abstracts, 111 relevant clinical trials were retrieved for detailed assessment, including ten randomized trials. As the scheme depicts, seven studies[10-14,17,18] met our inclusion criteria and were included in the quantitative synthesis, while the other three studies[19-21] were excluded.
Table 1 summarizes the characteristics of the included studies. Four different Gem-combination chemotherapy regimens were involved in the studies: Gem and cisplatin[10,11,18], Gem and S-1[12,13], Gem and oxaliplatin[17] and Gem and mitomycin C[14]. The effects of Gem-combination chemotherapy were compared with Gem monotherapy in three studies[10,12,18] and with other chemotherapy regimens in the remaining four studies[11,13,14,17]. The seven trials provided data from a total of 858 patients (mean: 123; range: 51 to 410). Males accounted for 17.9%-66% of all subjects, and the median patient age ranged from 47 to 75 years. All patients enrolled in the included studies had the following characteristics: histologically confirmed advanced local or metastatic biliary tract cancer not amenable to surgical resection; no previous chemotherapy; Eastern Cooperative Oncology Group Performance Status scores between 0 and 2; and adequate liver, renal and hematopoietic functions.
| Ref. | Year | Treatment | n | Male (%) | Age | DRR | DCR | PFS | OS |
| mean (range), yr | (%) | (%) | (mo) | (mo) | |||||
| Valle et al[10] | 2010 | GEM + CIS | 204 | 52.9 | 63.9 (32.8-81.9) | 21.6 | 81.4 | 8.0 | 11.7 |
| GEM | 206 | 52.4 | 63.2 (23.4-84.8) | 15.5 | 71.8 | 5.0 | 8.0 | ||
| Okusaka et al[18] | 2010 | GEM + CIS | 41 | 43.9 | 65 (43-80) | 19.5 | 68.3 | 5.8 | 11.2 |
| GEM | 42 | 50.0 | 66.5 (49-78) | 11.9 | 50.0 | 3.7 | 7.7 | ||
| Sasaki et al[12] | 2013 | GEM + S-1 | 30 | 53.3 | 68 (47-83) | 20.0 | 70.0 | 5.6 | 8.9 |
| GEM | 32 | 66.7 | 75 (55-86) | 9.4 | 62.5 | 4.3 | 9.2 | ||
| Kornek et al[14] | 2004 | GEM + MMC | 25 | 32.0 | 67 (44-75) | 21.7 | 60.9 | 4.2 | 6.7 |
| MMC + CAPE | 26 | 38.5 | 65 (45-75) | 33.3 | 70.8 | 5.3 | 9.3 | ||
| Sharma et al[17] | 2010 | GEM + OX | 26 | 19.2 | 49 | 30.7 | 68.7 | 8.5 | 9.5 |
| FUFA | 28 | 17.9 | 47 | 14.3 | 21.4 | 3.5 | 4.6 | ||
| Kang et al[11] | 2012 | GEM + CIS | 49 | 63.3 | 59 (32-77) | 19.6 | 71.7 | 5.7 | 10.1 |
| S-1 + CIS | 48 | 64.6 | 60 (36-77) | 23.8 | 85.7 | 5.4 | 9.9 | ||
| Morizane et al[13] | 2013 | GEM + S-1 | 50 | 54.0 | 66 (39-78) | 36.4 | 59.1 | 7.1 | 12.5 |
| S-1 | 51 | 47.1 | 62.5 (49-79) | 17.4 | 39.1 | 4.2 | 9.0 |
The details of grade 3-4 toxicity assessment are shown in Table 2 and include leukopenia, neutropenia, anemia, thrombocytopenia, increased alanine aminotransferase (ALT) level, nausea, vomiting, anorexia and diarrhea.
| Ref. | Treatment | Leukopenia | Neutropenia | Anemia | Thrombo-cytopenia | Increased ALT level | Nausea | Vomiting | Anorexia | Diarrhea |
| Valle et al[10] | GEM + CIS | 15.7 | 25.3 | 7.6 | 8.6 | 9.6 | 4.0 | 5.1 | 3.0 | NR |
| GEM | 9.5 | 16.6 | 3.0 | 6.5 | 17.1 | 3.5 | 5.5 | 2.5 | NR | |
| Okusaka et al[18] | GEM + CIS | 29.3 | 56.1 | 36.6 | 39.0 | 24.4 | 0.0 | 0.0 | 0.0 | 2.4 |
| GEM | 19.0 | 38.1 | 16.6 | 7.2 | 16.7 | 0.0 | 0.0 | 2.8 | 0.0 | |
| Sasaki et al[12] | GEM + S-1 | 33.0 | 33.0 | 10.0 | 7.0 | 3.0 | 3.0 | 0.0 | 3.0 | 0.0 |
| GEM | 19.0 | 22.0 | 6.0 | 6.0 | 0.0 | 0.0 | 0.0 | 6.0 | 0.0 | |
| Kornek et al[14] | GEM + MMC | 17.0 | 13.0 | 0.0 | 13.0 | 40.0 | 44.0 | NR | NR | 28.0 |
| MMC + CAPE | 17.0 | 17.0 | 0.0 | 17.0 | 45.0 | 42.0 | NR | NR | 28.0 | |
| Sharma et al[17] | GEM + OX | 38.5 | 38.5 | 38.5 | 10.0 | 15.4 | NR | 7.7 | NR | NR |
| FUFA | 7.1 | 7.1 | 7.1 | 2.0 | 0.0 | NR | 7.1 | NR | NR | |
| Kang et al[11] | GEM + CIS | 24.4 | 49.0 | 22.4 | 22.4 | 4.1 | 4.1 | 4.1 | 0.0 | 0.0 |
| S-1 + CIS | 0.0 | 31.8 | 2.3 | 4.5 | 0.0 | 2.1 | 0.0 | 0.0 | 4.3 | |
| Morizane et al[13] | GEM + S-1 | 29.4 | 60.7 | 11.8 | 11.8 | 13.7 | 2.0 | 2.0 | 7.8 | 2.0 |
| S-1 | 2.0 | 4.0 | 4.0 | 4.0 | 12.0 | 4.0 | 0.0 | 6.0 | 6.0 |
All seven eligible studies[10-14,17,18] were randomized, and two studies[13,14] described the method of randomization with definite descriptions. Allocation concealment was performed using a proprietary algorithm in one of the trials[13]. None of the trials reported double-blind procedures. Each trial included in the meta-analysis provided a detailed description of the number of and reasons for patient withdrawals and dropouts. Finally, two studies scored 4 points or above (Table 3).
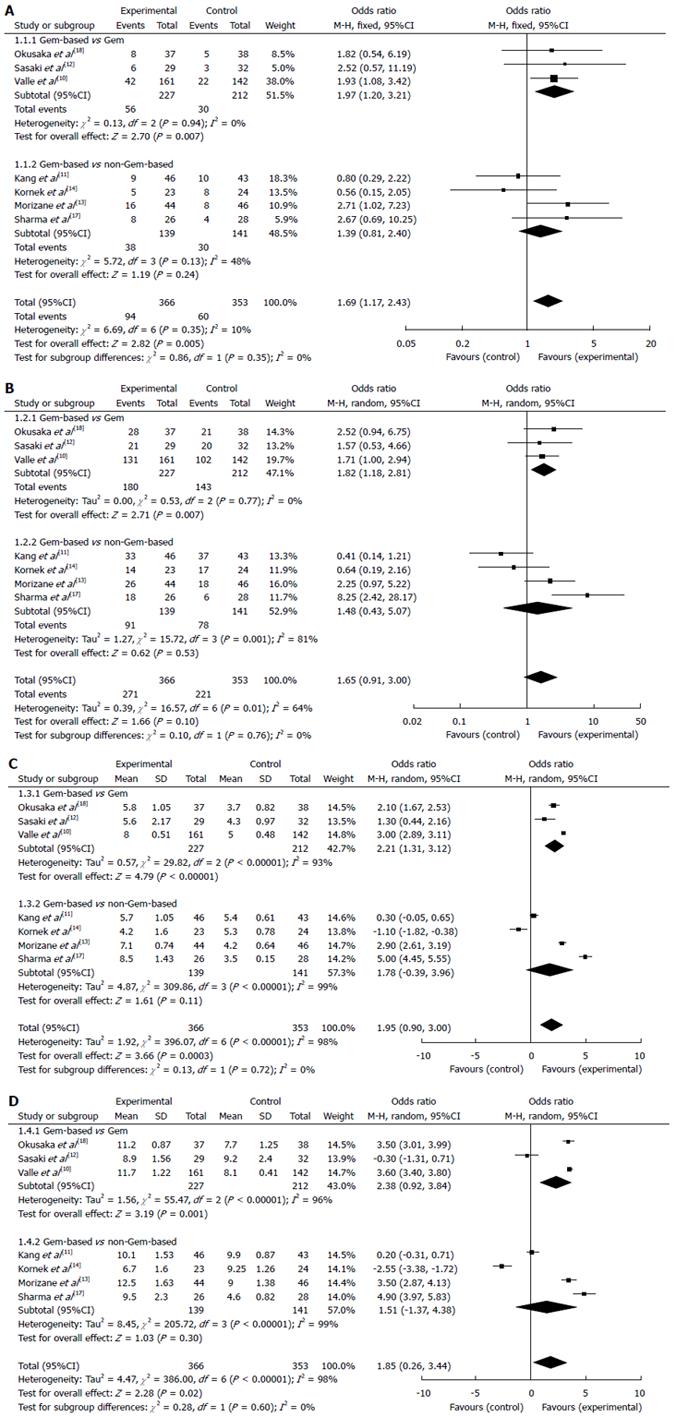
The DRRs were reported in all studies and ranged from 9.4%-36.4% without significant inter-study heterogeneity (Q = 6.69, df = 6, I² = 10%). The pooled OR of the DRR estimated by the fixed effects model was 1.69 (95%CI: 1.17-2.43) (Figure 2A). The overall analysis revealed that the patients treated with Gem-based combinations achieved significantly higher DRR compared with the patients who had not received this combination chemotherapy (P = 0.01). A subgroup analysis indicated that the DRR was significantly higher for the patients treated with Gem-based combination chemotherapy compared with the patients treated with Gem alone (OR = 1.97, 95%CI: 1.20-3.21; P = 0.01). The OR for the non-Gem-based chemotherapy subgroup was 1.39 (95%CI: 0.82-2.40), which was not statistically significant.
The DCR in the studies ranged from 21.4%-86.0%. Significant heterogeneity was detected between the studies (Q = 16.57, df = 6, I² = 64%; P = 0.01), therefore, a random effects model was used for the meta-analysis of the DCR. The total OR for the DCR was 1.65 (95%CI: 0.91-3.00; P = 0.10) (Figure 2B). The results of the subgroup analyses indicated that the patients treated with Gem-based combinations had significantly higher DCRs compared with the patients treated with Gem alone (OR = 1.82, 95%CI: 1.18-2.81; P = 0.01). The DCR of the non-Gem-based chemotherapy subgroup was lower compared with the Gem-based combination chemotherapy group (OR = 1.48, 95%CI: 0.43-5.07), but the difference was not significant.
The median PFS of the included studies ranged from 3.5-8.5 mo. After data pooling, significant heterogeneity was observed among the studies (Q = 396.07, df = 6, I² = 98%; P = 0.00), therefore, a random effects model was used for the meta-analysis of the median PFS. The overall MD for the PFS was 1.95 (95%CI: 0.90-3.00; P = 0.00) (Figure 2C), which suggests that Gem-based combination chemotherapy significantly improved patient PFS. A subgroup analysis also revealed that the PFS was significantly longer for the patients treated with Gem-based combination chemotherapy compared with the patients treated with Gem alone (MD = 2.21, 95%CI: 1.31-3.12; P = 0.00). The MD for Gem-based vs non-Gem-based chemotherapy was 1.78 (95%CI: -0.39-3.96), but was not significantly different.
The median OS ranged from 4.6-12.5 mo. Significant heterogeneity was detected between the studies (Q = 386.00, df = 6, I² = 98%; P = 0.00), therefore, a random effects model was used for the meta-analysis of OS. The overall MD for the OS was 1.85 (95%CI: 0.26-3.44; P = 0.02) (Figure 2D), which indicated that Gem-based combination chemotherapy significantly prolonged the overall survival time. A subgroup analysis revealed that the OS was significantly longer for the patients treated with Gem-based combination therapy compared with the patients treated with Gem alone (MD = 2.38, 95%CI: 0.92-3.84; P = 0.00). The OS of the Gem-based combination chemotherapy group was longer compared with the non-Gem-based chemotherapy group (MD = 1.51, 95%CI: -1.37-4.38), but the difference was not significant.
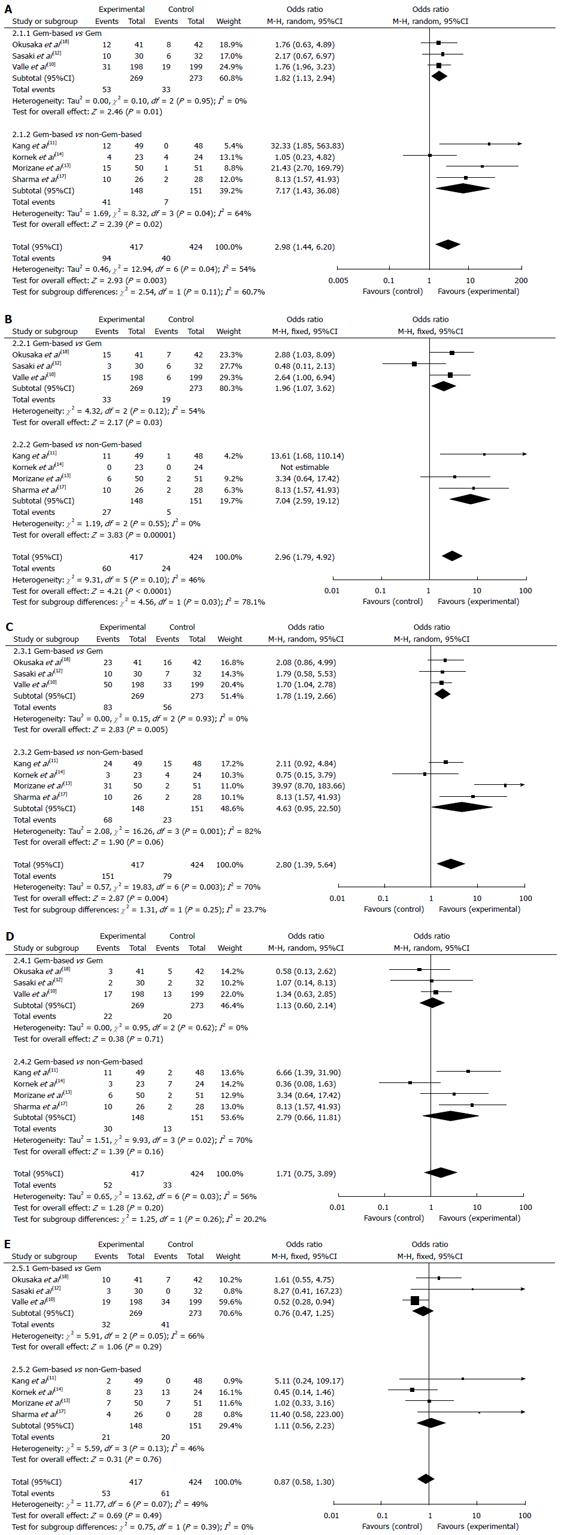
The results of the meta-analysis of the main toxicities are presented in Figure 3. The ORs for grade 3-4 hematological toxicities analyzed in this study were higher in the Gem-based combination chemotherapy group compared with the Gem monotherapy and non-Gem-based chemotherapy groups. The incidence of leukopenia (OR = 2.98, 95%CI: 1.44-6.20; P = 0.00), anemia (OR = 2.96, 95%CI: 1.79-4.92; P = 0.00) and neutropenia (OR = 2.80, 95%CI: 1.39-5.64; P = 0.00) were all significantly different between the treatment groups, whereas no significant difference was noted for thrombocytopenia (OR = 1.71; 95%CI: 0.75-3.89). No significant difference in the increased ALT level (OR = 0.87; 95%CI: 0.58-1.30) was found between the treatment groups.
In the subgroup analysis, the ORs were 1.82-7.01 for leukopenia, 1.96-7.04 for anemia, 1.78-4.63 for neutropenia, 1.13-2.79 for thrombocytopenia, and 0.76-1.11 for the increased ALT level. There were significant differences between the Gem-based combination chemotherapy group and the Gem monotherapy group in leukopenia (P = 0.01), anemia (P = 0.03) and neutropenia (P = 0.01), but not thrombocytopenia or the increased ALT level. The ORs of leukopenia (OR = 7.17; P = 0.02) and anemia (OR = 7.04; P = 0.00) in the Gem-based combination chemotherapy group were over seven times greater than those in the non-Gem-based chemotherapy subgroup; no significant differences were observed for the other toxicities.
Because significant heterogeneity was detected among the studies for the DCR, PFS and OS, we performed a sensitivity analysis to explore the heterogeneity by omitting one study at a time and calculating pooled ORs for the remainder of the studies. The study by Kang et al[11] may have been the key contributor to the inter-study heterogeneity in the DCR meta-analysis. No heterogeneity was observed after excluding this study (Q = 9.26, df = 5, I² = 46%; P = 0.10); the pooled OR for the DCR was 2.03 (95%CI: 1.21-3.40). The direction and magnitude of the pooled OR for the PFS and the pooled MD for the OS did not vary markedly with the removal of any study, which indicated good reliability (data not shown).
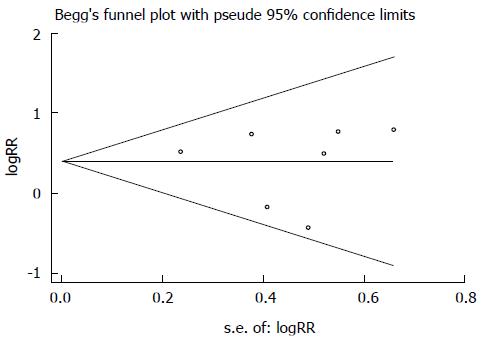
Egger’s test was used to investigate potential publication bias; there was no evidence of bias for the DRR (P = 1.00), DCR (P = 0.23), PFS (P = 0.55), or OS (P = 1.00). Additionally, publication bias was not indicated by a Begg’s funnel plot (Figure 4).
BTC is a heterogeneous group of relatively rare tumors, which often have extremely poor prognoses. Most patients present with locally advanced or metastatic disease and are candidates for surgical resection; as a result, many patients must rely on palliative chemotherapy as the only treatment option. During the last decade, several randomized controlled clinical trials have evaluated gemcitabine in combination with various agents in an attempt to improve the prognosis of advanced BTC. In this meta-analysis, we systematically evaluated the efficacy and safety of Gem-based combination chemotherapy in the treatment of advanced BTC. Our findings suggest that patients treated with Gem-based combination chemotherapy may experience better survival outcomes compared with patients not treated with this combination. However, Gem-combination chemotherapy regimens were not identical in all included studies; thus, our findings must be considered in light of this limitation.
Our overall analysis revealed that the patients treated with Gem-based combination chemotherapy had a significantly greater DRR as well as a longer PFS and OS compared with the patients not treated with this combination. Heterogeneity among the studies may be a reason for the lack of statistically significant data related to the DCR. Furthermore, the subgroup analysis revealed that the treatment with Gem-based combination chemotherapy was associated with significantly better DRR, DCR, PFS and OS outcomes compared with treatment with Gem alone. Our findings are consistent with those of Eckel et al[22], who conducted a meta-analysis of chemotherapy trials for advanced BTC treatment and reported that Gem-based combination chemotherapy (Gem-cisplatin or Gem-oxaliplatin) was associated with the highest DRRs and tumor control rates. However, their meta-analysis was not restricted to randomized trials. Another recent meta-analysis by Yang et al[23] including three randomized trials indicated that the patients with advanced BTC who were treated with Gem-based combination chemotherapy (Gem plus platinum agents) experienced better survival outcomes compared with the patients not treated with this chemotherapy combination. However, the analysis involved studies that evaluated Gem plus platinum chemotherapy; thus, new treatments, such as Gem plus S-1, were not included. A review published by Serrano et al[24] also suggested that Gem alone or in combination with other agents showed a better response rate that correlated with the time to progression. Additionally, regimens that contained two drugs induced higher response rates compared with single agent treatments. In our study, the subgroup analysis also indicated that Gem-based combination chemotherapy provides some benefit over non-Gem-based chemotherapy, but the result was not significant. The consistency between our findings and previous analyses further supports the use of Gem-based combination chemotherapy as a first-line treatment for advanced BTC.
We also assessed the five most common toxicities related to the chemotherapeutic treatment of advanced BTC, which are leukopenia, neutropenia, anemia, thrombocytopenia and an increased ALT level. The analysis indicated that the incidence of grade 3-4 hematological toxicities, including leukopenia, neutropenia and anemia, were significantly higher following Gem-based combination chemotherapy than following Gem monotherapy or non-Gem-based chemotherapy. There was no significant difference in the rates of thrombocytopenia or the increased ALT level. Furthermore, a subgroup analysis showed that the ORs of leukopenia, neutropenia and anemia after treatment with Gem-based combination chemotherapy were almost twice those of Gem monotherapy. Additionally, the ORs of leukopenia and neutropenia in the Gem-based combination chemotherapy were more than seven times those of the non-Gem-based chemotherapy. The results presented here suggest that Gem-based combination chemotherapy induced more toxicity compared with Gem alone or non-Gem-based chemotherapy. However, all severe hematological toxicities (grade 3 or 4) are infrequent and reversible, and these results are consistent with a previous study[24].
Several limitations of our meta-analysis should be mentioned. First, only randomized trials were selected; thus, the total included sample size was small (seven studies). Additionally, our selections may have been subjected to some bias, although we observed none. Second, our data are based on the published data for which the patient outcomes according to the type of BTC were not available. This may limit our capacity to fully explore the effects of Gem-based combination chemotherapy in different types of BTC. Another limitation was that the included studies did not have homogenous characteristics with respect to the regimens used for Gem-based combination chemotherapy or the competing regimen. Furthermore, the patient demographics between the included studies were different. Due to the limitations mentioned above, the results of this meta-analysis should be interpreted with care.
In conclusion, the results of our meta-analysis of randomized trials suggest that the treatment of advanced BTC with Gem-based combination chemotherapy is associated with significantly better survival outcomes compared with treatment with Gem alone or non-Gem-based chemotherapy. Major hematological toxicities associated with Gem-based combination chemotherapy were generally manageable and acceptable. Therefore, Gem-based combination chemotherapy should be considered as a standard first-line treatment for advanced BTC. In the future, larger multicenter randomized controlled trials should be designed to examine the efficacy and safety of Gem-based combination chemotherapy.
Biliary tract cancer (BTC) is a heterogeneous group of relatively rare tumors that often have extremely poor prognoses. Gemcitabine (Gem) is the treatment of choice for patients with advanced BTC. The results from several phase II studies suggest that Gem, alone or in combination with other agents, has been relatively effective for treating BTC. However, most of these studies were small, single-arm and nonrandomized trials.
The data from the largest randomized trial to date indicated that the overall survival of BTC patients was significantly higher in the Gem and cisplatin arm vs the Gem single-agent arm. Since that time, several randomized trials comparing Gem-based combination chemotherapy with other regimens have been published. However, the results of these trials were conflicting, which has made the role of Gem-based combination chemotherapy controversial.
Based on this meta-analysis, Gem-based combination chemotherapy was superior in disease response rate, progression-free survival and overall survival to the radiation therapy group or chemotherapy group alone. Similar results were indicated in the subgroup analyses. The Gem-based combination chemotherapy group had significantly more grade 3-4 treatment-related hematologic and non-hematologic toxicities than Gem alone or non-Gem-based chemotherapy groups. These findings were not presented clearly in previous systematic reviews.
The results of this meta-analysis of randomized trials suggest that Gem-based combination chemotherapy can improve the prognosis of patients with advanced BTC, though it may also increase the treatment-related toxicity.
This is a well-written manuscript analyzing therapeutic management of advanced biliary tract cancer. In this manuscript, the authors compared the efficacy and safety of Gem-based combination chemotherapy with Gem alone or non-Gem-based chemotherapy. The data were collected and analyzed effectively.
P- Reviewer: Cordelier P, Matrisian LM, Tagliaferri P S- Editor: Nan J L- Editor: AmEditor E- Editor: Wang CH
| 1. | Geynisman DM, Catenacci DV. Toward personalized treatment of advanced biliary tract cancers. Discov Med. 2012;14:41-57. [PubMed] |
| 2. | Lazaridis KN, Gores GJ. Cholangiocarcinoma. Gastroenterology. 2005;128:1655-1667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 344] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 3. | Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145:1215-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 923] [Cited by in RCA: 966] [Article Influence: 80.5] [Reference Citation Analysis (0)] |
| 4. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8406] [Cited by in RCA: 8970] [Article Influence: 690.0] [Reference Citation Analysis (0)] |
| 5. | Ciombor KK, Goff LW. Advances in the management of biliary tract cancers. Clin Adv Hematol Oncol. 2013;11:28-34. [PubMed] |
| 6. | Yang JD, Kim B, Sanderson SO, Sauver JS, Yawn BP, Larson JJ, Therneau TM, Roberts LR, Gores GJ, Kim WR. Biliary tract cancers in Olmsted County, Minnesota, 1976-2008. Am J Gastroenterol. 2012;107:1256-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 7. | Khan SA, Davidson BR, Goldin RD, Heaton N, Karani J, Pereira SP, Rosenberg WM, Tait P, Taylor-Robinson SD, Thillainayagam AV. Guidelines for the diagnosis and treatment of cholangiocarcinoma: an update. Gut. 2012;61:1657-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 593] [Article Influence: 45.6] [Reference Citation Analysis (1)] |
| 8. | Castro MP. Efficacy of gemcitabine in the treatment of patients with gallbladder carcinoma: a case report. Cancer. 1998;82:639-641. [PubMed] |
| 9. | Valle JW. Advances in the treatment of metastatic or unresectable biliary tract cancer. Ann Oncol. 2010;21 Suppl 7:vii345-vii348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2617] [Cited by in RCA: 3165] [Article Influence: 211.0] [Reference Citation Analysis (1)] |
| 11. | Kang MJ, Lee JL, Kim TW, Lee SS, Ahn S, Park do H, Lee SS, Seo DW, Lee SK, Kim MH. Randomized phase II trial of S-1 and cisplatin versus gemcitabine and cisplatin in patients with advanced biliary tract adenocarcinoma. Acta Oncol. 2012;51:860-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Sasaki T, Isayama H, Nakai Y, Ito Y, Yasuda I, Toda N, Kogure H, Hanada K, Maguchi H, Sasahira N. A randomized phase II study of gemcitabine and S-1 combination therapy versus gemcitabine monotherapy for advanced biliary tract cancer. Cancer Chemother Pharmacol. 2013;71:973-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 13. | Morizane C, Okusaka T, Mizusawa J, Takashima A, Ueno M, Ikeda M, Hamamoto Y, Ishii H, Boku N, Furuse J. Randomized phase II study of gemcitabine plus S-1 versus S-1 in advanced biliary tract cancer: a Japan Clinical Oncology Group trial (JCOG 0805). Cancer Sci. 2013;104:1211-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 14. | Kornek GV, Schuell B, Laengle F, Gruenberger T, Penz M, Karall K, Depisch D, Lang F, Scheithauer W. Mitomycin C in combination with capecitabine or biweekly high-dose gemcitabine in patients with advanced biliary tract cancer: a randomised phase II trial. Ann Oncol. 2004;15:478-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 101] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12275] [Cited by in RCA: 12882] [Article Influence: 444.2] [Reference Citation Analysis (1)] |
| 16. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 46470] [Article Influence: 2112.3] [Reference Citation Analysis (3)] |
| 17. | Sharma A, Dwary AD, Mohanti BK, Deo SV, Pal S, Sreenivas V, Raina V, Shukla NK, Thulkar S, Garg P. Best supportive care compared with chemotherapy for unresectable gall bladder cancer: a randomized controlled study. J Clin Oncol. 2010;28:4581-4586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 212] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 18. | Okusaka T, Nakachi K, Fukutomi A, Mizuno N, Ohkawa S, Funakoshi A, Nagino M, Kondo S, Nagaoka S, Funai J. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer. 2010;103:469-474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 570] [Cited by in RCA: 568] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 19. | Valle JW, Wasan H, Johnson P, Jones E, Dixon L, Swindell R, Baka S, Maraveyas A, Corrie P, Falk S. Gemcitabine alone or in combination with cisplatin in patients with advanced or metastatic cholangiocarcinomas or other biliary tract tumours: a multicentre randomised phase II study - The UK ABC-01 Study. Br J Cancer. 2009;101:621-627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 208] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 20. | Lee J, Park SH, Chang HM, Kim JS, Choi HJ, Lee MA, Jang JS, Jeung HC, Kang JH, Lee HW. Gemcitabine and oxaliplatin with or without erlotinib in advanced biliary-tract cancer: a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2012;13:181-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 346] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 21. | Neoptolemos JP, Moore MJ, Cox TF, Valle JW, Palmer DH, McDonald AC, Carter R, Tebbutt NC, Dervenis C, Smith D. Effect of adjuvant chemotherapy with fluorouracil plus folinic acid or gemcitabine vs observation on survival in patients with resected periampullary adenocarcinoma: the ESPAC-3 periampullary cancer randomized trial. JAMA. 2012;308:147-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 463] [Article Influence: 35.6] [Reference Citation Analysis (1)] |
| 22. | Eckel F, Schmid RM. Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Br J Cancer. 2007;96:896-902. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 332] [Cited by in RCA: 352] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 23. | Yang R, Wang B, Chen YJ, Li HB, Hu JB, Zou SQ. Efficacy of gemcitabine plus platinum agents for biliary tract cancers: a meta-analysis. Anticancer Drugs. 2013;24:871-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Serrano A, Gerson R. Chemotherapy with gemcitabine in advanced biliary tract carcinoma. Rev Recent Clin Trials. 2008;3:70-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |