Published online Dec 21, 2014. doi: 10.3748/wjg.v20.i47.17894
Revised: April 3, 2014
Accepted: May 29, 2014
Published online: December 21, 2014
Processing time: 335 Days and 1.6 Hours
AIM: To investigate whether hypoxia inducible factor (HIF)-1α modulates vasculogenic mimicry (VM) by upregulating VE-cadherin expression in esophageal squamous cell carcinoma (ESCC).
METHODS: Esophageal squamous cancer cell lines Eca109 and TE13 were transfected with plasmids harboring small interfering RNAs targeting HIF-1α or VE-cadherin. The proliferation and invasion of esophageal carcinoma cells were detected by MTT and Transwell migration assays. The formation of tubular networks of cells was analyzed by 3D culture in vitro. BALB/c nude mice were used to observe xenograft tumor formation. The relationship between the expression of HIF-1α and VE-cadherin, ephrinA2 (EphA2) and laminin5γ2 (LN5γ2) was measured by Western blot and real-time polymerase chain reaction.
RESULTS: Knockdown of HIF-1α inhibited cell proliferation (32.3% ± 6.1% for Eca109 cells and 38.6% ± 6.8% for TE13 cells, P < 0.05). Both Eca109 and TE13 cells formed typical tubular networks. The number of tubular networks markedly decreased when HIF-1α or VE-cadherin was knocked down. Expression of VE-cadherin, EphA2 and LN5γ2 was dramatically inhibited, but the expression of matrix metalloproteinase 2 had no obvious change in HIF-1α-silenced cells. Knockdown of VE-cadherin significantly decreased expression of both EphA2 and LN5γ2 (P < 0.05), while HIF-1α expression was unchanged. The time for xenograft tumor formation was 6 ± 1.2 d for Eca109 cells and Eca109 cells transfected with HIF-1α Neo control short hairpin RNA (shRNA) vector, and 8.4 ± 2.1 d for Eca109 cells transfected with an shRNA against HIF-1α. Knockdown of HIF-1α inhibited vasculogenic mimicry (VM) and tumorigenicity in vivo.
CONCLUSION: HIF-1α may modulate VM in ESCC by regulating VE-cadherin expression, which affects VM formation through EphA2 and LN5γ2.
Core tip: Hypoxia-inducible factor (HIF) is a key factor in regulating and promoting tumor progression. Angiogenesis and vasculogenic mimicry (VM) may play an important role in tumor acquisition of increased blood supply. We investigated the role of HIF-1α in the formation of VM in esophageal squamous cell carcinoma (ESCC). We showed that HIF-1α may upregulate the expression of VE-cadherin to modulate VM in ESCC, which may be related to changes in ephrin A2 and laminin 5γ2 protein expression. These results may have implications for the treatment of malignant tumor diseases.
- Citation: Tang NN, Zhu H, Zhang HJ, Zhang WF, Jin HL, Wang L, Wang P, He GJ, Hao B, Shi RH. HIF-1α induces VE-cadherin expression and modulates vasculogenic mimicry in esophageal carcinoma cells. World J Gastroenterol 2014; 20(47): 17894-17904
- URL: https://www.wjgnet.com/1007-9327/full/v20/i47/17894.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i47.17894
As tumors grow, their microenvironment becomes increasingly hypoxic. Under hypoxic conditions, a signaling pathway involving a crucial oxygen response regulator, termed hypoxia-inducible factor (HIF), is switched on. The α subunit of HIF-1 (HIF-1α) is a nuclear factor that is generally present in mammals, and is a well-established mediator in cancer response to hypoxia[1]. HIF-1α is degraded shortly after expression in the cytoplasm under normoxic conditions. However, HIF-1α protein can be translocated into the nucleus where it is combined with the β subunit of HIF-1 to form the HIF-1 heterodimer under hypoxic conditions. Research has shown that HIF-1α is related to angiogenesis and vasculogenic mimicry (VM)[2,3].
As we all know, angiogenesis is not the only mechanism by which tumors acquire a blood supply. Highly aggressive and metastatic melanoma cells can form vascular channel-like structures that are independent of angiogenesis. This phenomenon is called VM[3]. Tumor cell VM describes the functional plasticity of aggressive cancer cells forming de novo vascular networks. The initial morphological and molecular characterization of VM in human melanoma showed that the tumor cells coexpressed endothelial and tumor markers and formed channels, networks, and tubular structures. This provides a perfusion pathway for rapidly growing tumors, transporting fluid from leaky vessels, and/or connecting with endothelial-lined vasculature as well as an escape route for metastasis.
Recent research has suggested that tumors can be viewed as highly heterogeneous populations derived from one common progenitor. As suggested by Grunewald et al[4], although the degree to which cancer cells resemble endothelial cells is debatable, cancer cells can directly line the lumen of functional tumor blood vessels. Moreover, like the foragers in ant colonies, these cancer cells do not reproduce, but instead enable tumor growth indirectly by attraction of heterotypic tissues through chemotactic substances [e.g., vascular endothelial growth factor (VEGF)], in the same way that ants attract and recruit nestmates and even prey by odor trails and pheromones. Since the introduction of VM, many studies have contributed mechanistic insights into VM in a variety of cancers. In particular, critical VM-modulating genes are associated with vascular [VE-cadherin, ephrinA2 (EphA2) and VEGF] and hypoxia-related (HIF and Twist1) signaling pathways.
HIF-1α-siRNA significantly suppressed the VM networks under either normoxic or hypoxic conditions in gallbladder carcinoma[5]. Su et al[6] have suggested that a hypoxic microenvironment increases HIF-1α expression and induces the formation of VM channels to acquire an adequate blood supply in ovarian cancer cells.
VE-cadherin is a master gene for both tumor angiogenesis and VM[7-9]. Overexpression of VE-cadherin in various vasculogenic tumor cells has been implicated in tumor neovascularization, growth, and progression[10]. Accordingly, VE-cadherin is proposed as a target for antiangiogenic drug discovery and anti-cancer therapy[11]. HIF-1 is combined with the core recognition sequence 5’-RCGTG-3’ of the promoter sequence of hypoxia-inducible genes to promote transcription and translation of these genes[12]. There is the 5’-ACGTG-3’ sequence in the promote region of VE-cadherin gene. Therefore, we speculate that VE-cadherin may be one target gene of HIF-1α, which plays an important role in the development of VM in esophageal squamous cell carcinoma (ESCC).
This study was designed to observe the formation of vascular-network-like structures in ESCC cell lines and the impact of HIF-1α and VE-cadherin on VM in ESCC. Furthermore, the possible molecular mechanism by which HIF-1α modulates VM in ESCC cells was investigated.
ESCC cell lines Eca109 and TE13 were obtained from Cell Resource Center of Shanghai Life Science Institute. In former work, we established Eca109 and TE13 cells stably transfected with an short hairpin (sh)RNA targeting HIF-1α, which were designated as Eca109/HIF-1α shRNA cells and TE13/HIF-1α shRNA cells, respectively. The protein gel blot results demonstrated that compared to untransfected cells or cells transfected with HIF-1α Neo control shRNA vector, HIF-1α level was significantly decreased in shRNA-transfected cells[13]. Eca109 and TE13 cells were incubated in Dulbecco’s Modified Eagle’s Medium (DMEM; Hyclone, Logan, UT, United States) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Hyclone) at 37 °C in a humidified atmosphere containing 5% CO2/95% air, or hypoxic treatment was given by placing cells in a hypoxia chamber flushed with a gas mixture comprising 1% O2/5% CO2/94% N2. Eca109/HIF-1α shRNA and TE13/HIF-1α shRNA cells were cultured in the same environment.
A set of three shRNA constructs directed against human VE-cadherin mRNA and one negative control (Neo) were purchased from Shanghai Shengneng Gaming Biological Technology Company. Eca109 and TE13 cells were transfected with the VE-cadherin shRNA constructs or VE-cadherin control construct using Lipofectamine 2000 reagent (Invitrogen, United States) according to the manufacturer’s instructions. After transfection, 400 μg/mL G418 (Sigma, United States) was added to medium to select stable knockdown cells. The clones were characterized by real-time polymerase chain reaction (PCR) and Western blot to assess the level of silencing of VE-cadherin.
The stable cell lines in which HIF-1α was efficiently knocked down were named Eca109/shVE-cad derived from the Eca109 cell line and TE13/shVE-cad derived from the TE13 cell line, and the stable control cell lines were named Eca109/shVE-cad Neo and TE13/shVE-cad Neo.
The sequences of the three shRNA constructs against human VE-cadherin mRNA and the negative control were as follows: pGCsi-VE-cadherin 1: 5’-TGC TGA TGT CTT GCA GAG TGA CCA GCG TTT TGG CCA CTG ACT GAC GCT GGT CAC TGC AAG ACA T-3’ and 5’-CCT GAT GTC TTG CAG TGA CCA GCG TCA GTC AGT GGC CAA AAC GCT GGT CAC TCT GCA AGA CAT C-3’; pGCsi-VE-cadherin 2: 5’-TGC TGT AAG ATG GCT ACC ACT GCC TGG TTT TGG CCA CTG ACT GAC CAG GCA GTT AGC CAT CTT A-3’ and 5’-CCT GTA AGA TGG CTA ACT GCC TGG TCA GTC AGT GGC CAA AAC CAG GCA GTG GTA GCC ATC TTA C-3’; and pGCsi- VE-cadherin 3: 5’-TGCTG AAA TGT ACT GCG CGT GGA GAC GTT TTG GCC ACT GAC TGA CGT CTC CAC GCA GTA CAT TT-3’ and 5’-CCTG AAA TGT ACT GCG TGG AGA CGT CAG TCA GTG GCC AAA ACG TCT CCA CGC GCA GTA CAT TTc-3’.
Cells were seeded into 96-well plates at 1 × 104 cells/well (100 μL) and cultured for 7 d. In the following days, the medium was removed and 20 μL MTT solution (500 μg/mL) was added to each well, followed by 4 h incubation. MTT solution was replaced by DMSO to dissolve blue formazan crystals and absorbance was measured at 570 nm using a microplate reader.
Cells were seeded into 100-mm dishes and allowed to grow to 90% confluence. The cells were collected by digestion with EDTA-free trypsin (Invitrogen). The cell pellet was washed with cold phosphate-buffered saline (PBS) twice and resuspended in 250 μL Annexin V binding buffer (10 mmol/L HEPES pH 7.4, 150 mmol/L NaCl, 2.5 nmol/L CaCl2, 1 mmol/L MgCl2 and 4% bovine serum albumin). The cells were stained with Annexin V fluorescein isothiocyanate (FITC) for 15 min in the dark and subjected to flow cytometry analysis within 1 h.
Matrigel (300 μL/hole) was added to 24-well plates on ice and then incubated at 37 °C for 30 min. Tumor cells (5 × 105/mL) were then seeded onto the gels and incubated at 37 °C in 5% CO2/95% air. The cells were maintained in DMEM supplemented with 10% FBS. The tube-like structures in tumor cells were observed 12 h later. Five visual fields (up, down, left, right and center) were randomly chosen from each hole under an inverted microscope (Carl Zeiss, GER) to count the number of tube-like structures. The VM channels were identified by scanning at low power (magnification × 100 and magnification × 200). Ten non-overlapping fields at magnification × 400 were chosen to determine the median value of the VM channels. The number of VM channels was assessed using an ocular grid and the forbidden lines method to facilitate the counting.
The cells were washed twice with ice-cold PBS and harvested in 100-200 μL cold lysis buffer [20 mmol/L Tris-HCl (pH 7.5), 2% SDS, 4 mmol/L EDTA, 1 mmol/L PMSF and 10 U/mL aprotinin and 1 %( v/v) Triton X-100]. Lysates were kept on ice for 30 min and sonicated for 24 s using ultrasonic cell disrupter JY96-II (Ningbo, China). The extract was then centrifuged at 12000 rpm for 10 min at 4 °C and the supernatant was collected. Protein concentrations were determined using a BCA protein assay kit (Pierce, Rockford, IL, United States). Protein (40 μg) was loaded in each lane and separated by 10% SDS-polyacrylamide gel electrophoresis at 40 mA for 90 min. The protein was transferred electrophoretically to a polyvinylidene difluoride (PVDF) membrane (Roche, CH) at 100 V for 70 min, which was blocked with 5% non-fat milk in TBS-T (100 mmol/L Tris-HCl, pH 7.5, 150 mmol/L NaCl, 0.1% Tween-20) at room temperature for 1 h. Subsequently, the membrane was incubated with the indicated primary antibodies [(EphA2 1:500, HIF-1α 1:500, VE-cadherin 1:500, LN5γ2 1:200, matrix metalloproteinase (MMP)2 1:2000, β-actin 1:8000, α-tubulin 1:5000)] overnight at 4 °C and then incubated with the secondary antibodies for 1 h at 37 °C. Bands were visualized using the ECL Western Blotting Detection System (Pierce) according to the manufacturer’s instructions.
Protocols used in the animal experiment have been approved by the institutional animal ethics committee. All procedures were performed on thirty 6-wk-old female BALB/c nude mice (Nanjing Medical University Animal Centre). The mice were randomly divided into three groups (n = 10 for each). For tumor formation evaluation, 4 × 106 cells/0.2 mL (Eca109, Eca109/Neo and Eca109/shHIF) were suspended and injected subcutaneously near the shoulder and back area. Mice in Group A, B and C received an injection of Eca109 cells, Eca109/Neo cells, and Eca109/shHIF cells, respectively. Tumor size was determined by caliper measurements of length and width. Tumor volume was calculated using the following formula: (length × width)2/2. Four weeks after injection, the mice were sacrificed. Tumors were harvested, fixed with formalin and embedded in paraffin.
Formalin-fixed, paraffin-embedded sections were deparaffinized, hydrated in a graded ethanol series, and rinsed with PBS. Antigens were retrieved by heating sections in a steam cooker for 30 min. Endogenous peroxide was inactivated with 3% H2O2 inhibitor in PBS for 12 min. Nonspecific binding was blocked in 5% horse serum and 1% goat serum for 20 min. Slides were incubated overnight at 4 °C with Gal-3 (1:1000), interleukin-8 (1:25; Biosource International, Camarillo CA, United States), or MMP-2 (1:400; Chemicon, Temecula, CA, United States) antibody, and next day with a peroxidase-labeled anti-rabbit antibody (1:500; Jackson Immunoresearch,United States) for 1 h at room temperature. Signaling was detected with 3, 3-diaminobenzidine (DAB; Phoenix Bio-technologies, San Antonio, TX, United States) substrate for 6 min, and the slides were counterstained with Gill’s No. 3 hematoxylin (Sigma) for 20 s.
C918 xenograft specimens were fixed in 10% neutral buffered formalin and embedded in paraffin. Paraffin-embedded specimens were cut into serial 5-μm sections. The sections were deparaffinized, rehydrated, and subjected to immunohistochemical and periodic acid Schiff (PAS) double staining. Immunohistochemistry was conducted with a mouse monoclonal antibody against the endothelium marker CD34 (1:50 dilution; Zhong Shan Goldenbridge, Beijing, China) to identify the endothelium. DAB chromogen was used for immunohistochemistry. CD34 staining helped to distinguish the PAS-positive network of VM from endothelium-lined microvessels. Tissues were stained with PAS to identify the matrix-associated vascular channels of uveal melanoma. Quantification of VM was performed as follows: CD34-PAS dual stained sections were viewed at magnification × 400. The channels defined as VM were lined by PAS-positive material with red cells in the center of the channels, but not lined by CD34-positive endothelial cells. The mean VM count of 10 areas was calculated as the VM density (VMD) for each section. The mean VMD from five xenograft specimens in the genistein and control groups was obtained as the final VMD count.
Data were evaluated for statistical significance by one-way ANOVA with SPSS software version 11.0. All data are expressed as mean ± SE, accompanied by the number of experiments performed independently. P < 0.05 was considered statistically significant.
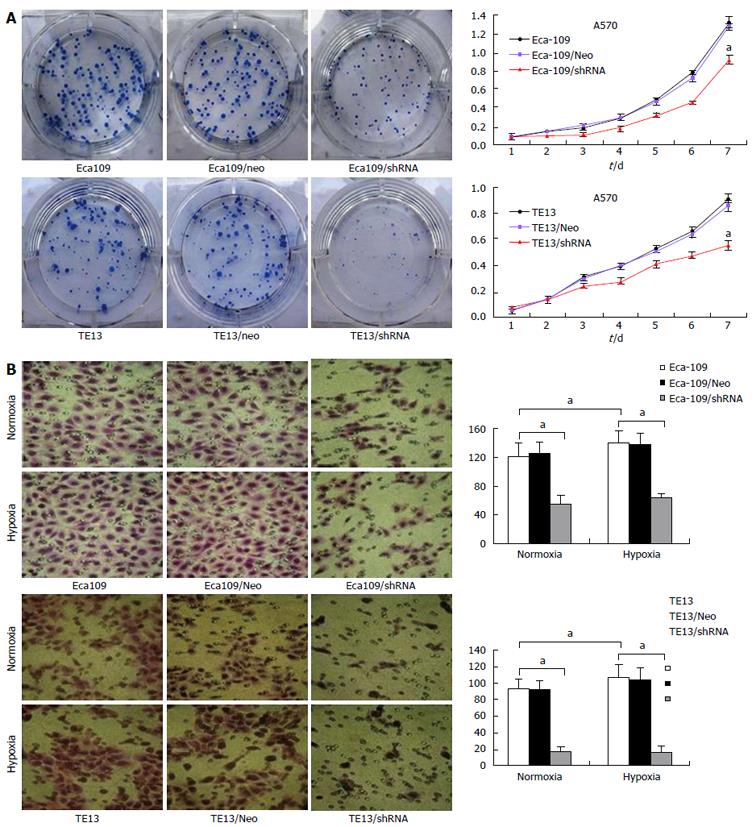
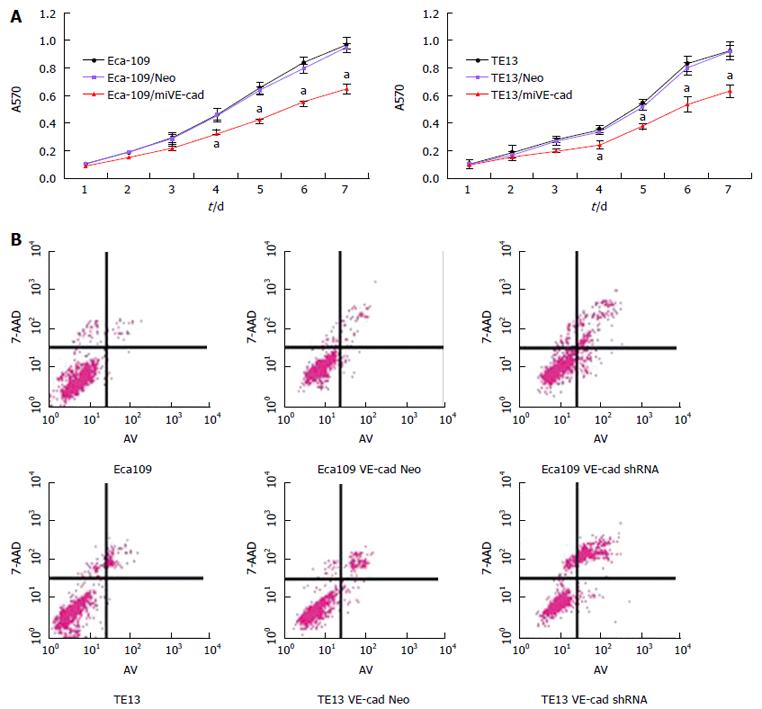
MTT and Transwell migration assays demonstrated that Eca109/HIF-1α shRNA and TE13/HIF-1α shRNA cells proliferated and invaded slower than normal Eca109 and TE13 cells or Eca109 HIF-1α Neo and TE13 HIF-1α Neo cells (Figure 1). On day 7, knockdown of HIF-1α led to 32.3% ± 6.1% inhibition of Eca109 cell proliferation and 38.6% ± 6.8% inhibition of TE13 cell proliferation (Figure 1A). These results suggest that HIF-1α could promote the proliferation and invasion of esophageal carcinoma cells (P < 0.05).
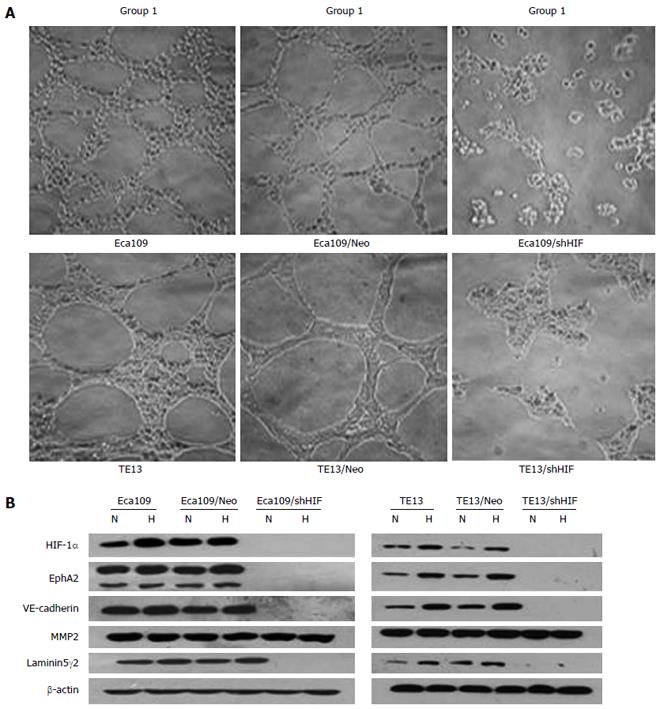
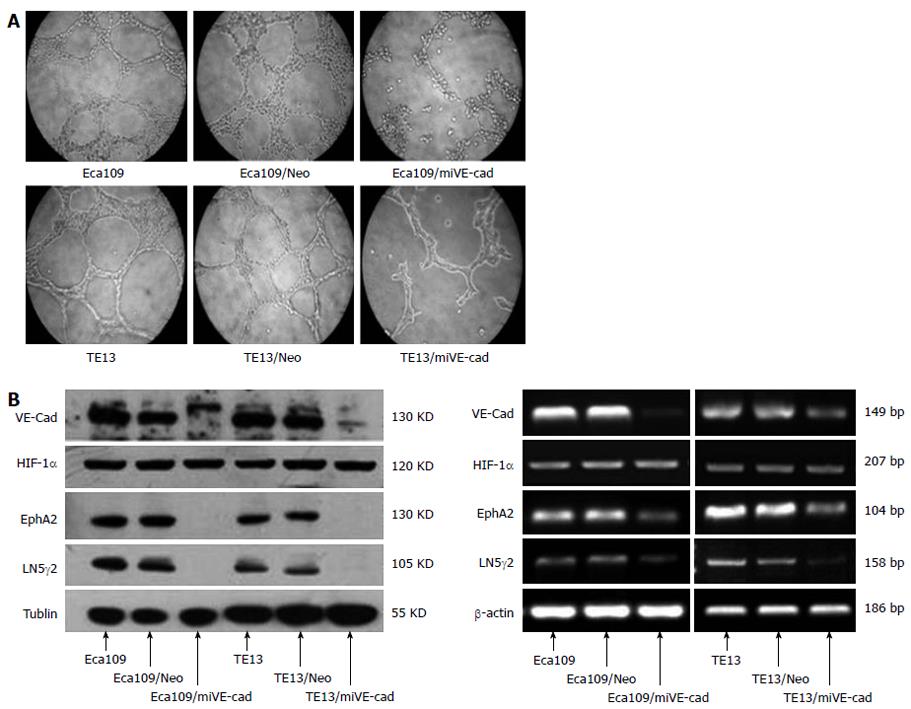
Eca109 and TE13 cells on Matrigel could connect with each other, and then formed a vascular network-like structure. The channel-forming abilities of Eca109/shHIF and TE13/shHIF cells in vitro were significantly inhibited, which demonstrated that the cyclic structure fractured and the number of tubular structures in the stable transfection group was markedly less than that in the control group (Figure 2A). The expression of HIF-1α, EphA2, VE-cadherin and laminin (LN)5γ2 proteins in TE13 and TE13/Neo (Eca109 and Eca109/Neo) cells under hypoxic conditions was increased compared with those under normoxia (P < 0.05), and expression of MMP2 had an increasing but nonsignificant trend (P > 0.05). Expression of HIF-1α, EphA2, VE-cadherin and LN5γ2 was notably inhibited in Eca109/shHIF and TE13/shHIF cells under normoxic conditions (P < 0.05) and expression of MMP2 did not change obviously. However, expression of HIF-1α and the four VM-related genes in TE13/shHIF and Eca109/shHIF cells under hypoxia was not increased compared with that under normoxia (P > 0.05) (Figure 2B).
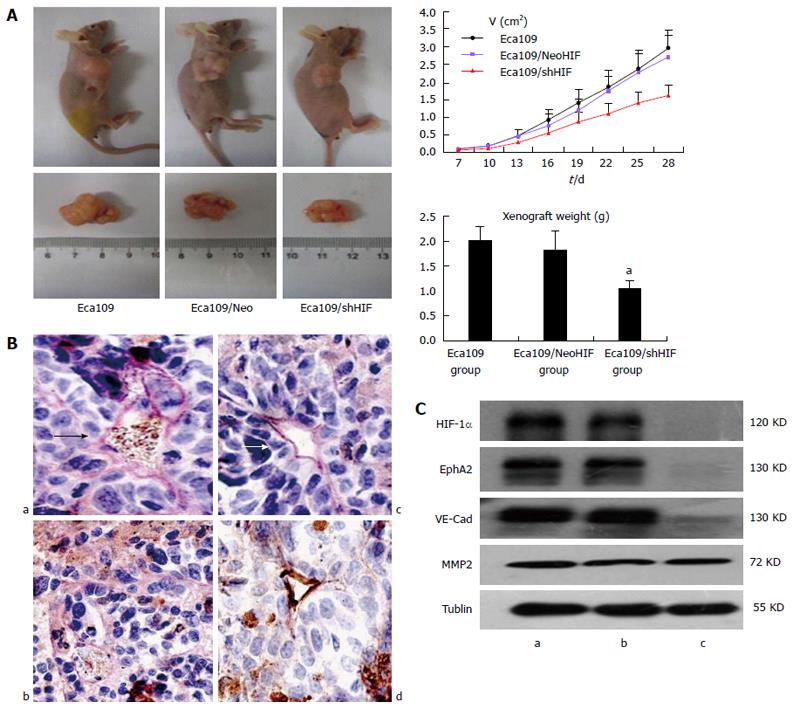
To validate the above in vitro findings in vivo, we established xenograft tumors by subcutaneous injection of HIF-1α knockdown Eca109 cells or the corresponding control cells into the flanks of BALB/c nude mice. The time for tumor formation was 6 ± 1.2 d for Eca109 and Eca109 HIF-1α Neo cells, and 8.4 ± 2.1 d for Eca109 HIF-1α shRNA cells. Four weeks after tumor formation, the size and weight of tumors derived from Eca109/shHIF-1α cells were significantly smaller (1.61 ± 0.29 cm3 and 1.04 ± 0.16 g) than those derived from Eca109 cells (2.96 ± 0.69 and 2.02 ± 0.28 g), and Eca109/HIF-1α Neo cells (2.69 ± 0.63 cm3 and 1.83 ± 0.39 g) (Figure 3A) (P < 0.05). Knockdown of HIF-1α significantly inhibited xenograft growth in vivo.
As shown in Figure 3B, the VM structure was found in Eca109 and Eca109/Neo shHIF cells cultured in 3D collagen gels more than in Eca109/shHIF cells. We examined the effects of HIF-1α knockdown on the expression of HIF-1α, EphA2, VE-cadherin and MMP2 in vivo. Protein gel blot analysis demonstrated that the expression levels of EphA2 and VE-cadherin were significantly lower in tumor tissues derived from Eca109/shHIF-1α cells than in those derived from Eca109 and Eca109 HIF-1α Neo control cells (Figure 3C). However, MMP2 protein expression was similar among the three groups. Quantitative PCR results for HIF-1α, EphA2, VE-cadherin and MMP2 in the Eca109 group were 1.0 ± 0.106, 1.0 ± 0.123, 1.0 ± 0.114 and 1.0 ± 0.172, respectively. In the Eca109/NeoHIF group, the PCR results for HIF-1α, EphA2, VE-cadherin and MMP2 were 1.103 ± 0.118, 1.078 ± 0.091, 0.937 ± 1.083 and 0.911 ± 1.106, respectively (P > 0.05). In the Eca109/shHIF group, the PCR results for HIF-1α, EphA2 and VE-cadherin were 0.684 ± 0.105, 0.713 ± 0.112 and 0.629 ± 0.094, respectively (P < 0.05) and for MMP2, it was 0.957 ± 0.162 (P > 0.05). These studies support the findings that knockdown of HIF-1α inhibits EphA2 and VE-cadherin expression in Eca109 cells in vivo.
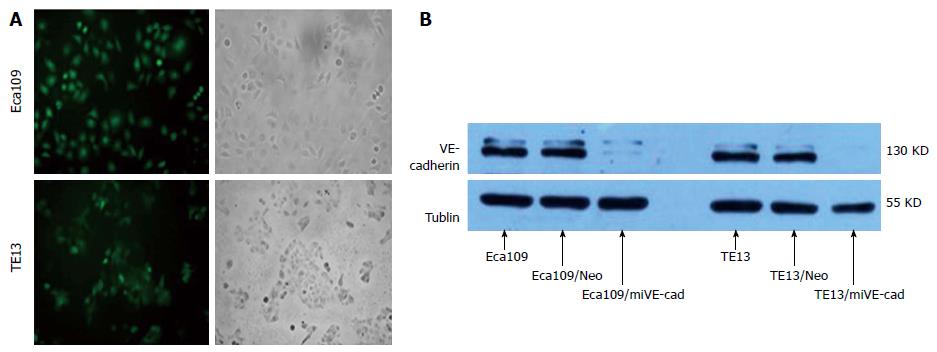
Green fluorescence was observed by inverted fluorescence microscopy in Eca109/siVE-cad and TE13/siVE-cad cells (Figure 4A), which were G418-resistant monoclones. These results revealed that Eca109/siVE-cad and TE13/siVE-cad cells had stable expression of green fluorescent protein (GFP) gene inserted in plasmid pGCsi-VE-cad. Western blot analysis indicated that expression of VE-cadherin protein was inhibited > 90% compared to untransfected cells or cells transfected with VE-cad Neo control shRNA vector (Figure 4B).
MTT assay demonstrated that Eca109/shVE-cad and TE13/shVE-cad cells proliferated slower than normal Eca109 and TE13 cells or Eca109/Neo VE-cad and TE13/Neo VE-cad cells. On day 7, HIF-1α knockdown led to 36.8% ± 6.7% inhibition of Eca109 cell proliferation and 31.0% ± 6.2% inhibition of TE13 cell proliferation (Figure 5A). These results suggest that VE-cadherin could promote the proliferation of ESCC cells (P < 0.05).
The apoptosis rate in different groups of ESCC cells was determined by Annexin V-FITC as follows: Eca109 6.77% ± 1.56%, Eca109/Neo 7.85% ± 1.47%, Eca109/shVE-cad 20.29% ± 5.23%; TE13 10.39% ± 3.08%, TE13/Neo 11.65% ± 3.79%, and TE13/shVE-cad 29.95% ± 7.39% (Figure 5B). Compared to control groups, the apoptosis rate in VE-cadherin knockdown cells was significantly increased in ESCC cells. These data indicate that VE-cadherin had an inhibitory effect on apoptosis of ESCC cells (P < 0.05)
The channel-forming ability of Eca109/shVE-cad and TE13/shVE-cad cells in vitro was significantly inhibited compared with Eca109 and TE13 cells. This result demonstrated that the cyclic structure fractured and the number of tubular structures in the stable transfection group was markedly less than that in the control group (Figure 6A). The mRNA and protein expression of VE-cadherin, EphA2, and LN5γ2 was notably inhibited in Eca109/shVE-cad and TE13/shVE-cad cells under normoxic conditions (P < 0.05) and expression of HIF-1α did not change obviously (Figure 6B).
Hypoxia is a unique microenvironment in solid tumors, including ESCC[14]. The particular characteristics of the tumor microenvironment have the potential to strongly promote tumor growth, metastasis and angiogenesis and induce drug resistance. HIF-1α is a key transcription factor in tumor development and only accumulates in hypoxic tumors[15,16].
Under normoxic conditions, the HIF-1α subunit is rapidly degraded via the von Hippel-Lindau tumor suppressor gene product (pVHL)-mediated ubiquitin-proteasome pathway. Under hypoxic conditions, the translationally controlled tumor protein decreases the protein level of VHL and increases the protein level of HIF1α, therefore, HIF-1α subunit becomes stable[17,18]. Recent studies have demonstrated that the inhibition of HIF-1α expression could inhibit the development of lung, liver, stomach, and other tumors[19-21], which indicate that targeting HIF-1α appears to be effective for cancer treatment.
In our previous study, we found that HIF-1α expression significantly increased in hypoxia compared with normoxia and inhibition of HIF-1α expression by YC-1 could inhibit the development of ESCC[13]. In this study, we found that shRNA-mediated knockdown of HIF-1α inhibited cell proliferation and migration in both hypoxia and normoxia. We used a xenograft nude mouse model to prove that shRNA-mediated HIF-1α knockdown suppressed the tumorigenicity of ESCC cells in vivo. These results indicated that HIF-1α could become an important target for ESCC therapy.
In 1999, Maniotis et al[3] found that tissue sections from highly aggressive and metastatic melanoma contained patterned networks of interconnected loops of extracellular matrix, in which endothelial cells were not identified and tumor cells mimicked endothelial cells - a process called VM. Wang et al[22] reported that VM can supplement the function of blood vessels to transport nutrients and oxygen to maintain the growth of tumor cells in malignant tumors[23]. Studies revealed that the presence of VM was associated with the expression of MMP-2, MMP-14, EphA2 and LN5γ2 in medulloblastoma[22,24,25]. At present, VM has been found in many cancers, such as malignant melanoma, osteosarcoma, ovarian cancer and liver cancer[26-28]. VM is also present in ESCC[29]. As a new blood supply pathway, VM tends to exist in highly malignant tumor tissues[30] and patients with VM often have a poor prognosis[31-33]. Some molecular mechanisms of VM have been investigated, however, the exact mechanism and the key signaling pathway have not yet been elucidated.
In the present study, we found that VM exists in human ESCC and HIF-1α plays an important role in the development of VM in human ESCC, which has not been reported. Our data also showed that targeting HIF-1α or VE-cadherin effectively inhibited formation of VM in human esophageal cancer cells, indicating that HIF-1α and VE-cadherin may be targeted for anti-ESCC therapy.
Recent publications have implied that development of VM in malignant carcinoma is closely related to VE-cadherin, EphA2, LN5γ2 and MMP-2[34-36]. Hess et al[37] brought forward the hypothesis that VE-cadherin and EphA2 activate the phosphoinositide 3-kinase and focal adhesion kinase (FAK) signaling pathways. These pathways further activate MT1-MMP, MMP-2 and then Laminin 5γ2 (LN5γ2) to promote the formation of VM[37]. However, in our study, the expression of VE-cadherin and EphA2 was inhibited after silencing of HIF-1α, while expression of MMP-2 was not significantly affected. The expression of LN5γ2 gene was almost completely inhibited in Eca109/shHIF and TE13/shHIF cells. When VE-cadherin was silenced, expression of LN5γ2 and EphA2 was inhibited, while expression of HIF-1α was unchanged. These results suggested that VE-cadherin is the downstream gene of HIF-1α and regulates VM in ESCC through EphA2 and LN5γ2 genes but not MMP2 gene.
In conclusion, the present study indicated that human ESCC cell lines could form vascular network-like structures, and HIF-1α plays an important role in the signal transduction pathway of the VM. The possible mechanism is that a hypoxic microenvironment causes greater expression of HIF-1α, and higher transcriptional activity of HIF-1α promotes the formation of VM by modulating VE-cadherin, EphA2 and LN5γ2 gene expression directly or indirectly to provide a blood supply to the tumor. Antiangiogenic drugs alone cannot completely block tumor blood supply. Thus, treatment strategies for carcinoma that presents with VM should take the latter into account, as well as targeting endothelium-dependent vessels.
The incidence of esophageal carcinoma is currently rising faster than any other cancer in the world, although the cause of this increase is largely unknown. Progression of this disease is associated with angiogenesis, a crucial event in tumor growth and metastasis.
Vasculogenic mimicry (VM) is a common event in highly malignant tumor tissue, with the function of blood vessels to transport nutrients and oxygen to maintain the growth of tumor cells. Hypoxia inducible factor (HIF)-1α and VE-cadherin, the major endothelial adhesion molecules controlling blood vessel formation, are overexpressed in esophageal squamous cell carcinoma (ESCC). Whether hypoxia induces VM formation via upregulation of VE-cadherin by HIF-1α in ESCC has not been confirmed. In this study, we demonstrated that HIF-1α may upregulate the expression of VE-cadherin to accommodate the ability of forming VM in ESCC.
Recent reports have highlighted the importance of VM. VM is closely related to VE-cadherin, ephrin A2 (EphA2) and laminin 5γ2 (LN5γ2) in malignant carcinoma. This is believed to be the first study to report that VM also exists in human ESCC and HIF-1α plays an important role in VM development. This study also showed that targeting HIF-1α or VE-cadherin effectively inhibited formation of VM in human esophageal cancer cell lines.
By understanding how VE-cadherin is induced and by blocking its expression, this study may represent a future strategy for therapeutic intervention in the treatment of patients with ESCC.
HIF is a key factor in regulating and promoting tumor progression. In this process, angiogenesis and VM may play an important role in helping tumors acquire more blood supply. VE-cadherin, EphA2 and LN5γ2 proteins are all involved in the process of VM.
The authors discussed the role of HIF-1α in the formation of VM in ESCC. They showed that HIF-1α may increase expression levels of EphA2 and LN5γ2 by upregulating VE-cadherin expression in ESCC during formation of VM. The results are interesting and may also have implications in the treatment of malignant tumor disease.
P- Reviewer: Grunewald TGP, Shehata MMM, Rodriguez-Manzaneque JC S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Zhu P, Ning Y, Yao L, Chen M, Xu C. The proliferation, apoptosis, invasion of endothelial-like epithelial ovarian cancer cells induced by hypoxia. J Exp Clin Cancer Res. 2010;29:124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Michaylira CZ, Nakagawa H. Hypoxic microenvironment as a cradle for melanoma development and progression. Cancer Biol Ther. 2006;5:476-479. [PubMed] |
| 3. | Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, Pe’er J, Trent JM, Meltzer PS, Hendrix MJ. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol. 1999;155:739-752. [PubMed] |
| 4. | Grunewald TG, Herbst SM, Heinze J, Burdach S. Understanding tumor heterogeneity as functional compartments--superorganisms revisited. J Transl Med. 2011;9:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Sun W, Shen ZY, Zhang H, Fan YZ, Zhang WZ, Zhang JT, Lu XS, Ye C. Overexpression of HIF-1α in primary gallbladder carcinoma and its relation to vasculogenic mimicry and unfavourable prognosis. Oncol Rep. 2012;27:1990-2002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Su M, Wei W, Xu X, Wang X, Chen C, Su L, Zhang Y. Role of hCG in vasculogenic mimicry in OVCAR-3 ovarian cancer cell line. Int J Gynecol Cancer. 2011;21:1366-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Zhao N, Sun BC, Sun T, Ma YM, Zhao XL, Liu ZY, Dong XY, Che N, Mo J, Gu Q. Hypoxia-induced vasculogenic mimicry formation via VE-cadherin regulation by Bcl-2. Med Oncol. 2012;29:3599-3607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Zhang W, Wang LJ, Xiao F, Wei Y, Ke W, Xin HB. Intermedin: a novel regulator for vascular remodeling and tumor vessel normalization by regulating vascular endothelial-cadherin and extracellular signal-regulated kinase. Arterioscler Thromb Vasc Biol. 2012;32:2721-2732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Vickerman V, Kamm RD. Mechanism of a flow-gated angiogenesis switch: early signaling events at cell-matrix and cell-cell junctions. Integr Biol (Camb). 2012;4:863-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 10. | Liu R, Cao Z, Tu J, Pan Y, Shang B, Zhang G, Bao M, Zhang S, Yang P, Zhou Q. Lycorine hydrochloride inhibits metastatic melanoma cell-dominant vasculogenic mimicry. Pigment Cell Melanoma Res. 2012;25:630-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Cao Z, Yu D, Fu S, Zhang G, Pan Y, Bao M, Tu J, Shang B, Guo P, Yang P. Lycorine hydrochloride selectively inhibits human ovarian cancer cell proliferation and tumor neovascularization with very low toxicity. Toxicol Lett. 2013;218:174-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 12. | Semenza GL. HIF-1, O(2), and the 3 PHDs: how animal cells signal hypoxia to the nucleus. Cell. 2001;107:1-3. [PubMed] |
| 13. | Zhu H, Feng Y, Zhang J, Zhou X, Hao B, Zhang G, Shi R. Inhibition of hypoxia inducible factor 1α expression suppresses the progression of esophageal squamous cell carcinoma. Cancer Biol Ther. 2011;11:981-987. [PubMed] |
| 14. | Natsuizaka M, Naganuma S, Kagawa S, Ohashi S, Ahmadi A, Subramanian H, Chang S, Nakagawa KJ, Ji X, Liebhaber SA. Hypoxia induces IGFBP3 in esophageal squamous cancer cells through HIF-1α-mediated mRNA transcription and continuous protein synthesis. FASEB J. 2012;26:2620-2630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Grosso S, Doyen J, Parks SK, Bertero T, Paye A, Cardinaud B, Gounon P, Lacas-Gervais S, Noël A, Pouysségur J. MiR-210 promotes a hypoxic phenotype and increases radioresistance in human lung cancer cell lines. Cell Death Dis. 2013;4:e544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 186] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 16. | Liu Z, Jia X, Duan Y, Xiao H, Sundqvist KG, Permert J, Wang F. Excess glucose induces hypoxia-inducible factor-1α in pancreatic cancer cells and stimulates glucose metabolism and cell migration. Cancer Biol Ther. 2013;14:428-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 17. | Lee JW, Bae SH, Jeong JW, Kim SH, Kim KW. Hypoxia-inducible factor (HIF-1)alpha: its protein stability and biological functions. Exp Mol Med. 2004;36:1-12. [PubMed] |
| 18. | Chen K, Chen S, Huang C, Cheng H, Zhou R. TCTP increases stability of hypoxia-inducible factor 1α by interaction with and degradation of the tumour suppressor VHL. Biol Cell. 2013;105:208-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Fu L, Chen W, Guo W, Wang J, Tian Y, Shi D, Zhang X, Qiu H, Xiao X, Kang T. Berberine Targets AP-2/hTERT, NF-κB/COX-2, HIF-1α/VEGF and Cytochrome-c/Caspase Signaling to Suppress Human Cancer Cell Growth. PLoS One. 2013;8:e69240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 20. | Li W, Chen YQ, Shen YB, Shu HM, Wang XJ, Zhao CL, Chen CJ. HIF-1α knockdown by miRNA decreases survivin expression and inhibits A549 cell growth in vitro and in vivo. Int J Mol Med. 2013;32:271-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Liu XQ, Xiong MH, Shu XT, Tang RZ, Wang J. Therapeutic delivery of siRNA silencing HIF-1 alpha with micellar nanoparticles inhibits hypoxic tumor growth. Mol Pharm. 2012;9:2863-2874. [PubMed] |
| 22. | Wang SY, Yu L, Ling GQ, Xiao S, Sun XL, Song ZH, Liu YJ, Jiang XD, Cai YQ, Ke YQ. Vasculogenic mimicry and its clinical significance in medulloblastoma. Cancer Biol Ther. 2012;13:341-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Rodríguez MI, Peralta-Leal A, O’Valle F, Rodriguez-Vargas JM, Gonzalez-Flores A, Majuelos-Melguizo J, López L, Serrano S, de Herreros AG, Rodríguez-Manzaneque JC. PARP-1 regulates metastatic melanoma through modulation of vimentin-induced malignant transformation. PLoS Genet. 2013;9:e1003531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 24. | Vartanian A, Gatsina G, Grigorieva I, Solomko E, Dombrovsky V, Baryshnikov A, Stepanova E. The involvement of Notch signaling in melanoma vasculogenic mimicry. Clin Exp Med. 2013;13:201-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Kirschmann DA, Seftor EA, Hardy KM, Seftor RE, Hendrix MJ. Molecular pathways: vasculogenic mimicry in tumor cells: diagnostic and therapeutic implications. Clin Cancer Res. 2012;18:2726-2732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 223] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 26. | Xu Y, Li Q, Li XY, Yang QY, Xu WW, Liu GL. Short-term anti-vascular endothelial growth factor treatment elicits vasculogenic mimicry formation of tumors to accelerate metastasis. J Exp Clin Cancer Res. 2012;31:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 135] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 27. | Ma JL, Han SX, Zhu Q, Zhao J, Zhang D, Wang L, Lv Y. Role of Twist in vasculogenic mimicry formation in hypoxic hepatocellular carcinoma cells in vitro. Biochem Biophys Res Commun. 2011;408:686-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 28. | Itzhaki O, Greenberg E, Shalmon B, Kubi A, Treves AJ, Shapira-Frommer R, Avivi C, Ortenberg R, Ben-Ami E, Schachter J. Nicotinamide inhibits vasculogenic mimicry, an alternative vascularization pathway observed in highly aggressive melanoma. PLoS One. 2013;8:e57160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 29. | Chai DM, Bao ZQ, Hu JG, Ma L, Feng ZZ, Tao YS. Vasculogenic mimicry and aberrant expression of HIF-lα/E-cad are associated with worse prognosis of esophageal squamous cell carcinoma. J Huazhong Univ Sci Technolog Med Sci. 2013;33:385-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Li M, Gu Y, Zhang Z, Zhang S, Zhang D, Saleem AF, Zhao X, Sun B. Vasculogenic mimicry: a new prognostic sign of gastric adenocarcinoma. Pathol Oncol Res. 2010;16:259-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 31. | Vartanian AA, Stepanova EV, Gutorov SL, Solomko ESh, Grigorieva IN, Sokolova IN, Baryshnikov AY, Lichinitser MR. Prognostic significance of periodic acid-Schiff-positive patterns in clear cell renal cell carcinoma. Can J Urol. 2009;16:4726-4732. [PubMed] |
| 32. | Baeten CI, Hillen F, Pauwels P, de Bruine AP, Baeten CG. Prognostic role of vasculogenic mimicry in colorectal cancer. Dis Colon Rectum. 2009;52:2028-2035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 33. | Niu YJ, Liu FL, Yang Y, Yuan CY. [Relationship between vasculogenic mimicry and clinical pathological characters in retinoblastoma]. Zhonghua Yanke Zazhi. 2009;45:318-322. [PubMed] |
| 34. | Sun T, Zhao N, Zhao XL, Gu Q, Zhang SW, Che N, Wang XH, Du J, Liu YX, Sun BC. Expression and functional significance of Twist1 in hepatocellular carcinoma: its role in vasculogenic mimicry. Hepatology. 2010;51:545-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 271] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 35. | Liu T, Sun B, Zhao X, Gu Q, Dong X, Yao Z, Zhao N, Chi J, Liu N, Sun R. HER2/neu expression correlates with vasculogenic mimicry in invasive breast carcinoma. J Cell Mol Med. 2013;17:116-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 36. | Lu XS, Sun W, Ge CY, Zhang WZ, Fan YZ. Contribution of the PI3K/MMPs/Ln-5γ2 and EphA2/FAK/Paxillin signaling pathways to tumor growth and vasculogenic mimicry of gallbladder carcinomas. Int J Oncol. 2013;42:2103-2115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 37. | Hess AR, Seftor EA, Gruman LM, Kinch MS, Seftor RE, Hendrix MJ. VE-cadherin regulates EphA2 in aggressive melanoma cells through a novel signaling pathway: implications for vasculogenic mimicry. Cancer Biol Ther. 2006;5:228-233. [PubMed] |