Published online Dec 14, 2014. doi: 10.3748/wjg.v20.i46.17661
Revised: July 28, 2014
Accepted: August 13, 2014
Published online: December 14, 2014
Processing time: 112 Days and 8.3 Hours
Familial adenomatous polyposis is associated with a high incidence of malignancies in the upper gastrointestinal tract (particularly ampullary adenocarcinomas). However, few reports have described a correlation between familial adenomatous polyposis and gallbladder neoplasms. We present a case of a 60-year-old woman with familial adenomatous polyposis who presented with an elevated mass in the neck of the gallbladder (measuring 16 mm × 8 mm in diameter) and multiple small cholecystic polyps. She had undergone a total colectomy for ascending colon cancer associated with familial adenomatous polyposis 22 years previously. The patient underwent laparoscopic cholecystectomy under a preoperative diagnosis of multifocal gallbladder polyps. Pathologic examination of the resected gallbladder revealed more than 70 adenomatous lesions, a feature consistent with adenoma of the gallbladder. This case suggests a requirement for long-term surveillance of the biliary system in addition to the gastrointestinal tract in patients with familial adenomatous polyposis.
Core tip: Familial adenomatous polyposis (FAP) is associated with a high incidence of malignancies in the upper gastrointestinal tract. However, few reports have described a correlation between FAP and gallbladder neoplasms. This case, along with the other previously reported cases of FAP-associated extraintestinal neoplasia, suggests a requirement for long-term surveillance of the biliary system in addition to the gastrointestinal tract in patients with FAP.
- Citation: Mori Y, Sato N, Matayoshi N, Tamura T, Minagawa N, Shibao K, Higure A, Nakamoto M, Taguchi M, Yamaguchi K. Rare combination of familial adenomatous polyposis and gallbladder polyps. World J Gastroenterol 2014; 20(46): 17661-17665
- URL: https://www.wjgnet.com/1007-9327/full/v20/i46/17661.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i46.17661
Familial adenomatous polyposis (FAP) is an inherited autosomal dominant disease characterized by the development of hundreds to thousands of adenomas in the colonic mucosa. Patients with FAP are at a high risk of developing adenocarcinomas during the second and third decades of life unless prophylactic colectomy is performed[1]. The frequency of FAP is 1/5000 and 1/17000 in American and Japanese populations, respectively[2]. The disease is caused by a germline mutation of the adenomatous polyposis coli (APC) gene located on chromosome 5q21[3]. Patients with FAP are known to develop malignancies in the upper gastrointestinal tract, in particular ampullary adenocarcinomas[4-8]. In contrast, few case reports have described an association between FAP and biliary system neoplasia[9-12]. We present a rare case of a patient with FAP who developed multifocal gallbladder polyps.
A 60-year-old woman was admitted to our hospital with the chief complaint of right hypochondralgia after meals. She had undergone total colectomy for ascending colon cancer associated with FAP 22 years previously. Histopathology of the resected colon revealed moderately differentiated adenocarcinoma invading up to the muscularis propria in the cecum and multiple (over 100) adenomas throughout the colon. There was no lymph node metastasis at the time of colectomy.
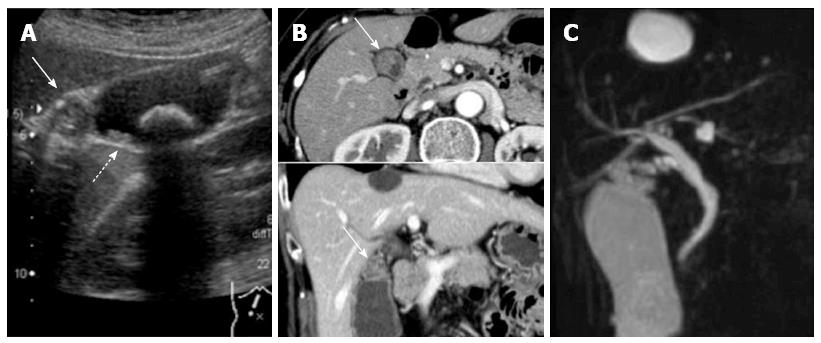
Upon presentation, a physical examination revealed no tenderness or palpable masses in the abdomen. Laboratory data, including blood cell counts, serum biochemistry, and tumor marker levels (carcinogenic embryonic antigen and carbohydrate antigen 19-9), did not show any abnormalities. Abdominal ultrasound showed an isoechoic mass in the neck of the gallbladder and multiple small polyps, measuring between 3 and 16 mm in diameter, throughout the gallbladder (Figure 1A). A gallbladder stone, measuring 22 mm in diameter, was also identified. CT showed a contrast-enhanced mass in the neck of the gallbladder (Figure 1B). There was no visible lymphadenopathy. Magnetic resonance cholangiopancreatography showed no other abnormalities of the biliary and pancreatic ductal systems (Figure 1C).
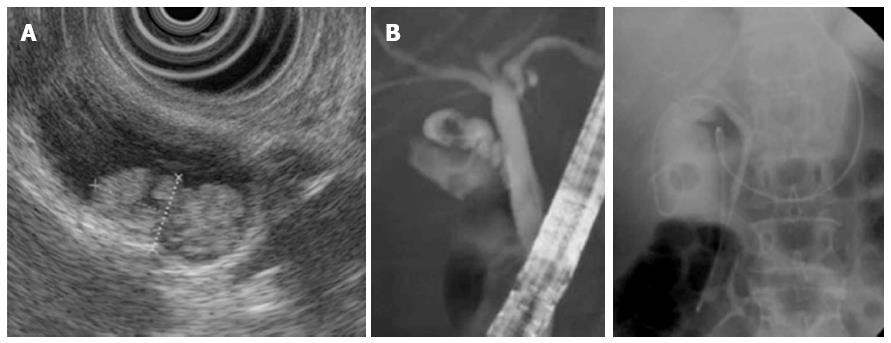
Endoscopic ultrasound revealed a hyperechoic papillary mass, measuring 16 mm × 8 mm in diameter, in the neck of the gallbladder as well as multiple small polyps (Figure 2A). Endoscopic naso-gallbladder drainage was performed to enable cytologic examination of the bile (Figure 2B). The biliary amylase level was 289 IU/L. Cytology of the bile was negative for cancer cells. These findings together led to a tentative preoperative diagnosis of multifocal gallbladder polyps.
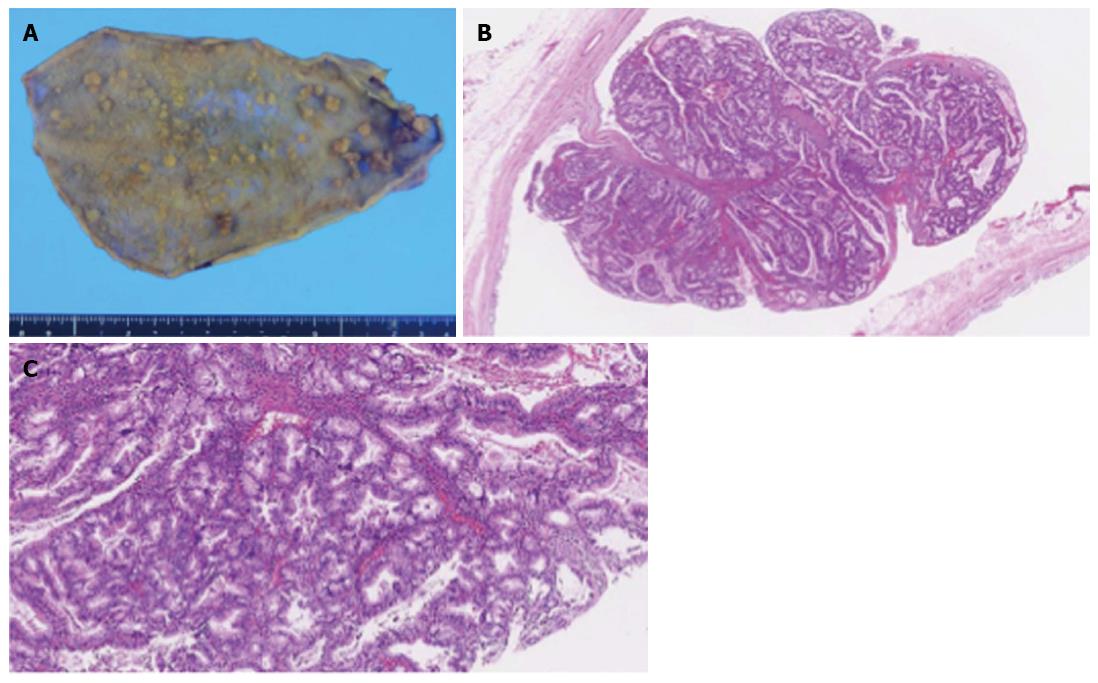
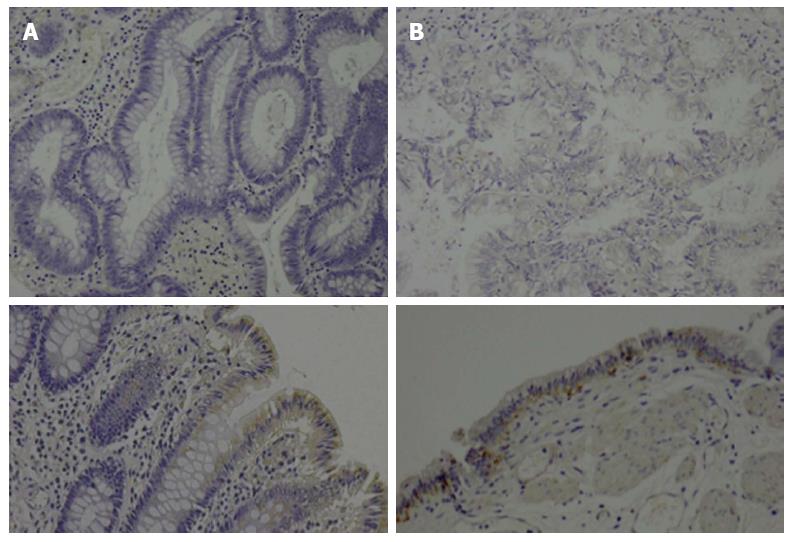
The patient underwent laparoscopic cholecystectomy. Pathologic examination of the resected specimen showed more than 70 polypoid lesions of various sizes. They were composed of proliferative lobules of closely packed pyloric-type glands displaying low- to intermediate-grade dysplasia without stromal invasion; the lesions were consistent with multiple tubular adenomas (Figure 3). Immunohistochemical analysis of APC protein expression in the previously resected colonic polyps and the gallbladder polyps revealed loss of expression in both lesions compared with the corresponding normal mucosa, which was weakly positive for APC in both locations (Figure 4). The patient’s postoperative course was uneventful, and she has been well for 15 mo without any signs of biliary tract cancer.
Early screening for FAP and prophylactic colectomies for affected patients have decreased the mortality rate of colorectal cancer[1]. Many reports have suggested an association between FAP and extracolonic lesions such as neoplasms of the stomach, ampulla of Vater, and small intestine[4-8]. However, few reports have described a correlation between FAP and polyposis or carcinoma in the hepatopancreaticobiliary tract. Okamura et al[13] described a case of hepatocellular adenoma concomitant with FAP. In a large cohort study, Giardiello et al[14] demonstrated that the relative risk of pancreatic cancer for patients with FAP was 4.46 (95%CI: 1.2-11.4). Maire et al[15] reported the first case of intraductal papillary mucinous neoplasm of the pancreas in a patient with FAP. In addition, two FAP cases associated with neoplasia of the common bile duct (a carcinoma and an adenoma) were described by Järvinen et al[16].
Gallbladder adenomas are histologically classified into tubular, papillary, tubulopapillary, and villous adenomas[17]; adenomas can transform into adenocarcinomas through an adenoma-carcinoma sequence. Twelve cases of gallbladder neoplasms (adenomas or adenocarcinomas) have been previously reported in association with FAP[12]. Of these 12 patients, two had malignancies; one was adenocarcinoma in situ, and the other was an invasive adenocarcinoma. Gallbladder stones were present in 70% of the cases. In our patient, the resected gallbladder showed approximately 70 polypoid lesions with a maximum diameter of 16 mm. These lesions were classified as adenomas, showing low- to intermediate-grade dysplasia without malignant cells. Nugent et al[18] reported that 40% of gallbladder specimens from patients with FAP revealed dysplasia, including epithelial dysplasia, microadenomas, and adenomatous polyps.
The germline loss of function mutation of the tumor suppressor gene APC that causes FAP results in the constitutive activation of β-catenin; this culminates in cellular proliferation, resulting in adenoma formation[19]. In our case, the adenomatous polyps from both the colon and the gallbladder were negative for APC expression by immunohistochemistry, suggesting biallelic inactivation of APC during neoplastic progression in both organs. Therefore, we considered the gallbladder adenomas that developed in this case to be a phenotype of FAP, rather than a coincidence.
Our case represents a rarely described combination of FAP and gallbladder polyposis. This case, along with the other previously reported cases of FAP-associated extraintestinal neoplasia, suggests a requirement for long-term surveillance of the biliary system in addition to the gastrointestinal tract in patients with FAP.
A 60-year-old woman with a history of total colectomy for familial adenomatous polyposis (FAP) presented with right hypochondralgia.
Multifocal tubular adenomas of the gallbladder polyps.
Gallbladder cancer, cholesterol polyps.
White blood cell, 7800/μL; hemoglobin, 12.8 g/dL; carcinoembryonic antigen, 2.6 ng/mL, carbohydrate antigen 19-9, 25.0 U/mL; serum chemistry including liver function test were within normal limits.
Endoscopic ultrasound revealed a hyperechoic papillary mass, measuring 16 mm × 8 mm in diameter, in the neck of the gallbladder, as well as multiple small polyps.
Pathologic examination showed multiple tubular adenomas, and immunohistochemical analysis of adenomatous polyposis coli protein expression in the previously resected colonic polyps and the gallbladder polyps revealed loss of expression in both lesions compared with the weakly positive expression in the corresponding normal mucosa.
The patient underwent laparoscopic cholecystectomy.
This case suggests a requirement for long-term surveillance of the biliary system in addition to the gastrointestinal tract in patients with FAP.
This article applies clinical significance in the guidance of diagnosis and treatment of FAP.
P- Reviewer: Kai K, Zhu YF S- Editor: Ding Y L- Editor: AmEditor E- Editor: Ma S
| 1. | Bussey HJ, Veale AM, Morson BC. Genetics of gastrointestinal polyposis. Gastroenterology. 1978;74:1325-1330. [PubMed] |
| 2. | Nishisho I, Nakamura Y, Miyoshi Y, Miki Y, Ando H, Horii A, Koyama K, Utsunomiya J, Baba S, Hedge P. Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science. 1991;253:665-669. [PubMed] |
| 3. | Bodmer WF, Bailey CJ, Bodmer J, Bussey HJ, Ellis A, Gorman P, Lucibello FC, Murday VA, Rider SH, Scambler P. Localization of the gene for familial adenomatous polyposis on chromosome 5. Nature. 1987;328:614-616. [PubMed] |
| 4. | Jones TR, Nance FC. Periampullary malignancy in Gardner’s syndrome. Ann Surg. 1977;185:565-573. [PubMed] |
| 5. | Watanabe H, Enjoji M, Yao T, Ohsato K. Gastric lesions in familial adenomatosis coli: their incidence and histologic analysis. Hum Pathol. 1978;9:269-283. [PubMed] |
| 6. | Yamada A, Watabe H, Iwama T, Obi S, Omata M, Koike K. The prevalence of small intestinal polyps in patients with familial adenomatous polyposis: a prospective capsule endoscopy study. Fam Cancer. 2014;13:23-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Jaganmohan S, Lynch PM, Raju RP, Ross WA, Lee JE, Raju GS, Bhutani MS, Fleming JB, Lee JH. Endoscopic management of duodenal adenomas in familial adenomatous polyposis--a single-center experience. Dig Dis Sci. 2012;57:732-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Skipworth JR, Morkane C, Raptis DA, Vyas S, Olde Damink SW, Imber CJ, Pereira SP, Malago M, West N, Phillips RK. Pancreaticoduodenectomy for advanced duodenal and ampullary adenomatosis in familial adenomatous polyposis. HPB (Oxford). 2011;13:342-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Lees CD, Hermann RE. Familial polyposis coli associated with bile duct cancer. Am J Surg. 1981;141:378-380. [PubMed] |
| 10. | Bombi JA, Rives A, Astudillo E, Pera C, Cardesa A. Polyposis coli associated with adenocarcinoma of the gallbladder. Report of a case. Cancer. 1984;53:2561-2563. [PubMed] |
| 11. | Komorowski RA, Tresp MG, Wilson SD. Pancreaticobiliary involvement in familial polyposis coli/Gardner’s syndrome. Dis Colon Rectum. 1986;29:55-58. [PubMed] |
| 12. | Horne J, Jaynes E, Carr N. Unicryptal gallbladder adenomas in a patient with Gardner’s syndrome. Pathol Res Pract. 2013;209:527-529. [PubMed] |
| 13. | Okamura Y, Maeda A, Matsunaga K, Kanemoto H, Furukawa H, Sasaki K, Yamaguchi S, Uesaka K. Hepatocellular adenoma in a male with familial adenomatous polyposis coli. J Hepatobiliary Pancreat Surg. 2009;16:571-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Giardiello FM, Offerhaus GJ, Lee DH, Krush AJ, Tersmette AC, Booker SV, Kelley NC, Hamilton SR. Increased risk of thyroid and pancreatic carcinoma in familial adenomatous polyposis. Gut. 1993;34:1394-1396. [PubMed] |
| 15. | Maire F, Hammel P, Terris B, Olschwang S, O’Toole D, Sauvanet A, Palazzo L, Ponsot P, Laplane B, Lévy P. Intraductal papillary and mucinous pancreatic tumour: a new extracolonic tumour in familial adenomatous polyposis. Gut. 2002;51:446-449. [PubMed] |
| 16. | Järvinen HJ, Nyberg M, Peltokallio P. Biliary involvement in familial adenomatosis coli. Dis Colon Rectum. 1983;26:525-528. [PubMed] |
| 17. | Hamilton S. Pathology and genetics of tumours of the digestive system. Lyon: Iarc 2000; 201-213. |
| 18. | Nugent KP, Spigelman AD, Talbot IC, Phillips RK. Gallbladder dysplasia in patients with familial adenomatous polyposis. Br J Surg. 1994;81:291-292. [PubMed] |
| 19. | Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM, Bos JL. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319:525-532. [PubMed] |