Published online Dec 14, 2014. doi: 10.3748/wjg.v20.i46.17603
Revised: March 17, 2014
Accepted: June 17, 2014
Published online: December 14, 2014
Processing time: 378 Days and 0.2 Hours
AIM: To present and integrate findings of studies investigating the effects of laparoscopic cholecystectomy on various aspects of lung function.
METHODS: We extensively reviewed literature of the past 24 years concerning the effects of laparoscopic cholecystectomy in comparison to the open procedure on many aspects of lung function including spirometric values, arterial blood gases, respiratory muscle performance and aspects of breathing control, by critically analyzing physiopathologic interpretations and clinically important conclusions. A total of thirty-four articles were used to extract information for the meta-analysis concerning the impact of the laparoscopic procedure on lung function and respiratory physiopathology. The quality of the literature reviewed was evaluated by the number of their citations and the total impact factor of the corresponding journals. A fixed and random effect meta-analysis was used to estimate the pooled standardized mean difference of studied parameters for laparoscopic (LC) and open (OC) procedures. A crude comparison of the two methods using all available information was performed testing the postoperative values expressed as percentages of the preoperative ones using the Mann-Whitney two-sample test.
RESULTS: Most of the relevant studies have investigated and compared changes in spirometric parameters.The median percentage and interquartile range (IQR) of preoperative values in forced vital capacity (FVC), forced expiratory volume in 1 s and forced expiratory flow (FEF) at 25%-75% of FVC (FEF25%-75%) expressed as percentage of their preoperative values 24 h after LC and OC were respectively as follows: [77.6 (73.0, 80.0) L vs 55.4 (50.0, 64.0) L, P < 0.001; 76.0 (72.3, 81.0) L vs 52.5 (50.0, 56.7) L, P < 0.001; and 78.8 (68.8, 80.9) L/s vs 60.0 (36.1, 66.1) L/s, P = 0.005]. Concerning arterial blood gases, partial pressure of oxygen [PaO2 (kPa)] at 24 or 48 h after surgical treatment showed reductions that were significantly greater in OC compared with LC [LC median 1.0, IQR (0.6, 1.3); OC median 2.4, IQR (1.2, 2.6), P = 0.019]. Fewer studies have investigated the effect of LC on respiratory muscle performance showing less impact of this surgical method on maximal respiratory pressures (P < 0.01); and changes in the control of breathing after LC evidenced by increase in mean inspiratory impedance (P < 0.001) and minimal reduction of duty cycle (P = 0.01) compared with preoperative data.
CONCLUSION: Laparoscopic cholecystectomy seems to be associated with less postoperative derangement of lung function compared to the open procedure.
Core tip: Laparoscopic cholecystectomy does not damage the abdominal muscles and diaphragmatic function is significantly less affected compared to the open method. This procedure is accompanied by a lower impact on respiratory function and better oxygenation. Mobilization can occur within the first twenty-four h postoperatively, thus preventing the formation of atelectasis. Atelectasis is known to be the primary cause of infective sequelae, which are associated with increased morbidity and mortality especially for patients with deranged lung function.
- Citation: Bablekos GD, Michaelides SA, Analitis A, Charalabopoulos KA. Effects of laparoscopic cholecystectomy on lung function: A systematic review. World J Gastroenterol 2014; 20(46): 17603-17617
- URL: https://www.wjgnet.com/1007-9327/full/v20/i46/17603.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i46.17603
According to the literature[1-7], patients who undergo major surgical procedures often develop postoperative respiratory complications including atelectasis and infection. In particular, for upper abdominal surgery, the morbidity and mortality range from 20%-25%[1,4] and 3%-5%[7], respectively. The aforementioned complications are also more frequently associated with operations in the upper abdomen than in the lower abdomen[6-11]. In 1933 Beecher[8,12] was the first to study changes in lung volumes in a pool of sixty-four patients between laparotomy and recovery by using a nitrogen powered device. Specifically, he observed that variables such as residual volume (RV), functional residual capacity, total lung capacity and vital capacity (VC) were reduced after surgical intervention, all of which gradually recovered within a week[8,12]. Compared to patients recovering from lower abdominal surgery, the change in VC was more acute for patients recovering from upper abdominal surgery[8,12]. Moreover, it was reported for the first time[8,12] that changes in lung volumes were accompanied by a pattern of rapid shallow breathing, namely a decrease in tidal volume along with an increase in breathing frequency without any changes in minute ventilation.
The impact of upper abdominal surgery on respiratory function parameters, including lung volumes, flow rates, arterial blood gases and diaphragmatic function, was investigated in a number of studies[2,3,9,11,13-15] published during the seventies and eighties. Since 1990 the laparoscopic surgical method has been introduced for asymptomatic cholecystitis and cholelithiasis and the first laparoscopic cholecystectomy (LC) was performed in Lyon, France, by Philip Mouret in 1987[16]. During the last twenty-four years a number of comparative studies[17-36] have been published in the literature on LC and open cholecystectomy (OC), in which the influence of each surgical method on morbidity related to both respiratory functions and recovery outcomes of patients were discussed. In this systematic review a three-fold aim is presumed: (1) to perform a systematic review of comparative studies between LC and OC, published in the relevant literature since 1990, by examining changes in the pulmonary function, breathing pattern and oxygenation status; (2) to present an overall physiopathological interpretation of the findings from the literature search; and (3) to present guidelines addressed to general physicians and surgeons for the management of cholecystectomy candidates with impaired lung functions.
Two different literature searches were conducted for this review, and both searches covered from 1st January 1990 to present. The PubMed (National Library of Medicine/National Institute of Health, United States) database was used.
The key words and research terms used for the selection of articles were determined by one of the investigators (Bablekos GD). The methodology concerning this approach is described as follows. The aim was to explore comparative studies between laparoscopic and open cholecystectomy focusing on their influences on the respiratory performance and oxygenation status in operated patients from the early postoperative period to their return to usual daily activities. Particularly for the first literature search a generic term such as open was used, and (laparosc* or endosc* or minimally invasive) was used as the prerequisite term. Then, the prerequisite term was combined with the following terms with the AND operator: Abdomen/Viscera (Mesh) or surgery (Mesh)/surgery or [digestive system (Mesh) + surgery] or cholecystectomy (Mesh), Laparoscopic (Mesh) or Endoscopy (Mesh), Digestive System (Mesh) or Digestive system Surgical procedures (Mesh) or abdominal pain (Mesh) or abdominal wall (Mesh). The results from the above searches were combined with the following terms with the AND operator: Alveolar-arterial difference or arterio-alveolar difference, Oxygen content, Oxygenation status, Respiratory Physiological Phenomena (Mesh), Respiratory Function Tests (Mesh), Hydrogen Ion Concentration (Mesh), Respiratory function, Respiratory physiopathology, Partial Pressure (Mesh), Blood gas analysis (Mesh). All results were combined with the OR operator and forty-one articles were detected. Twenty studies[17-32,36-39] were selected by the primary investigator (Bablekos GD) for review and analysis because their titles and abstracts were related to our study and could be used to support the medical interpretation of the findings resulting from the search process. The descriptions of the baseline characteristics for the aforementioned 20 articles, along with their reference number as an exponent, are provided in Table 1.
| Ref. | Baseline characteristics |
| Frazee et al[17] | Comparative measurements of FVC, FEV1 and FEF25%-75% variables preoperatively and on the 1st postoperative day after LC and OC |
| Hall et al[18] | Comparison of the incidence of postoperative pulmonary complications (collapse/consolidation, unexplained temperature > 38 °C and positive sputum microbiology) after LC and OC |
| Coskun et al[19] | Comparative measurements of the FVC, FEV1, Tiffeneau index, PEF and MEF25% variables before and 24 h after LC and OC |
| Damiani et al[20] | Comparative meta-analytic study focusing on the evaluation of the Tiffenneau index after LC and OC |
| Osman et al[21] | Comparative measurements for FVC, FEV1, Tiffenneau index and ABGs variables preoperatively and on the first day after LC and OC |
| Putensen-Himmer et al[22] | Comparative measurements for FVC, FEV1, FRC and ABGs variables preoperatively and up to the 3rd postoperative day after LC and OC |
| Mealy et al[23] | Comparative measurements for FVC, FEV1, PF, ABGs, urinary cortisol, vanillylmandelic acid, metanephrines and nitrogen loss, CRP, ESR and pain analogue scale preoperatively and up to 48 h after LC and OC |
| Williams et al[24] | Comparative measurements for FVC, FEV1 and Maximum Forced Expiratory Flow Rate preoperatively and after LC and OC, according to patient’s cooperation |
| Gunnarsson et al[25] | Comparative measurements for FVC, FEV1 and ABGs variables before surgery and 2 h and the first day after LC and OC |
| Karayiannakis et al[26] | Comparative measurements for FVC, FEV1, FRC, FEF25%-75% and ABGs variables preoperatively and on the second day after LC and OC |
| Hendolin et al[27] | Comparative measurements for FVC, FEV1, Peak Flow Velocity and arterial oxygen tension variables and measurements for plasma concentrations of catecholamines, cortisol and glucose preoperatively, in the recovery room and on the first day after LC and OC |
| Hasukić et al[28] | Comparative measurements for FVC, FEV1, FEF25%-75%, Peak Expiratory Flow and ABGs variables preoperatively and on the first day after LC and OC |
| Bablekos et al[29] | Comparative measurements of lung volumes (FVC, VC , ERV, IC, FRC, RV/TLC variables), flow rates (FEV1, Tiffenneau index, PEF, FEF25%-75% variables) and ABGs parameters preoperatively, on the 2nd and on the 8th day after LC and OC |
| Ravimohan et al[30] | Comparative measurements for FVC, FEV1, FEF25%-75%, PEF, Tiffenneau index and ABGs parameters preoperatively, on the first and on the sixth postoperative day after LC and OC |
| Bablekos et al[31] | Comparative measurements of Control of Breathing indices (VT, BF, TI, TI/TTOT, Po.1, Zminsp) and airway resistance (Raw) preoperatively, two days and eight days after LC and OC |
| McMahon et al[32] | Minute ventilation, arterial carbon dioxide tension, end-tidal CO2 tension, peak airway pressure and arterial oxygen levels were studied just before operation and at the time of gallbladder removal during LC and OC |
| Mimica et al[36] | Examination of the influence of physical therapy on both the values of respiratory parameters, such as FVC, FEV1, Tiffenneau index, and ABGs variables preoperatively and to the sixth day after LC and OC |
| Farrow et al[37] | The authors showed that LC is associated with significantly less morbidity compared with OC. Variables such as FVC and FEV1 along with the occurrence of postoperative pulmonary complications and narcotic doses were studied preoperatively to the third day after LC and OC |
| Redmond et al[38] | Parameters determining the immune function such as monocyte superoxide anion (O2-) and tumor necrosis factor release, neutrophil O2- levels and chemotaxis, serum cortisol and CRP were studied prior to surgery and on the first and third days after LC and OC |
| Kimberley et al[39] | FVC, FEV1, Maximum voluntary HGS and MIP were studied preoperatively and on the first day after LC and OC |
The second literature search was performed with different combinations. The main items were cholecystectomy and laparoscopic with subheadings such as adverse effects and contra-indications. By using AND the above items were combined with the following Mesh terms: (1) respiration (subheadings: abnormalities, pathology, physiology and physiopathology); (2) lung (subheadings: abnormalities, injuries, pathology, physiology and physiopathology); (3) respiratory Physiological Phenomena (subheadings: abnormalities, adverse effects, complications, pathology, physiology and physiopathology); (4) respiratory function tests; and (5) respiratory mechanics. The results were combined with OR (Boolean operator) which resulted in forty-eight articles. Eight works[34,40-46] out of the 48 relative to our topic were selected and used by the primary investigator (Bablekos GD) in writing this review and their baseline characteristics along with the reference number as an exponent, are presented in Table 2.
| Ref. | Baseline characteristics |
| Johnson et al[44] | Preoperative and postoperative measurements 24 h after LC of VC, FRC, arterial PO2 and chest-X-ray atelectasis. |
| Poulin et al[45] | Postoperative values of FVC and FEV1 variables measured on the first day after LC compared with values of the respective pulmonary function indices recorded on the first day after upper abdominal surgery and cholecystectomy. |
| Schulze et al[43] | Assessments of pain scores, peak flow values and subjective feeling of fatigue preoperatively, 6 h postoperatively and daily during the first week after operation for patients having undergone LC |
| Schauer et al[40] | Comparative measurements for FVC, FEV1, FEF25%-75%, Tiffenneau Index, FEFMAX, total lung capacity and oxygen saturation preoperatively to ten days after surgery between LC and OC |
| Saunders et al[41] | Measurements of FVC and the potential emergence of respiratory and gastrointestinal disturbances preoperatively to the first postoperative day between LC and OC |
| Torrington et al[42] | Comparative evaluations for FVC and FEV1 and arterial blood gases between LC and OC preoperatively and 24 h after surgery |
| Chumillas et al[34] | Comparative examination for FVC, FEV1 and arterial oxygenation values between LC and OC, from preoperatively up to 48 h after surgery |
| Hasukić et al[46] | Comparative measurements for FVC, FEV1, FEF25%-75% and arterial oxygenation preoperatively and 24 h after LC (included in the statistical analysis) |
Six additional studies[33,47-51], which were used in the PhD Thesis of the primary investigator (Bablekos GD), were also included in this review. The baseline characteristics of these works and their reference number as an exponent are given in Table 3.
| Ref. | Baseline characteristics |
| Chuter et al[50] | Parameters of respiratory pattern such as minute ventilation, tidal volume, the contribution of chest wall (VC/VT) to tidal volume and the contribution of the abdominal wall (Vab/VT) to tidal volume were studied preoperatively, on the first and on the third day after OC |
| Rademaker et al[47] | FVC, FEV1 and PEF were examined in a half sitting position preoperatively, and 24 h postoperatively in patients having undergone elective LC and OC while the effects of thoracic epidural analgesia after LC were also studied |
| McMahon et al[51] | FVC, FEV1, PEF, postoperative pain scores, analgesic consumption and oxygen saturation were examined preoperatively, on the first postoperative day and on the second postoperative day between patients who underwent LC and OC. The OC was performed with minilaparotomy surgical approach |
| Freeman and Armstrong[48] | Measurements of FVC, FEV1, Tiffenneau index, FRC, TLC, inspiratory and expiratory mouth pressures were examined preoperatively, and 24 h postoperatively between LC and OC |
| Rovina et al[49] | Measurements of FVC, FEV1, Tiffenneau index, blood gases indices, maximum static inspiratory (PImax) and maximum expiratory (PEmax) muscle pressures were studied preoperatively, on the first postoperative day and on the second postoperative day between LC and OC |
| Mimica et al[33] | Spirometric parameters (FVC, FVE1, Tiffenneau index), arterial blood gases, abdominal circumference, intestinal peristalsis and defecation were studied preoperatively, to the sixth postoperative day between LC and OC |
A total of thirty-four articles were used to extract information for the meta-analysis and writing of the impact of LC on lung function and respiratory physiopathology.
Moreover, to determine the quality of the literature reviewed in our study, we determined the number of citations of these articles since 1/1/1990 and the total impact factor (IF) of their corresponding journals. The number of citations was found to be 1502 in 927 overall hits on February 28th 2014 while the total IF was calculated to 69.176. Both citations and impact-factors were provided by the Hellenic National Documentation Center.
Most of the studies reported the mean values and standard deviations of forced vital capacity (FVC) and forced expiratory volume in 1 s (FEV1) preoperatively and 24 h postoperatively. One study reported the postoperative values 6 h later and 4 studies reported the values 48 h after operation. A few studies also reported values at 3 and 6 d after operation. Seven studies reported postoperative values expressed as percentages of the preoperative values. There were also 3 studies that did not report the values of FVC and FEV1.
We used a fixed and random effect meta-analysis to separately estimate the pooled standardized mean difference (SMD) for laparoscopic and open cholecystectomies for studies that reported preoperative and postoperative values.
To use all available information we also performed a crude comparison of the two methods by testing the postoperative values expressed as percentages of the preoperative values using the Mann-Whitney two-sample test.
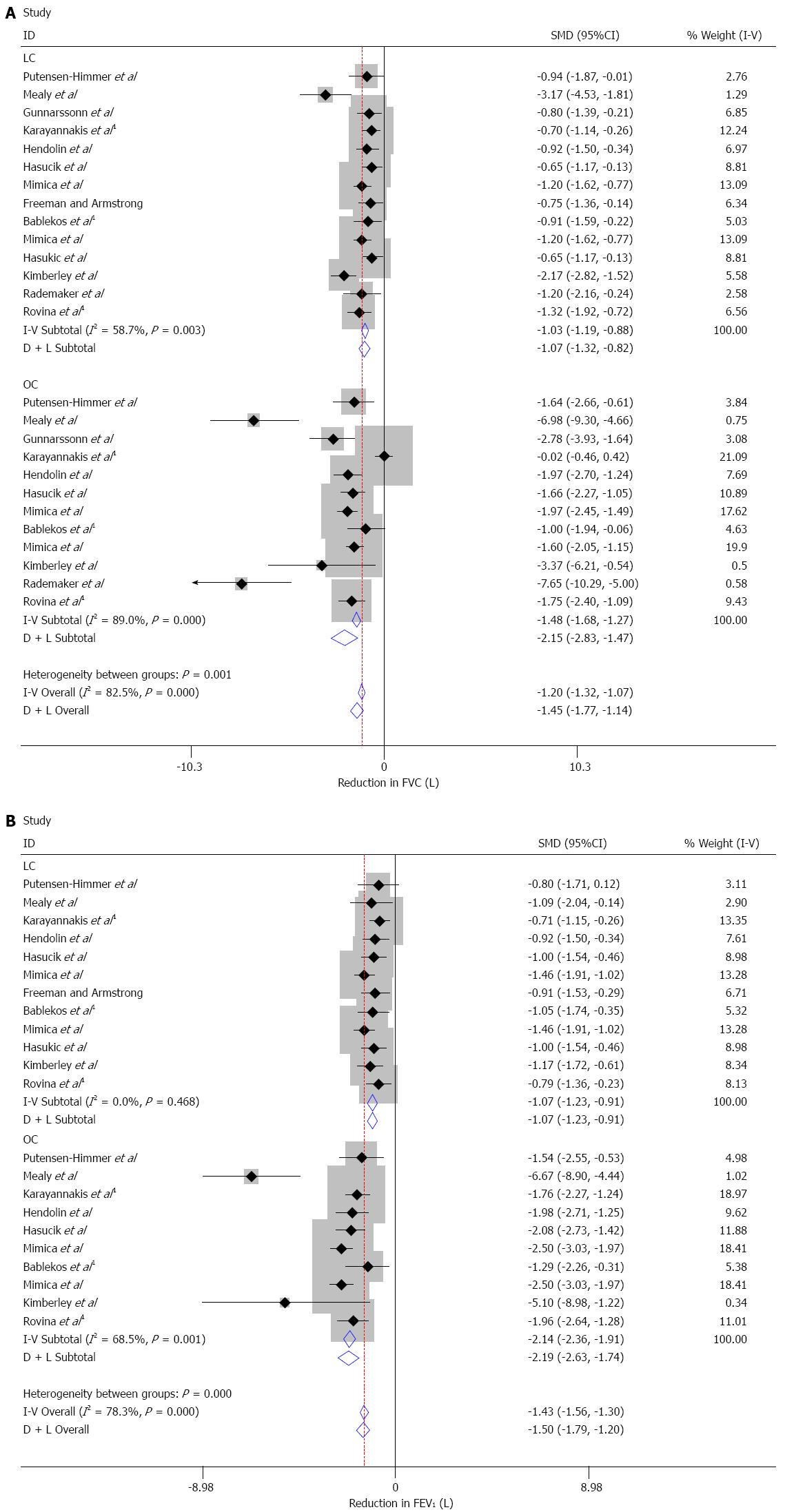
The descriptive statistics for FVC, FEV1 and FEF25%-75% (maximal-mid expiratory flow rate) values in laparoscopic and open cholecystectomies are shown in Table 4. The reduction in both FVC and FEV1 variables is greater in open cholecystectomy and is significantly different from those in laparoscopies (P < 0.001). Moreover, according to a number of comparative studies[17,24,26,28,29,30,40,46] between LC and OC, the reduction in the FEF25%-75% variable is also significantly greater in open compared with laparoscopic cholecystectomies (P = 0.005).
| Variables | Studies (n) | % of preoperative value [Median (IQR)] | P value | |
| LC | OC | |||
| FVC (L) | 21 | 77.6 (73.0, 80.0) | 55.4 (50.0, 64.0) | < 0.001 |
| FEV1 (L) | 19 | 76.0 (72.3, 81.0) | 52.5 (50.0, 56.7) | < 0.001 |
| FEF25%-75% (L/s) | 8 | 78.8 (68.8, 80.9) | 60.0 (36.1, 66.1) | 0.005 |
Figure 1 show the individual and pooled standardized mean differences between the preoperative and 24 h postoperative values in both types of cholecystectomies. The differences in the open procedure are more heterogeneous between studies (I2 = 82.5%, P < 0.001 and I2 = 68.5%, P = 0.001 in FVC and FEV1, respectively). The results confirm that the reduction is greater in the open cholecystectomy where the SMD in FVC is -1.45 [95%CI: -1.77-(-1.14)] compared to -1.07 [95%CI: -1.32-(-0.82)] for laparoscopic whereas for FEV1 the respective values are -2.19 [95%CI: -2.63-(-1.74) and -1.07 [95%CI: -1.23-(-0.91)].
Additionally, statistical analyses of a number of comparative studies[22,23,25-29,32-34,38,48,49] between laparoscopic and open cholecystectomies for PaO2(kPa) 24 or 48 h after surgical treatment showed reductions that were significantly greater in OC compared with LC [LC median 1.0, IQR (0.6, 1.3); OC median 2.4, IQR (1.2, 2.6), P-value = 0.019].
Significant heterogeneity between groups reflects the different effect of each treatment. The heterogeneity within each treatment group should be investigated, but there is in all studies lack of consistent information focusing on explanative factors. The most reported variable in papers is age, which is comparable. By visual inspection of the forest plots we can identify the studies responsible for heterogeneity.
In FVC variable (Table 5), by removing Mealy et al[23] and Kimberley et al[39] from the LC group; and Karayiannakis et al[26] and Rademaker et al[47] from the OC group there is no significant heterogeneity (I2 = 0.0% and 6.8%; P value = 0.645 and 0.379, respectively for LC and OC). The pooled standardized mean difference (SMD) changed slightly from -1.074 to -0.933 in the LC and from -2.152 to -1.773 in the OC group. The overall SMD changed from -1.455 to -1.245.
| Study | SMD | 95%CI | Weight | |
| LC | ||||
| Putensen-Himmer et al[22] | -0.937 | -1.866-(-0.009) | 3.0% | |
| Gunnarsson et al[25] | -0.803 | -1.392-(-0.214) | 7.4% | |
| 1Karayiannakis et al[26] | -0.697 | -1.138-(-0.257) | 13.1% | |
| Hendolin et al[27] | -0.920 | -1.504-(-0.336) | 7.5% | |
| Hasukić et al[28] | -0.649 | -1.169-(-0.130) | 9.5% | |
| Mimica et al[33] | -1.197 | -1.624-(-0.771) | 14.1% | |
| Freeman et al[48] | -0.752 | -1.364-(-0.139) | 6.8% | |
| 1Bablekos et al[29] | -0.905 | -1.593-(-0.218) | 5.4% | |
| Mimica et al[36] | -1.197 | -1.624-(-0.771) | 14.1% | |
| Hasukić et al[46] | -0.649 | -1.169-(-0.130) | 9.5% | |
| Rademaker et al[47] | -1.198 | -2.158-(-0.238) | 2.8% | |
| 1Rovina et al[49] | -1.320 | -1.922-(-0.718) | 7.1% | |
| Sub-total | ||||
| I-V pooled SMD | -0.933 | -1.093-(-0.773) | 100.0% | |
| D + L pooled SMD | -0.933 | -1.093-(-0.773) | ||
| OC | ||||
| Putensen-Himmer et al[22] | -1.637 | -2.664-(-0.610) | 5.0% | |
| Gunnarsson et al[25] | -2.785 | -3.932-(-1.637) | 4.0% | |
| Hendolin et al[27] | -1.970 | -2.695-(-1.244) | 9.9% | |
| Hasukić et al[28] | -1.656 | -2.266-(-1.046) | 14.0% | |
| Mimica et al[33] | -1.973 | -2.452-(-1.493) | 22.7% | |
| 1Bablekos et al[29] | -1.000 | -1.935-(-0.065) | 6.0% | |
| Mimica et al[36] | -1.596 | -2.048-(-1.145) | 25.6% | |
| Kimberley et al[39] | -3.373 | -6.206-(-0.540) | 0.7% | |
| 1Rovina et al[49] | -1.748 | -2.403-(-1.092) | 12.2% | |
| Sub-total | ||||
| I-V pooled SMD | -1.771 | -1.999-(-1.542) | 100.0% | |
| D + L pooled SMD | -1.773 | -2.014-(-1.531) | ||
| Overall | ||||
| I-V pooled SMD | -1.208 | -1.339-(-1.077) | ||
| D + L pooled SMD | -1.245 | -1.468-(-1.022) | ||
| Test(s) of heterogeneity | ||||
| Heterogeneity statistic | Degrees of freedom | P value | I2 | |
| LC | 8.75 | 11 | 0.645 | 0.0% |
| OC | 8.59 | 8 | 0.379 | 6.8% |
| Overall | 52.01 | 20 | < 0.001 | 61.5% |
In FEV1 variable (Table 6), there was no significant heterogeneity in the LC group. By removing Mealy et al[23] from the OC group there is no significant heterogeneity (I2 = 36.4%; P value = 0.127). The SMD in the OC group was slightly changed from -2.186 to -2.059 and the overall SMD from -1.497 to -1.422.
| Study | SMD | 95%CI | Weight | |
| LC | ||||
| Putensen-Himmer et al[22] | -0.796 | -1.710-(0.118) | 3.1% | |
| Mealy et al[23] | -1.090 | -2.036-(-0.143) | 2.9% | |
| 1Karayiannakis et al[26] | -0.706 | -1.147-(-0.265) | 13.4% | |
| Hendolin et al[27] | -0.920 | -1.504-(-0.336) | 7.6% | |
| Hasukić et al[28] | -1.000 | -1.538-(-0.462) | 9.0% | |
| Mimica et al[33] | -1.463 | -1.906-(-1.021) | 13.3% | |
| Freeman et al[48] | -0.913 | -1.535-(-0.290) | 6.7% | |
| 1Bablekos et al[29] | -1.045 | -1.744-(-0.346) | 5.3% | |
| Mimica et al[36] | -1.463 | -1.906-(-1.021) | 13.3% | |
| Hasukić et al[46] | -1.000 | -1.538-(-0.462) | 9.0% | |
| Kimberley et al[39] | -1.167 | -1.725-(-0.608) | 8.3% | |
| 1Rovina et al[49] | -0.794 | -1.360-(-0.229) | 8.1% | |
| Sub-total | ||||
| I-V pooled SMD | -1.068 | -1.229-(-0.907) | 100.0% | |
| D + L pooled SMD | -1.068 | -1.229-(-0.907) | ||
| OC | ||||
| Putensen-Himmer et al[22] | -1.540 | -2.550-(-0.529) | 5.0% | |
| 1Karayiannakis et al[26] | -1.756 | -2.273-(-1.238) | 19.2% | |
| Hendolin et al[27] | -1.980 | -2.707-(-1.253) | 9.7% | |
| Hasukić et al[28] | -2.078 | -2.732-(-1.424) | 12.0% | |
| Mimica et al[33] | -2.500 | -3.026-(-1.974) | 18.6% | |
| 1Bablekos et al[29] | -1.286 | -2.257-(-0.314) | 5.4% | |
| Mimica et al[36] | -2.500 | -3.026-(-1.974) | 18.6% | |
| Kimberley et al[39] | -5.099 | -8.978-(-1.220) | 0.3% | |
| 1Rovina et al[49] | -1.964 | -2.644-(-1.285) | 11.1% | |
| Sub-total | ||||
| I-V pooled SMD | -2.091 | -2.318-(-1.865) | 100.0% | |
| D + L pooled SMD | -2.059 | -2.359-(-1.760) | ||
| Overall | ||||
| I-V pooled SMD | -1.412 | -1.543-(-1.280) | ||
| D + L pooled SMD | -1.422 | -1.688-(-1.156) | ||
| Test(s) of heterogeneity | ||||
| Heterogeneity statistic | Degrees of freedom | P value | I2 | |
| LC | 10.70 | 11 | 0.468 | 0.0% |
| OC | 12.58 | 8 | 0.127 | 36.4% |
| Overall | 75.31 | 20 | < 0.001 | 73.4% |
In any case, the random effects pooled estimate is appropriate when significant heterogeneity is evident.
Before analyzing and commenting on the search results of the relevant literature, we first present an overview of the physiopathological mechanisms concerning both the diaphragmatic function impairment and the influence of anesthesia or analgesia after upper abdominal surgery.
According to Ford et al[3], disturbances in the respiratory muscle activity were observed after surgical removal of the gallbladder. Postoperatively, in addition to reduced lung volumes, a weakened diaphragmatic function was detected during inspiration and was associated with a rapid shallow breathing pattern and a paradoxical abdominal motion, especially in the case of sedatives administration[3]. A number of studies[14,15,52-54] also showed that for patients who underwent upper abdominal surgery, compared with the abdominal cavity, the thoracic wall provided major postoperative contributions in respiratory movement. This result implied a clear translocation of the respiratory drive from the diaphragm to other respiratory muscles. In their experimental study in dogs, Farkas and De Troyer[55] found that inspiration following upper abdomen surgery is accompanied by diminished diaphragmatic activity. This produced changes in the trans-diaphragmatic pressure resulting in a decreased diaphragmatic oscillations width, which is attributed to a restricted respiratory effort rather than intra-operative muscle damage. This assumption is supported by two studies[14,15] showing that after upper abdominal surgery, (1) the artificial stimulation of phrenic nerves produced normal trans-diaphragmatic pressure[15]; and (2) diaphragmatic pressures developed during inspiration were shown to be decreased[14]. As for why general anesthesia and analgesia had no effects, the diaphragmatic activity is not affected by perceptible postoperative pain[14]. However, a reflex inhibition regarding diaphragmatic motility seems to occur with the aid of the abdominal region nerves being activated during the operation[56]. Additionally, in anesthetized patients, touch and scrape between the abdominal peritoneum and gallbladder brings about a provisional breathlessness[56]. According to three other studies, the electrical stimulation of splanchnic nerves produces breathlessness in dogs[57], cats[58] and humans[59]. Furthermore, Prabhakar et al[60] found that the electric stimulation of the mesenterium in cats shifts the respiration pattern from a diaphragmatic to an inter-costal type. The above studies[56-60] concluded that the electrical stimulation of abdominal splanchnic nerves during inspiration produced the following changes: (1) an impediment of the entry of the tidal volume; (2) a decrease in the phrenic nerve activity; and (3) an increase in the inter-costal muscles activity. Moreover, the mechanical stimulation of the gallbladder due to surgical manipulations such as compression or extension may reduce both the electro-myographic activity and motility of the diaphragm during spontaneous inspiration[61]. As a result, the ability for expansion of thoracic cavity during inspiration was barely influenced compared with the abdominal cavity whose expansion was strongly diminished[61]. Therefore the width of diaphragmatic oscillations dependent on the intra-abdominal pressure was significantly decreased in comparison with the ones dependent on the intra-pleural pressure, which established a selective reduction of diaphragmatic activity[61]. In the same work[61], it was found that the inhibition of diaphragmatic function persisted for 250 ms after stimulus withdrawal, which demonstrated that diaphragmatic oscillations were of neural origin. According to Briscoe[62], diaphragmatic motility depends on two segments of the muscle the costal and crural segment. It has been shown that during upper abdominal surgery, the functional inhibition of either the costal or the crural segment of the diaphragm produces a severe disorder of the total diaphragmatic activity[62]. The aforementioned diaphragmatic disturbances have an important role in the emergence of atelectatic areas; thus it will be useful to discuss its mechanisms from the relevant literature. These atelectatic areas are attributed to respiratory muscles dysfunction following operations in the upper abdomen. Specifically, when the thorax is postoperatively expanded with the aid of inter-costal muscles rather than the diaphragm, this motion is simultaneously associated with the transposition of the abdominal cavity towards the thoracic cavity[63], resulting in both a redistribution of ventilation from the lower towards the upper parts of the lungs with a reduction in tidal volume (VT) inflow[63]. In addition, according to Farkas and De Troyer[55], the following changes were observed in laparotomic surgical procedures: (1) surgically activated abdominal muscles decreased the functional residual capacity (FRC) below the level measured at the end of a passive expiration (i.e., when the thoracic and lung elastic recoil are in equilibrium); and (2) diminished diaphragmatic activity. These changes[55] reduced the ability to cough after upper abdominal surgery by increasing the possibility of atelectasis especially in the lower lung regions. Given the aforementioned observations, the clinical question of whether the pattern of breathing is dysfunctional after upper abdominal operations is posed. Despite the relative hindrance that this particular pattern of breathing imposes on lung function and gas exchange, the relative immobility of the abdominal wall can be beneficial for recovery from abdominal wall trauma and for the prevention of peritoneal infection.
The following section discusses the contributions of medicines, anesthesia and analgesia in the development of respiratory physiopathology. Systemic parameters such as sepsis[64], hypophosphatemia[65] and hypocalcemia[66,67] were found to deteriorate postoperative respiratory function by weakening diaphragmatic activity. The impact of sepsis in diaphragmatic dysfunction was shown to be prevented with indomethacin[68]. For upper abdominal surgery, Jansen et al[69] observed that the administration of doxapram, a respiratory activity stimulating agent, significantly reduced postoperative pulmonary complications. It has been suggested that doxapram increases minute ventilation without affecting diaphragmatic function[69]. In addition, inotropic factors, caffeine, aminophylline and b2-agonists enhance diaphragmatic contractility by simultaneously averting muscle fatigue, whereas medicines such as halothane and sodium pentothal have the opposite effect[11,61,70,71].
General anesthesia contributes to the deterioration of postoperative respiratory function[72,73] due to the following reasons: (1) tracheal intubation, particularly if frequent, increases the dead space leading to further bronchoconstriction which is accompanied by a reduction in V/Q values and shunt establishment; (2) the interaction between anesthetic agents and myochalasis; and (3) the intake of an anesthetic gas mixture at high oxygen concentrations which increases shunt areas in lung parenchyma by diminishing the V/Q rate. The above parameters decrease both lung volumes and compliance, thus contributing to atelectasis formation[72,73]. Intercostal muscles activity was also found to be reduced when using benzodiazepines and narcotics for anesthesia because these chemical agents decreased the threshold of the central respiratory drive[71,73]. Moreover, in the early postoperative period, during the excretion of anesthetic agents, a type of general hypoxia is usually established and is accompanied by an elevation of either the metabolic rate or tissue temperature; both effects are associated with an increased oxygen consumption and carbon dioxide production[74]. Additionally, during the upper postoperative period, the administration of analgesia for pain relief could potentially lead to hypoventilation, a diminished sensitivity of the respiratory center to carbon dioxide stimulation, an intensification of obstructive breathlessness, the repression of cough reflex and irregular mucus effacement[74]. In turn, these conditions could induce hypoxemia and/or hypercapnia in the postoperative period. In 1991 Baron et al[75] observed that to relieve postoperative pain, the use of epidural anesthesia intra-operatively rather than after surgery, had a similar percentage of complications as the effects of general anesthesia. However in their experimental study in humans, Pansard et al[76] observed that the paradoxical pattern of breathing, especially present in upper abdominal surgery, could be totally or partially reversed by injecting epidural a 0.5% anesthesia solution (in bupivacaine) in thoracic nerves roots up to the fourth thoracic vertebrae. It was further observed that a lower concentration (i.e., 0.25% in bupivacaine) despite reducing postoperative pain, did not change the paradoxical pattern of breathing[76]. The higher bupivacaine dose restored both the tidal volume and breathing frequency to their preoperative values, thus diminishing the work of breathing[76]. Moreover, according to two studies[7,77], lengthening the duration of anesthesia contributed to the development of postoperative complications. Additionally, since 1920[62] a number of respiratory disturbances following upper abdominal surgery have been reported in the literature. Studies[78-82] published between 1962 and 1976 noted the necessity of spirometry for screening high risk patients who were prone to develop respiratory complications after upper abdominal surgery. By examining the issue of postoperative respiratory complications, Stein et al[78] and Stein et al[81], for the first time in the relevant literature, recommended a model of appropriate preoperative pulmonary management for patients undergoing upper abdominal surgery. The term “respiratory complication” was also defined so that one or more of the following clinical pathologic conditions could be included[78,81]: (1) bronchitis deterioration; (2) purulent saliva and fever; (3) intense cough; (4) intense breathlessness; (5) formation of atelectatic areas regardless of their shape and extent in lung parenchyma; and (6) pneumonia.
Because our literature searches spanned from 1990 to the present, a large number of studies used the following variables for the preoperative or postoperative investigation of respiratory functions in patients of upper abdominal surgery: (1) FVC/L; (2) FEV1/L; (3) FEF25%-75% L/s; (4) PEF L/s; (5) the FEV1 to FVC percentage ratio or Tiffenneau index; (6) PaO2 (kPa); and (7) PaCO2 (kPa). According to our analysis of the collated data, it appears that laparoscopic cholecystectomy is better for postoperative lung function compared with open cholecystectomy. The following section analyzes the factors and mechanisms of respiratory physiology which contribute to the improved respiratory performance and the clinical outcome of patients undergoing laparoscopic surgery for gallbladder removal.
During normal inspiration the intra-abdominal pressure increases along with an expansion of the thoracic cavity which causes an outward movement of the abdominal wall[83]. However, in upper abdominal operations, pain due to surgical incisions is likely the cause of the observed postoperative shallow breathing pattern[17]. This breathing pattern moderates the patient’s discomfort but limits the aforementioned outward movement of the abdominal wall[17] by decreasing either the postoperative vital (VC) or the FRC values[2,13,84]. Additional studies[24,84,85] have shown that lower FRC values (relative to FRC values at the end of a relaxed expiration) are suggestive of a small-airway closure which could induces hypoxemia and atelectasis especially under postoperative conditions. A possible mechanism that causes these postoperative alveolar volume changes could be due to disorders present during respiration[84]. These disorders are characterized by decreased lung surface tension which leads to alveolar wall collapse, a decrease in the FRC and hypoxemia[84]. Various studies since the early nineties have investigated the impact of these disorders on pulmonary function and the role of abdominal trauma in postoperative morbidity during both open upper abdominal surgery and newer operations such as laparoscopic cholecystectomy[37,86]. Moreover, according to the literature, changes observed in metabolic and immune functions appears to be associated with the extent of the surgical trauma[87]. An earlier study showed that minimally invasive (compared with open) surgical procedures induced less postoperative pain, leading to early discharges and returns to usual daily activities[88]. The above findings were well correlated with a second study[23] which observed significantly higher postoperative inflammatory responses as evidenced by both the erythrocyte sedimentation rate 24 h postoperatively and C-reactive protein production 24 and 48 h postoperatively, in patients undergoing open (compared with laparoscopic) cholecystectomy. However, in a separate study[38], although there were no significant postoperative differences in metabolic responses between laparoscopic and open cholecystectomy, immune functions were significantly better preserved in the laparoscopic group of patients. Additionally, in the open cholecystectomy group a significantly higher incidence of postoperative septic complications was observed. The differences in postoperative metabolic and immune functions between the two groups was attributed to endotoxin effects rather than wound factors[38].
With respect to the pulmonary function variables we have reviewed the reduction in FVC from the very early postoperative period precedes the decrease in FRC detected following upper abdominal surgery[84]. The FEV1 and PEF parameters both represent the expiratory capacity and are related to the cough potentiality[24] that is involved in atelectasis formation. The FEF25%-75% variable reflects the average flow corresponding to the middle 50% of the FVC respiratory index[89]. Generally, there is a strong relationship between the FEV1 and FEF25%-75% indices; however, in some cases the forced expiratory flow rate may be decreased while the FEV1 is at a normal value[89]. It is also well known that the Tiffeneau Index, or the ratio between FEV1 and FVC, contributes to the differential diagnosis between airflow limitation and airway restriction[28,40]. Additionally, similar to open upper abdominal surgery[55-62,76] laparoscopic cholecystectomy also induces a restrictive pattern regarding postoperative diaphragmatic motion[18]. A pneumoperitoneum induced by carbon dioxide brings about a distention of the peritoneal cavity which limits the diaphragmatic motility while simultaneously increasing the dead space and leading to a ventilation-perfusion mismatch[90]. According to a few studies[32,91] the insufflated carbon dioxide is absorbed from the peritoneum covering the abdominal cavity, thereby contributing to respiratory acidosis and hypercarbia disorders. Another issue that should be clarified concerning upper abdominal surgery is that general anesthesia - by itself[40,92] - or in combination with thoracic epidural analgesia[47] has no influence on postoperative lung function regardless of the type of cholecystectomy procedure. Additionally, a number of comparative studies[17,19,21-30,33,34,36,39,40,49] between open and laparoscopic cholecystectomy groups of patients examined lung function preoperatively to the 12th postoperative day and showed that most of the measured variables had returned to their preoperative levels between the 4th and 10th postoperative days[21,29-31,33,36,40]. For the early postoperative period[22,24,25,27,33,36] (i.e., the first[17,19,21-23,25,27,28,30,33,36,39,40,48], the second[19,26,29,34,40,49] and the third[22,33,36] postoperative days) significant differences were observed when compared with preoperative values for both laparoscopic and open surgical techniques. Statistically significant differences were also found between the two groups[17,19,21-28,33,34,36,39,40,49] in favor of the laparoscopic method. According to the literature, the main cause for the decreased values of pulmonary function indices observed in laparoscopic or open cholecystectomy patients is diaphragmatic dysfunction which appears to be related to the upper abdominal incision. The extent of the surgical upper abdominal trauma is an important factor influencing the postoperative pain intensity, the demand for postoperative analgesia and the stimulation of the parietal peritoneum covering the upper abdominal cavity[93]. The closer the surgical incision is to the diaphragmatic muscle (either sub-costal or midline), the greater the reduction in postoperative pulmonary function variables, particularly for lung volumes[13,79]. Because the anatomic site of the gallbladder is the same for that of the abdominal cavity, manipulations for its surgical removal (whether laparoscopic[94,95] or open[3,9,11,14,15,61]) induces local phrenic nerve stimuli by eliciting a reflex inhibition of diaphragmatic activity. In addition, the extent of the local stimulation of the parietal peritoneum due to surgical manipulations during gallbladder removal affects the degree[3,14] of the aforementioned respiratory reflex inhibition. These works[3,14] suggest that a better postoperative pulmonary function is observed for patients undergoing laparoscopic than open cholecystectomy because of the significantly smaller surgical trauma in the upper abdomen. A meta-analytic study concerning the Tiffeneau Index[20] also supports this conclusion. After a meta-analysis of thirteen related articles a better postoperative respiratory performance was observed for the patients of laparoscopic cholecystectomy[20]. In addition to these conclusions, two studies[29,31] examined pulmonary function and control of breathing indices between the two surgical procedures preoperatively to the eighth postoperative day (i.e., when patients usually returned to their daily activities). According to inspiratory capacity (IC) measurements, on the 8th postoperative day, laparoscopic patients presented an overall improved respiratory performance when compared with open cholecystectomy patients; the expiratory activity was found to be better for the open group of patients[29]. Additionally, the control of breathing indices such as duty cycle (TI/TTOT) and airway resistance (raw, in centimeters of water. L-1.s) for only the laparoscopic cholecystectomy group were found to be significantly reduced and increased respectively, compared with their preoperative values[31]. Other investigators examined the duty cycle preoperatively[94,96], 3 h[94] and 24 h[96] after laparoscopic cholecystectomy, while airway resistance was studied preoperative[26] and two days[26] following surgery in both laparoscopic and open cholecystectomy patients. Statistically significant differences were found for the TI/TTOT variable 3 h[94] after laparoscopic cholecystectomy. Given that the duty cycle detects the degree of airway obstruction[97] and that high airway resistance values are compatible with an aggravating total work of breathing[98], the findings[31] for TI/TTOT and Raw measurements on the 8th day after laparoscopic cholecystectomy are indicative of small but persistent changes reflected in both diaphragmatic dysfunction and airway obstruction. These findings can be attributed to a prolonged influence of intra-abdominal pressure in respiratory mechanics[31], which correlates well with the fact that on the 8th postoperative day open cholecystectomy patients were free of pain attributed to surgical trauma and wound, and presented a better expiratory performance when compared with the laparoscopic cholecystectomy group[29]. Significant changes found in the respiratory pattern on the first day after OC were attributed to a decrease of the abdominal wall compartment contribution to the tidal volume (Vab/VT) and was accompanied by an increase in the chest wall compartment (Vc/VT)[50]. The better expiratory activity[29] observed in OC patients compared with LC patients can be explained by the improved recovery of the abdominal wall motility eight days after surgery, in contrast with the small but persisting changes in the breathing pattern for LC patients[31]. Other studies[41,42] published in the mid nineties that measured the potential emergence of compromised ventilation[41], gastrointestinal dysfunction[41], atelectasis formation[42] and perseverance of sub-diaphragmatic free air[42], all following LC, brought up issues concerning the candidates selection for laparoscopies.
For the issue of postoperative pain after upper abdominal surgery a number of previous studies[51,99-103] highlighted the noticeable analgesic effect of bupivacaine administration via intercostal-interpleural or thoracic epidural infusions. When comparing the postoperative pain scores and analgesia requirements between laparoscopic and open cholecystectomy group, the laparoscopic method was found to be better[23,51,93,104,105]. The role of postoperative analgesia in local pain relief, regardless of the surgical method, has been supported in the literature[51,105]. Recent works[106,107] suggested newer alternatives for the control of postoperative pain especially for laparoscopic cholecystectomy patients. It was found that a single-dose of dexamethasone administered prior to surgery brought about significantly decreased pain scores, occurrences of nausea and vomiting and use of antiemetic medication within the first and second postoperative days[106]. After the laparoscopic procedure, the active aspiration of carbon dioxide, insufflated for the creation of a pneumoperitoneum, resulted in the improvement of abdominal and shoulder postoperative pain; the use of postoperative analgesia also decreased[107]. Additionally the use of isothermic rather than hypothermic carbon dioxide for creating a pneumoperitoneum did not significantly affect the postoperative lung volumes and flow rates. This result suggests that for patients with deranged lung function are candidates for laparoscopic surgical treatment[108].
Additionally, it was found[36] that postoperative physical therapy for open cholecystectomy patients resulted in decreased pain scores and increased respiratory functions for half an hour after each session; these are factors that may aid in reducing patient recovery time.
A number of studies[22,23,25-29,32-34,38,48,49] observed better oxygenation in laparoscopic compared with open cholecystectomy patients. The administration of analgesia to attenuate postoperative pain, which was more intense after open cholecystectomy[51,105], and the dysregulated breathing pattern due to the open upper abdominal surgical trauma[15,51,55,56] have more significant effects on patient oxygenation for laparoscopic cholecystectomy. Despite the contribution of postoperative analgesia in limiting pain after cholecystectomy[105] the use of opiates caused hypoventilation and hypoxemia[100,108,109]. Moreover, the diaphragmatic dysfunction resulting from the upper abdominal surgery altered the ability to sigh[14], which requires a rapid shallow breathing pattern associated with a small airway closure[85]. This closure is implicated in an intra-pulmonary shunt formation and precludes the emergence of hypoxemia[85]. According to a recent work[110], atelectatic areas in lung parenchyma are not favorable for shunt formation. In a previous study[111] healthy individuals undergoing laparoscopic cholecystectomy with intra-abdominal pressure values between 11 and 13 mmHg were considered to have a protective effect against pulmonary shunts. With respect to: (1) the conclusions of the above studies[110,111]; (2) the beneficial role of the absence of a surgical incision close to diaphragmatic muscles; and (3) the significantly smaller surgical trauma, there is a sufficient explanation for the better postoperative oxygenation status observed in laparoscopically treated patients. These factors account for: (1) the earlier mobilization[43]; (2) the lower pain scores[43]; (3) the smaller changes in postoperative lung function[44]; and (4) the lower incidence in pulmonary complications[45]. These observations were made during the first three years after 1990, following the widespread adoption of laparoscopic cholecystectomy, despite the formation of a pneumoperitoneum, which resulted in a significant increase in carbon dioxide burden necessitating an increase in minute ventilation to recuperate during gas elimination through the peritoneum[32,35].
Taking into account: (1) the extensive review of the relevant literature concerning the impact of laparoscopic cholecystectomy on lung function; (2) our results from statistical analyses of basic variables of respiratory function, such as FVC (L), FEV1 (L), FEF25%-75% (L/s), PaO2 (kPa), between laparoscopic and open cholecystectomy; and (3) the agreement of our findings with a meta-analytic study[20] concerning the Tiffenneau index expressed as the FEV1/FVC (%) ratio, we conclude that laparoscopic cholecystectomy has less impact on postoperative lung function and is preferable compared with open cholecystectomy, even for patients with relatively deranged lung function due to the following reasons: (1) the absence of an extensive surgical incision close to the diaphragm which has a strong influence in postoperative function; (2) the negligible potential for a postoperative shunt formation; (3) the significantly lower postoperative pain intensity because of better oxygenation; (4) the earlier mobilization which prevents atelectasis; and (5) the proposed alternatives for preventing harmful effects on postoperative lung function resulting from pneumoperitoneum.
We thank Mr. Antonios Kardasis, Information Specialist, Hellenic National Documentation Centre, EKT/NHRF, 48 Vas. Constantinou Ave GR-11635 Athens, Greece, for his valuable contribution to the literature search and for locating the article citations and impact-factors of the corresponding journals.
There have been plenty of studies evaluating and comparing laparoscopic cholecystectomy to the classical open procedure. In this meta-analytic review pathophysiologic medical interpretation of the postoperative effects of laparoscopic cholecystectomy on pulmonary function, is attempted. Data resulted from a thorough investigation of the studies in the relevant literature from 1990 to present. The authors’ aim was the analysis and medical interpretation of the acquired information in order to elucidate the effect of laparoscopic compared to open cholecystectomy on postoperative lung function and evaluate the method of choice for upper abdominal surgery in candidates with impaired lung function.
The hotspots of our study focus on the following aspects: (1) study of basic spirometric variables such as forced vital capacity (L), forced expiratory volume in 1 s and maximal-mid expiratory flow rate (L/s) postoperatively, between laparoscopic and open cholecystectomy; (2) study of the arterial oxygenation after laparoscopic and open cholecystectomy; and (3) the extensive and critical review of changes concerning breathing control and respiratory muscle performance indices in the sequel of laparoscopic and open cholecystectomy.
The overall panoramic presentation of the information concerning pathophysiologic changes imposed to respiratory function as a result of the widespread use of the new surgical method, which is critically discussed in comparison with the open classical technique.
Presentation of evidence to general physicians and surgeons for the evaluation and management of candidates for upper abdominal surgery particularly those with impaired lung function.
The authors perform a thorough literature review to integrate findings of studies concerning the effects of laparoscopic cholecystectomy on lung function and comparative findings in relation with the open surgical procedure.
P- Reviewer: Chiu CC, Li JD S- Editor: Gou SX L- Editor: A E- Editor: Ma S
| 1. | Celli BR, Rodriguez KS, Snider GL. A controlled trial of intermittent positive pressure breathing, incentive spirometry, and deep breathing exercises in preventing pulmonary complications after abdominal surgery. Am Rev Respir Dis. 1984;130:12-15. [PubMed] |
| 2. | Craig DB. Postoperative recovery of pulmonary function. Anesth Analg. 1981;60:46-52. [PubMed] |
| 3. | Ford GT, Whitelaw WA, Rosenal TW, Cruse PJ, Guenter CA. Diaphragm function after upper abdominal surgery in humans. Am Rev Respir Dis. 1983;127:431-436. [PubMed] |
| 4. | Hall JC, Tarala RA, Hall JL, Mander J. A multivariate analysis of the risk of pulmonary complications after laparotomy. Chest. 1991;99:923-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 118] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Tahir AH, George RB, Weill H, Adriani J. Effects of abdominal surgery upon diaphragmatic function and regional ventilation. Int Surg. 1973;58:337-340. [PubMed] |
| 6. | Tisi GM. Preoperative evaluation of pulmonary function. Validity, indications, and benefits. Am Rev Respir Dis. 1979;119:293-310. [PubMed] |
| 7. | Wightman JA. A prospective survey of the incidence of postoperative pulmonary complications. Br J Surg. 1968;55:85-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 189] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Beecher HK. The measured effect of laparotomy on the respiration. J Clin Invest. 1933;12:639-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Dureuil B, Cantineau JP, Desmonts JM. Effects of upper or lower abdominal surgery on diaphragmatic function. Br J Anaesth. 1987;59:1230-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 90] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Ford GT, Guenter CA. Toward prevention of postoperative pulmonary complications. Am Rev Respir Dis. 1984;130:4-5. [PubMed] |
| 11. | Road JD, Burgess KR, Whitelaw WA, Ford GT. Diaphragm function and respiratory response after upper abdominal surgery in dogs. J Appl Physiol Respir Environ Exerc Physiol. 1984;57:576-582. [PubMed] |
| 12. | Beecher HK. Effect of laparotomy on lung volume. Demonst ration of a new type of pulmonary collapse. J Clin Invest. 1933;12:651-658. [PubMed] |
| 13. | Latimer RG, Dickman M, Day WC, Gunn ML, Schmidt CD. Ventilatory patterns and pulmonary complications after upper abdominal surgery determined by preoperative and postoperative computerized spirometry and blood gas analysis. Am J Surg. 1971;122:622-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 188] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Simonneau G, Vivien A, Sartene R, Kunstlinger F, Samii K, Noviant Y, Duroux P. Diaphragm dysfunction induced by upper abdominal surgery. Role of postoperative pain. Am Rev Respir Dis. 1983;128:899-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 15. | Dureuil B, Viirès N, Cantineau JP, Aubier M, Desmonts JM. Diaphragmatic contractility after upper abdominal surgery. J Appl Physiol (1985). 1986;61:1775-1780. [PubMed] |
| 16. | Dubois F, Icard P, Berthelot G, Levard H. Coelioscopic cholecystectomy. Preliminary report of 36 cases. Ann Surg. 1990;211:60-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 536] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 17. | Frazee RC, Roberts JW, Okeson GC, Symmonds RE, Snyder SK, Hendricks JC, Smith RW. Open versus laparoscopic cholecystectomy. A comparison of postoperative pulmonary function. Ann Surg. 1991;213:651-653; discussion 653-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 169] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 18. | Hall JC, Tarala RA, Hall JL. A case-control study of postoperative pulmonary complications after laparoscopic and open cholecystectomy. J Laparoendosc Surg. 1996;6:87-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Coskun I, Hatipoglu AR, Topaloglu A, Yoruk Y, Yalcinkaya S, Caglar T. Laparoscopic versus open cholecystectomy: effect on pulmonary function tests. Hepatogastroenterology. 2000;47:341-342. [PubMed] |
| 20. | Damiani G, Pinnarelli L, Sammarco A, Sommella L, Francucci M, Ricciardi W. Postoperative pulmonary function in open versus laparoscopic cholecystectomy: a meta-analysis of the Tiffenau index. Dig Surg. 2008;25:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Osman Y, Fusun A, Serpil A, Umit T, Ebru M, Bulent U, Mete D, Omer C. The comparison of pulmonary functions in open versus laparoscopic cholecystectomy. J Pak Med Assoc. 2009;59:201-204. [PubMed] |
| 22. | Putensen-Himmer G, Putensen C, Lammer H, Lingnau W, Aigner F, Benzer H. Comparison of postoperative respiratory function after laparoscopy or open laparotomy for cholecystectomy. Anesthesiology. 1992;77:675-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 79] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Mealy K, Gallagher H, Barry M, Lennon F, Traynor O, Hyland J. Physiological and metabolic responses to open and laparoscopic cholecystectomy. Br J Surg. 1992;79:1061-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 103] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Williams MD, Sulentich SM, Murr PC. Laparoscopic cholecystectomy produces less postoperative restriction of pulmonary function than open cholecystectomy. Surg Endosc. 1993;7:489-492; discussion 493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Gunnarsson L, Lindberg P, Tokics L, Thorstensson O, Thörne A. Lung function after open versus laparoscopic cholecystectomy. Acta Anaesthesiol Scand. 1995;39:302-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Karayiannakis AJ, Makri GG, Mantzioka A, Karousos D, Karatzas G. Postoperative pulmonary function after laparoscopic and open cholecystectomy. Br J Anaesth. 1996;77:448-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 71] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Hendolin HI, Pääkönen ME, Alhava EM, Tarvainen R, Kemppinen T, Lahtinen P. Laparoscopic or open cholecystectomy: a prospective randomised trial to compare postoperative pain, pulmonary function, and stress response. Eur J Surg. 2000;166:394-399. [PubMed] |
| 28. | Hasukić S, Mesić D, Dizdarević E, Keser D, Hadziselimović S, Bazardzanović M. Pulmonary function after laparoscopic and open cholecystectomy. Surg Endosc. 2002;16:163-165. [PubMed] |
| 29. | Bablekos GD, Roussou T, Rasmussen T, Vassiliou MP, Behrakis PK. Postoperative changes on pulmonary function after laparoscopic and open cholecystectomy. Hepatogastroenterology. 2003;50:1193-1200. [PubMed] |
| 30. | Ravimohan SM, Kaman L, Jindal R, Singh R, Jindal SK. Postoperative pulmonary function in laparoscopic versus open cholecystectomy: prospective, comparative study. Indian J Gastroenterol. 2005;24:6-8. [PubMed] |
| 31. | Bablekos GD, Michaelides SA, Roussou T, Charalabopoulos KA. Changes in breathing control and mechanics after laparoscopic vs open cholecystectomy. Arch Surg. 2006;141:16-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | McMahon AJ, Baxter JN, Kenny G, O’Dwyer PJ. Ventilatory and blood gas changes during laparoscopic and open cholecystectomy. Br J Surg. 1993;80:1252-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 45] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 33. | Mimica Z, Biocić M, Bacić A, Banović I, Tocilj J, Radonić V, Ilić N, Petricević A. Laparoscopic and laparotomic cholecystectomy: a randomized trial comparing postoperative respiratory function. Respiration. 2000;67:153-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Chumillas MS, Ponce JL, Delgado F, Viciano V. Pulmonary function and complications after laparoscopic cholecystectomy. Eur J Surg. 1998;164:433-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 35. | Iwasaka H, Miyakawa H, Yamamoto H, Kitano T, Taniguchi K, Honda N. Respiratory mechanics and arterial blood gases during and after laparoscopic cholecystectomy. Can J Anaesth. 1996;43:129-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 36. | Mimica Z, Pogorelić Z, Srsen D, Perko Z, Stipić R, Dujmović D, Tocilj J, Ujević D. The effect of analgesics and physical therapy on respiratory function after open and laparoscopic cholecystectomy. Coll Antropol. 2008;32:193-199. [PubMed] |
| 37. | Farrow HC, Fletcher DR, Jones RM. The morbidity of surgical access: a study of open versus laparoscopic cholecystectomy. Aust N Z J Surg. 1993;63:952-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 38. | Redmond HP, Watson RW, Houghton T, Condron C, Watson RG, Bouchier-Hayes D. Immune function in patients undergoing open vs laparoscopic cholecystectomy. Arch Surg. 1994;129:1240-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 143] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 39. | Kimberley NA, Kirkpatrick SM, Watters JM. Alterations in respiratory mechanics after laparoscopic and open surgical procedures. Can J Surg. 1996;39:312-316. [PubMed] |
| 40. | Schauer PR, Luna J, Ghiatas AA, Glen ME, Warren JM, Sirinek KR. Pulmonary function after laparoscopic cholecystectomy. Surgery. 1993;114:389-397; discussion 397-399. [PubMed] |
| 41. | Saunders CJ, Leary BF, Wolfe BM. Is outpatient laparoscopic cholecystectomy wise? Surg Endosc. 1995;9:1263-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 42. | Torrington KG, Bilello JF, Hopkins TK, Hall EA. Postoperative pulmonary changes after laparoscopic cholecystectomy. South Med J. 1996;89:675-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 43. | Schulze S, Thorup J. Pulmonary function, pain, and fatigue after laparoscopic cholecystectomy. Eur J Surg. 1993;159:361-364. [PubMed] |
| 44. | Johnson D, Litwin D, Osachoff J, McIntosh D, Bersheid B, Church D, Yip R, Gallagher C. Postoperative respiratory function after laparoscopic cholecystectomy. Surg Laparosc Endosc. 1992;2:221-226. [PubMed] |
| 45. | Poulin EC, Mamazza J, Breton G, Fortin CL, Wabha R, Ergina P. Evaluation of pulmonary function in laparoscopic cholecystectomy. Surg Laparosc Endosc. 1992;2:292-296. [PubMed] |
| 46. | Hasukić S, Mesić D. Postoperative pulmonary changes after laparoscopic cholecystectomy. Med Arh. 2001;55:91-93. [PubMed] |
| 47. | Rademaker BM, Ringers J, Odoom JA, de Wit LT, Kalkman CJ, Oosting J. Pulmonary function and stress response after laparoscopic cholecystectomy: comparison with subcostal incision and influence of thoracic epidural analgesia. Anesth Analg. 1992;75:381-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 78] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 48. | Freeman JA, Armstrong IR. Pulmonary function tests before and after laparoscopic cholecystectomy. Anaesthesia. 1994;49:579-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 49. | Rovina N, Bouros D, Tzanakis N, Velegrakis M, Kandilakis S, Vlasserou F, Siafakas NM. Effects of laparoscopic cholecystectomy on global respiratory muscle strength. Am J Respir Crit Care Med. 1996;153:458-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 50. | Chuter TA, Weissman C, Starker PM. Respiratory patterns after cholecystectomy. Effects of posture and CO2 stimulation. Chest. 1991;100:23-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 51. | McMahon AJ, Russell IT, Ramsay G, Sunderland G, Baxter JN, Anderson JR, Galloway D, O’Dwyer PJ. Laparoscopic and minilaparotomy cholecystectomy: a randomized trial comparing postoperative pain and pulmonary function. Surgery. 1994;115:533-539. [PubMed] |
| 52. | Gibson GJ, Pride NB, Davis JN, Loh LC. Pulmonary mechanics in patients with respiratory muscle weakness. Am Rev Respir Dis. 1977;115:389-395. [PubMed] |
| 53. | Davis J, Goldman M, Loh L, Casson M. Diaphragm function and alveolar hypoventilation. Q J Med. 1976;45:87-100. [PubMed] |
| 54. | Chuter TA, Weissman C, Starker PM, Gump FE. Effect of incentive spirometry on diaphragmatic function after surgery. Surgery. 1989;105:488-493. [PubMed] |
| 55. | Farkas GA, De Troyer A. Effects of midline laparotomy on expiratory muscle activation in anesthetized dogs. J Appl Physiol (1985). 1989;67:599-605. [PubMed] |
| 56. | Reeve EB, Nanson EM, Rundle FF. Observations on inhibitory respiratory reflexes during abdominal surgery. Clin Sci. 1951;10:65-87. [PubMed] |
| 57. | Kostreva DR, Hopp FA, Zuperku EJ, Igler FO, Coon RL, Kampine JP. Respiratory inhibition with sympathetic afferent stimulation in the canine and primate. J Appl Physiol Respir Environ Exerc Physiol. 1978;44:718-724. [PubMed] |
| 58. | Downman CB. Skeletal muscle reflexes of splanchnic and intercostal nerve origin in acute spinal and decerebrate cats. J Neurophysiol. 1955;18:217-235. [PubMed] |
| 59. | Kruta V, Bedrna J, Prochazka J, Volf J. [Influence of afferent stimulation of the splanchnic nerve on respiratory movements in man]. Arch Int Physiol. 1950;58:90-100. [PubMed] |
| 60. | Prabhakar NR, Marek W, Loeschcke HH. Altered breathing pattern elicited by stimulation of abdominal visceral afferents. J Appl Physiol (1985). 1985;58:1755-1760. [PubMed] |
| 61. | Ford GT, Grant DA, Rideout KS, Davison JS, Whitelaw WA. Inhibition of breathing associated with gallbladder stimulation in dogs. J Appl Physiol (1985). 1988;65:72-79. [PubMed] |
| 62. | Briscoe JC. The mechanism of post-operative massive collapse of the lungs. Q J Med. 1920;13:293-U9. |
| 63. | Roussos C, Engel LA. Thorax-Lung interactions. The Thorax. New York: Marcel Dekker 1985; 667-700. |
| 64. | Boczkowski J, Dureuil B, Branger C, Pavlovic D, Murciano D, Pariente R, Aubier M. Effects of sepsis on diaphragmatic function in rats. Am Rev Respir Dis. 1988;138:260-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 82] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 65. | Aubier M, Murciano D, Lecocguic Y, Viires N, Jacquens Y, Squara P, Pariente R. Effect of hypophosphatemia on diaphragmatic contractility in patients with acute respiratory failure. N Engl J Med. 1985;313:420-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 290] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 66. | Aubier M, Viires N, Piquet J, Murciano D, Blanchet F, Marty C, Gherardi R, Pariente R. Effects of hypocalcemia on diaphragmatic strength generation. J Appl Physiol (1985). 1985;58:2054-2061. [PubMed] |
| 67. | Viirès N, Murciano D, Seta JP, Dureuil B, Pariente R, Aubier M. Effects of Ca2+ withdrawal on diaphragmatic fiber tension generation. J Appl Physiol (1985). 1988;64:26-30. [PubMed] |
| 68. | Boczkowski J, Dureuil B, Pariente R, Aubier M. Preventive effects of indomethacin on diaphragmatic contractile alterations in endotoxemic rats. Am Rev Respir Dis. 1990;142:193-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 69. | Jansen JE, Sorensen AI, Naesh O, Erichsen CJ, Pedersen A. Effect of doxapram on postoperative pulmonary complications after upper abdominal surgery in high-risk patients. Lancet. 1990;335:936-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 70. | Aubier M. Pharmacotherapy of respiratory muscles. Clin Chest Med. 1988;9:311-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 71. | Tusiewicz K, Bryan AC, Froese AB. Contributions of changing rib cage--diaphragm interactions to the ventilatory depression of halothane anesthesia. Anesthesiology. 1977;47:327-337. [PubMed] |
| 72. | Wahba RW. Perioperative functional residual capacity. Can J Anaesth. 1991;38:384-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 149] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 73. | Weissman C. Perioperative respiratory physiology. J Crit Care. 1991;6:160-171. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 74. | Gamsu G, Singer MM, Vincent HH, Berry S, Nadel JA. Postoperative impairment of mucous transport in the lung. Am Rev Respir Dis. 1976;114:673-679. [PubMed] |
| 75. | Baron JF, Bertrand M, Barré E, Godet G, Mundler O, Coriat P, Viars P. Combined epidural and general anesthesia versus general anesthesia for abdominal aortic surgery. Anesthesiology. 1991;75:611-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 124] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 76. | Pansard JL, Clergue F, Lesage R, Viars P. The effects of epidural thoracic bupivacaine 0.5% on the inspiratory force after upper abdominal surgery. Annales Francaises d’Anesthesie et de Reanimation. 1991;10:R59. |
| 77. | Rehder K, Sessler AD, Marsh HM. General anesthesia and the lung. Am Rev Respir Dis. 1975;112:541-563. [PubMed] |
| 78. | Stein M, Koota GM, Simon M, Frank HA. Pulmonary evaluation of surgical patients. JAMA. 1962;181:765-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 126] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 79. | Diament ML, Palmer KN. Spirometry for preoperative assessment of airways resistance. Lancet. 1967;1:1251-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 80. | Nealon TF, McNeil AG. Management of operations in the pulmonary cripple. Surg Clin North Am. 1967;47:1223-1234. [PubMed] |
| 81. | Stein M, Cassara EL. Preoperative pulmonary evaluation and therapy for surgery patients. JAMA. 1970;211:787-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 82. | Williams CD, Brenowitz JB. “Prohibitive” lung function and major surgical procedures. Am J Surg. 1976;132:763-766. [PubMed] |
| 83. | Derenne JP, Macklem PT, Roussos C. The respiratory muscles: mechanics, control, and pathophysiology. Am Rev Respir Dis. 1978;118:119-133. [PubMed] |
| 84. | Ali J, Weisel RD, Layug AB, Kripke BJ, Hechtman HB. Consequences of postoperative alterations in respiratory mechanics. Am J Surg. 1974;128:376-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 138] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 85. | Alexander JI, Spence AA, Parikh RK, Stuart B. The role of airway closure in postoperative hypoxaemia. Br J Anaesth. 1973;45:34-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 107] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 86. | Fletcher DR, Jones RM. Perforated duodenal ulcer and common bile duct stone--appropriate conditions for laparoscopic surgery. Med J Aust. 1991;155:208. [PubMed] |
| 87. | Guillou PJ. Biological variation in the development of sepsis after surgery or trauma. Lancet. 1993;342:217-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 75] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 88. | Grace PA, Quereshi A, Coleman J, Keane R, McEntee G, Broe P, Osborne H, Bouchier-Hayes D. Reduced postoperative hospitalization after laparoscopic cholecystectomy. Br J Surg. 1991;78:160-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 167] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 89. | West John B. Respiratory Physiology-the Essentials, 9th edition. Chapter 7: Mechanics of Breathing-How the Lung is Supported and Moved. Philadelphia: Wolters Kluwer/Lippincot Williams & Wilkins 2012; 118. |
| 90. | Baxter JN, O’Dwyer PJ. Pathophysiology of laparoscopy. Br J Surg. 1995;82:1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 91. | Safran DB, Orlando R. Physiologic effects of pneumoperitoneum. Am J Surg. 1994;167:281-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 163] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 92. | Bartlett RH. Pulmonary pathophysiology in surgical patients. Surg Clin North Am. 1980;60:1323-1338. [PubMed] |
| 93. | Soper NJ, Barteau JA, Clayman RV, Ashley SW, Dunnegan DL. Comparison of early postoperative results for laparoscopic versus standard open cholecystectomy. Surg Gynecol Obstet. 1992;174:114-118. [PubMed] |
| 94. | Erice F, Fox GS, Salib YM, Romano E, Meakins JL, Magder SA. Diaphragmatic function before and after laparoscopic cholecystectomy. Anesthesiology. 1993;79:966-975; discussion 27A-28A. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 54] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 95. | Sharma RR, Axelsson H, Oberg A, Jansson E, Clergue F, Johansson G, Reiz S. Diaphragmatic activity after laparoscopic cholecystectomy. Anesthesiology. 1999;91:406-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 96. | Shulman SM, Chuter T, Weissman C. Dynamic respiratory patterns after laparoscopic cholecystectomy. Chest. 1993;103:1173-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 97. | Tobin MJ, Chadha TS, Jenouri G, Birch SJ, Gazeroglu HB, Sackner MA. Breathing patterns. 2. Diseased subjects. Chest. 1983;84:286-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 229] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 98. | Daubenspeck JA. Mechanical aspects of loaded breathing. The Thorax. New York, NY: Marcel Dekker Inc 1995; 953-985. |
| 99. | Cuschieri RJ, Morran CG, Howie JC, McArdle CS. Postoperative pain and pulmonary complications: comparison of three analgesic regimens. Br J Surg. 1985;72:495-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 96] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 100. | Catley DM, Thornton C, Jordan C, Lehane JR, Royston D, Jones JG. Pronounced, episodic oxygen desaturation in the postoperative period: its association with ventilatory pattern and analgesic regimen. Anesthesiology. 1985;63:20-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 258] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 101. | Frank ED, McKay W, Rocco A, Gallo JP. Interpleural bupivacaine for postoperative analgesia following cholecystectomy: a randomized prospective study. Reg Anesth. 1990;15:26-30. [PubMed] |
| 102. | VadeBoncouer TR, Riegler FX, Gautt RS, Weinberg GL. A randomized, double-blind comparison of the effects of interpleural bupivacaine and saline on morphine requirements and pulmonary function after cholecystectomy. Anesthesiology. 1989;71:339-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 103. | Hendolin H, Lahtinen J, Länsimies E, Tuppurainen T, Partanen K. The effect of thoracic epidural analgesia on respiratory function after cholecystectomy. Acta Anaesthesiol Scand. 1987;31:645-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 46] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 104. | Joris J, Cigarini I, Legrand M, Jacquet N, De Groote D, Franchimont P, Lamy M. Metabolic and respiratory changes after cholecystectomy performed via laparotomy or laparoscopy. Br J Anaesth. 1992;69:341-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 207] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 105. | Squirrell DM, Majeed AW, Troy G, Peacock JE, Nicholl JP, Johnson AG. A randomized, prospective, blinded comparison of postoperative pain, metabolic response, and perceived health after laparoscopic and small incision cholecystectomy. Surgery. 1998;123:485-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 61] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 106. | Sistla S, Rajesh R, Sadasivan J, Kundra P, Sistla S. Does single-dose preoperative dexamethasone minimize stress response and improve recovery after laparoscopic cholecystectomy? Surg Laparosc Endosc Percutan Tech. 2009;19:506-510. [PubMed] |
| 107. | Atak I, Ozbagriacik M, Akinci OF, Bildik N, Subasi IE, Ozdemir M, Ayta NI. Active gas aspiration to reduce pain after laparoscopic cholecystectomy. Surg Laparosc Endosc Percutan Tech. 2011;21:98-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 108. | Uzunkoy A, Ozgonul A, Ceylan E, Gencer M. The effects of isothermic and hypothermic carbon dioxide pneumoperitoneum on respiratory function test results. J Hepatobiliary Pancreat Surg. 2006;13:567-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 109. | Arunasalam K, Davenport HT, Painter S, Jones JG. Ventilatory response to morphine in young and old subjects. Anaesthesia. 1983;38:529-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 110. | Hedenstierna G, Rothen HU. Respiratory function during anesthesia: effects on gas exchange. Compr Physiol. 2012;2:69-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 111. | Andersson L, Lagerstrand L, Thörne A, Sollevi A, Brodin LA, Odeberg-Wernerman S. Effect of CO(2) pneumoperitoneum on ventilation-perfusion relationships during laparoscopic cholecystectomy. Acta Anaesthesiol Scand. 2002;46:552-560. [PubMed] |