Published online Dec 14, 2014. doi: 10.3748/wjg.v20.i46.17588
Revised: May 18, 2014
Accepted: July 15, 2014
Published online: December 14, 2014
Processing time: 324 Days and 19 Hours
AIM: To determine the prevalence, demographic, clinical and histopathologic features of heterotopic gastric mucosa (HGM) in Chinese patients.
METHODS: Patients referred to three endoscopy units were enrolled in this study. The macroscopic characteristics of HGM were documented. Biopsies were obtained and observed using hematoxylin and eosin staining. Helicobacter pylori colonization was examined by Whartin-Starry staining.
RESULTS: HGM was observed in 420 Chinese patients, yielding a prevalence of 0.4%. The majority of patients had a single patch (300/420; 71.4%), while the remainder had two (84/420; 20%) or multiple patches (36/420; 8.6%). The size of the patches and the distance from the patch to the frontal incisor teeth varied significantly. The large majority of HGM patches were flat (393/420; 93.6%), whereas the remaining patches were slightly elevated. The primary histological characteristic was fundic-type (216/420; 51.4%) within the HGM patch, and antral- (43/420; 10.2%) and transitional-type (65/420; 15.5%) mucosa were also observed. The prevalence of intestinal metaplasia was 3.1% (13/420) and the prevalence of dysplasia was 1.4% (6/420), indicating the necessity for endoscopic follow-up in patients with HGM. Esophageal and extraesophageal complaints were also observed in patients with HGM. Dysphagia and epigastric discomfort (odds ratios: 6.836 and 115.826, respectively; Ps < 0.05) were independent risk factors for HGM.
CONCLUSION: Clinical complaints should be considered to improve the detection rate of HMG. The prevalence of intestinal metaplasia and dysplasia also indicates a need for endoscopic follow-up.
Core tip: This study reports the prevalence, demographic, clinical and histologic features of heterotopic gastric mucosa (HGM) in Chinese patients. HGM was not commonly observed in the study population. Although malignant transformation was rare, intestinal metaplasia and dysplasia within HGM should be carefully followed-up. Some clinical symptoms, although not specific, were helpful in screening for HGM.
- Citation: Fang Y, Chen L, Chen DF, Ren WY, Shen CF, Xu Y, Xia YJ, Li JW, Wang P, Zhang AR, Shao SZ, Yu XN, Peng GY, Fang DC. Prevalence, histologic and clinical characteristics of heterotopic gastric mucosa in Chinese patients. World J Gastroenterol 2014; 20(46): 17588-17594
- URL: https://www.wjgnet.com/1007-9327/full/v20/i46/17588.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i46.17588
Heterotopic gastric mucosa (HGM) is an area of heterotopic columnar mucosal islands located in the postcricoid portion of the cervical esophagus or below the level of the upper esophageal sphincter[1]. The majority of researchers consider HGM to be the remnant of the esophageal columnar embryologic lining due to underdevelopment of squamous epithelium during the fetal period. However, some researchers consider that HGM may share a similar pathogenesis to that of Barrett’s esophagus and is associated with gastroesophageal reflux[2]. A possible explanation for the origin of HGM was recently postulated, suggesting that HGM might develop from mucus gland cysts within the cervical esophagus after eruption[3]. The symptoms of esophageal HGM are diverse and primarily comprised of chest pain, throat discomfort, dysphagia, globus sensation, and laryngopharyngeal or supraesophageal symptoms[4-7]. Although most esophageal HGM patches do not lead to obvious health problems, a few case reports have indicated that these lesions can further develop into a web, stricture, ulcer, perforation, esophagotracheal fistula, Barrett’s esophagus or adenocarcinoma[8-12].
The endoscopic prevalence of HGM ranges from 0.1 to 11%, with the majority of reports indicating the prevalence to be between 1% and 2%[5,13-16]. The significant discrepancy may be the result of differences in the experience and special interests of endoscopists[17]. The clinical significance and prevalence of esophageal HGM are still largely uncertain due to the limited number of subjects in most studies and the discrepant focus on these lesions by endoscopists. Here we present a prospective, multicenter cohort study to determine the prevalence of esophageal HGM in a Chinese population, identify the macroscopic and histologic features, and evaluate the associations of HGM with demographic and clinical characteristics.
A total of 101395 patients referred to three endoscopy units (Southwest Hospital and Daping Hospital of The Third Military Medical University, and The Affiliated Hospital of The Armed Police Medical College, China) for elective endoscopy were enrolled in this study between February 2008 and June 2010. The patients had not undergone previous endoscopies and were included in the study if they were willing to participate and gave written informed consent. Patients undergoing emergency endoscopy, or those with abnormal coagulation and other contraindications for biopsy were excluded. Symptoms, particularly involving esophageal and laryngopharyngeal regions were recorded using questionnaires. Symptoms included heartburn and regurgitation, throat discomfort, dysphagia, retrosternal pain and epigastric discomfort. This study was approved by the ethics committees of The Third Military Medical University and The Affiliated Hospital of The Armed Police Medical College.
Patients were fasted for at least 12 h and procedures were performed with an Olympus GIF Q-260 endoscope (Olympus, Tokyo, Japan) under pharyngeal anesthesia with lidocaine, and gastrointestinal peristalsis and salivation were reduced with an injection of anisodamine. The endoscopists were not instructed to search for HGM. The number of HGM patches in the cervical esophagus was recorded. The greatest diameter of the largest HGM patch was evaluated using open biopsy forceps. The distance from the largest HGM patch to the frontal incisor teeth was documented. The HGM patch was classified as flat or slightly elevated. Other features, such as the shape, color and border of the HGM patch, were also documented.
A minimum of four biopsies were harvested and fixed in neutral formalin solution when the HGM patch was identified under endoscopy. Biopsies were stained with hematoxylin and eosin and Wharthin-Starry [for detection of Helicobacter pylori (H. pylori)], and examined by two pathologists. The histology of HGM was classified on the basis of the contents of parietal and chief cells as follows: (1) fundic-type (predominantly parietal cells); (2) antral-type (predominantly chief cells); and (3) transitional-type (mixture chief and parietal cells). The occurrence of intestinal metaplasia, dysplasia and chronic inflammation within HGM patches was documented, and the prevalence of gastroesophageal reflux and Barrett’s esophagus in patients with HGM was recorded.
Two-way tables were analyzed by the Pearson χ2 test, and multiple logistic regression analysis was performed using SPSS version 19.0 software (IBM, Armonk, NY, United States). A P < 0.05 was considered statistically significant.
HGM patches were identified in 420 of the 101395 patients undergoing endoscopy for a prevalence of 0.41% in this Chinese population. Demographic characteristics of the patients with and without HGM patches are listed in Table 1. The gender ratio of male to female was 65.5:34.5 in 420 patients with HGM and 67.8:32.2 in patients without HGM, which were not significantly different. In addition, there was no significant difference in the mean age of patients with and without HGM.
| Characteristic | HGM(+) | HGM(-) | P |
| Male | 275 | 68423 | - |
| Female | 145 | 32552 | - |
| Male/Female | 65.5/34.5 | 67.8/32.2 | 0.31 |
| Age range (yr) | 22-80 | 19-85 | - |
| Mean age (yr) | 42.3 | 46.9 | 0.10 |
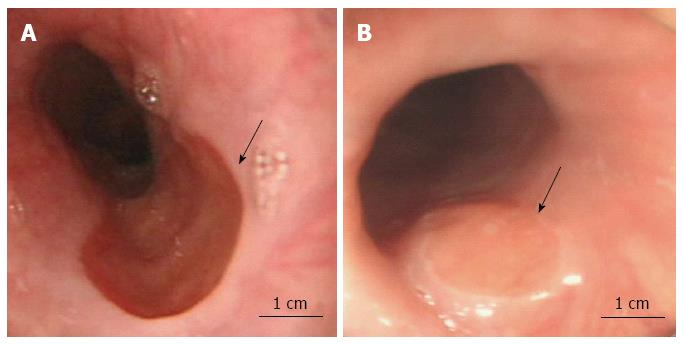
The morphologic evaluation of HGM patches by endoscopy revealed that 300/420 patients (71.4%) had a single patch, 84/420 patients (20.0%) had two patches, and 36/420 patients (8.6%) had multiple patches. The diameter of HGM patches ranged from 0.3 to 3.0 cm, with a mean of 1.1 cm. The distance from the largest HGM patch to the frontal incisor teeth ranged between 14 and 22 cm, with a mean of 18.8 cm. In 393/420 (93.6%) cases, the endoscopists characterized HGM patches as flat, whereas in 27/420 (6.4%) cases, HGM patches were characterized as slightly elevated (Figure 1). In the majority of patients, the HGM patch appeared as a round, oval or irregular smooth patch of pink or dark red color with longitudinal extension on the anterior or posterior wall with a clear border. No cases of ulceration, stricture, perforation or bleeding in the HGM patches were observed.
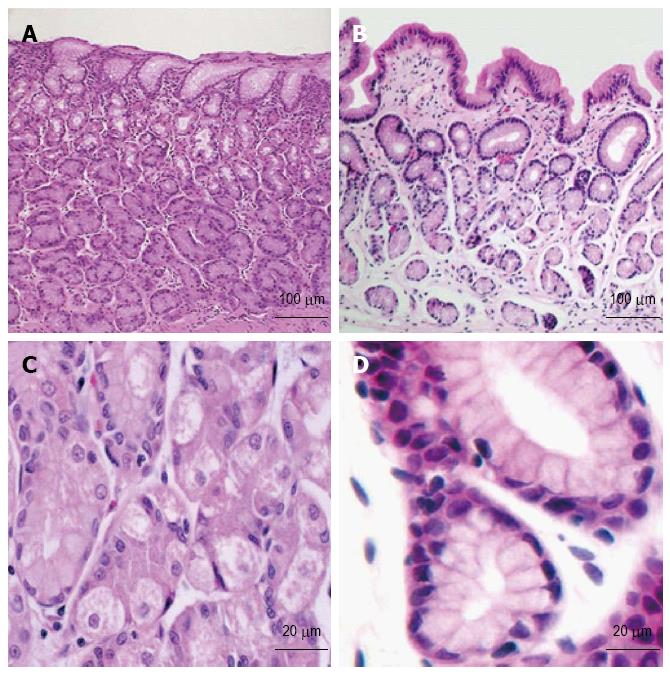
Histopathologic characteristics of the HGM patches are listed in Table 2. The HGM patches were classified as fundic-type in 216/420 patients (51.4%), antral-type in 43/420 patients (10.2%), and transitional-type in 65/420 patients (15.5%) (Figure 2). Intestinal metaplasia was observed in 13/420 patients (3.1%), and 6/420 patients (1.4%) showed mild dysplasia within the HGM patch. A total of 42/420 patients (10.0%) were diagnosed with H. pylori colonization, and 35/420 cases (8.3%) showed mucosal atrophy within the HGM patch. More than half of the patients (55.9%) showed mild or moderate chronic inflammation within the HGM patch. None of the H. pylori-positive HGM patients showed dysplasia (0/42) or intestinal metaplasia (0/42), though 47.6% (20/42) showed chronic inflammation. The results show that 11.2% (47/420) of patients with HGM suffered from gastroesophageal reflux disease, and 0.47% (2/420) suffered from Barrett’s esophagus.
| Histopathologic characteristics | n (%) |
| Pathologic classification | |
| Fundic-type | 216 (51.4) |
| Antral-type | 43 (10.2) |
| Transitional-type | 65 (15.5) |
| Chronic inflammation | 235 (55.9) |
| Atrophy | 35 (8.3) |
| Intestinal metaplasia | 13 (3.1) |
| Dysplasia | 6 (1.4) |
| Helicobacter pylori | 42 (10.0) |
| Gastroesophageal reflux disease | 47 (11.2) |
| Barrett’s esophagus | 2 (0.5) |
The occurrence of clinical symptoms in patients with and without HGM is listed in Table 3. A total of 38/420 patients (9.0%) with HGM complained of heartburn and regurgitation, which was significantly higher than patients without heterotopic gastric inlet patches (6.2%) (P = 0.02). Throat discomfort was documented in 24/420 (5.7%) patients with, and 3.6% without, HGM patches (P = 0.02). The prevalence of dysphagia was significantly higher in patients with HGM compared to those without (9.3% vs 3.2%; P = 0.00). A significantly larger number of patients with HGM complained of epigastric discomfort (61.9 vs 10.0; P = 0.00). The presence of retrosternal pain was similar in patients with and without HGM. A large number (289/420) of HGM patients showed esophageal or laryngeal symptoms (e.g., heartburn and regurgitation, throat discomfort, dysphagia, retrosternal pain, epigastric discomfort).
| Clinical symptoms | HGM(+)n (%) | HGM(-)n (%) | P |
| Heartburn and regurgitation | 38 (9) | 6261 (6.2) | 0.02 |
| Throat discomfort | 24 (5.7) | 3635 (3.6) | 0.02 |
| Dysphagia | 39 (9.3) | 3231 (3.2) | 0.00 |
| Retrosternal pain | 17 (4.0) | 4847 (4.1) | 0.47 |
| Epigastric discomfort | 260 (61.9) | 10098 (10.0) | 0.00 |
Multivariate logistic regression analysis was performed to determine the risk factors associated with clinical symptoms in the presence of HGM patches (Table 4). Men had a significantly reduced risk of HGM compared to women (P = 0.00). Furthermore, dysphagia (P = 0.00) and epigastric discomfort (P = 0.00) were significant independent risk factors for HGM. Heartburn, regurgitation and retrosternal pain were not associated with HGM.
The clinical significance of HGM has recently been discussed in numerous published reports. However, most studies only included a limited number of patients, due to lack of persuasion. To our knowledge, our study includes the largest number of subjects in the literature. An HGM prevalence rate of 0.4% was observed in our study, which is consistent with the 0.3%-0.4% reported in a previous study conducted by Wang et al[18] in China. HGM patches are always located near the upper esophageal sphincter and are easily ignored by endoscopists if the endoscope is withdrawn quickly and without care[15]. Maconi et al[17] demonstrated a prevalence of 2.27% when endoscopists were informed about the presence of HGM patches, and a prevalence of 0.3% when they were not informed about these lesions. Thus, the low prevalence of HGM in our study may partly be due to the fact that endoscopists were not instructed to search for HGM lesions. Furthermore, HGM patches were equally distributed between male and female patients in our study, and there was no significant difference in the mean age of patients with and without HGM, consistent with previous reports[6,19].
In the present study, the presence of a single patch under endoscopy was more common than that of double or multiple patches within the upper esophagus, which is consistent with previous studies[14,20]. The size of patches (0.3-3.0 cm) and their distance from the frontal incisor teeth (14-22 cm) varied significantly, but were in accordance with previous publications[5,16,21]. The patch surface may be smooth and flat, slightly raised or depressed with a heaped margin[21]. The patch can appear as a sessile polyp in rare cases[22]. Our study showed that the majority of HGM patches appeared flat, while only a small portion were identified as slightly elevated.
The histopathology of HGM is usually observed as fundic-type, characterized by oxyntic features other than antral- or transitional-type[21], which is in accordance with the results of our study where more than half of the heterotopic patches were identified as fundic-type. Although intestinal metaplasia or malignant transformation of HGM patches are considered rare events and have only been published as case reports[23-25], the prevalence of intestinal metaplasia and dysplasia in our series of 420 patients suggests that these are not infrequent events.
H. pylori colonization within HGM is considered to be part of H. pylori-positive gastritis. There are no reports of exclusive H. pylori colonization in HGM. The prevalence of H. pylori colonization within HGM is more frequent in the proximal esophagus, and ranges between 5.3% and 18% in the literature[26,27]. Although we did not detect H. pylori colonization in the gastric mucosa in our study, the prevalence was 10% in patients with HGM, which is in accordance with previous studies[13,28]. In addition, chronic inflammation is reportedly present within most of the cervical inlet patches[5,14]. Our findings similarly revealed that more than half of patients with HGM patches suffered from chronic inflammation.
Acid production and/or H. pylori colonization within HGM patches can potentially lead to esophageal web, stricture, ulcer, perforation or bleeding, which are rarely reported in the literature[10,29,30]. In our study, no such complications were observed in patients with HGM patches. Although mucosal atrophy is very rarely observed within HGM patches, we observed a prevalence of 8.3% in our study. The factors leading to such a high prevalence of mucosal atrophy within HGM patches are unknown and may be associated with chronic inflammation, H. pylori infection, and even racial difference.
The results of this study showed a significant difference between patients with and without HGM regarding heartburn and regurgitation, throat discomfort, dysphagia and epigastric discomfort, but not retrosternal pain. Previous studies indicated that esophageal symptoms (e.g., heartburn, regurgitation, chest pain, dysphagia, and globus sensation) and extraesophageal symptoms (e.g., cough, asthma, and wheezing) differ between those with and without patches[19,20]. Although these symptoms are not specific for the diagnosis of HGM, they are potentially regarded as evidence for further endoscopic examination. It is important to investigate the association between HGM and these symptoms from a clinical point of view. It is known that dysphagia, globus sensation and respiratory symptoms (e.g., cough, hoarseness) are commonly observed with HGM[7,31-33]. In our study, dysphagia and epigastric discomfort were identified as independent risk factors for HGM; however, male gender was associated with a reduced risk of HGM. Other symptoms, such as throat discomfort, heartburn, regurgitation and retrosternal pain were not associated with the presence of HGM.
Although our study indicated a relatively high occurrence of intestinal metaplasia and malignant transformation, a very small number of these lesions have been reported in other studies. Therefore, whether patients with HGM require follow-up by endoscopy is still a controversial issue and requires further consideration.
In conclusion, the prevalence of HGM patches is an infrequent anomaly based on our multicenter prospective cohort study in a Chinese population, although the endoscopists were not instructed to focus on these lesions. Because it is easy to ignore HGM patches in the upper esophagus, an endoscopic examination should be thorough and performed carefully. Biopsy was reasonable once the HGM patch was identified. Although malignant transformation of a heterotopic patch is rare, the prevalence of intestinal metaplasia and dysplasia indicates a need for endoscopic follow-up in patients with HGM. Clinical complaints should be a focus of attention to increase the rate of HGM detection in light of our findings.
Heterotopic gastric mucosa (HGM) can cause diverse symptoms, such as chest pain, discomfort of throat, dysphagia, globus sensation, and laryngopharyngeal or supraesophageal symptoms. In a minority of cases, HGM can further develop into a web, stricture, ulcer, perforation, esophagotracheal fistula, Barrett’s esophagus or adenocarcinoma. The clinical significance and prevalence of HGM are still largely unknown due to the limited number of subjects in most studies and discrepant focus on this lesion by endoscopists.
This article focuses on the prevalence of HGM in a Chinese population, evaluates the association of HGM with demographic and clinical characteristics, and identifies its macroscopic and histologic features. The authors conducted a prospective multi-center cohort study without special focus of the endoscopist on HGM.
This study included a large number of subjects from multiple medical centers in China. It focused on clinical features, pathologic and endoscopic characteristics of HGM, and proposed certain sensitive factors involving clinical symptoms (e.g., chest pain, discomfort of throat, dysphagia, and globus sensation) for the diagnosis of HGM.
This study provides guidance in the diagnosis of HGM. As it is easy to ignore HGM patches in the upper esophagus, a careful and thorough endoscopic examination should be performed. Biopsy is reasonable once the patch is found. Although malignant transformation of heterotopic patches is rare, endoscopic follow-up is necessary for intestinal metaplasia and dysplasia. Attention should be paid to the patient’s complaints so as to increase the detectable rate of HGM.
HGM is an area of heterotopic columnar mucosal islands in the postcricoid portion of the cervical esophagus or below the level of the upper esophageal sphincter. HGM is thought to be the remnant of the esophageal columnar embryologic lining due to the underdevelopment of squamous epithelium during the fetal period, or associated with Barrett’s esophagus and gastroesophageal reflux, or developed from mucus gland cysts within the cervical esophagus after eruption.
This article is a well-written piece of work concerning the epidemiology of HGM in a Chinese population. This manuscript is well-designed, the clinical and laboratory studies are described in detail, and the results are presented clearly.
P- Reviewer: Reis H, Yang ZM S- Editor: Qi Y L- Editor: AmEditor E- Editor: Liu XM
| 1. | Truong LD, Stroehlein JR, McKechnie JC. Gastric heterotopia of the proximal esophagus: a report of four cases detected by endoscopy and review of literature. Am J Gastroenterol. 1986;81:1162-1166. [PubMed] |
| 2. | von Rahden BH, Stein HJ, Becker K, Liebermann-Meffert D, Siewert JR. Heterotopic gastric mucosa of the esophagus: literature-review and proposal of a clinicopathologic classification. Am J Gastroenterol. 2004;99:543-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 109] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 3. | Meining A, Bajbouj M. Erupted cysts in the cervical esophagus result in gastric inlet patches. Gastrointest Endosc. 2010;72:603-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Chong VH, Jalihal A. Cervical inlet patch: case series and literature review. South Med J. 2006;99:865-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Akbayir N, Alkim C, Erdem L, Sökmen HM, Sungun A, Başak T, Turgut S, Mungan Z. Heterotopic gastric mucosa in the cervical esophagus (inlet patch): endoscopic prevalence, histological and clinical characteristics. J Gastroenterol Hepatol. 2004;19:891-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 62] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Poyrazoglu OK, Bahcecioglu IH, Dagli AF, Ataseven H, Celebi S, Yalniz M. Heterotopic gastric mucosa (inlet patch): endoscopic prevalence, histopathological, demographical and clinical characteristics. Int J Clin Pract. 2009;63:287-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Alaani A, Jassar P, Warfield AT, Gouldesbrough DR, Smith I. Heterotopic gastric mucosa in the cervical oesophagus (inlet patch) and globus pharyngeus--an under-recognised association. J Laryngol Otol. 2007;121:885-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Balon JM, Mariette C, Fabre S, Tiret E, Triboulet JP. [Primary adenocarcinoma of the cervical esophagus arising from heterotopic gastric mucosa]. Gastroenterol Clin Biol. 2003;27:836-838. [PubMed] |
| 9. | Waring JP, Wo JM. Cervical esophageal web caused by an inlet patch of gastric mucosa. South Med J. 1997;90:554-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Sánchez-Pernaute A, Hernando F, Díez-Valladares L, González O, Pérez Aguirre E, Furió V, Remezal M, Torres A, Balibrea JL. Heterotopic gastric mucosa in the upper esophagus (“inlet patch”): a rare cause of esophageal perforation. Am J Gastroenterol. 1999;94:3047-3050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Kohler B, Köhler G, Riemann JF. Spontaneous esophagotracheal fistula resulting from ulcer in heterotopic gastric mucosa. Gastroenterology. 1988;95:828-830. [PubMed] |
| 12. | Melato M, Ferlito A. Heterotopic gastric mucosa of the tongue and the oesophagus. ORL J Otorhinolaryngol Relat Spec. 1975;37:244-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Tang P, McKinley MJ, Sporrer M, Kahn E. Inlet patch: prevalence, histologic type, and association with esophagitis, Barrett esophagus, and antritis. Arch Pathol Lab Med. 2004;128:444-447. [PubMed] |
| 14. | Weickert U, Wolf A, Schröder C, Autschbach F, Vollmer H. Frequency, histopathological findings, and clinical significance of cervical heterotopic gastric mucosa (gastric inlet patch): a prospective study in 300 patients. Dis Esophagus. 2011;24:63-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Azar C, Jamali F, Tamim H, Abdul-Baki H, Soweid A. Prevalence of endoscopically identified heterotopic gastric mucosa in the proximal esophagus: endoscopist dependent? J Clin Gastroenterol. 2007;41:468-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Baudet JS, Alarcón-Fernández O, Sánchez Del Río A, Aguirre-Jaime A, León-Gómez N. Heterotopic gastric mucosa: a significant clinical entity. Scand J Gastroenterol. 2006;41:1398-1404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Maconi G, Pace F, Vago L, Carsana L, Bargiggia S, Bianchi Porro G. Prevalence and clinical features of heterotopic gastric mucosa in the upper oesophagus (inlet patch). Eur J Gastroenterol Hepatol. 2000;12:745-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 77] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Wang GQ, Dawsey SM, Zhou MH, Lewin KJ. Gastric heterotopia in the upper esophagus (inlet patch) in endoscopic surveys in northern China. J Clin Gastroenterol. 1994;19:321-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Jacobs E, Dehou MF. Heterotopic gastric mucosa in the upper esophagus: a prospective study of 33 cases and review of literature. Endoscopy. 1997;29:710-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Rosztóczy A, Izbéki F, Németh IB, Dulic S, Vadászi K, Róka R, Gecse K, Gyökeres T, Lázár G, Tiszlavicz L. Detailed esophageal function and morphological analysis shows high prevalence of gastroesophageal reflux disease and Barrett’s esophagus in patients with cervical inlet patch. Dis Esophagus. 2012;25:498-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Borhan-Manesh F, Farnum JB. Incidence of heterotopic gastric mucosa in the upper oesophagus. Gut. 1991;32:968-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 97] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Raine CH. Ectopic gastric mucosa in the upper esophagus as a cause of dysphagia. Ann Otol Rhinol Laryngol. 1983;92:65-66. [PubMed] |
| 23. | Song ZY, Huang X, Qian KD, Peng JP, Sun AW, Zhang YY, Zhao ZG. [Clinical analysis of 39 cases of heterotopic gastric mucosa in the upper esophagus]. Zhonghua Yixue Zazhi. 2005;85:244-247. [PubMed] |
| 24. | Klaase JM, Lemaire LC, Rauws EA, Offerhaus GJ, van Lanschot JJ. Heterotopic gastric mucosa of the cervical esophagus: a case of high-grade dysplasia treated with argon plasma coagulation and a case of adenocarcinoma. Gastrointest Endosc. 2001;53:101-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Pech O, May A, Gossner L, Vieth M, Trump F, Stolte M, Ell C. Early stage adenocarcinoma of the esophagus arising in circular heterotopic gastric mucosa treated by endoscopic mucosal resection. Gastrointest Endosc. 2001;54:656-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Flejou JF, Potet F, Molas G, Bogomoletz WV, Nasca S, Rigaud C, Feydy P, Ectors N, Geboes K. Campylobacter-like organisms in heterotopic gastric mucosa of the upper oesophagus. J Clin Pathol. 1990;43:961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Borhan-Manesh F, Farnum JB. Study of Helicobacter pylori colonization of patches of heterotopic gastric mucosa (HGM) at the upper esophagus. Dig Dis Sci. 1993;38:142-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 28] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Gutierrez O, Akamatsu T, Cardona H, Graham DY, El-Zimaity HM. Helicobacter pylori and hetertopic gastric mucosa in the upper esophagus (the inlet patch). Am J Gastroenterol. 2003;98:1266-1270. [PubMed] |
| 29. | Jerome-Zapadka KM, Clarke MR, Sekas G. Recurrent upper esophageal webs in association with heterotopic gastric mucosa: case report and literature review. Am J Gastroenterol. 1994;89:421-424. [PubMed] |
| 30. | Byrne M, Sheehan K, Kay E, Patchett S. Symptomatic ulceration of an acid-producing oesophageal inlet patch colonized by helicobacter pylori. Endoscopy. 2002;34:514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Bajbouj M, Becker V, Eckel F, Miehlke S, Pech O, Prinz C, Schmid RM, Meining A. Argon plasma coagulation of cervical heterotopic gastric mucosa as an alternative treatment for globus sensations. Gastroenterology. 2009;137:440-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 32. | Lancaster JL, Gosh S, Sethi R, Tripathi S. Can heterotopic gastric mucosa present as globus pharyngeus? J Laryngol Otol. 2006;120:575-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 33. | Silvers WS, Levine JS, Poole JA, Naar E, Weber RW. Inlet patch of gastric mucosa in upper esophagus causing chronic cough and vocal cord dysfunction. Ann Allergy Asthma Immunol. 2006;96:112-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |