Published online Dec 7, 2014. doi: 10.3748/wjg.v20.i45.17107
Revised: June 22, 2014
Accepted: July 16, 2014
Published online: December 7, 2014
Processing time: 200 Days and 19.8 Hours
AIM: To investigate the potential association of circulating zonulin with the stage of liver disease in obese children with biopsy-confirmed nonalcoholic fatty liver disease (NAFLD).
METHODS: A case-control study was performed. Cases were 40 obese children with NAFLD. The diagnosis of NAFLD was based on magnetic resonance imaging (MRI) with high hepatic fat fraction (HFF ≥ 5%), and confirmed by liver biopsy with ≥ 5% of hepatocytes containing macrovesicular fat. Controls were selected from obese children with normal levels of aminotransferases, and without MRI evidence of fatty liver as well as of other causes of chronic liver diseases. Controls were matched (1-to 1) with the cases on age, gender, pubertal stage and as closely as possible on body mass index- standard deviation score. All participants underwent clinical examination, laboratory tests including zonulin, inflammatory and metabolic parameters, and MRI for measurement of HFF and visceral adipose tissue.
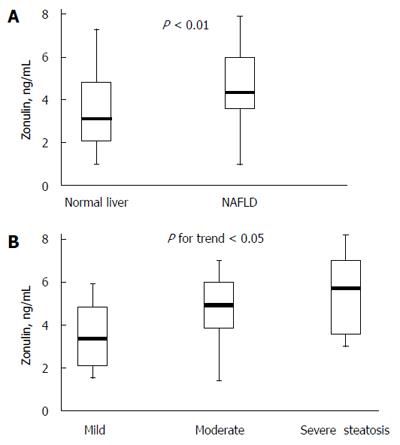
RESULTS: Zonulin values were significantly greater in obese subjects with NAFLD than in those without NAFLD [median (interquartile range), 4.23 (3.18-5.89) vs 3.31 (2.05-4.63), P < 0.01]. In patients with NAFLD, zonulin concentrations increased significantly with the severity of steatosis and the Spearman’s coefficient revealed a positive correlation between zonulin values and steatosis (r = 0.372, P < 0.05); however, we did not find a significant correlation between zonulin and lobular inflammation (P = 0.23), ballooning (P = 0.10), fibrosis score (P = 0.18), or presence of nonalcoholic steatohepatitis (P = 0.17). Within the entire study population, zonulin levels were positively associated with gamma-glutamyl transferase, 2-h insulin, HFF, and negatively associated with whole-body insulin sensitivity index (WBISI), after adjustment for age, gender and pubertal status. When the associations were restricted to the group of NAFLD patients, 2-h insulin, hepatic fat, and WBISI retained statistical significance.
CONCLUSION: Circulating zonulin is increased in children and adolescents with NAFLD and correlates with the severity of steatosis.
Core tip: Alteration in gut microbiota followed by impairment of intestinal wall integrity may play an important role in the pathogenesis of nonalcoholic fatty liver disease (NAFLD). Zonulin is a mediator known to regulate intestinal permeability by modulating intracellular tight junctions. We showed that zonulin concentrations are increased in obese children with biopsy-proven NAFLD and correlate with the severity of steatosis, but not with the presence of nonalcoholic steatohepatitis (NASH), lobular inflammation or fibrosis score. These findings may well fit with the recent theory suggesting that simple steatosis and NASH are different and not necessarily inter-related diseases.
- Citation: Pacifico L, Bonci E, Marandola L, Romaggioli S, Bascetta S, Chiesa C. Increased circulating zonulin in children with biopsy-proven nonalcoholic fatty liver disease. World J Gastroenterol 2014; 20(45): 17107-17114
- URL: https://www.wjgnet.com/1007-9327/full/v20/i45/17107.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i45.17107
Paralleling the worldwide epidemic of childhood obesity, nonalcoholic fatty liver disease (NAFLD) has become the most common cause of chronic liver disease in children and adolescents[1]. NAFLD is a spectrum of fat-associated liver conditions that can result in end-stage liver disease and the need for liver transplantation[2]. Simple steatosis, or fatty liver, occurs early in NAFLD and may progress to nonalcoholic steatohepatitis (NASH), fibrosis and cirrhosis with increased risk of hepatocellular carcinoma[2]. Growing evidence supports the concept that NAFLD is a highly heritable disease in which genetic variations and environmental factors closely interact to determine the disease phenotype and progression to the more advanced forms of the disease[3]. Recently, it has been suggested that alteration in gut microbiota followed by impairment of intestinal wall integrity may play an important role in the pathogenesis of NAFLD[4,5]. Miele et al[6] provided the first evidence that NAFLD in adult subjects is associated with increased gut permeability and small intestinal bacterial overgrowth (SIBO) and that these factors are associated with the severity of hepatic steatosis[6]. Specifically, the increased permeability appeared to be caused by disruption of intercellular tight junctions in the intestine.
Zonulin is a mediator known to regulate intestinal permeability by modulating intracellular tight junctions (TJ)[7-9]. Human zonulin is a 47-kDa protein that increases intestinal permeability in nonhuman primate intestinal epithelia[8], participates in the development of intestinal innate immunity[10], and is overexpressed in autoimmune disorders in which TJ dysfunction is central, including celiac disease[11,12] and type 1 diabetes[13]. Circulating zonulin is considered as a useful marker of intestinal permeability[8,14]. In humans, elevated serum zonulin levels have been demonstrated to correlate with increased intestinal permeability associated with altered genetic expression of intestinal TJ proteins[13]. Recently published studies have shown higher circulating zonulin levels in obese than in nonobese adults and in adults with glucose intolerance compared with a group with normal glucose tolerance[15,16]. The current study was therefore designed to investigate the potential association of circulating zonulin with the stage of liver disease in obese children with biopsy-confirmed NAFLD.
The study population consisted of obese children [body mass index (BMI) above the 95th percentile for age and gender] seen in the Hepatology outpatient Clinic of the Department of Pediatrics, Sapienza University of Rome, Italy, for chronically (at least 6 mo) elevated aminotransferase levels. The diagnosis of NAFLD was based on magnetic resonance imaging (MRI) with high hepatic fat fraction (HFF ≥ 5%)[17], and confirmed by liver biopsy. Other causes of chronic liver disease, including hepatic virus infections (hepatitis A-E and G, cytomegalovirus, and Epstein-Barr virus), autoimmune hepatitis, metabolic liver disease, α-1-antitrypsin deficiency, cystic fibrosis, Wilson’s disease, hemochromatosis, and celiac disease were excluded with appropriate tests. Other exclusion criteria were history of type 1 or type 2 diabetes, renal disease, food allergy, total parenteral nutrition, smoking or alcohol consumption, and use of hepatotoxic medications.
For comparative purposes, we enrolled a group of control subjects, matched for age, gender, pubertal stage and as closely as possible for BMI-standard deviation score (SDS). The group was composed of obese children with normal levels of aminotransferases, and without MRI evidence of fatty liver (HFF < 5%) as well as of other causes of chronic liver disease (see above). Controls were also excluded if they had history of type 1 or type 2 diabetes, renal disease, food allergy, smoking or alcohol consumption. Finally, the use of anti-inflammatory drugs, antibiotics or probiotics was considered among the exclusion criteria for both cases and controls.
The research protocol was approved by the local Ethics Committee (Policlinico Umberto I Hospital, Rome, Italy) and written informed consent was obtained from the next of kin, caretakers, or guardians on behalf of the children enrolled in this study, in accordance with principles of Helsinki Declaration.
All participants underwent physical examination including measurements of weight, standing height, BMI, waist circumference (WC), and determination of the stage of puberty. The pubertal stage was categorized into two groups (prepubertal: boys with pubic hair and gonadal stage I, and girls with pubic hair stage and breast stage I; pubertal: boys with pubic hair and gonadal stage ≥ II and girls with pubic hair stage and breast stage ≥ II). The degree of obesity was quantified using Cole’s least mean-square method, which normalizes the skewed distribution of BMI and expresses BMI as SDS[18].
Blood samples were taken from each subject, after an overnight fast, for estimation of glucose, insulin, C peptide, total cholesterol, high-density lipoprotein cholesterol (HDL-C), triglycerides, alanine aminotransferase (ALT), aspartate aminotransferase, gamma-glutamyl transferase (GGT), high-sensitive C reactive protein (HSCRP), and zonulin. An oral glucose tolerance test (OGTT) was performed for all obese children with the administration of glucose at 1.75 g per kg of body weight (maximum dose 75 g); blood samples were obtained at 0 min and every 30 min thereafter for 180 min for determination of serum glucose and insulin. Estimates of insulin sensitivity were calculated using the homeostasis model assessment of insulin resistance (HOMA-IR), defined by fasting insulin and fasting glucose and whole-body insulin sensitivity index (WBISI), based on mean values of insulin and glucose obtained from OGTT and the corresponding fasting values[19].
1-wk weighted dietary and physical activity records were used. Depending on the age of the child, the parent or the child weighted and recorded all foods and fluids consumed using electronic food scales. Nutrient analyses were obtained from the Food Composition Database for Epidemiological Studies in Italy (Banca Dati di Composizione degli Alimenti per Studi Epidemiologici in Italia-BDA). The subjects’ physical activity was assessed with a questionnaire, in which the length of time spent playing sport, watching television, playing video games, and so on were recorded.
Abdominal MRI studies were performed on a 1.5T Siemens Avanto magnet (Siemens AG, Erlangen, Germany). The hepatic fat content (% HFF) was measured by MRI as previously described and validated[17]. The three-point chemical-shift-fat-water separation method (fat-only dataset) was used to measure abdominal fat mass distribution[20]. MRI results were interpreted by an experienced radiologist who was blinded to the clinical, laboratory and/or histologic findings.
The clinical indication for biopsy was either to assess the presence of NASH and degree of fibrosis or other likely independent or competing liver diseases. Percutaneous needle liver biopsy was performed as previously described[17]. The main histologic features of NAFLD were scored according to the scoring system developed by the NASH Clinical Research Network (CNR)[21]: steatosis [grade 0 (< 5% macrovesicular fat), grade 1 (mild = 5%-33%), grade 2 (moderate = 34%-66%), and grade 3 (severe > 66%)], portal inflammation (0-2), lobular inflammation (0-3), ballooning degeneration (0-2), and fibrosis (stage 0 to 4). As recommended by a recent NASH CRN article[22], a microscopic diagnosis, based on overall injury pattern (i.e., steatosis, hepatocyte ballooning, and inflammation), as well as the presence of additional lesions (i.e., zonality of lesions, portal inflammation, and fibrosis), has been assigned to each case[23]. Accordingly, biopsies were subdivided into not-NASH and definite NASH subcategories[23].
Serum zonulin concentrations were measured by ELISA (Immundiagnostik AG, Bensheim, Germany). The Elisa kit used for zonulin measurement only detects the active (uncleaved) form of zonulin. The lower limit of detection was 0.23 ng/mL. Intra- and interassay coefficients of variation were 3%-7% and 5%-12%, respectively. All remaining analyses were conducted by COBAS 6000 (Roche Diagnostics). While insulin and C peptide concentrations were measured on cobas e 601 module (Electrochemiluminescence Technology, Roche Diagnostics), the remaining analytes on cobas e 501 clinical chemistry module (Photometric Technology).
Statistical analyses were performed using the SPSS package. The data are expressed as frequencies, mean with SD, or median with interquartile range (IQR), as appropriate. The differences between cases and control children in quantitative variables were evaluated by t-test or Mann–Whitney U-test, as appropriate. Proportions were compared by the χ2 test. The Spearman’s correlation coefficient and multiple regression analyses were used to evaluate the relationship between zonulin values and the other continuous variables. For comparison of the mean of zonulin between the NAFLD and control groups of children, the power was calculated after the results were obtained. With sample sizes n = 40 and n = 40 for the two study groups, and assuming the observed standard deviations, this study had a power of 84% to detect the observed difference statistically significantly at the 5% level. A P value of less than 0.05 was considered to be statistically significant.
Forty obese children with NAFLD and 40 obese children without evidence of liver disease were enrolled. The mean age of cases and controls was 11.10 (SD 3.1) years. Both cases and controls included 15 girls and 25 boys, and five prepubertal children. The mean BMI-SDS of cases and controls was 2.15 (SD 0.50) and 2.10 (SD 0.32), respectively. The clinical and laboratory characteristics for cases and controls are shown in Table 1. By definition, HFF values were significantly different between the two groups. WC and visceral adipose tissue (VAT) were significantly greater in obese subjects with NAFLD than in those without NAFLD. NAFLD patients had higher values for triglycerides, HSCRP, fasting insulin, C peptide, HOMA-IR, 2-h insulin, and HbA1c, and lower WBISI values compared to subjects with no liver involvement. As expected, children with NAFLD had higher liver enzymes compared to those without NAFLD. There were no differences between children with and without NAFLD with respect to subcutaneous adipose tissue (SAT), total cholesterol and HDL-C, fasting glucose, and 2-h glucose.
| No NAFLD (n = 40) | NAFLD (n = 40) | P value | |
| Age, yr1 | 11.10 (3.1) | 11.10 (3.1) | |
| Male gender1 | 25 (62.5) | 25 (62.5) | |
| BMI-SD score | 2.10 (0.32) | 2.15 (0.50) | 0.5600 |
| Waist circumference, cm | 92 (12) | 97 (12)a | 0.0270 |
| Abdominal fat | |||
| Visceral adipose tissue, cm2 | 284 (217-502) | 447 (337-676) | 0.0040 |
| Subcutaneous adipose tissue, cm2 | 1648 (1301-2503) | 1828 (1629-2471) | 0.2200 |
| Hepatic fat fraction | 1.5% (1.0%-3.0%) | 16.0% (10.0%-30.0%) | < 0.0001 |
| Triglycerides, mg/dL | 81 (52-114) | 96 (68-149) | 0.0490 |
| Total cholesterol, mg/dL | 154 (142-185) | 149 (130-179) | 0.1100 |
| HDL-C, mg/dL | 49 (43-54) | 45 (37-53) | 0.3100 |
| Aspartate aminotransferase, U/L | 25 (11) | 34 (26) | 0.0370 |
| Alanine aminotransferase, U/L | 26 (17) | 50 (43) | < 0.0001 |
| γ-glutamyl transferase, U/L | 15 (8) | 23 (12) | < 0.0001 |
| Fasting glucose, mg/dL | 85 (6) | 83 (8) | 0.3300 |
| 2-h glucose, mg/dL | 94 (13) | 93 (14) | 0.8700 |
| Fasting insulin, μU/mL | 11 (8-15) | 20 (15-28) | 0.0010 |
| 2-h insulin, μU/mL | 35 (23-63) | 67 (29-107) | 0.0070 |
| Fasting C peptide, pmol/L | 780 (576-887) | 1075 (816-1302) | < 0.0001 |
| HOMA-IR values | 2.24 (1.80-3.17) | 4.11 (2.95-6.67) | 0.0030 |
| WBISI | 5.84 (3.16-6.87) | 2.57 (1.58-5.36) | 0.0020 |
| HbA1c | 5.0% (0.32) | 5.4% (0.47) | 0.0170 |
| HSCRP, μg/L | 1800 (1000-3200) | 3000 (1500-4325) | 0.0490 |
| Zonulin, ng/mL | 3.31 (2.05-4.63) | 4.23 (3.18-5.89) | 0.0090 |
As shown in Table 2, the daily energy intake and the percentages of fat, protein, and carbohydrate in the diet were similar between obese children with NAFLD and those without liver involvement. No differences were found when comparing intake of saturated fat between patients with and without NAFLD; however, NAFLD children had a lower intake of mono and polyunsaturated fatty acids. Furthermore, intake of sugar-sweetened beverages was significantly higher in NAFLD patients than the control group. The energy expenditure expressed as metabolic equivalents was comparable between the two groups.
| No NAFLD(n = 40) | NAFLD(n = 40) | |
| Diet | ||
| Energy intake, kcal/d | 1633 (1380-1840) | 1641 (1341-1964) |
| Protein, % kcal | 18 (15-20) | 17 (16-19) |
| Fat, % kcal | 38 (35-46) | 39 (36-46) |
| Carbohydrates, % kcal | 40 (36-46) | 41 (35-46) |
| Fiber, g/kcal | 11 (9-15) | 10 (7-13) |
| Saturated fatty acids, g/d | 20 (16-24) | 24 (15-33) |
| Mono unsaturated fatty acids, g/d | 42 (29-46) | 34 (26-47)a |
| Polyunsaturated fatty acids, g/d | 10 (9-12) | 9 (7-10)a |
| Sugar-sweetened beverage, kcal/da | 30 (13-46) | 118 (10-162)a |
| Daily physical activity level-MET | 3.0 (2.4-3.3) | 3.5 (2.8-4.6) |
Histopathological features associated with NAFLD are reported in Table 3. The hepatic fat content averaged 46%, with about two thirds of cases having more than 33% fat accumulation. Some degree of fibrosis was present in 75% of patients (40%, stage 1; 32.5%, stage 2; 2.5%, stage 3). Twenty-five (62.5%) patients had definite-NASH, while 15 (37.5%) not-NASH.
| Histopathological features | Degree or stage | No NASH(n = 15) | NASH(n = 25) |
| Steatosis | 1 | 8 (53.3) | 3 (12.0) |
| 2 | 5 (33.3) | 8 (32.0) | |
| 3 | 2 (13.3) | 14 (56.0) | |
| Lobular inflammation | 0 | 1 (6.7) | 0 |
| 1 | 10 (66.6) | 8 (32.0) | |
| 2 | 4 (26.6) | 12 (48.0) | |
| 3 | 0 | 5 (20) | |
| Ballooning | 0 | 4 (26.6) | 0 |
| 1 | 10 (66.6) | 18 (72.0) | |
| 2 | 1 (6.7) | 7 (28.0) | |
| Fibrosis | 0 | 3 (20.0) | 7 (28.0) |
| 1 | 8 (53.3) | 8 (32.0) | |
| 2 | 4 (26.6) | 9 (36.0) | |
| 3 | 0 | 1 (4.0) | |
| NAS, mean ± SD | 2.60 ± 0.41 | 5.45 ± 0.63 |
Zonulin values were significantly greater in obese subjects with NAFLD than in those without NAFLD (Figure 1A). Among patients of the NAFLD group, zonulin concentrations increased significantly with the severity of steatosis (Figure 1B) and the Spearman’s coefficient revealed a positive correlation between zonulin values and steatosis (r = 0.372, P < 0.05); however, we did not find a significant correlation between zonulin and lobular inflammation (P = 0.23), ballooning (P = 0.10), fibrosis score (P = 0.18), or presence of NASH (P = 0.17).
Within the entire study population, zonulin levels were positively associated with GGT (standardized β coefficient, 0.229, P < 0.01), 2-h insulin (standardized β coefficient, 0.340, P < 0.01), HFF (standardized β coefficient, 0.471, P < 0.01), and negatively associated with WBISI (standardized β coefficient, - 0.236, P < 0.05), after adjustment for age, gender and pubertal status. No relationship was found between zonulin concentrations and BMI-SDS, VAT, SAT, ALT, total cholesterol and HDL-C, triglycerides, fasting and 2-h glucose. Serum zonulin was not related to daily energy intake nor to fat, protein and carbohydrate (including sugar- intake sweetened beverages). When the associations were restricted to the group of NAFLD patients, 2-h insulin (standardized β coefficient, 0.394, P < 0.01), hepatic fat (standardized β coefficient, 0.516, P < 0.01), and WBISI (standardized β coefficient, -0.352, P < 0.05) retained statistical significance.
A multiple regression analysis was also used to assess the independent association of NAFLD with circulating zonulin concentrations in the whole obese population and in biopsy-proven NAFLD, respectively, after adjustment for age, gender, pubertal status, and clinical and metabolic variables (including BMI-SDS, abdominal fat, and WBISI). HFF (standardized β coefficient, 0.447, P < 0.01) remained significantly associated with circulating zonulin values. In biopsy-proven NAFLD, hepatic fat was significantly associated with zonulin values (standardized β coefficient, 0.415, P < 0.05).
The main findings of this study are that circulating zonulin, a known mediator of intestinal permeability, modulating intracellular tight junctions, is increased in children and adolescents with NAFLD and correlates with the severity of steatosis.
Recently, several lines of evidence suggest a strong interaction between gut flora and liver[4,24,25]. Due to its anatomical position, the liver receives 70% of its blood supply from the intestine through the portal vein, and so it is the first line of defence against gut-derived antigens, and one of the organs most exposed to gut-derived toxic factors, such as bacteria and bacterial products[24]. The gut epithelium plays a central role in demarcating microbes in the gut from the host immune system. Gut epithelial cells are linked to one another with tight junctions, which play a pivotal role in maintaining the integrity of the intestinal barrier. Occurrence of SIBO and increased intestinal permeability due to “leaky” tight junctions has been reported in patients with NAFLD[4,6]. Miele et al[6] demonstrated an increased intestinal permeability and tight junction alterations in 35 adult patients with biopsy-proven NAFLD compared with healthy subjects. Furthermore, both gut permeability and the prevalence of SIBO correlated with the severity of steatosis, but not with the presence of NASH. In the current study involving children with biopsy-proven NAFLD, we also found a positive correlation of zonulin levels with severity of steatosis, but not with the presence of NASH, lobular inflammation or fibrosis score. Thus, the lack of association between intestinal permeability and steatohepatitis or fibrosis as shown currently in children and previously in adults may well fit with the recent theory suggesting that simple steatosis and NASH are different and not necessarily inter-related diseases[26].
Of note, our results and those of Miele et al[6] differ from those reported in the study by Giorgio et al[27], in which children with biopsy-proven NAFLD had a significant increase in gut permeability that positively correlated with liver disease severity. In the study performed by Miele et al[6] in adults, the 51Cr-EDTA excretion test was preferred over the lactulose-mannitol bowel permeability test utilized by Giorgio et al[27] in children, because the latter test is influenced by the presence of SIBO, one of the measured outcomes of the article[6]. Many clinical factors may influence the results of the permeability tests, including food allergy, diabetes, and BMI[28,29]. A higher BMI is associated with higher glomerular filtration rate (GFR)[30]. In obese subjects, the values for GFR have been reported to exceed by 61% the values for GFR of the control group and by 32% the value of renal plasma flow, suggestive of glomerular hyperfiltration[31]. The obesity-related glomerular hyperfiltration ameliorated after weight loss[31]. It is a possible pitfall when non-metabolized sugar intestinal permeability tests are performed in subjects with excess of weight: could a higher amount of excreted sugar be a consequence of higher intestinal absorption (due to high intestinal permeability) or of a higher glomerular hyperfiltration? This has not been investigated in the study by Giorgio et al[27]. Moreover, food allergy was not adopted as exclusion criterion. Therefore, differences in patients’ clinical and metabolic characteristics, differences in methods for evaluating intestinal permeability, and differences in analysis of the data make comparisons of the results of the 3 studies difficult.
In the present study, we have chosen to measure circulating zonulin, a protein recently found to reflect intestinal permeability. Zonulin has been studied as a marker for increased intestinal permeability in other disease entities such as type 1 diabetes or obesity-associated insulin resistance[13,15,16]. Sapone et al[13] showed that patients with type 1 diabetes and their relatives had elevated serum zonulin levels that correlated with increased intestinal permeability. Zak-Gołąb et al[15] assessed plasma zonulin, haptoglobin and proinflammatory cytokines levels in relation to composition of gut microbiota in obese and normal weight subjects. They showed that circulating zonulin was higher in obese subjects and proportional to total bacteria count[15]. Additionally, they observed that plasma zonulin was associated with fat percentage in diet and fiber intake in relation to daily energy consumption. No association was found between zonulin level and insulin resistance (scored as HOMA-IR) or with lipid levels. In subjects with glucose intolerance, Moreno-Navarrete et al[16] found that circulating zonulin was strongly associated with insulin resistance and obesity. However, these associations disappeared in multiple regression analysis. In our study, we found a relationship between zonulin levels and insulin sensitivity in the entire population as well as in the NAFLD group, after adjustment for age, gender and pubertal status, but failed to show an association between zonulin and total and abdominal obesity. In addition, circulating zonulin concentration was not associated with daily energy consumption and diet composition, though cases and controls differed in some specific dietary intake such as mono and polyunsaturated fatty acids and consumption of sugar-sweetened beverages. Dietary analysis was based on a 1-wk dietary records done the week prior to the study, and we cannot exclude that a more prolonged assessment of dietary intake might have given different results. Therefore, differences in patients’ clinical and metabolic characteristics may again explain the discrepant results among the studies.
This study has a few potential limitations, including the lack of characterization of gut microbiota, and the lack of endotoxin assessment. Moreover, we did not include a control group of children with either gut disease or fatty liver disease due to non-NAFLD etiology. Finally, no intestinal biopsies to evaluate the correlation between circulation zonulin and integrity of tight junctions within the small intestine were performed.
In conclusion, we have demonstrated that serum zonulin concentration is increased in children and adolescents with NAFLD and correlates with the severity of steatosis. However, the cross-sectional design of our study precludes the establishment of causal or temporal relationships between increased zonulin concentrations and severity of steatosis and the presence of NASH. Follow-up studies may be helpful in elucidating the cause-and-effect relations and the underlying mechanisms.
In parallel with epidemic obesity, nonalcoholic fatty liver disease (NAFLD) has emerged as the leading cause of chronic liver disease in both pediatric and adult patients worldwide. Recently, it has been suggested that alteration in gut microbiota followed by impairment of intestinal wall integrity may play an important role in the pathogenesis of NAFLD. Zonulin is a mediator known to regulate intestinal permeability by modulating intracellular tight junctions.
A better understanding of the role of gut-liver axis in the pathogenesis of obesity and NAFLD may open a new frontier to fight a highly prevalent condition like NAFLD.
Recently, several lines of evidence suggest a strong interaction between gut flora and liver. Due to its anatomical position, the liver receives 70% of its blood supply from the intestine through the portal vein, and so it is the first line of defence against gut-derived antigens, and is one of the organs most exposed to gut-derived toxic factors, such as bacteria and bacterial products. The gut epithelium plays a central role in demarcating microbes in the gut from the host immune system. Gut epithelial cells are linked to one another with tight junctions, which play a pivotal role in maintaining the integrity of the intestinal barrier. In this study, authors showed that circulating zonulin, a known mediator of intestinal permeability, modulating intracellular tight junctions, is increased in children and adolescents with NAFLD and correlates with the severity of steatosis. These data may suggest that increased intestinal permeability may be the condition sine qua non for the hypothesis of the contribution of gut-liver axis to the development of NAFLD.
Despite intense research efforts, universally accepted therapies for NAFLD, besides lifestyle modification focusing on weight reduction, are lacking. The possibility of manipulating gut microbiota and therefore intestinal permeability by means of agents such as probiotics may open novel opportunities.
NAFLD comprises a disease spectrum ranging from simple fatty liver to nonalcoholic steatohepatitis, with varying degrees of inflammation and fibrosis, progressing to end-stage liver disease with cirrhosis and hepatocellular carcinoma. Zonulin was the first tight junction-associated protein to be identified, and it is now widely considered to be an excellent marker for detecting intact cell-to-cell contacts and assessing tight junction integrity.
The study investigated the relation between zonulin, a marker of intestinal permeability, and stages of hepatic disease in obese children with biopsy confirmed NAFLD. They observed correlation between zonulin and steatosis severity but no correlation with inflammation, ballooning, fibrosis score and nonalcoholic steatohepatitis (NASH) diagnosis. They suggested that their findings add data to the hypothesis that simple steatosis and NASH are different, not necessarily inter-related diseases. This is a well performed study.
P- Reviewer: Cardoso CRL, Schaffler A, Yang SC S- Editor: Gou SX L- Editor: A E- Editor: Liu XM
| 1. | Mencin AA, Lavine JE. Advances in pediatric nonalcoholic fatty liver disease. Pediatr Clin North Am. 2011;58:1375-1392, x. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 2. | Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3655] [Cited by in RCA: 3719] [Article Influence: 161.7] [Reference Citation Analysis (2)] |
| 3. | Wree A, Broderick L, Canbay A, Hoffman HM, Feldstein AE. From NAFLD to NASH to cirrhosis-new insights into disease mechanisms. Nat Rev Gastroenterol Hepatol. 2013;10:627-636. [PubMed] |
| 4. | Duseja A, Chawla YK. Obesity and NAFLD: the role of bacteria and microbiota. Clin Liver Dis. 2014;18:59-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 5. | Miele L, Marrone G, Lauritano C, Cefalo C, Gasbarrini A, Day C, Grieco A. Gut-liver axis and microbiota in NAFLD: insight pathophysiology for novel therapeutic target. Curr Pharm Des. 2013;19:5314-5324. [PubMed] |
| 6. | Miele L, Valenza V, La Torre G, Montalto M, Cammarota G, Ricci R, Mascianà R, Forgione A, Gabrieli ML, Perotti G. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877-1887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1133] [Cited by in RCA: 1101] [Article Influence: 68.8] [Reference Citation Analysis (1)] |
| 7. | Fasano A. Regulation of intercellular tight junctions by zonula occludens toxin and its eukaryotic analogue zonulin. Ann N Y Acad Sci. 2000;915:214-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 8. | Wang W, Uzzau S, Goldblum SE, Fasano A. Human zonulin, a potential modulator of intestinal tight junctions. J Cell Sci. 2000;113 Pt 24:4435-4440. [PubMed] |
| 9. | Tripathi A, Lammers KM, Goldblum S, Shea-Donohue T, Netzel-Arnett S, Buzza MS, Antalis TM, Vogel SN, Zhao A, Yang S. Identification of human zonulin, a physiological modulator of tight junctions, as prehaptoglobin-2. Proc Natl Acad Sci USA. 2009;106:16799-16804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 319] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 10. | El Asmar R, Panigrahi P, Bamford P, Berti I, Not T, Coppa GV, Catassi C, Fasano A. Host-dependent zonulin secretion causes the impairment of the small intestine barrier function after bacterial exposure. Gastroenterology. 2002;123:1607-1615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 256] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 11. | Drago S, El Asmar R, Di Pierro M, Grazia Clemente M, Tripathi A, Sapone A, Thakar M, Iacono G, Carroccio A, D’Agate C. Gliadin, zonulin and gut permeability: Effects on celiac and non-celiac intestinal mucosa and intestinal cell lines. Scand J Gastroenterol. 2006;41:408-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 341] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 12. | Fasano A, Not T, Wang W, Uzzau S, Berti I, Tommasini A, Goldblum SE. Zonulin, a newly discovered modulator of intestinal permeability, and its expression in coeliac disease. Lancet. 2000;355:1518-1519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 436] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 13. | Sapone A, de Magistris L, Pietzak M, Clemente MG, Tripathi A, Cucca F, Lampis R, Kryszak D, Cartenì M, Generoso M. Zonulin upregulation is associated with increased gut permeability in subjects with type 1 diabetes and their relatives. Diabetes. 2006;55:1443-1449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 381] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 14. | Smecuol E, Sugai E, Niveloni S, Vázquez H, Pedreira S, Mazure R, Moreno ML, Label M, Mauriño E, Fasano A. Permeability, zonulin production, and enteropathy in dermatitis herpetiformis. Clin Gastroenterol Hepatol. 2005;3:335-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Zak-Gołąb A, Kocełak P, Aptekorz M, Zientara M, Juszczyk L, Martirosian G, Chudek J, Olszanecka-Glinianowicz M. Gut microbiota, microinflammation, metabolic profile, and zonulin concentration in obese and normal weight subjects. Int J Endocrinol. 2013;2013:674106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 16. | Moreno-Navarrete JM, Sabater M, Ortega F, Ricart W, Fernández-Real JM. Circulating zonulin, a marker of intestinal permeability, is increased in association with obesity-associated insulin resistance. PLoS One. 2012;7:e37160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 212] [Article Influence: 16.3] [Reference Citation Analysis (1)] |
| 17. | Pacifico L, Martino MD, Catalano C, Panebianco V, Bezzi M, Anania C, Chiesa C. T1-weighted dual-echo MRI for fat quantification in pediatric nonalcoholic fatty liver disease. World J Gastroenterol. 2011;17:3012-3019. [PubMed] |
| 18. | Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10513] [Cited by in RCA: 10502] [Article Influence: 420.1] [Reference Citation Analysis (0)] |
| 19. | Yeckel CW, Weiss R, Dziura J, Taksali SE, Dufour S, Burgert TS, Tamborlane WV, Caprio S. Validation of insulin sensitivity indices from oral glucose tolerance test parameters in obese children and adolescents. J Clin Endocrinol Metab. 2004;89:1096-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 279] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 20. | Lê KA, Ventura EE, Fisher JQ, Davis JN, Weigensberg MJ, Punyanitya M, Hu HH, Nayak KS, Goran MI. Ethnic differences in pancreatic fat accumulation and its relationship with other fat depots and inflammatory markers. Diabetes Care. 2011;34:485-490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 106] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 21. | Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313-1321. [PubMed] |
| 22. | Brunt EM, Kleiner DE, Wilson LA, Belt P, Neuschwander-Tetri BA. Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology. 2011;53:810-820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 997] [Cited by in RCA: 952] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 23. | Brunt EM. Nonalcoholic steatohepatitis: definition and pathology. Semin Liver Dis. 2001;21:3-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 636] [Cited by in RCA: 617] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 24. | Compare D, Coccoli P, Rocco A, Nardone OM, De Maria S, Cartenì M, Nardone G. Gut--liver axis: the impact of gut microbiota on non alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis. 2012;22:471-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 25. | Vajro P, Paolella G, Fasano A. Microbiota and gut-liver axis: their influences on obesity and obesity-related liver disease. J Pediatr Gastroenterol Nutr. 2013;56:461-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 323] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 26. | Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52:1836-1846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1543] [Cited by in RCA: 1820] [Article Influence: 121.3] [Reference Citation Analysis (0)] |
| 27. | Giorgio V, Miele L, Principessa L, Ferretti F, Villa MP, Negro V, Grieco A, Alisi A, Nobili V. Intestinal permeability is increased in children with non-alcoholic fatty liver disease, and correlates with liver disease severity. Dig Liver Dis. 2014;46:556-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 137] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 28. | Perrier C, Corthésy B. Gut permeability and food allergies. Clin Exp Allergy. 2011;41:20-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 132] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 29. | Salles Teixeira TF, Boroni Moreira AP, Silva Souza NC, Frias R, Gouveia Peluzio Mdo C. Intestinal permeability measurements: general aspects and possible pitfalls. Nutr Hosp. 2014;29:269-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 30. | Bosma RJ, van der Heide JJ, Oosterop EJ, de Jong PE, Navis G. Body mass index is associated with altered renal hemodynamics in non-obese healthy subjects. Kidney Int. 2004;65:259-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 99] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 31. | Chagnac A, Weinstein T, Herman M, Hirsh J, Gafter U, Ori Y. The effects of weight loss on renal function in patients with severe obesity. J Am Soc Nephrol. 2003;14:1480-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 389] [Article Influence: 17.7] [Reference Citation Analysis (0)] |