Published online Dec 7, 2014. doi: 10.3748/wjg.v20.i45.17049
Revised: June 30, 2014
Accepted: August 13, 2014
Published online: December 7, 2014
Processing time: 195 Days and 14.8 Hours
AIM: To analyze the role of CYLD for receptor-mediated cell death of murine hepatocytes in acute liver injury models.
METHODS: Hepatocyte cell death in CYLD knockout mice (CYLD-/-) was analyzed by application of liver injury models for CD95- (Jo2) and tumor necrosis factor (TNF)-α- [D-GalN/lipopolysaccharide (LPS)] induced apoptosis. Liver injury was assessed by measurement of serum transaminases and histological analysis. Apoptosis induction was quantified by cleaved PARP staining and Western blotting of activated caspases. Nuclear factor (NF)-κB, ERK, Akt and jun amino-terminal kinases signaling were assessed. Primary Hepatocytes were isolated by two step-collagenase perfusion and treated with recombinant TNF-α and with the CD95-ligand Jo2. Cell viability was analyzed by MTT-assay.
RESULTS: Livers of CYLD-/- mice showed increased anti-apoptotic NF-κB signaling. In both applied liver injury models CYLD-/- mice showed a significantly reduced apoptosis sensitivity. After D-GalN/LPS treatment CYLD-/- mice exhibited significantly lower levels of alanine aminotransferase (ALT) (295 U/L vs 859 U/L, P < 0.05) and aspartate aminotransferase (AST) (560 U/L vs 1025 U/L, P < 0.01). After Jo injection CYLD-/- mice showed 2-fold lower ALT (50 U/L vs 110 U/L, P < 0.01) and lower AST (250 U/L vs 435 U/L, P < 0.01) serum-levels compared to WT mice. In addition, isolated CYLD-/- primary murine hepatocytes (PMH) were less sensitive towards death receptor-mediated apoptosis and showed increased levels of Bcl-2, XIAP, cIAP1/2, survivin and c-FLIP expression upon TNF- and CD95-receptor triggering, respectively. Inhibition of NF-κB activation by the inhibitor of NF-κB phosphorylation inhibitor BAY 11-7085 inhibited the expression of anti-apoptotic proteins and re-sensitized CYLD-/- PMH towards TNF- and CD95-receptor mediated cell death.
CONCLUSION: CYLD is a central regulator of apoptotic cell death in murine hepatocytes by controlling NF-κB dependent anti-apoptotic signaling.
Core tip: Activation of death receptors, such as CD95 (Fas/APO-1) and tumor necrosis factor (TNF)-R1, is involved in the pathophysiology of acute and chronic liver diseases. Inactivation of the deubiquitinase CYLD is accompanied by increased survival of different cell types. However, the role of CYLD in death receptor-mediated apoptosis of hepatocytes has not been addressed so far. The study showed for the first time that CYLD negative hepatocytes are less sensitive to CD95 and TNF-R-mediated apoptosis, at least in part via triggering nuclear factor-κB signaling leading to induction of anti-apoptotic proteins. Inhibition of CYLD might represent a therapeutic approach to protect hepatocytes from death receptor-mediated apoptosis.
- Citation: Urbanik T, Koehler BC, Wolpert L, Elßner C, Scherr AL, Longerich T, Kautz N, Welte S, Hövelmeyer N, Jäger D, Waisman A, Schulze-Bergkamen H. CYLD deletion triggers nuclear factor-κB-signaling and increases cell death resistance in murine hepatocytes. World J Gastroenterol 2014; 20(45): 17049-17064
- URL: https://www.wjgnet.com/1007-9327/full/v20/i45/17049.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i45.17049
Dysregulation of apoptosis is mechanistically important in the pathogenesis of liver diseases. Hepatocytes can undergo apoptosis via an extrinsic, death receptor-mediated pathway, or alternatively, intracellular stress can activate the intrinsic pathway of apoptosis. Both pathways converge on mitochondrial activation, which is a prerequisite for hepatocyte apoptosis[1]. The integrity of the outer mitochondrial membrane is regulated by the Bcl-2 protein family, which is divided into anti- and pro-apoptotic members[2]. Persistent apoptosis is a feature of chronic liver diseases. Acute liver failure (ALF) is characterized by massive apoptosis and is associated with life threatening consequences[3]. It is one of the most challenging gastrointestinal emergencies encountered in clinical practice and carries a high mortality rate worldwide[4]. Autoimmune hepatitis, viral hepatitis, alcohol consumption and hepatotoxins have been identified as triggers of ALF. Therapeutic approaches for delaying or reversing liver failure apart from orthotopic liver transplantation are rare. Understanding of the mechanisms of hepatocyte survival and cell death pathways would offer potential therapeutic targets.
Targeting ubiquitin related posttranslational modifications of signaling molecules is a novel approach in the treatment of several human diseases[5]. Ubiquitination controls the half-life of proteins, but also acts as modulator of the enzymatic activity or docking of regulatory proteins. The functional outcome of ubiquitination processes is determined by the linkage type of single or poly-ubiquitin chains: Lysine 48 (K-48)-linked polyubiquitination mainly targets proteins for proteasomal degradation, whereas lysine 63 (K-63)-linked polyubiquitination primarily leads to non-proteasomal modifications such as subcellular localization or protein-protein interactions. Ubiquitination is a dynamic process that can be counterbalanced by deubiquitinating enzymes including the tumor suppressor CYLD[6].
The ubiquitin C-terminal hydrolase domain of CYLD allows the enzyme to remove K-63-linked ubiquitin chains, e.g., from signaling molecules involved in the nuclear factor (NF)-κB pathway, the stress-activated protein kinases (SAPK)/Jun amino-terminal kinases (JNK) and Akt pathway[6,7]. Increased NF-κB activation promotes cell survival, at least in part via induction of anti-apoptotic Bcl-2 family members[8,9] as well as several inhibitor of apoptosis (IAP) proteins[10,11]. A loss of CYLD expression or its function was shown to increase NF-κB signaling in several cell types including hepatocytes[12,13].
Here we show for the first time that livers of CYLD-/- mice are less sensitive to CD95 and TNF-R-mediated apoptosis, at least in part via triggering NF-κB signaling leading to induction of anti-apoptotic proteins such as survivin. Therefore, inhibition of CYLD might represent a therapeutic approach to protect hepatocytes from death receptor-mediated apoptosis.
CYLD-/- mice were generated and genotyping was performed as previously described[14]. Animals were bred and housed at the animal facility of the University of Mainz in a standard laboratory animal environment (fresh filtered air, 15 changes per hour; temperature, 21 ± 2 °C; humidity, 50% ± 20%; and 12:12-h light:dark cycle). All experiments were done in accordance with the governmental and institutional guidelines and were performed under the written approval of the state animal care commission (Regierungspräsidium Koblenz, Germany).
Acute liver injury was induced in 8-10 wk old mice by i.p. injection of Jo2 antibody (0.5 μg/g bodyweight, BD Pharmingen, Heidelberg, Germany) or D-galactosamine (D-GalN; 0.75 mg/g bodyweight, Carl Roth, Karlsruhe, Germany) and lipopolysaccharide (LPS; 2.5 μg/g bodyweight, Sigma-Aldrich, Hamburg, Germany). 3 and 5 h after D-GalN/LPS and Jo2 injection, respectively, mice were anaesthetized by i.p. injection of Ketamine/Xylazine (350 mg/kg/55mg/kg bodyweight, Sigma-Aldrich), blood for measurement of serum transaminase levels was collected via cardiac puncture and mice were scarified by cervical dislocation.
Isolation of total RNA and cDNA synthesis was performed as previously described[2]. Quantitative real-time polymerase chain reaction (q-RT PCR) was performed using primer assay kits (Qiagen, Hilden, Germany). Data acquisition and determination of gene expression was performed using the LightCycler software package (Roche). Each PCR reaction was run in duplicates. mRNA expression was normalized to the expression of the housekeeping gene glycerinaldehyde-3-phosphate-dehydrogenase.
Blood was collected by cardiocentesis. Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were measured in the Institute of Clinical and Laboratory Medicine at the University Hospital Mainz by standard procedures.
Hepatocytes were isolated by a two-step perfusion technique and cultured as previously described[2]. After 24 h, cells were treated with TNF-α (Biomol, Hamburg, Germany), Jo2 (BD Pharmingen), actinomycin D (Carl Roth), BAY 11-7085 (Enzo-Life-Science, Lörrach, Germany) and YM155 (Selleckchem, Houston, United States). Cell viability was determined using a colorimetric 3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay.
Initially, livers were assessed visually. To investigate liver architecture and tumor histology, 3 μm thick sections were made from formalin-fixed paraffin-embedded liver tissues and were stained with hematoxylin and eosin (HE). Modified Gomori (Gom) staining was used to assess fibrotic remodeling and architectural distortion. For detection of cleaved poly (ADP-ribose) polymerase (cl. PARP) and RelA frozen liver tissues were sectioned (10 μm) and further proceeded using the NovoLink™ Min Polymer Detection System (Leica Microsystems, Wetzlar, Germany) according to the manufacturer’s instructions. For quantification six fields of view per liver section of 5 mice were counted. The primary antibodies anti-cleaved PARP [E51] (Abcam, Cambridge, United Kingdom) and anti-NF-κB p65 antibodies (Santa Cruz Biotechnology, Heidelberg, Germany) were used.
Tissue lysis, protein extraction and preparation of nuclear and cytosolic extracts were performed as previously described[2,15]. Protein concentration was determined by Bradford protein assay (Bio-Rad, Munich, Germany). SDS-PAGE and Western blotting were performed according to standard procedures. Immunodetection was performed using primary the antibodies: CYLD (E-4), IκB-α (C-21), p-IκB-α (B-9), NF-κB p65, NF-κB1 p105/p50 (NLS), ERK2, cIAP1/2 (Santa Cruz Biotechnology, Heidelberg, Germany), NF-κB2 p100/p52, RelB, c-Rel (G57), p-JNK, JNK, p-ERK, p-Akt, Akt, caspase-8, cleaved caspase-8 (Asp387), caspase-9, caspase-3, cleaved caspase-3 (Asp175), Bid, Bcl-2, Bcl-xL, XIAP (Cell Signalling, Frankfurt, Germany), Bid cleaved (Ab-1, tBid, Merck Chemicals, Nottingham, United Kingdom), anti-c-FLIP mAb NF6 (a kind gift from Prof. P.H. Krammer, DKFZ Heidelberg), Mcl-1 (Rockland, Gilbertsville, United States) and α-Tubulin (Sigma-Aldrich, Munich, Germany). For densitometric analysis ImageJ software was used. Band density was measured relative to the corresponding controls (set to 1) and then adjusted to tubulin as loading control.
To quantify NF-κB transcription factor activation, the TransAM NF-κB Family Transcription Factor Assay Kit (Active Motiv, La Hulpe, Belgium) was used according to the manufacturer’s instructions. The assay is based on immobilized oligonucleotides containing NF-κB consensus sites. For each well, 3 μg nuclear cell extract were used.
Cells were lysed in buffer containing 20 mmol/L Tris/HCl pH 8.0, 5 mmol/L EDTA, 0.5% Triton X-100 and 1x complete protease inhibitor cocktail (Roche, Mannheim, Germany). Protein concentration was equilibrated by Bradford assay (Bio-Rad, Munich, Germany). Lysates were incubated in reaction buffer (25 mmol/L HEPES pH 7.5, 50 mmol/L NaCl, 10% glycerol, 0.05% CHAPS, and 5 mmol/L DTT) in the presence of 50 μmol/L caspase-3 fluorogenic substrate (Ac-DEVD-AFC) (Biomol). Assays were performed in black Maxisorb microtiter plates (Nunc, Langenselbold, Germany), generation of free AFC at 37 °C was measured using a fluorometric plate reader set to an excitation wavelength of 400 nm and emission wavelength of 505 nm.
For comparison of experimental groups, the nonparametric Mann-Whitney U-test was applied. Statistical differences of in vitro PMH experiments were determined by standard analysis of variance (ANOVA) following Post-Hoc tests. A P-value less than 0.05 was considered as significant.
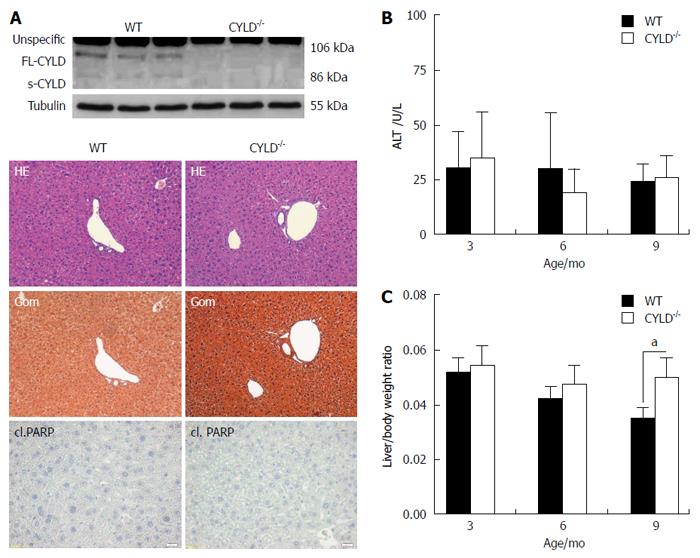
To explore the function of CYLD in vivo, CYLD-deficient mice were generated as previously described[14]. The absence of FL- (full length) CYLD and CYLD splice variants in liver tissues from CYLD deficient mice (CYLD-/-) mice were asserted by Western blotting (Figure 1A, upper panel). Livers from CYLD-/- mice were macroscopically normal. Pathohistological analysis revealed a regular liver architecture. Histological staining of cl. PARP indicated no spontaneous apoptotic liver damage in CYLD-/- mice at the age of 3, 6 and 9 mo (Figure 1A, lower panel). ALT serum level as marker for hepatocyte damage was not different from WT animals (Figure 1B). Interestingly, CYLD-/- mice showed a significantly higher liver/body weight ratio at the age of 9 mo compared to WT [0.05 vs 0.035; P < 0.05; (Figure 1C)], while their body weight was comparable to WT animals (data not shown). To assess proliferation of hepatocytes, BrdU was administered i.p. in 8 wk old animals. Proliferation rates in WT and CYLD-/- mice were not significantly different (data not shown).
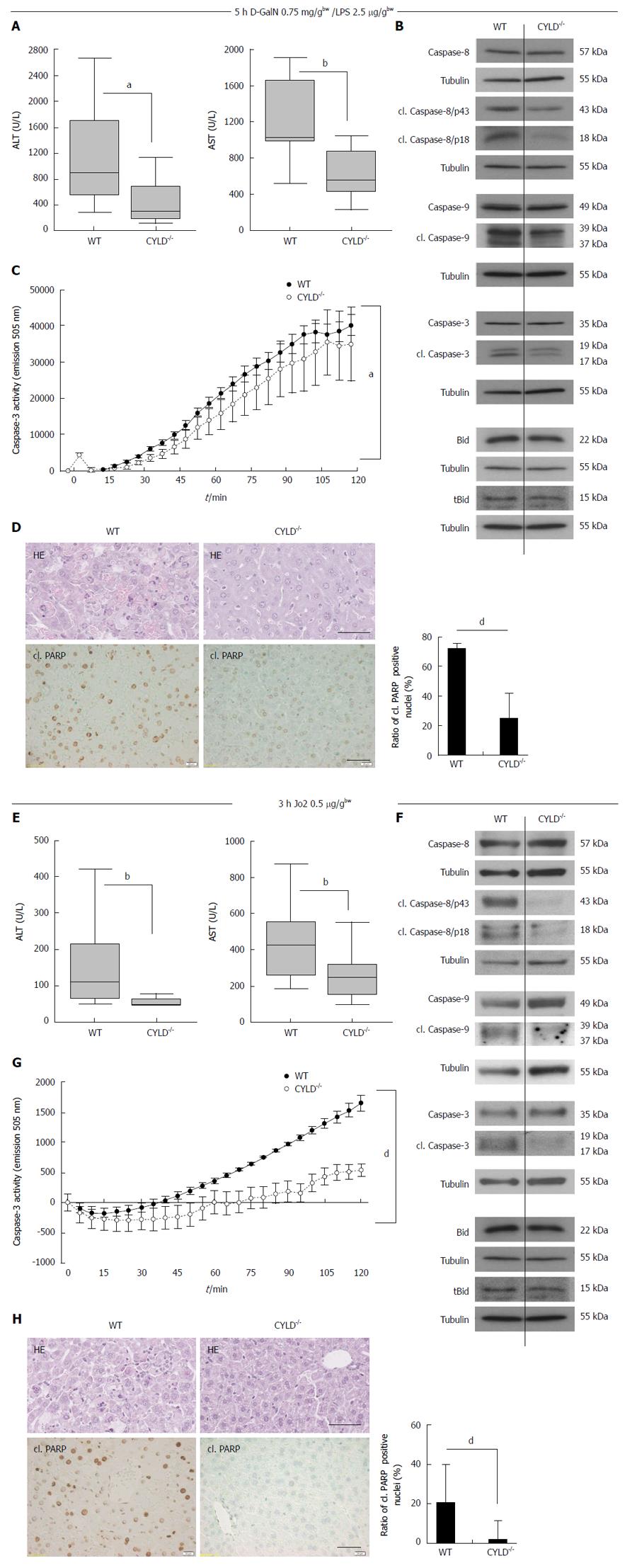
CYLD-/- hepatocytes did not show differences in TNF-R1 and CD95 surface expression compared to WT (data not shown). To assess a potential impact of the CYLD deletion on liver injury resulting from activation of TNF-α signaling, WT and CYLD-/- mice were injected i.p. with D-GalN and LPS[16]. Triggering of TNF-R1 by LPS-induced TNF-α leads to apoptotic liver injury upon D-GalN-mediated inhibition of NF-κB[1]. 5 h after D-GalN/LPS treatment, CYLD-/- mice exhibited significantly lower levels of ALT (295 U/L vs 859 U/L, P < 0.05) and AST (560 U/L vs 1025 U/L, P < 0.01, Figure 2A). Activation of caspase-8, -9 and -3 was reduced compared to WT as indicated by less detection of the respective cleaved (cl.) forms (Figure 2B). In line with this, caspase-3 activity assay showed significantly reduced substrate turnover in liver lysates of CYLD-/- mice compared to WT (Figure 2C). Analysis of Bid cleavage revealed slightly lower tBid levels after D-GalN/LPS treatment (Figure 2B). In pooled liver lysates from untreated mice no cl. caspase-3 levels were detectable (data not shown), confirming the results of immunohistological analysis of cl. PARP (Figure 1A, lower panel).
CYLD-/- mice showed significantly less hepatocyte damage compared to WT mice after D-GalN/LPS induced liver damage, which was further confirmed by a significant lower apoptosis rate of CYLD-/- hepatocytes compared to WT as indicated by less cl. PARP positive nuclei (24.8% vs 62.3%, P < 0.001, Figure 2D).
Administration of the agonistic CD95 receptor antibody Jo2 is an established model to induce acute liver injury[17]. To further examine the role of CYLD in acute liver injury, Jo2 was injected i.p. in WT and CYLD-/- mice. Liver injury was assessed by measurement of serum transaminases 3 h after injection: CYLD-/- mice showed 2-fold lower ALT (50 U/L vs 110 U/L, P < 0.01) and lower AST (250 U/L vs 435 U/L, P < 0.01) serum-levels compared to WT mice (Figure 2E). As in the D-GalN/LPS injury model CYLD-/- mice showed less caspase activation and liver cell damage compared to WT (Figure 2F-H).
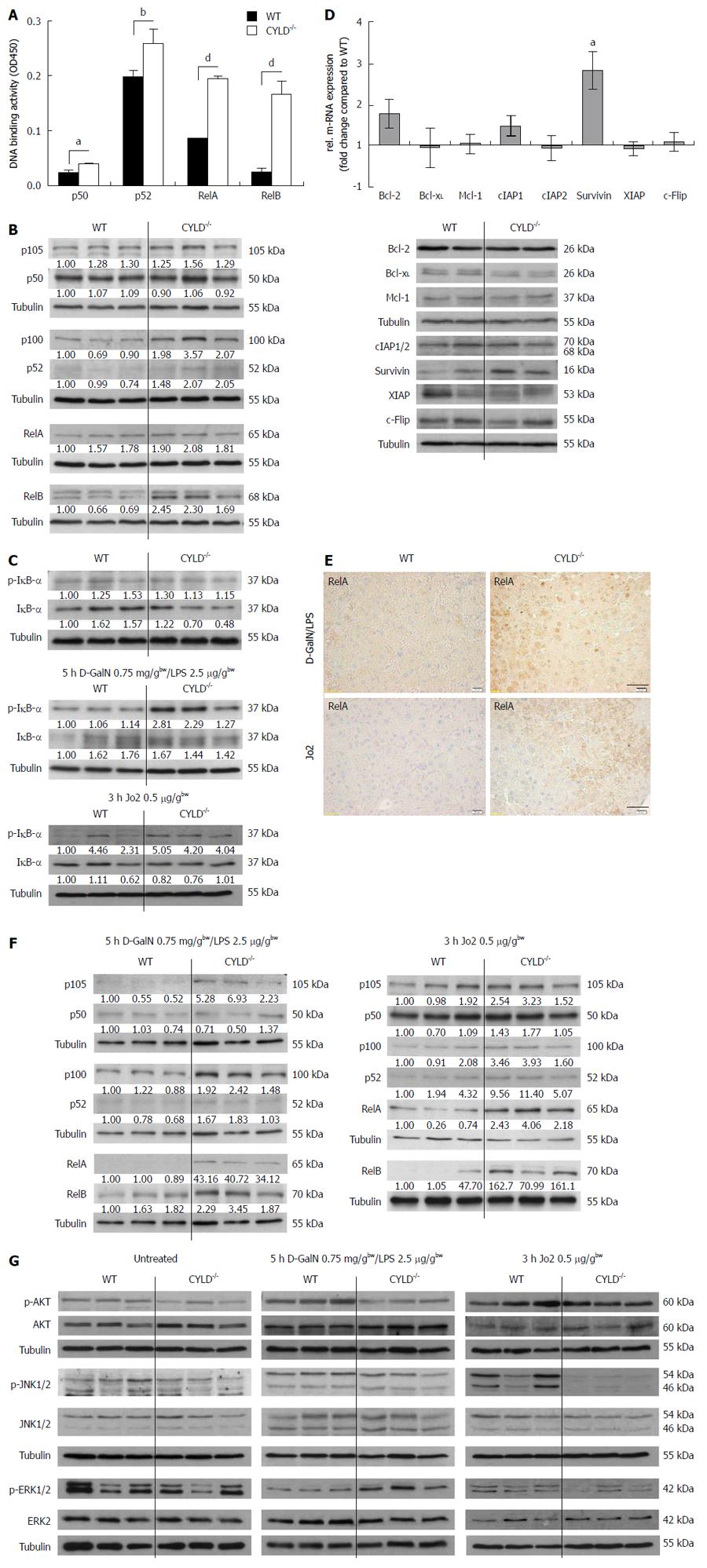
NF-κB is known as a crucial decider of life and death[18]. Because CYLD acts as a negative regulator in NF-κB signaling[6], we analyzed livers of CYLD-/- mice for altered NF-κB activity. Freshly isolated CYLD-/- primary murine hepatocytes (PMH) showed a significantly increased basal NF-κB activation level. P50 activity levels were 1.6-fold and the activity of the p50 dimerisation partner RelA was 2.3-fold increased in CYLD-/- PMH (Figure 3A).In addition, analysis of the non-canonical NF-κB pathway revealed higher basal p52 and RelB activity levels. We found a 1.3-fold increase of p52 and a 6.7-fold higher basal RelB activity in CYLD-/- compared to WT PMH (Figure 3A). Protein analysis of whole liver lysates demonstrated increased expression levels of p100 and p52. P105 and p50 were expressed equally compared to WT. RelB was most profoundly expressed in CYLD-/- compared to WT livers (Figure 3B). IκB-α phosphorylation was not substantially increased in CYLD-/- but total IκB-α levels were slightly reduced (Figure 3C, upper panel).
Q-RT PCR experiments revealed increased levels of the NF-κB inducible genes survivin: 2.8-fold and Bcl-2: 1.8-fold (P < 0.05). The mRNA-expression levels of Bcl-xL, c-IAP1/2, XIAP and c-Flip were not significantly different compared to WT (Figure 3D, upper panel).
Protein expression analysis confirmed higher expression levels of survivin. Bcl-xL was slightly lower expressed in CYLD-/- compared to WT livers. The expression of other analyzed anti-apoptotic proteins was not significantly different (Figure 3D, lower panel).
After 5 h D-GalN/LPS treatment IκB-α phosphorylation was substantially increased in CYLD-/- but IκB-α degradation was not different compared to WT livers (Figure 3C, middle panel). Immunohistochemical RelA stainings revealed highly increased nuclear RelA levels in CYLD-/- livers after D-GalN/LPS treatment (Figure 3E, upper panel). Additionally, liver lysates of D-GalN/LPS treated CYLD-/- mice showed increased expression levels of the NF-κB precursor proteins p105 and p100. However, only the active subunit p52 showed increased expression levels. RelA and RelB expression levels were increased compared to WT (Figure 3F, left panel).
Following 3 h CD95 triggering in vivo, p-IκB-α levels were increased and total IκB-α levels were slightly reduced in CYLD-/- compared to WT mice (Figure 3C, lower panel). Immunohistochemical RelA staining of liver sections substantiated the increased NF-κB activation in CYLD-/- livers after Jo2 treatment (Figure 3E, lower panel). Expression levels of NF-κB subunits (RelA, p52 and RelB) were substantially elevated after Jo2 treatment (Figure 3F, right panel).
Further analysis of signaling events relevant for hepatocyte survival showed reduced Akt and slightly reduced JNK activation in untreated livers of CYLD-/- mice. The levels of phosphorylated ERK were not different compared to WT (Figure 3G, left panel). We observed considerably lower Akt activation and increased ERK activation in livers of CYLD-/- mice after D-GalN/LPS treatment. JNK activation was slightly reduced in CYLD-/- livers compared to WT (Figure 3G, middle panel).
Interestingly, we found decreased ERK and JNK activation in CYLD-/- mice after Jo2 administration. Akt activation was not different compared to WT (Figure 3G, right panel).
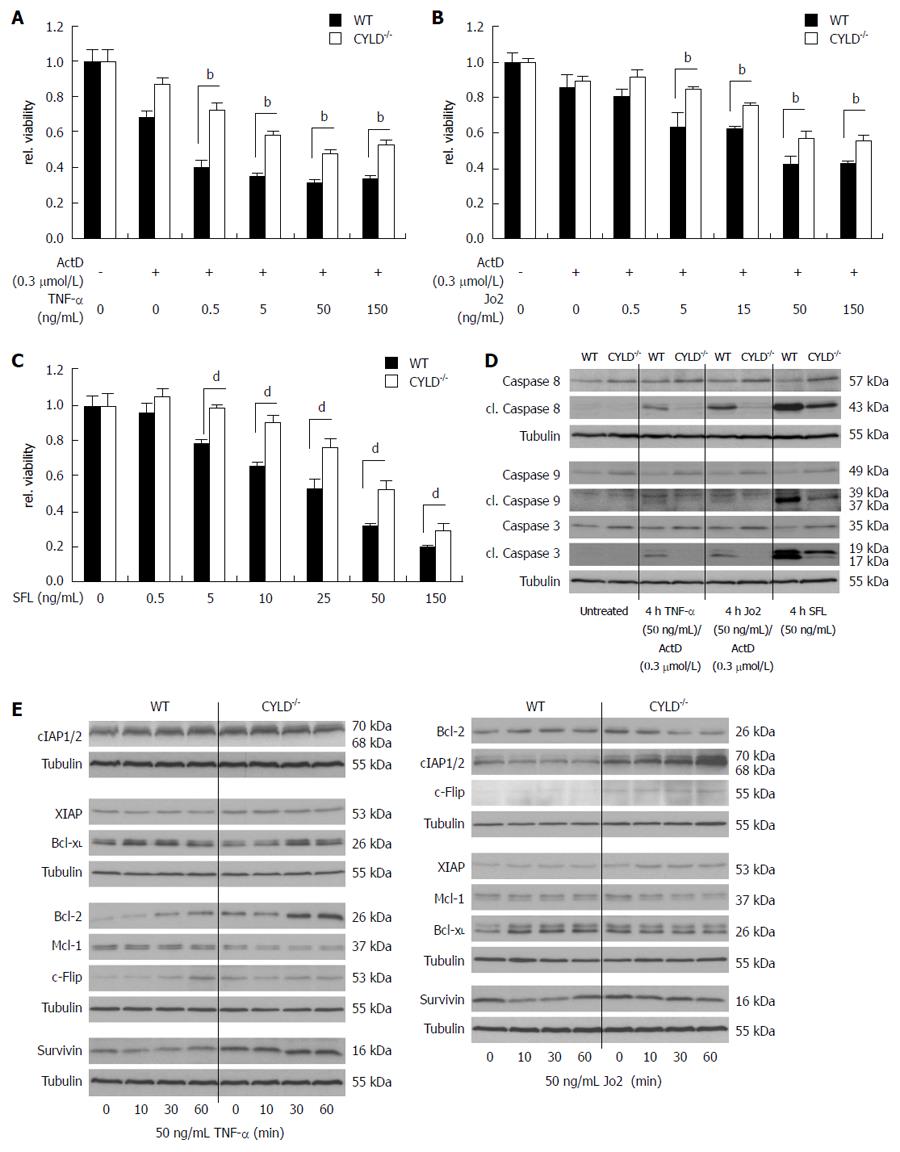
To confirm reduced apoptosis sensitivity of hepatocytes, we isolated PMH from WT and CYLD-/- mice and treated them with increasing concentrations of TNF-α and Jo2. For receptor-mediated cell death induction in vitro, co-treatment with mRNA/protein synthesis inhibitors, like actinomycin D, was necessary as described[19]. In line with the in vivo results, freshly isolated primary hepatocytes of CYLD-/- mice were less sensitive towards 24 h TNF-α and Jo2 treatment (Figure 4A and B). To induce apoptosis without the need of mRNA/protein synthesis inhibitors, FasL oligomer (SFL) was applied. Again, CYLD deficient hepatocytes were less sensitive compared to WT hepatocytes (Figure 4C). Western blot analysis of caspase activation clearly demonstrates reduced levels of cl. caspase-8, -9 and -3 in CYLD-/- hepatocytes compared to WT after treatment with TNF-α/ActD, Jo2/ActD and SFL for 4 h (Figure 4D).
To explore the role of NF-κB activation for reduced sensitivity of CYLD negative hepatocytes, we next analyzed the expression of NF-κB regulated anti-apoptotic genes after stimulation with TNF-α and Jo2. TNF-α treatment for up to 1 h showed stronger induction of Bcl-2 expression in CYLD-/- compared to WT hepatocytes. Moreover, XIAP, survivin and cIAP1/2 were increasingly expressed in CYLD-/- hepatocytes, but were not further induced by TNF-α. Interestingly, Bcl-xL and Mcl-1 expression were decreased in CYLD-/- compared to WT hepatocytes (Figure 4E, left panel).
Stimulation with Jo2 led to a pronounced induction of cIAP1/2 in CYLD-/- hepatocytes, which we could not observe in WT. XIAP and c-FLIP were increased in CYLD-/- hepatocytes. Bcl-2 and Bcl-xL expression levels were higher in WT hepatocytes after stimulation with Jo2 compared to CYLD-/- hepatocytes (Figure 4E, right panel), whereas unstimulated CYLD-/- PMH exhibited higher basal levels of Bcl-2.
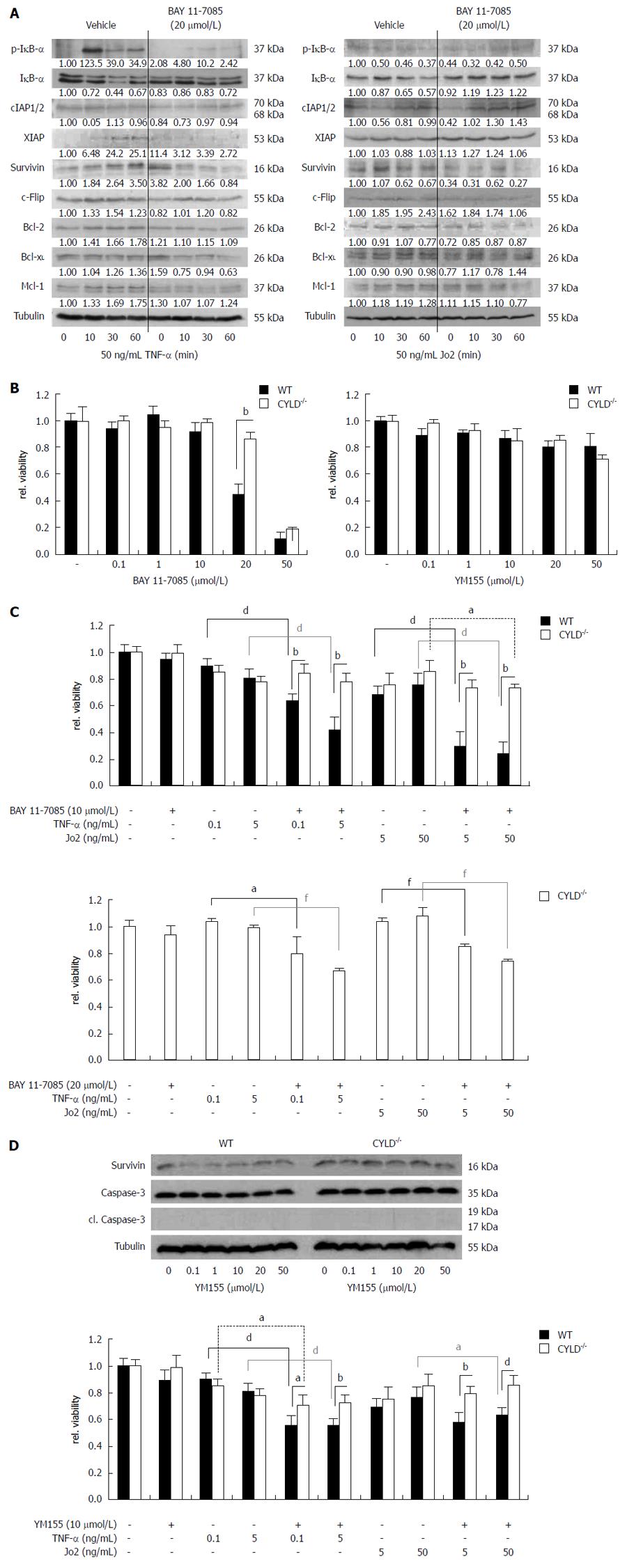
Next we examined whether inhibition of NF-κB leads to sensitization of PMH towards death receptor-induced cell death. For chemical inhibition we used BAY 11-7085, an irreversible inhibitor of IκB-α phosphorylation[20]. BAY 11-7085 efficiently blocked phosphorylation of IκB-α triggered by TNF-α treatment. Control treated CYLD-/- PMH showed strong IκB-α phosphorylation and increased degradation. Pre-incubation of CYLD-/- hepatocytes with BAY 11-7085 reduced basal and TNF-α induced expression levels of anti-apoptotic, NF-κB regulated target genes including cIAP1/2, XIAP, survivin, c-FLIP, Bcl-2, Bcl-xL and Mcl-1 (Figure 5A, left panel).
Stimulation of CYLD-/- PMH with Jo2 for up to 1h did not induce phosphorylation of IκB-α. However, after a short induction of total IκB-α expression after 10 min Jo2 treatment, which is a well known event[21], it degraded only in mock treated CYLD-/- PMH. In contrast, 4 h pre-incubation with BAY 11-7085 led to increased basal IκB-α expression levels and reduced IκB-α degradation after Jo2 treatment. BAY 11-7085 blocked basal expression and/or the induction of the anti-apoptotic proteins survivin, c-FLIP, Bcl-2, Bcl-xL and Mcl-1 after CD95 triggering. Interestingly, XIAP expression was increased after NF-κB inhibition. In line with our results from TNF-α stimulation experiments, BAY 11-7085 was capable of reducing cIAP1/2 levels in untreated CYLD-/- PMH, but did not repress increased expression after CD95 triggering (Figure 5A, right panel).
On the basis of these results we next tried to compare TNF-α and Jo2 triggered cell death induction in WT and CYLD-/- PMH in combined treatment with BAY 11-7085. Interestingly, WT PMH showed significantly higher cell death sensitivity towards BAY 11-7085 treatment alone (Figure 5B, left panel). 4 h pre-incubation with BAY 11-7085 in a non-toxic concentration (10 μmol/L) significantly sensitized WT PMH towards TNF-α (0.1, 0.5 ng/mL) and Jo2 (5, 50 ng/mL). CYLD-/- PMH could only be significantly sensitized for 50 ng/mL Jo2 treatment (Figure 5C, upper panel). Doubling BAY 11-7085 concentration to 20 μmol/L made CYLD-/- PMH also significantly sensitive for lower concentrations of the death receptor agonists (Figure 5C, lower panel).
Absence of survivin renders the liver more sensitive to Fas[22]. To elucidate the role of one distinct NF-κB regulated anti-apoptotic protein in the increased cell death resistance of CYLD negative hepatocytes, we treated WT and CYLD-/- PMH with YM155, an inhibitor of endogenous survivin expression[23]. 24 h YM155 treatment alone did not induce caspase-3 activation and did not reduce viability in concentrations up to 50 μmol/L, in both WT and CYLD-/- PMH (Figure 5B and D, right panel). YM155 caused a remarkable reduction of survivin protein levels in WT but not in CYLD-/- PMH (Figure 5D, upper panel). The combined treatment of 10 μmol/L YM155 with TNF-α or Jo2 reduced cell viability only in WT PMH (Figure 5D, lower panel).
Increased hepatocyte apoptosis via death receptors such as CD95 and TNF-R1 plays a prominent role in liver diseases[24]. Previous reports show that NF-κB activation counterbalances CD95- and TNF-R1-mediated death pathways[4]. NF-κB participates in the induction of a wide variety of cellular genes involved in immunity, inflammation and regulation of apoptosis[11,25]. Inhibition of NF-κB subunits is an interesting approach to manipulate pathophysiological processes but its inactivation can also exert a deleterious role in hepatic diseases[26]. Thus, there is a need for further basic research in understanding cell death and survival pathways to elucidate novel therapeutic targets for liver injury treatment.
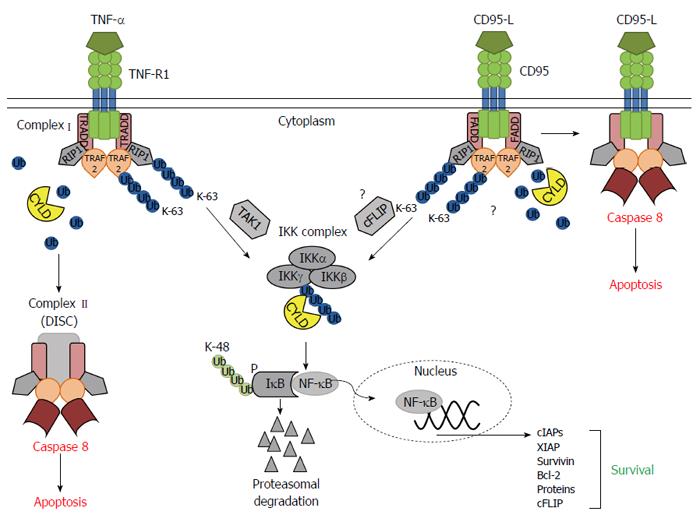
The deubiquitinase CYLD removes K-63-linked polyubiquitin chains from distinct proteins involved in NF-κB signaling[27,28]. Reduced CYLD expression was shown to increase the survival of several cell types[12,13]. Only little is known about the role of CYLD for cell death sensitivity of hepatocytes. We recently showed that mice lacking exon 7 and 8 of the CYLD gene exhibit an increased sensitivity towards chemical-induced hepatocarcinogenesis and postulated the involvement of an impaired apoptosis machinery promoting carcinogenesis in the liver[13]. In contrast, a conditional knockout of exon 9 of the CYLD gene resulted in massive apoptosis induction in hepatocytes[29]. However, in both studies on liver specific CYLD knockouts the role of the remaining splice variants remains elusive and needs to be further clarified (Figure 6).
In the present study, we show that complete deletion of CYLD protected hepatocytes from TNF-R- and CD95-mediated apoptosis via promoting anti-apoptotic NF-κB signaling. In contrast to studies on liver specific knockout models[13,29], CYLD-/- livers showed no obvious changes in liver architecture. However, the weight of CYLD-/- livers was significantly increased at the age of 9 mo. We postulate that increased liver weight of CYLD-/- mice might be a consequence of an increased life span of CYLD-/- hepatocytes by alterations in survival related signal transduction.
Accordingly to the known role of CYLD as negative regulator of NF-κB[6], we detected increased NF-κB activity in CYLD-/- hepatocytes. We next analyzed CYLD-/- livers for expression of NF-κB regulated anti-apoptotic genes. Members of the IAP protein family, such as survivin, were shown to be regulated by NF-κB. Survivin has the capacity to directly bind to and inhibit caspase-3 activation and thus, to suppress cell death[30]. Interestingly, we detected significantly increased expression of survivin in CYLD-/- livers and consequently reduced levels of cleaved caspase 3.
CYLD was additionally shown to negatively regulate Akt activation in lung injury[7] as well as JNK activation in melanoma[31]. In contrast, CYLD-/- livers showed decreased Akt and JNK activation, which points to organ specific functions of CYLD in this context and excludes Akt and JNK activation as potential anti-apoptotic mechanisms in our CYLD knockout model.
To evaluate the function of CYLD in receptor-mediated apoptosis in hepatocytes and liver injury in vivo, we administered CD95-agonistic antibodies. Hepatocytes are acutely sensitive to CD95-induced apoptosis and triggering of CD95 is an established in vivo model to induce ALF[32]. CYLD-/- mice clearly showed a decreased apoptosis sensitivity. Analysis of survival related signaling revealed unaltered Akt, slightly reduced ERK and profoundly decreased JNK activation in CYLD-/- livers. In other studies CYLD knockdown was shown to increase JNK activity by increased K-63 ubiquitination of TRAF2[33]. In contrast, it was demonstrated that CYLD was able to remove K-48 linked ubiquitin chains from TRAF2 and prevent proteolytic degradation of TRAF2[34]. This discrepancy has, thus far been unexplained but might be an explanation for the reduced JNK activity in CYLD-/- livers.
CD95 triggering is known as a weak trigger for NF-κB[18]. However, we detected increased NF-κB activation in CYLD-/- livers after Jo2 treatment indicating that loss of CYLD promotes CD95-induced NF-κB activation. Analysis of NF-κB dependent anti-apoptotic genes after CD95 triggering showed profoundly aggravated expression of IAPs and c-FLIP in CYLD-/- PMH.
To extend the analysis of the apoptosis sensitivity of CYLD deficient hepatocytes, we also applied the D-GalN/LPS model. Injection of D-GalN and LPS triggers TNF- dependent apoptosis in hepatocytes[16,35]. In line with the findings in the Jo2-model, CYLD-/- mice showed a reduced sensitivity towards TNF-R mediated apoptosis. NF-κB induction after in vivo TNF-R-triggering was even higher compared to the Jo2 model and likewise clearly increased in CYLD-/- livers compared to WT. In contrast to the Jo2 injury model, CYLD-/- livers showed increased ERK activation. ERK is known to repress CYLD via induction of the transcriptional repressor Snail in melanoma[36]. We cannot exclude that ERK activation contributes to increased survival of CYLD-/- hepatocytes after TNF-R activation in the in vivo-model.
Interestingly, in the D-GalN/LPS injury model we detected reduced Akt and JNK activation in CYLD-/- livers. While transient JNK activation may be beneficial for hepatocyte survival, sustained JNK activity can trigger cell death[37]. Inhibition of JNK decreased apoptosis sensitivity of hepatocytes towards TNF-α[38]. Thus, reduced JNK activity might be an additional survival factor for CYLD-/- hepatocytes.
Our analysis of survival signaling revealed differences between WT and CYLD-/- livers in Akt, ERK and JNK activation in respective to the applied injury models. However, in both models we consistently observed an increased NF-κB activation in CYLD-/- livers, notably at the basal level.
To exclude immunoregulatory effects, like an altered TNF-α or IL-6 release of CYLD negative immune cells, we furthermore verified our findings in vitro after isolation of PMH. Consistent with the in vivo results we detected reduced apoptosis sensitivity of CYLD-/- PMH towards TNF-α and CD95 triggering.
The anti-apoptotic activity of NF-κB depends on gene induction[18]. Comparison of the inducing potential of NF-κB regulated genes after death receptor triggering revealed impressive induction of Bcl-2 as well as increased basal expression levels of IAPs and c-FLIP in CYLD-/- PMH. Under basal conditions hepatic Bcl-2 expression is very low[39]. In CYLD-/- hepatocytes Bcl-2 could be dramatically increased by TNF-α treatment. Triggering of CD95 profoundly induced cIAP1/2 in CYLD-/- but not in WT PMH. cIAPs are known to mediate anti-apoptotic effects of NF-κB, e.g., in Jurkat cells[40]. Furthermore, it was shown that cIAP2 prevents apoptosis in rat hepatocytes[41]. Finally, our study included the application of the IκB-α phosphorylation inhibitor BAY 11-7085[20] and YM155 as an inhibitor of survivin expression[23]. BAY 11-7085 was able to re-sensitize CYLD-/- PMH towards TNF-α- and Jo2-induced cell death without co-treatment with transcription/translation inhibitors. This was accompanied by a reduction of NF-κB regulated anti-apoptotic protein expression and, additionally, by blockage of their induction after TNF-R and CD95 triggering. In addition to the anti-apoptotic NF-κB activity, we exemplarily investigated the involvement of NF-κB regulated survivin expression. Treatment with YM155 led only in WT PMH to remarkable decreased survivin levels. This raises the question about altered survivin protein stability in CYLD-/- cells and has to be addressed in future studies. Importantly, in comparison to CYLD-/-, WT PMH were much more sensitive towards co-treatment with BAY 11-7085/YM155 and TNF-α or Jo2, which underlines the relevance of anti-apoptotic NF-κB signaling for the increased resistance of CYLD-/- hepatocytes towards death receptor agonists.
Our study demonstrates for the first time that deletion of CYLD increases resistance of murine hepatocytes towards TNF-α and CD95 induced apoptosis and points to a crucial role of increased anti-apoptotic NF-κB signaling following CYLD deletion. Thus, inhibition of CYLD represents a potential approach for the treatment of acute and chronic liver injury triggered by death receptor-induced apoptosis of hepatocytes.
The tumor suppressor gene CYLD, involved in the control of nuclear factor (NF)-κB signaling, was initially identified as mutated in patients suffering from familial cylindromatosis. The product of the CYLD gene contains an ubiquitin C-terminal hydrolase domain allowing it to act as a Deubiquitinating enzyme (DUB) in removing K-63-linked poly-ubiquitin chains from distinct proteins involved in the NF-κB survival signaling pathway.
Persistent apoptosis is a feature of chronic liver diseases. Acute liver failure is characterized by massive apoptosis and is associated with life threatening consequences. Therapeutic approaches for delaying or reversing liver failure apart from orthotopic liver transplantation are rare. Understanding of the mechanisms of hepatocyte survival and cell death pathways would offer potential therapeutic targets.
The study showed for the first time that CYLD negative hepatocytes are less sensitive to CD95 and TNF-R-mediated apoptosis, at least in part via triggering NF-κB signaling leading to induction of anti-apoptotic proteins.
The identification here about the relevance of CYLD in resistance of murine hepatocytes towards CD95 and TNF-R-induced apoptosis will help to improve our understanding of the mechanisms of acute and chronic liver injury. Inhibition of CYLD might represent a therapeutic approach to protect hepatocytes from death receptor-mediated apoptosis.
This study evaluated the CYLD's function in the murine hepatocytes apoptotisis network which controlled by NF-kB. The apoptosis related factors including Bcl-2, XIAP, cIAP and survivin were assessed after hepatocyte cell death in CYLD knockout mice. Subsequently, the study speculates CYLD regulate NF-kB dependent anti-apoptotic pathway. An excellent work had been done in this study.
P- Reviewer: Dai ZJ, Kuo SM, Novo E S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH
| 1. | Malhi H, Gores GJ. Cellular and molecular mechanisms of liver injury. Gastroenterology. 2008;134:1641-1654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 437] [Cited by in RCA: 413] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 2. | Vick B, Weber A, Urbanik T, Maass T, Teufel A, Krammer PH, Opferman JT, Schuchmann M, Galle PR, Schulze-Bergkamen H. Knockout of myeloid cell leukemia-1 induces liver damage and increases apoptosis susceptibility of murine hepatocytes. Hepatology. 2009;49:627-636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 133] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 3. | Lee WM, Squires RH, Nyberg SL, Doo E, Hoofnagle JH. Acute liver failure: Summary of a workshop. Hepatology. 2008;47:1401-1415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 566] [Cited by in RCA: 513] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 4. | Zou SS, Yang W, Yan HX, Yu LX, Li YQ, Wu FQ, Tang L, Lin Y, Guo LN, Zhou HB. Role of β-Catenin in regulating the balance between TNF-α- and Fas-induced acute liver injury. Cancer Lett. 2013;335:160-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Jankowska E, Stoj J, Karpowicz P, Osmulski PA, Gaczynska M. The proteasome in health and disease. Curr Pharm Des. 2013;19:1010-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Massoumi R. CYLD: a deubiquitination enzyme with multiple roles in cancer. Future Oncol. 2011;7:285-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 7. | Lim JH, Jono H, Komatsu K, Woo CH, Lee J, Miyata M, Matsuno T, Xu X, Huang Y, Zhang W. CYLD negatively regulates transforming growth factor-β-signalling via deubiquitinating Akt. Nat Commun. 2012;3:771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 125] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 8. | Lee HH, Dadgostar H, Cheng Q, Shu J, Cheng G. NF-kappaB-mediated up-regulation of Bcl-x and Bfl-1/A1 is required for CD40 survival signaling in B lymphocytes. Proc Natl Acad Sci USA. 1999;96:9136-9141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 448] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 9. | Zong WX, Edelstein LC, Chen C, Bash J, Gélinas C. The prosurvival Bcl-2 homolog Bfl-1/A1 is a direct transcriptional target of NF-kappaB that blocks TNFalpha-induced apoptosis. Genes Dev. 1999;13:382-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 565] [Cited by in RCA: 571] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 10. | Stehlik C, de Martin R, Kumabashiri I, Schmid JA, Binder BR, Lipp J. Nuclear factor (NF)-kappaB-regulated X-chromosome-linked iap gene expression protects endothelial cells from tumor necrosis factor alpha-induced apoptosis. J Exp Med. 1998;188:211-216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 503] [Cited by in RCA: 517] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 11. | Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS. NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680-1683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2159] [Cited by in RCA: 2189] [Article Influence: 81.1] [Reference Citation Analysis (0)] |
| 12. | Hövelmeyer N, Wunderlich FT, Massoumi R, Jakobsen CG, Song J, Wörns MA, Merkwirth C, Kovalenko A, Aumailley M, Strand D. Regulation of B cell homeostasis and activation by the tumor suppressor gene CYLD. J Exp Med. 2007;204:2615-2627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 82] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Urbanik T, Boger RJ, Longerich T, Becker K, Ehrenberg KR, Hövelmeyer N, Hahn M, Schuchmann M, Jäger D, Waisman A. Liver specific deletion of CYLDexon7/8 induces severe biliary damage, fibrosis and increases hepatocarcinogenesis in mice. J Hepatol. 2012;57:995-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Massoumi R, Chmielarska K, Hennecke K, Pfeifer A, Fässler R. Cyld inhibits tumor cell proliferation by blocking Bcl-3-dependent NF-kappaB signaling. Cell. 2006;125:665-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 404] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 15. | Urbanik T, Köhler BC, Boger RJ, Wörns MA, Heeger S, Otto G, Hövelmeyer N, Galle PR, Schuchmann M, Waisman A. Down-regulation of CYLD as a trigger for NF-κB activation and a mechanism of apoptotic resistance in hepatocellular carcinoma cells. Int J Oncol. 2011;38:121-131. [PubMed] |
| 16. | Galanos C, Freudenberg MA, Reutter W. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc Natl Acad Sci USA. 1979;76:5939-5943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 720] [Cited by in RCA: 711] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 17. | Song E, Lee SK, Wang J, Ince N, Ouyang N, Min J, Chen J, Shankar P, Lieberman J. RNA interference targeting Fas protects mice from fulminant hepatitis. Nat Med. 2003;9:347-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 839] [Cited by in RCA: 813] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 18. | Karin M, Lin A. NF-kappaB at the crossroads of life and death. Nat Immunol. 2002;3:221-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2154] [Cited by in RCA: 2186] [Article Influence: 95.0] [Reference Citation Analysis (1)] |
| 19. | Alessenko AV, Boikov PYa GN, Khrenov AV, Loginov AS, Makarieva ED. Mechanisms of cycloheximide-induced apoptosis in liver cells. FEBS Lett. 1997;416:113-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Pierce JW, Schoenleber R, Jesmok G, Best J, Moore SA, Collins T, Gerritsen ME. Novel inhibitors of cytokine-induced IkappaBalpha phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J Biol Chem. 1997;272:21096-21103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 852] [Cited by in RCA: 892] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 21. | Pinna F, Sahle S, Beuke K, Bissinger M, Tuncay S, D’Alessandro LA, Gauges R, Raue A, Timmer J, Klingmüller U. A Systems Biology Study on NFκB Signaling in Primary Mouse Hepatocytes. Front Physiol. 2012;3:466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Conway EM, Pollefeyt S, Steiner-Mosonyi M, Luo W, Devriese A, Lupu F, Bono F, Leducq N, Dol F, Schaeffer P. Deficiency of survivin in transgenic mice exacerbates Fas-induced apoptosis via mitochondrial pathways. Gastroenterology. 2002;123:619-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | Coumar MS, Tsai FY, Kanwar JR, Sarvagalla S, Cheung CH. Treat cancers by targeting survivin: just a dream or future reality? Cancer Treat Rev. 2013;39:802-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 120] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 24. | Faubion WA, Gores GJ. Death receptors in liver biology and pathobiology. Hepatology. 1999;29:1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 127] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 25. | Gilmore TD, Koedood M, Piffat KA, White DW. Rel/NF-kappaB/IkappaB proteins and cancer. Oncogene. 1996;13:1367-1378. [PubMed] |
| 26. | Muriel P. NF-kappaB in liver diseases: a target for drug therapy. J Appl Toxicol. 2009;29:91-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 134] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 27. | Brummelkamp TR, Nijman SM, Dirac AM, Bernards R. Loss of the cylindromatosis tumour suppressor inhibits apoptosis by activating NF-kappaB. Nature. 2003;424:797-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 757] [Cited by in RCA: 758] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 28. | Glittenberg M, Ligoxygakis P. CYLD: a multifunctional deubiquitinase. Fly (Austin). 2007;1:330-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Nikolaou K, Tsagaratou A, Eftychi C, Kollias G, Mosialos G, Talianidis I. Inactivation of the deubiquitinase CYLD in hepatocytes causes apoptosis, inflammation, fibrosis, and cancer. Cancer Cell. 2012;21:738-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 30. | Tamm I, Wang Y, Sausville E, Scudiero DA, Vigna N, Oltersdorf T, Reed JC. IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res. 1998;58:5315-5320. [PubMed] |
| 31. | Ke H, Augustine CK, Gandham VD, Jin JY, Tyler DS, Akiyama SK, Hall RP, Zhang JY. CYLD inhibits melanoma growth and progression through suppression of the JNK/AP-1 and β1-integrin signaling pathways. J Invest Dermatol. 2013;133:221-229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 32. | Galle PR, Hofmann WJ, Walczak H, Schaller H, Otto G, Stremmel W, Krammer PH, Runkel L. Involvement of the CD95 (APO-1/Fas) receptor and ligand in liver damage. J Exp Med. 1995;182:1223-1230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 551] [Cited by in RCA: 539] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 33. | Reiley W, Zhang M, Sun SC. Negative regulation of JNK signaling by the tumor suppressor CYLD. J Biol Chem. 2004;279:55161-55167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 116] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 34. | Xue L, Igaki T, Kuranaga E, Kanda H, Miura M, Xu T. Tumor suppressor CYLD regulates JNK-induced cell death in Drosophila. Dev Cell. 2007;13:446-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 35. | Leist M, Gantner F, Bohlinger I, Tiegs G, Germann PG, Wendel A. Tumor necrosis factor-induced hepatocyte apoptosis precedes liver failure in experimental murine shock models. Am J Pathol. 1995;146:1220-1234. [PubMed] |
| 36. | Massoumi R, Kuphal S, Hellerbrand C, Haas B, Wild P, Spruss T, Pfeifer A, Fässler R, Bosserhoff AK. Down-regulation of CYLD expression by Snail promotes tumor progression in malignant melanoma. J Exp Med. 2009;206:221-232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 174] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 37. | De Smaele E, Zazzeroni F, Papa S, Nguyen DU, Jin R, Jones J, Cong R, Franzoso G. Induction of gadd45beta by NF-kappaB downregulates pro-apoptotic JNK signalling. Nature. 2001;414:308-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 590] [Cited by in RCA: 610] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 38. | Schwabe RF, Uchinami H, Qian T, Bennett BL, Lemasters JJ, Brenner DA. Differential requirement for c-Jun NH2-terminal kinase in TNFalpha- and Fas-mediated apoptosis in hepatocytes. FASEB J. 2004;18:720-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 119] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 39. | de la Coste A, Fabre M, McDonell N, Porteu A, Gilgenkrantz H, Perret C, Kahn A, Mignon A. Differential protective effects of Bcl-xL and Bcl-2 on apoptotic liver injury in transgenic mice. Am J Physiol. 1999;277:G702-G708. [PubMed] |
| 40. | Chu ZL, McKinsey TA, Liu L, Gentry JJ, Malim MH, Ballard DW. Suppression of tumor necrosis factor-induced cell death by inhibitor of apoptosis c-IAP2 is under NF-kappaB control. Proc Natl Acad Sci USA. 1997;94:10057-10062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 695] [Cited by in RCA: 727] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 41. | Schoemaker MH, Ros JE, Homan M, Trautwein C, Liston P, Poelstra K, van Goor H, Jansen PL, Moshage H. Cytokine regulation of pro- and anti-apoptotic genes in rat hepatocytes: NF-kappaB-regulated inhibitor of apoptosis protein 2 (cIAP2) prevents apoptosis. J Hepatol. 2002;36:742-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 125] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 42. | Sun SC. CYLD: a tumor suppressor deubiquitinase regulating NF-kappaB activation and diverse biological processes. Cell Death Differ. 2010;17:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 337] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 43. | Peter ME, Krammer PH. The CD95(APO-1/Fas) DISC and beyond. Cell Death Differ. 2003;10:26-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 804] [Cited by in RCA: 777] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 44. | Neumann L, Pforr C, Beaudouin J, Pappa A, Fricker N, Krammer PH, Lavrik IN, Eils R. Dynamics within the CD95 death-inducing signaling complex decide life and death of cells. Mol Syst Biol. 2010;6:352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 113] [Article Influence: 7.5] [Reference Citation Analysis (0)] |