Published online Nov 28, 2014. doi: 10.3748/wjg.v20.i44.16639
Revised: June 4, 2014
Accepted: July 11, 2014
Published online: November 28, 2014
Processing time: 280 Days and 6.3 Hours
Alcoholic liver disease (ALD) is the commonest cause of cirrhosis in many Western countries and it has a high rate of morbidity and mortality. The pathogenesis is characterized by complex interactions between metabolic intermediates of alcohol. Bacterial intestinal flora is itself responsible for production of endogenous ethanol through the fermentation of carbohydrates. The intestinal metabolism of alcohol produces a high concentration of toxic acetaldehyde that modifies gut permeability and microbiota equilibrium. Furthermore it causes direct hepatocyte damage. In patients who consume alcohol over a long period, there is a modification of gut microbiota and, in particular, an increment of Gram negative bacteria. This causes endotoxemia and hyperactivation of the immune system. Endotoxin is a constituent of Gram negative bacteria cell walls. Two types of receptors, cluster of differentiation 14 and Toll-like receptors-4, present on Kupffer cells, recognize endotoxins. Several studies have demonstrated the importance of gut-liver axis and new treatments have been studied in recent years to reduce progression of ALD modifying gut microbiota. It has focused attention on antibiotics, prebiotics, probiotics and synbiotics.
Core tip: A close anatomical and functional relationship between gut and liver exists. Blood circulated in the portal vein transfers various toxic compounds for filtration by liver. Endotoxin is a lipopolysaccharide derived from the cell wall of Gram negative bacteria presents in the intestine, which is absorbed from intestinal epithelium and transported to the liver and Kupffer cells through the portal vein. A qualitative (dysbiosis) and quantitative (bacterial overgrowth) alteration of intestinal microbiome are the causes of an increase of endotoxins and subsequently, liver damage. The new treatments try to contrast dysbiosis and bacterial overgrowth decreasing evolution of alcohol liver disease.
- Citation: Malaguarnera G, Giordano M, Nunnari G, Bertino G, Malaguarnera M. Gut microbiota in alcoholic liver disease: Pathogenetic role and therapeutic perspectives. World J Gastroenterol 2014; 20(44): 16639-16648
- URL: https://www.wjgnet.com/1007-9327/full/v20/i44/16639.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i44.16639
Alcoholic liver disease (ALD) is the cause of a high rate of morbidity and mortality worldwide. It is the commonest cause of cirrhosis in many Western countries[1]. It accounted for 3.8% of all deaths in 2004[2]. ALD consists of several types of disease such as fatty liver (steatosis), steatohepatitis, fibrosis, cirrhosis and ultimately hepatocarcinoma (HCC). Steatosis is reversible with alcohol abstention, but it is considered a risk factor for progression to fibrosis and cirrhosis[3,4].
The metabolism of alcohol is also regulated by intestinal bacteria (“bacteriocolonic” metabolism of ethanol). In 1984 Bode et al[5] demonstrated a qualitative and quantitative significant difference between flora in people with alcoholism and gut microflora of a control group. Intestinal homeostasis is influenced by several factors such as gut motility, gastric acidity, immunological defence factors, bile salts, and colonic pH[6].
The liver strategic position confers it with the important role of translating physiological and pathological processes within the gastrointestinal tract into metabolic and immunologic outcomes[7].
Alcoholic steatohepatitis (ASH) and severe ALD occur in approximately 30% of heavy drinkers[8]. The pathogenesis of ALD is a dynamic and unknown process characterized by several interactions that involve the immune system and metabolic intermediates of alcohol. The poor understanding of these interactions contrasted with the progress in developing specific treatments for ALD[9-11]. Ethanol metabolism-associated oxidative stress, abnormal methionine metabolism, ethanol-mediated induction of leakage of gut endotoxins, and activation of Kupffer cells are all involved in the pathogenesis of ALD[12-14].
The fermentation of carbohydrates made by bacterial intestinal flora is itself responsible for production of endogenous ethanol. This is strongly enhanced in the presence of gut dysmotility (e.g., from obesity, diabetes, or chronic alcohol use) or an excess of carbohydrates in the diet[15]. The intestinal oxidation of alcohol results in increasing concentrations of acetaldehyde[16,17], the first and most toxic product of ethanol metabolism responsible for alteration of intestinal permeability (gut leakiness) and microbiota homeostasis.
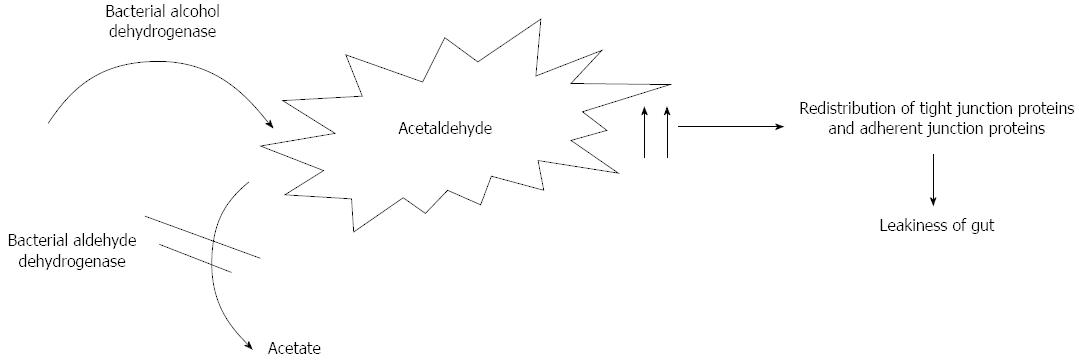
Apart from the liver, several organs contribute to ethanol metabolism resulting in acetaldehyde production, such as the pancreas, gastrointestinal tract, heart and brain[18-20]. Acetaldehyde is produced by bacterial alcohol dehydrogenase[21] and metabolised by aldehyde dehydrogenase in the colon[22]. In a recent study Kwon et al[23] evaluated the role of aldehyde dehydrogenase 2 deficiency in mouse in the progression of alcohol liver disease. They showed the role of acetaldehyde in hepatic inflammation and fibrosis.
Acetaldehyde is itself responsible for mitochondrial dysfunction and altered acetaldehyde metabolism that leads to its accumulation. It determines direct hepatocyte damage forming adducts with proteins and DNA by the interactions with amino, hydroxyl, and sulfhydryl groups[24]. Acetaldehyde is also responsible for increased paracellular intestinal permeability because of a redistribution of tight junction proteins (occluding and ZO-1) and adherent junction (E-cadherin and β-catenin) proteins inhibiting their phosphorylation by protein tyrosine phosphatase[25-27] (Figure 1).
The leakiness of gut activates the transcription of nuclear factor kappaB (NF-κB) gene and over-expression of nitric oxide (NO) synthesis.
NO is synthesized from L-arginine by nitric oxide synthases (NOS). Three isoforms of nitric oxide synthases exist: neuronal NOS (nNOS), endothelial NOS (eNOS), defined as constitutive NOS (cNOS), and inducible NOS (iNOS)[28]. NO production by cNOS is responsible for epithelial cell barrier integrity[29,30]. Otherwise NO produced by iNOS occurs in inflammation and it may contribute to aggravate integrity of the intestinal barrier[31].
iNOS is expressed in endothelial cells, hepatocytes, macrophages, neutrophils, and many other cell types[32]. An increased expression of iNOS and consequent production of NO is responsible for an augmented nitration and oxidation of tubulin. This leads to a decreased stability of tubulin and damage of the microtubule cytoskeleton with disruption of barrier function. Besides, the increased synthesis of NO results in oxidative stress in hepatocytes[33,34].
Epidermal growth factor (EGF) contrasts this process, promoting growth and differentiation of gastrointestinal mucosa. EGF stabilizes the cytoskeleton through down regulation of activity of iNOS[35,36].
The intestinal microflora changes after fetal development and the major changes occur after weaning[37]. The microbiota is composed by more than 500 species of bacteria; some of them are fixed in the intestine, while the others only pass through the intestine[38]. According to the study by Neish, 109 CFU/mL and 1012 CFU/mL of bacteria may be found, respectively, in the terminal ileum and colon. Gram negative bacteria and anaerobes are dominant species in the intestinal lumen which are estimated to be 100 to 1000 times more than aerobic ones. Bacteroides, Porphyromonas, Bifidobacterium, Lactobacillus, Clostridium and Escherichia coli (E. coli) are the most frequent ones[39]. The intestine also provides residence to more than 15 species-level bacteria phylotypes and in a healthy state they have a symbiotic relationship with its host. However, in each person, the pattern of the microorganism population is unique and different[40] (Table 1).
| Gastrointestinal tract | Microbiota |
| Oesophagus | Streptococcus, Prevotella, Veilonella |
| Stomach | Streptococcus, Staphylococcus, Lactobacillus, Helicobacter pylori |
| Duodenum | Streptococcus, Staphylococcus, Lactobacillus, Helicobacter pylori, Veilonella, Yeasts |
| Jeiunum | Streptococcus, Staphylococcus, Lactobacillus, Helicobacter pylori, Veilonella, Yeasts |
| Ileum | Bifidobacterium, Bacteroides, Veilonella, Clostridium, Enterobacteriacea |
| Colon | Bacteroides, Bifidobacterium, Clostridium, Streptococcus, Ruminococcus, Peptostreptococcus, Eubacterium, Faecalibacterium |
There is a close anatomical and functional relationship between the gut and the liver known as the gut-liver axis and in patients with liver cirrhosis, the intestinal balance is compromised.
Blood circulating in the portal vein transfers various toxic compounds such as bacteria and their derivatives (ethanol, ammonia, and acetaldehyde) for filtration by liver and modulates Kupffer cells activity and cytokine production. The increase of pathogen-associated molecular patterns and accumulation of metabolites in the liver can cause the liver harm. In return, the liver secretes bile acids to the intestine and modulates its activities[41].
Alterations in the type and amount of microorganisms are important elements in the dysfunctions of the liver; in fact liver disease causes quantitative (bacterial overgrowth) and qualitative (dysbiosis) changes in the intestinal microflora[42].
Dysbiosis is the alteration of intestinal homeostasis. Several studies have shown the role of continuous ethanol assumption in the breakdown of this balance. Bull-Otterson et al[43] studied the temporal effects of chronic ethanol consumption on commensally intestinal bacteria in a mouse model. They demonstrated that alcohol consumption over a long period elevates the growth of Gram negative bacteria and causes a decrease of both Bacteriodetes and Firmicutes, and an increase of Actinobacteria and Proteobacteria. Proteobacteria are Gram negative bacteria and include several pathogenic species such as Salmonella, Helicobacter, Vibrio and Escherichia, one of the main bacteria in the gut. Similar results were obtained by Mutlu et al[44]. They noted higher levels of Proteobacteria and lower abundance of Bacteroidetes in subjects with chronic alcohol consumption.
The breakdown of microbiota balance is responsible for different negative consequences (endotoxemia, translocation of lipopolysaccharides) that leads to hyperactivation of the immune system.
LPS is a constituent of the wall of Gram-negative bacteria[45] which induces macrophages to release proinflammatory cytokines, such as IL-1β and tumour necrosis factor (TNF)[46].
Endotoxin is a LPS, a component of the outer membrane Gram negative bacteria present in the gut. Generally only a little part of endotoxin is absorbed from the intestinal epithelial lining reaching the liver and the Kupffer cells inside the portal vein. In chronic alcohol consumption, bowel flora releases a bigger amount of endotoxins, responsible for the altered intestinal barrier and activation of the inflammatory process that leads to the progression of ALD[47], cirrhosis and HCC[48].
Hyper-permeability of the intestine following alcohol consumption leads to endotoxemia, which is filtrated by the liver and triggers the proinflammatory pathways for causing ASH.
Endotoxemia is responsible for elevated plasma levels of LPS-binding protein (LBP). The augmentation of endotoxins can prime and activate both hepatic and extra hepatic macrophages to overproduce inflammatory cytokines such as TNF-α, IL-6, IL-1 and IL-8[12].
Systemic endotoxemia and the cytokines produced in the inflammatory process increase intestinal permeability altering tight junctions. This leads to endotoxins passing into the circulation, creating a vicious cycle[49,50].
ALD also results in quantitative alterations of the intestinal microbioma. The small intestinal bacterial overgrowth (SIBO) is another cause of bacterial translocation.
To make a diagnosis of SIBO, it is necessary to find ≥ 1 × 103 bacteria (i.e., CFU) per mL of proximal jejunal aspiration[51]. Bacterial overgrowth is advantaged by intestinal stasis that permits the proliferation of coliform bacteria[52]. Therefore, the bacteria generally recognized as SIBO are gram negative aerobes and anaerobes such as E. coli, Enterococcus spp. and Proteus mirabilis[53,54]. The main causes of SIBO are gastric achlorhydria, gastrocolic or coloenteric fistula and small intestine motility disorder[52]. Ethanol decreases intestinal motility which favours proliferation of luminal bacteria[16].
Chronic ethanol consumption has been associated with immune suppression and increased morbidity and mortality[55]. Alcohol ingestion alters both the innate and adaptive immune system.
Ethanol increases the susceptibility of the gastrointestinal tract to bacteria through the suppression of natural killer cell activity and antibody-dependent cell-mediated cytotoxicity by lymphocytes[56,57].
The host immune system has an important role in the defence of the intestine. Several molecules are responsible for the limited expansion of pathogenic microorganisms, such as reactive oxygen species, IgA, β-defensins and cryptidins[39,58]. The innate immune system is also composed by Toll-like receptors (TLRs) that recognize specific pathogen-associated molecular patterns (PAMPs) such as LPS, lipoteichoic acid, peptidoglycan, unmethylated DNA and double-stranded RNA[59].
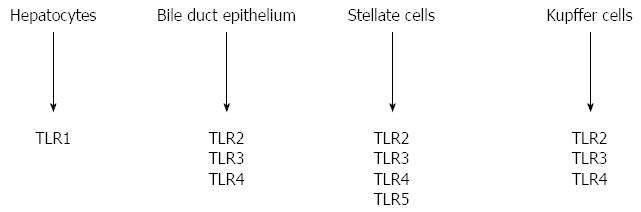
In humans 10 TLRs have been recognized. Multiple cells in the liver express significant levels of multiple TLRs and have long been recognized to be critical determinants in the pathogenesis of cirrhosis[60,61]. Every type of liver cell expresses specific TLR: TLR1 was found in hepatocytes, TLR2, 3, and 4 in stellate cells, bile duct epithelium and particularly in Kupffer cells. Bile duct epithelium expresses TLR5 too (Figure 2).
Endotoxins produced in the body cause an inflammatory reaction, activating Kupffer cells through their link with two types of receptors, cluster of differentiation 14 (CD-14) and TLR-4. These receptors are both essential to determine liver injury, but they present different structures. CD-14 is a surface receptor without a cytoplasmic domain, while TLR4 is a transmembrane protein with a cytoplasmic domain that can be associated with a soluble protein, MD-2, through a not covalently link.
CD14 binds LPS and this complex is recognized by TLR4. CD14 also has a soluble form that facilitates the transfer of LPS to the TLR4/MD-2 receptor complex[62]. The association between LPS and CD14 is facilitated by a soluble shuttle protein, LPS-binding protein (LBP)[63].
LPS recognition by TLR4 on macrophages and other cell types in the liver determines activation of downstream signaling pathways responsible for activation of transcription factors such as NF-κB and activator protein-1 (AP-1). This process causes an increased inflammatory cytokine production such as interferon gamma (IFNγ), TNF-α, interleukin-6 (IL-6), IL-1, chemokines and reactive oxygen species[64,65].
Furthermore LPS/TLR4 promotes fibrogenesis by sensitizing hepatic stellate cells (HSCs). The sensitised HSCs induce NF-κB activation, up-regulate gene expression of some chemokines (IL-8 and monocyte chemoattractant protein-1) and promote transforming growth factor beta (TGFβ) release by Kupffer cells[66]. The activated TLRs can enroll adapter molecules like myeloid differentiation factor-88 (MyD88)[67].
The CD14/TLR4 receptor complexes activate MyD88 dependent and MyD88 independent pathways that modulate survival and replication of apoptosis cells[68]. Furthermore, the MyD88-signaling pathway leads to production of oxidative stress and pro-inflammatory cytokines that causes hepatocellular damage[69-71].
The effects of alcohol are exerted on organs different from liver too. Blanco et al[69,70] demonstrated the role of ethanol in neuroinflammation. They showed that ethanol can directly induce downstream iNOS expression and activation of NF-κB through the translocation of TLR4 into lipid rafts. The activation of NF-signalling is also determined by acetaldehyde[71].
Abstinence from alcohol is the foundation for treatment of alcoholic liver diseases. In every stage of liver damage, the cessation or marked reduction in alcohol consumption has been demonstrated to improve the histology and/or survival of patients[72].
In patients with elevated alcohol ingestion, high levels of plasma endotoxin may be determined by: (1) excessive production of endotoxin in the intestine through overgrowth of intestinal bacteria; (2) gut permeability; and (3) delayed clearance of endotoxin by Kupffer cells. Actually, the main treatments, such as antibiotics, prebiotics, probiotics and synbiotics, try to prevent endotoxemia by inhibiting the intestinal Gram negative overgrowth and preserving intestinal permeability.
Acute and chronic ingestion of alcohol causes an increased endotoxin plasma level in humans and mice models[73,74]. Alcohol consumption causes changes in gut microbiota and it is associated with upper gastrointestinal bacterial overgrowth[75,76].
The antibiotic treatment controls large bowel bacterial overgrowth improving the prognosis of ALD[77]. However, despite improvement of liver function, prolonged use of antibiotics alters gut flora and this may favour pathogenic bacterial colonization.
Antibiotic treatment should be based on bacterial sensitivity testing to particular antibiotics, but this will require an excessive use of culture therefore it should be targeted at those intestinal bacteria generally responsible for SIBO[78,79].
Several antibiotics are considered suitable against overgrowth of Gram negative aerobes and anaerobes such as rifaximin, amoxicillin/clavulanate, metronidazole, ciprofloxacin, norfloxacin, and cephalexin. The fundamental role of rifaximin in the treatment of hepatic encephalopathy has been recently demonstrated, but it seems to have a role in treatment of ALD too[80].
The main advantage of rifaximin is that it is not absorbed and therefore presents few side effects. Furthermore there is little evidence for resistance[57,81-83].
Antibiotics produce quantitative alterations of intestinal microflora whereas prebiotics act against dysbiosis. The prebiotics promote selectively the growth of protective gut bacteria (Bifidobacteria and Lactobacilli) increasing the body’s natural resistance to invading pathogens[84].
Prebiotics are identified as “non digestible food ingredients that, when consumed in sufficient amounts, selective stimulate the growth and/or activity of one or a limited number of microbes in the colon, resulting in documented health benefits”[85].
They are complex carbohydrates that reach the small bowel because they cannot be metabolized by pancreatic and intestinal enzymes in gastrointestinal tract[86]. All prebiotics are resistant to gastric acidity but are susceptible to the metabolism by gut microbiota. While probiotics show strain specific beneficial effects, prebiotics of the same family present similar properties, though their degree of polymerisation distribution linkage type may differ[87].
The most commonly commercialized prebiotics are lactulose, fructo-oligosaccharides (FOS) and galacto-oligo-saccharides (GOS). GOS are nondigestible oligosaccharides derived from lactose, chains of galactose monomers that are naturally found in human milk. GOS, like other prebiotics, simulate pathogen binding sites present on the surface of gastrointestinal epithelial cells inhibiting enteric pathogen adhesion and successive infection[86,88-91].
FOS is naturally present in vegetables such as onions, asparagus, wheat, artichokes etc. They modulate gut microbiota, prevent pathogens adhesion and colonization, induce anti-inflammatory effects and regulate lipid and glucose metabolism. These prebiotics can exercise these effects thanks to their structural resistance to mammalian digestive enzymes.
Actually probiotics are defined as “monocultures or mixed culture of live microorganisms that, if administered to a person, positively influence the host by improving the properties of his/her own microflora”. Probiotics modulate intestinal microbiota, favouring an anti-inflammatory milieu that contrast bacterial translocation, endotoxin production and improve intestinal barrier integrity.
The mechanisms by which probiotics exert their effects are largely unknown. Different actions have been reported in literature. They control inflammation reducing gut pH and compete with pathogens for binding and receptor sites[92-94]. To do this, they have to show specific characteristics, in particular they should be resistant to bile, hydrochloric and pancreatic juice in order to reach the small bowel (Table 2). Tolerating stomach and duodenum conditions, probiotics can stimulate the immune system and improve intestinal function via adherence and colonization of the intestinal epithelium.
| Properties of ideal probiotic strains |
| Resistance to bile |
| Resistance to hydrochloric acid |
| Resistance to pancreatic juice |
| Ability to tolerate stomach and duodenum conditions and gastric transport |
| Stimulation of the immune system |
| Improvement of intestinal function via adhering and colonizing the intestinal epithelium |
| Competition with pathogens |
| Modulation of permeability |
| Anticarcinogenic and antipathogenic activity |
The most common probiotics are lactose-fermenting Lactobacilli and Bifidobacteria. Lactobacillus strains, LAP5 and LF33 exert their effects by inhibiting the growth of E. coli and Salmonella typhimurium in vitro[95]. Furthermore, other studies have demonstrated that Lactobacillus acidophilus strain NP51 reduces the number of E. coli O157:H7 in the fecal samples of beef cattle[96,97]. Bifidobacterium animalis MB5 and Lactobacillus rhamnosus GG protect intestinal cells from the inflammation caused by E. coli[98]. Finally it has been demonstrated that Lactobacillus GG, administered to rats, reduced plasma levels of endotoxin and severity of liver injury[99].
They have been reported to stabilize mucosal barrier function and modulate the gut microflora, limiting the growth of pathogenic bacteria, by acidifying the gut lumen, competing for nutrients, and producing antimicrobial substances[100-103].
Developing nutritional practices, mucosal barrier repairing, apoptosis prevention due to providing of short chain acids, and improving intestinal epithelial viability are other probiotic effects which stabilize physiological luminal permeability together with lowering ammonia adsorption[104]. These functions alleviate tight junction disturbance by pathogens[105], and are essential agents for lowering bacterial translocation. BT is also affected by probiotics because of their induction of anaerobes and gram positive bacteria growth, limiting gram negative bacteria, and preventing pathogen adherence[101].
Controlling flora bacteria quantity can lead to decreased endotoxins and other toxic compounds derived from bacteria such as ethanol, phenol, indoles which cause injury to the liver. Decreased levels of these substances in the liver result in lowering of proinflammatory production such as TNF-α, IL-6, and IFNγvia down-regulation of NF-κB[106]. On the other hand, they can depress urease activity of microflora bacteria followed by ammonia production and release into the portal system. Furthermore, probiotics decrease fecal pH value and reduce ammonia adsorption[107]. Therefore, probiotics determine an improvement in hepatic encephalopathy through a reduction of bacterial ammonia reaching the portal vein.
In 2010 Foster et al[108] demonstrated that probiotics effects on mental status were maintained during the wash out period.
The synbiotic is a compound of probiotics and prebiotics that exercises its beneficial effects stimulating the growth of protective intestinal bacteria[102,103,109].
Prebiotics stimulate the growth of beneficial bacteria (i.e., Bifidobacteria and Lactobacilli) in the gut and their effectiveness increases when they are used in association with probiotics[110,111]. On the other hand, the mixture of prebiotics and probiotics might enhance the survival and activity of probotics.
Several studies have demonstrated the central role of microbiota in the pathogenesis and development of liver disease[65,112]. For this reason therapeutic strategies to control ALD are focussed on the gut microbiome. Obviously the beneficial effects of probiotics depend upon a number of factors such as the duration, frequency and quantity of probiotics consumption and the health of the patients at the beginning of the treatment.
Probiotics have been shown to have several beneficial effects on intestinal function. They prolong remission in ulcerative colitis, maintaining and improving intestinal barrier integrity and stimulate mucosal immunity[113-116].
The treatment could prevent alcohol-induced gut leakiness and development of AHS and the possible mechanisms are the reduction of alcohol-induced intestinal and systemic oxidative stress. Actually the beneficial effects of probiotics in ALD are supported by numerous laboratory results and several studies have shown their potential, however, despite their demonstrated effects on intestinal barrier integrity, there is no high-quality clinical evidence. This is an important limit that does not always permit recommendation of the use of probiotics in clinical practice[117,118].
P- Reviewer: Breitkopf-Heinlein K, Gao B, Halsted CH, Spahr L S- Editor: Ding Y L- Editor: O’Neill M E- Editor: Zhang DN
| 1. | Lieber CS. Aetiology and pathogenesis of alcoholic liver disease. Baillieres Clin Gastroenterol. 1993;7:581-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373:2223-2233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2298] [Cited by in RCA: 2324] [Article Influence: 145.3] [Reference Citation Analysis (0)] |
| 3. | Lieber CS. Hyperuricemia induced by alcohol. Arthritis Rheum. 1965;8:786-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 22] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Teli MR, Day CP, Burt AD, Bennett MK, James OF. Determinants of progression to cirrhosis or fibrosis in pure alcoholic fatty liver. Lancet. 1995;346:987-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 320] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 5. | Bode JC, Bode C, Heidelbach R, Dürr HK, Martini GA. Jejunal microflora in patients with chronic alcohol abuse. Hepatogastroenterology. 1984;31:30-34. [PubMed] |
| 6. | Fooks LJ, Gibson GR. Probiotics as modulators of the gut flora. Br J Nutr. 2002;88 Suppl 1:S39-S49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 267] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 7. | Henao-Mejia J, Elinav E, Thaiss CA, Licona-Limon P, Flavell RA. Role of the intestinal microbiome in liver disease. J Autoimmun. 2013;46:66-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 129] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 8. | Gramenzi A, Caputo F, Biselli M, Kuria F, Loggi E, Andreone P, Bernardi M. Review article: alcoholic liver disease--pathophysiological aspects and risk factors. Aliment Pharmacol Ther. 2006;24:1151-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 125] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 9. | Lieber CS. Alcoholic liver disease: new insights in pathogenesis lead to new treatments. J Hepatol. 2000;32:113-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 165] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 10. | Beier JI, McClain CJ. Mechanisms and cell signaling in alcoholic liver disease. Biol Chem. 2010;391:1249-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 11. | Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1244] [Cited by in RCA: 1496] [Article Influence: 106.9] [Reference Citation Analysis (0)] |
| 12. | Thurman RG. II. Alcoholic liver injury involves activation of Kupffer cells by endotoxin. Am J Physiol. 1998;275:G605-G611. [PubMed] |
| 13. | Hoek JB, Pastorino JG. Ethanol, oxidative stress, and cytokine-induced liver cell injury. Alcohol. 2002;27:63-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 335] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 14. | Tsukamoto H, Takei Y, McClain CJ, Joshi-Barve S, Hill D, Schmidt J, Deaciuc I, Barve S, Colell A, Garcia-Ruiz C. How is the liver primed or sensitized for alcoholic liver disease? Alcohol Clin Exp Res. 2001;25:171S-181S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Zhu L, Baker SS, Gill C, Liu W, Alkhouri R, Baker RD, Gill SR. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology. 2013;57:601-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1015] [Cited by in RCA: 1282] [Article Influence: 106.8] [Reference Citation Analysis (1)] |
| 16. | Bode C, Bode JC. Effect of alcohol consumption on the gut. Best Pract Res Clin Gastroenterol. 2003;17:575-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 261] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 17. | Visapää JP, Tillonen J, Salaspuro M. Microbes and mucosa in the regulation of intracolonic acetaldehyde concentration during ethanol challenge. Alcohol Alcohol. 2002;37:322-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Zakhari S. Overview: how is alcohol metabolized by the body? Alcohol Res Health. 2006;29:245-254. [PubMed] |
| 19. | Vonlaufen A, Wilson JS, Pirola RC, Apte MV. Role of alcohol metabolism in chronic pancreatitis. Alcohol Res Health. 2007;30:48-54. [PubMed] |
| 20. | Edenberg HJ. The genetics of alcohol metabolism: role of alcohol dehydrogenase and aldehyde dehydrogenase variants. Alcohol Res Health. 2007;30:5-13. [PubMed] |
| 21. | Seitz HK, Oneta CM. Gastrointestinal alcohol dehydrogenase. Nutr Rev. 1998;56:52-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Nosova T, Jokelainen K, Kaihovaara P, Heine R, Jousimies-Somer H, Salaspuro M. Characteristics of aldehyde dehydrogenases of certain aerobic bacteria representing human colonic flora. Alcohol Alcohol. 1998;33:273-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Kwon HJ, Won YS, Park O, Chang B, Duryee MJ, Thiele GE, Matsumoto A, Singh S, Abdelmegeed MA, Song BJ. Aldehyde dehydrogenase 2 deficiency ameliorates alcoholic fatty liver but worsens liver inflammation and fibrosis in mice. Hepatology. 2014;60:146-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 153] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 24. | Lieber CS. Liver disease of the alcoholic: pathogenesis and metabolic complications. Ala J Med Sci. 1975;12:355-360. [PubMed] |
| 25. | Atkinson KJ, Rao RK. Role of protein tyrosine phosphorylation in acetaldehyde-induced disruption of epithelial tight junctions. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1280-G1288. [PubMed] |
| 26. | Seth A, Basuroy S, Sheth P, Rao RK. L-Glutamine ameliorates acetaldehyde-induced increase in paracellular permeability in Caco-2 cell monolayer. Am J Physiol Gastrointest Liver Physiol. 2004;287:G510-G517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 97] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 27. | Sheth P, Seth A, Atkinson KJ, Gheyi T, Kale G, Giorgianni F, Desiderio DM, Li C, Naren A, Rao R. Acetaldehyde dissociates the PTP1B-E-cadherin-beta-catenin complex in Caco-2 cell monolayers by a phosphorylation-dependent mechanism. Biochem J. 2007;402:291-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 75] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 28. | Kolios G, Valatas V, Ward SG. Nitric oxide in inflammatory bowel disease: a universal messenger in an unsolved puzzle. Immunology. 2004;113:427-437. [PubMed] |
| 29. | Takahashi S, Mendelsohn ME. Synergistic activation of endothelial nitric-oxide synthase (eNOS) by HSP90 and Akt: calcium-independent eNOS activation involves formation of an HSP90-Akt-CaM-bound eNOS complex. J Biol Chem. 2003;278:30821-30827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 157] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 30. | Vallance BA, Dijkstra G, Qiu B, van der Waaij LA, van Goor H, Jansen PL, Mashimo H, Collins SM. Relative contributions of NOS isoforms during experimental colitis: endothelial-derived NOS maintains mucosal integrity. Am J Physiol Gastrointest Liver Physiol. 2004;287:G865-G874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 31. | Kimura H, Nakajima T, Kagawa K, Deguchi T, Kakusui M, Katagishi T, Okanoue T, Kashima K, Ashihara T. Angiogenesis in hepatocellular carcinoma as evaluated by CD34 immunohistochemistry. Liver. 1998;18:14-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 80] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 32. | Lala PK, Orucevic A. Role of nitric oxide in tumor progression: lessons from experimental tumors. Cancer Metastasis Rev. 1998;17:91-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 33. | Banan A, Fields JZ, Decker H, Zhang Y, Keshavarzian A. Nitric oxide and its metabolites mediate ethanol-induced microtubule disruption and intestinal barrier dysfunction. J Pharmacol Exp Ther. 2000;294:997-1008. [PubMed] |
| 34. | Banan A, Keshavarzian A, Zhang L, Shaikh M, Forsyth CB, Tang Y, Fields JZ. NF-kappaB activation as a key mechanism in ethanol-induced disruption of the F-actin cytoskeleton and monolayer barrier integrity in intestinal epithelium. Alcohol. 2007;41:447-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 35. | Banan A, Farhadi A, Fields JZ, Zhang LJ, Shaikh M, Keshavarzian A. The delta-isoform of protein kinase C causes inducible nitric-oxide synthase and nitric oxide up-regulation: key mechanism for oxidant-induced carbonylation, nitration, and disassembly of the microtubule cytoskeleton and hyperpermeability of barrier of intestinal epithelia. J Pharmacol Exp Ther. 2003;305:482-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Banan A, Zhang LJ, Shaikh M, Fields JZ, Farhadi A, Keshavarzian A. Inhibition of oxidant-induced nuclear factor-kappaB activation and inhibitory-kappaBalpha degradation and instability of F-actin cytoskeletal dynamics and barrier function by epidermal growth factor: key role of phospholipase-gamma isoform. J Pharmacol Exp Ther. 2004;309:356-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 37. | Mackie RI, Sghir A, Gaskins HR. Developmental microbial ecology of the neonatal gastrointestinal tract. Am J Clin Nutr. 1999;69:1035S-1045S. [PubMed] |
| 38. | Saavedra JM, Tschernia A. Human studies with probiotics and prebiotics: clinical implications. Br J Nutr. 2002;87 Suppl 2:S241-S246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 81] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 39. | Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology. 2009;136:65-80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1071] [Cited by in RCA: 921] [Article Influence: 57.6] [Reference Citation Analysis (0)] |
| 40. | Guarner F, Malagelada JR. Gut flora in health and disease. Lancet. 2003;361:512-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2194] [Cited by in RCA: 2114] [Article Influence: 96.1] [Reference Citation Analysis (0)] |
| 41. | Cesaro C, Tiso A, Del Prete A, Cariello R, Tuccillo C, Cotticelli G, Del Vecchio Blanco C, Loguercio C. Gut microbiota and probiotics in chronic liver diseases. Dig Liver Dis. 2011;43:431-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 127] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 42. | Fouts DE, Torralba M, Nelson KE, Brenner DA, Schnabl B. Bacterial translocation and changes in the intestinal microbiome in mouse models of liver disease. J Hepatol. 2012;56:1283-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 252] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 43. | Bull-Otterson L, Feng W, Kirpich I, Wang Y, Qin X, Liu Y, Gobejishvili L, Joshi-Barve S, Ayvaz T, Petrosino J. Metagenomic analyses of alcohol induced pathogenic alterations in the intestinal microbiome and the effect of Lactobacillus rhamnosus GG treatment. PLoS One. 2013;8:e53028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 337] [Cited by in RCA: 427] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 44. | Mutlu EA, Gillevet PM, Rangwala H, Sikaroodi M, Naqvi A, Engen PA, Kwasny M, Lau CK, Keshavarzian A. Colonic microbiome is altered in alcoholism. Am J Physiol Gastrointest Liver Physiol. 2012;302:G966-G978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 600] [Cited by in RCA: 595] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 45. | Fadl AA, Sha J, Klimpel GR, Olano JP, Niesel DW, Chopra AK. Murein lipoprotein is a critical outer membrane component involved in Salmonella enterica serovar typhimurium systemic infection. Infect Immun. 2005;73:1081-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 46. | Fleming S, Toratani S, Shea-Donohue T, Kashiwabara Y, Vogel SN, Metcalf ES. Pro- and anti-inflammatory gene expression in the murine small intestine and liver after chronic exposure to alcohol. Alcohol Clin Exp Res. 2001;25:579-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 47. | Forsyth CB, Farhadi A, Jakate SM, Tang Y, Shaikh M, Keshavarzian A. Lactobacillus GG treatment ameliorates alcohol-induced intestinal oxidative stress, gut leakiness, and liver injury in a rat model of alcoholic steatohepatitis. Alcohol. 2009;43:163-172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 333] [Cited by in RCA: 321] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 48. | Gratz SW, Mykkanen H, El-Nezami HS. Probiotics and gut health: a special focus on liver diseases. World J Gastroenterol. 2010;16:403-410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 76] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 49. | Ma TY, Iwamoto GK, Hoa NT, Akotia V, Pedram A, Boivin MA, Said HM. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am J Physiol Gastrointest Liver Physiol. 2004;286:G367-G376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 613] [Cited by in RCA: 714] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 50. | Rao RK, Seth A, Sheth P. Recent Advances in Alcoholic Liver Disease I. Role of intestinal permeability and endotoxemia in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2004;286:G881-G884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 233] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 51. | Khoshini R, Dai SC, Lezcano S, Pimentel M. A systematic review of diagnostic tests for small intestinal bacterial overgrowth. Dig Dis Sci. 2008;53:1443-1454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 203] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 52. | Sachdev AH, Pimentel M. Gastrointestinal bacterial overgrowth: pathogenesis and clinical significance. Ther Adv Chronic Dis. 2013;4:223-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 131] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 53. | Bouhnik Y, Alain S, Attar A, Flourié B, Raskine L, Sanson-Le Pors MJ, Rambaud JC. Bacterial populations contaminating the upper gut in patients with small intestinal bacterial overgrowth syndrome. Am J Gastroenterol. 1999;94:1327-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 121] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 54. | Frissora CL, Cash BD. Review article: the role of antibiotics vs. conventional pharmacotherapy in treating symptoms of irritable bowel syndrome. Aliment Pharmacol Ther. 2007;25:1271-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 55. | Nelson S, Kolls JK. Alcohol, host defence and society. Nat Rev Immunol. 2002;2:205-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 184] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 56. | Jerrells TR, Peritt D, Marietta C, Eckardt MJ. Mechanisms of suppression of cellular immunity induced by ethanol. Alcohol Clin Exp Res. 1989;13:490-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 68] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 57. | Sibley D, Jerrells TR. Alcohol consumption by C57BL/6 mice is associated with depletion of lymphoid cells from the gut-associated lymphoid tissues and altered resistance to oral infections with Salmonella typhimurium. J Infect Dis. 2000;182:482-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 58. | Frank DN, Pace NR. Gastrointestinal microbiology enters the metagenomics era. Curr Opin Gastroenterol. 2008;24:4-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 258] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 59. | Akira S. TLR signaling. Curr Top Microbiol Immunol. 2006;311:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 259] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 60. | Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13:816-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1429] [Cited by in RCA: 1554] [Article Influence: 81.8] [Reference Citation Analysis (0)] |
| 61. | Miyake Y, Yamamoto K. Role of gut microbiota in liver diseases. Hepatol Res. 2013;43:139-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 62. | Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2839] [Cited by in RCA: 2908] [Article Influence: 83.1] [Reference Citation Analysis (0)] |
| 63. | Wright SD, Tobias PS, Ulevitch RJ, Ramos RA. Lipopolysaccharide (LPS) binding protein opsonizes LPS-bearing particles for recognition by a novel receptor on macrophages. J Exp Med. 1989;170:1231-1241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 227] [Cited by in RCA: 240] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 64. | Oliva J, Bardag-Gorce F, Li J, French BA, French SW. S-adenosylmethionine prevents the up regulation of Toll-like receptor (TLR) signaling caused by chronic ethanol feeding in rats. Exp Mol Pathol. 2011;90:239-243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 65. | Seki E, Schnabl B. Role of innate immunity and the microbiota in liver fibrosis: crosstalk between the liver and gut. J Physiol. 2012;590:447-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 340] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 66. | Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, Schwabe RF. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1361] [Cited by in RCA: 1556] [Article Influence: 86.4] [Reference Citation Analysis (1)] |
| 67. | Adams S. Toll-like receptor agonists in cancer therapy. Immunotherapy. 2009;1:949-964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 247] [Cited by in RCA: 229] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 68. | Inokuchi S, Tsukamoto H, Park E, Liu ZX, Brenner DA, Seki E. Toll-like receptor 4 mediates alcohol-induced steatohepatitis through bone marrow-derived and endogenous liver cells in mice. Alcohol Clin Exp Res. 2011;35:1509-1518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 69. | Blanco AM, Perez-Arago A, Fernandez-Lizarbe S, Guerri C. Ethanol mimics ligand-mediated activation and endocytosis of IL-1RI/TLR4 receptors via lipid rafts caveolae in astroglial cells. J Neurochem. 2008;106:625-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 70. | Blanco AM, Vallés SL, Pascual M, Guerri C. Involvement of TLR4/type I IL-1 receptor signaling in the induction of inflammatory mediators and cell death induced by ethanol in cultured astrocytes. J Immunol. 2005;175:6893-6899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 206] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 71. | Shen Z, Ajmo JM, Rogers CQ, Liang X, Le L, Murr MM, Peng Y, You M. Role of SIRT1 in regulation of LPS- or two ethanol metabolites-induced TNF-alpha production in cultured macrophage cell lines. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1047-G1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 149] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 72. | Parés A, Caballería J, Bruguera M, Torres M, Rodés J. Histological course of alcoholic hepatitis. Influence of abstinence, sex and extent of hepatic damage. J Hepatol. 1986;2:33-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 189] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 73. | Mathurin P, Deng QG, Keshavarzian A, Choudhary S, Holmes EW, Tsukamoto H. Exacerbation of alcoholic liver injury by enteral endotoxin in rats. Hepatology. 2000;32:1008-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 230] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 74. | Parlesak A, Schäfer C, Schütz T, Bode JC, Bode C. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J Hepatol. 2000;32:742-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 492] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 75. | Purohit V, Bode JC, Bode C, Brenner DA, Choudhry MA, Hamilton F, Kang YJ, Keshavarzian A, Rao R, Sartor RB. Alcohol, intestinal bacterial growth, intestinal permeability to endotoxin, and medical consequences: summary of a symposium. Alcohol. 2008;42:349-361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 251] [Cited by in RCA: 251] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 76. | Järveläinen HA, Fang C, Ingelman-Sundberg M, Lindros KO. Effect of chronic coadministration of endotoxin and ethanol on rat liver pathology and proinflammatory and anti-inflammatory cytokines. Hepatology. 1999;29:1503-1510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 92] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 77. | Bergheim I, Weber S, Vos M, Krämer S, Volynets V, Kaserouni S, McClain CJ, Bischoff SC. Antibiotics protect against fructose-induced hepatic lipid accumulation in mice: role of endotoxin. J Hepatol. 2008;48:983-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 402] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 78. | Singh VV, Toskes PP. Small Bowel Bacterial Overgrowth: Presentation, Diagnosis, and Treatment. Curr Treat Options Gastroenterol. 2004;7:19-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 79. | Quigley EM, Abu-Shanab A. Small intestinal bacterial overgrowth. Infect Dis Clin North Am. 2010;24:943-59, viii-ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 80. | Bass NM, Mullen KD, Sanyal A, Poordad F, Neff G, Leevy CB, Sigal S, Sheikh MY, Beavers K, Frederick T. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:1071-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 868] [Cited by in RCA: 868] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 81. | Koo HL, DuPont HL. Rifaximin: a unique gastrointestinal-selective antibiotic for enteric diseases. Curr Opin Gastroenterol. 2010;26:17-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 120] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 82. | Rabenstein T, Fromm MF, Zolk O. [Rifaximin--a non-resorbable antibiotic with many indications in gastroenterology]. Z Gastroenterol. 2011;49:211-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 83. | Di Stefano M, Malservisi S, Veneto G, Ferrieri A, Corazza GR. Rifaximin versus chlortetracycline in the short-term treatment of small intestinal bacterial overgrowth. Aliment Pharmacol Ther. 2000;14:551-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 94] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 84. | Cummings JH, Macfarlane GT. Gastrointestinal effects of prebiotics. Br J Nutr. 2002;87 Suppl 2:S145-S151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 176] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 85. | Ouwehand AC, Derrien M, de Vos W, Tiihonen K, Rautonen N. Prebiotics and other microbial substrates for gut functionality. Curr Opin Biotechnol. 2005;16:212-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 106] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 86. | Yan AW, Fouts DE, Brandl J, Stärkel P, Torralba M, Schott E, Tsukamoto H, Nelson KE, Brenner DA, Schnabl B. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology. 2011;53:96-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 668] [Cited by in RCA: 638] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 87. | Roberfroid MB. Inulin-type fructans: functional food ingredients. J Nutr. 2007;137:2493S-2502S. [PubMed] |
| 88. | Watzl B. Consumption of vegetables and fruits and risk of breast cancer. JAMA. 2005;293:2209-210; author reply 2210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 90. | Gibson GR. Prebiotics as gut microflora management tools. J Clin Gastroenterol. 2008;42 Suppl 2:S75-S79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 91. | Shoaf K, Mulvey GL, Armstrong GD, Hutkins RW. Prebiotic galactooligosaccharides reduce adherence of enteropathogenic Escherichia coli to tissue culture cells. Infect Immun. 2006;74:6920-6928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 259] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 92. | Kailasapathy K, Chin J. Survival and therapeutic potential of probiotic organisms with reference to Lactobacillus acidophilus and Bifidobacterium spp. Immunol Cell Biol. 2000;78:80-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 416] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 93. | Isolauri E, Juntunen M, Rautanen T, Sillanaukee P, Koivula T. A human Lactobacillus strain (Lactobacillus casei sp strain GG) promotes recovery from acute diarrhea in children. Pediatrics. 1991;88:90-97. [PubMed] |
| 94. | Isolauri E, Joensuu J, Suomalainen H, Luomala M, Vesikari T. Improved immunogenicity of oral D x RRV reassortant rotavirus vaccine by Lactobacillus casei GG. Vaccine. 1995;13:310-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 226] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 95. | Tsai CC, Hsih HY, Chiu HH, Lai YY, Liu JH, Yu B, Tsen HY. Antagonistic activity against Salmonella infection in vitro and in vivo for two Lactobacillus strains from swine and poultry. Int J Food Microbiol. 2005;102:185-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 88] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 96. | Younts-Dahl SM, Osborn GD, Galyean ML, Rivera JD, Loneragan GH, Brashears MM. Reduction of Escherichia coli O157 in finishing beef cattle by various doses of Lactobacillus acidophilus in direct-fed microbials. J Food Prot. 2005;68:6-10. [PubMed] |
| 97. | Peterson RE, Klopfenstein TJ, Moxley RA, Erickson GE, Hinkley S, Rogan D, Smith DR. Efficacy of dose regimen and observation of herd immunity from a vaccine against Escherichia coli O157: H7 for feedlot cattle. J Food Prot. 2007;70:2561-2567. [PubMed] |
| 98. | Roselli M, Finamore A, Britti MS, Mengheri E. Probiotic bacteria Bifidobacterium animalis MB5 and Lactobacillus rhamnosus GG protect intestinal Caco-2 cells from the inflammation-associated response induced by enterotoxigenic Escherichia coli K88. Br J Nutr. 2006;95:1177-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 137] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 99. | Nanji AA, Khettry U, Sadrzadeh SM. Lactobacillus feeding reduces endotoxemia and severity of experimental alcoholic liver (disease). Proc Soc Exp Biol Med. 1994;205:243-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 303] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 100. | Griffiths EA, Duffy LC, Schanbacher FL, Qiao H, Dryja D, Leavens A, Rossman J, Rich G, Dirienzo D, Ogra PL. In vivo effects of bifidobacteria and lactoferrin on gut endotoxin concentration and mucosal immunity in Balb/c mice. Dig Dis Sci. 2004;49:579-589. [PubMed] [DOI] [Full Text] |
| 101. | Gorbach SL. Probiotics and gastrointestinal health. Am J Gastroenterol. 2000;95:S2-S4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 175] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 102. | Malaguarnera M, Gargante MP, Malaguarnera G, Salmeri M, Mastrojeni S, Rampello L, Pennisi G, Li Volti G, Galvano F. Bifidobacterium combined with fructo-oligosaccharide versus lactulose in the treatment of patients with hepatic encephalopathy. Eur J Gastroenterol Hepatol. 2010;22:199-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 103. | Malaguarnera G, Leggio F, Vacante M, Motta M, Giordano M, Bondi A, Basile F, Mastrojeni S, Mistretta A, Malaguarnera M. Probiotics in the gastrointestinal diseases of the elderly. J Nutr Health Aging. 2012;16:402-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 104. | Kanauchi O, Fujiyama Y, Mitsuyama K, Araki Y, Ishii T, Nakamura T, Hitomi Y, Agata K, Saiki T, Andoh A. Increased growth of Bifidobacterium and Eubacterium by germinated barley foodstuff, accompanied by enhanced butyrate production in healthy volunteers. Int J Mol Med. 1999;3:175-179. [PubMed] |
| 105. | Johnson-Henry KC, Donato KA, Shen-Tu G, Gordanpour M, Sherman PM. Lactobacillus rhamnosus strain GG prevents enterohemorrhagic Escherichia coli O157: H7-induced changes in epithelial barrier function. Infect Immun. 2008;76:1340-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 205] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 106. | O’Sullivan DJ. Genomics can advance the potential for probiotic cultures to improve liver and overall health. Curr Pharm Des. 2008;14:1376-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 107. | Clausen MR, Mortensen PB. Lactulose, disaccharides and colonic flora. Clinical consequences. Drugs. 1997;53:930-942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 65] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 108. | Foster KJ, Lin S, Turck CJ. Current and emerging strategies for treating hepatic encephalopathy. Crit Care Nurs Clin North Am. 2010;22:341-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 109. | Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125:1401-1412. [PubMed] |
| 110. | Loguercio C, Federico A, Tuccillo C, Terracciano F, D’Auria MV, De Simone C, Del Vecchio Blanco C. Beneficial effects of a probiotic VSL#3 on parameters of liver dysfunction in chronic liver diseases. J Clin Gastroenterol. 2005;39:540-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 340] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 111. | Malaguarnera M, Vacante M, Antic T, Giordano M, Chisari G, Acquaviva R, Mastrojeni S, Malaguarnera G, Mistretta A, Li Volti G. Bifidobacterium longum with fructo-oligosaccharides in patients with non alcoholic steatohepatitis. Dig Dis Sci. 2012;57:545-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 333] [Article Influence: 25.6] [Reference Citation Analysis (1)] |
| 112. | Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S. Host-gut microbiota metabolic interactions. Science. 2012;336:1262-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2838] [Cited by in RCA: 3235] [Article Influence: 248.8] [Reference Citation Analysis (0)] |
| 113. | Bruzzese E, Raia V, Gaudiello G, Polito G, Buccigrossi V, Formicola V, Guarino A. Intestinal inflammation is a frequent feature of cystic fibrosis and is reduced by probiotic administration. Aliment Pharmacol Ther. 2004;20:813-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 135] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 114. | Resta-Lenert S, Barrett KE. Live probiotics protect intestinal epithelial cells from the effects of infection with enteroinvasive Escherichia coli (EIEC). Gut. 2003;52:988-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 428] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 115. | Sartor RB. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology. 2004;126:1620-1633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 726] [Cited by in RCA: 718] [Article Influence: 34.2] [Reference Citation Analysis (1)] |
| 116. | Versalovic J. Probiotics: intestinal gatekeeping, immunomodulation, and hepatic injury. Hepatology. 2007;46:618-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 117. | Compare D, Coccoli P, Rocco A, Nardone OM, De Maria S, Cartenì M, Nardone G. Gut--liver axis: the impact of gut microbiota on non alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis. 2012;22:471-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 324] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 118. | Frazier TH, Stocker AM, Kershner NA, Marsano LS, McClain CJ. Treatment of alcoholic liver disease. Therap Adv Gastroenterol. 2011;4:63-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |