Published online Nov 21, 2014. doi: 10.3748/wjg.v20.i43.16318
Revised: June 4, 2014
Accepted: June 25, 2014
Published online: November 21, 2014
Processing time: 221 Days and 1.8 Hours
AIM: To investigate the optimum period of treatment for post endoscopic submucosal dissection (ESD) ulcers.
METHODS: Patients who underwent ESD for gastric cancer were randomized to two groups and treated with esomeprazole 20 mg per day for 4 wk (4W group) or 2 wk (2W group). At 4 wk after ESD, we measured the size of the artificial ulcers by endoscopy and determined the ulcer healing rate, compared with the size of the ESD specimens. This randomized controlled trial study was approved by our ethics committee and registered in the UMIN Clinical Trial Registry.
RESULTS: A total of 60 consecutive patients were included in the study. All patients received rebamipide 300 mg per day for 4 wk. One patient in 2W group who showed bleeding within two weeks and received endoscopic treatment was excluded from further analysis. The numbers of patients with ulcers in the healing/scar stage in the 2W and 4W groups at 4 wk after ESD were 20/6 and 28/5, respectively, with no significant difference. The ulcer healing rate in the 2W and 4W groups were 96.1% [95% confidence interval (CI): 94.6%-97.55] vs 94.8% (95%CI: 92.6%-97.1%), respectively, with no statistical difference (UMIN000006951).
CONCLUSION: Two-wk treatment with a proton pump inhibitor is as effective as four-week treatment for healing post ESD ulcers.
Core tip: There is no consensus regarding the optimum period of treatment of patients with post endoscopic submucosal dissection (ESD) ulcers. 60 patients who underwent ESD for gastric cancer were randomized to two groups and treated with proton pump inhibitor for 4 wk (4W group) or 2 wk (2W group). The numbers of patients with ulcers in the healing/scar stage and the ulcer healing rate at 4 wk after ESD showed no statistical difference between 2W and 4W groups. Two-week treatment with a proton pump inhibitor is as effective as four-week treatment for healing post ESD ulcers.
- Citation: Arai M, Matsumura T, Okimoto K, Oyamada A, Saito K, Minemura S, Maruoka D, Tanaka T, Nakagawa T, Katsuno T, Yokosuka O. Two-week treatment with proton pump inhibitor is sufficient for healing post endoscopic submucosal dissection ulcers. World J Gastroenterol 2014; 20(43): 16318-16322
- URL: https://www.wjgnet.com/1007-9327/full/v20/i43/16318.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i43.16318
Recently, improvements in endoscopic treatment techniques and technology have enabled the development of a new method, endoscopic submucosal dissection (ESD). ESD is a less invasive treatment for early gastric cancer than gastrectomy[1-3]. In addition, we can obtain precise histological data following ESD. After ESD, delayed bleeding, which is defined as hematemesis or melena up to 30 d after the procedure, may be treated by emergency endoscopy. The frequency of delayed bleeding varies with the tumor location and size[4,5]. Proton pump inhibitors (PPIs) are used widely to avoid delayed bleeding. PPIs have a strong effect in up-regulating gastric pH and promoting the healing of gastric ulcers, but various side effects of PPIs also have been reported, for example, pneumonia, intestinal infection, osteoporosis, and microscopic colitis[6]. In addition, most PPIs are metabolized by the cytochrome P450 pathway, specifically CYP2C19 and CYP3A3, so that combined medication with PPIs and other drugs which are metabolized by the same pathway should be undertaken with care or avoided completely[7,8].
It is difficult to identify the occurrence timing of peptic ulcers associated with Helicobacter pylori (H. pylori) infection accurately and to compare the difference in ulcer healing between the H. pylori infected ulcer and the artificial ulcer after ESD directly. In contrast, Hashimoto et al[9,10] reported that the speed of healing of artificial ulcers was faster than that of ordinary peptic ulcers and showed that the pathophysiology of artificial ulcers which form after ESD might differ from peptic ulcers associated with H. pylori infection. Therefore, we suppose that the duration of PPI treatment for post ESD ulcers might be reduced to avoid the side effects of PPIs, unlike peptic ulcers associated with H. pylori infection. However, there is no consensus regarding the optimal period of PPI treatment of patients with artificial ulcers after ESD treatment. Therefore, we evaluated the optimal period of treatment of post ESD ulcers in a randomized controlled trial.
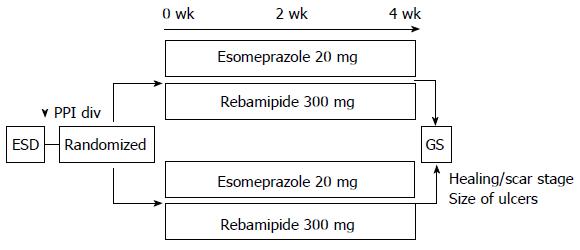
This study was a paralleled, randomized controlled trial that investigated the pharmacodynamic effect, efficacy and safety of a proton pump inhibitor in patients, following ESD treatment. Before ESD treatment, patients who were to be treated at Chiba University Hospital from January 2012 to March 2013 were recruited. Patients using antithrombotic drugs, with a tendency to bleed or on dialysis were excluded. Informed consent was obtained from all patients and this study was approved by the Ethics Committee of Chiba University Hospital (Registration number, UMIN000006951). In this study, patients with ESD were enrolled, randomized and treated with esomeprazole 20 mg per day, either for 4 wk (4W group) or 2 wk (2W group) (Figure 1). All patients received rebamipide 300 mg per day for 4 wk. Post procedure-related bleeding was recorded when hematemesis or melena were observed or the hemoglobin concentration decreased by more than 2 g/dL.
At 4 wk after ESD, we measured the size of the artificial ulcers by endoscopy and determined the ulcer healing rate and speed compared to the size of the ESD specimens. In addition, we calculated the ulcer healing speed (mm2/mo) of cases in the stage of healing, that is (ESD size) - (Ulcer size at 4 wk). The primary outcome variable was the ulcer healing rate in the 2W and 4W groups.
ESD was performed with a conventional single-channel endoscope (GIF-H260Z or -Q260J, Olympus, Tokyo, Japan) or 2-channel endoscope (2TQ260M, Olympus). We used an IT knife 2 (KD-611L, Olympus) and an electrosurgical current was applied with the use of an electrosurgical generator (VIO300D, ERBE). The injection solutions contained glycerin and hyaluronic acid sodium (0.4%) with 1% indigo carmine dye. The ulcers that developed after ESD were carefully examined endoscopically and any visible vessels were heat-coagulated using hemostatic forceps (FD-410LR, Olympus). Thereafter, the resected specimens were stretched, pinned flat on a cork board, and measured.
All data are represented as the mean ± SD. The unpaired t-test, χ2 test, and Mann-Whitney U test were used for statistical analyses as appropriate with the statistical program SPSS version 21 statistical analysis package (SAS Institute, Cary, NC, United States). A P value of less than 0.05 was considered statistically significant.
The clinical characteristics of the 60 patients after ESD treatment are shown in Table 1 and the average size of ESD in the 2W group was less than that of the 4W group (P = 0.048, Mann-Whitney U test) in spite of randomized assignation.
| Parameter | 2 wk(n = 27) | 4 wk(n = 33) | P value |
| Clinical background | |||
| Age (yr) | 73.3 ± 7.8 | 70.8 ± 9.3 | NS2 |
| Gender (male/female) | 17/10 | 19/14 | NS3 |
| Helicobacter pylori infection (+/-/unknown) | 18/8/1 | 25/6/2 | NS3 |
| Location (upper/middle/lower) | 3/10/14 | 1/14/18 | NS3 |
| Size of ESD (mm2) | 777.6 ± 437.8 | 1251.2 ± 918.6 | 0.0484 |
| Serum albumin (g/dL) | 4.1 ± 0.4 | 4.1 ± 0.4 | NS2 |
| Comorbidity | |||
| Diabetes (+/-) | 3/24 | 6/27 | NS3 |
| Hyperlipidemia (+/-) | 7/20 | 8/25 | NS3 |
| Hypertension (+/-) | 8/19 | 11/22 | NS3 |
| Status of ulcer at 4 wk after ESD1 | |||
| Stage of ulcer (active/healing/scar) | 0/20/6 | 0/28/5 | NS2 |
| Ulcer healing rate (%) (average, 95%CI) | 96.1 (94.6-97.5) | 94.8 (92.6-97.1) | NS3 |
Re-bleeding was observed in one patient and emergency endoscopy and endoscopic treatment was given within 2 wk of the ESD treatment. This patient was excluded from further analysis. The othes did not show post procedure-related bleeding. The numbers of patients in the ulcer healing (H)/scar (S) stage at 4 wk after ESD were 20/6 and 28/5 in the 2W and 4W groups, respectively, and this showed no significant difference (Table 1). There was no ulcer in the active stage. We evaluated the ulcer healing rate at 4 wk and these were 96.1% [95% confidence interval (CI): 94.6%-97.5%] vs 94.8% (95%CI: 92.6%-97.1%), respectively, which showed the non-inferiority of 2-wk treatment compared with 4-wk treatment. The drug compliance of all patients was over 80% of the established quantity. No accumulation of adverse events for a specific symptom, disease or laboratory abnormality was observed.
Because the median healing area in 48 patients at the H stage at 4 wk was 968.5 mm2, we designated this as rapid healing, that is good healing (≥ 968.5 mm2) in the H or S stage at 4 wk. We observed a significant difference between the rapid healing and non-rapid healing groups in the initial ulcer size (P < 0.001, Mann-Whitney U test) and the disease rate of hypertension (P = 0.049, χ2 test) (Table 2). In contrast, age, sex, tumor location in the stomach, H. pylori infection, ulcer healing rate, diabetes, hyperlipidemia, and the duration of PPI treatment did not predict the speed of ulcer healing. To adjust for the difference of ESD size between 2W and 4W groups, we selected the patients who were within the range (average ± 1 SD), and the size-matched analysis was also performed (Table 3). As a result, there was no significant difference in ulcer healing rate and speed.
| Parameter | Healing area in healing stage | P value | |
| ≥ 968.5 mm2 (n = 24) | < 968.5 mm2 (n = 24) | ||
| Age (yr) | 72.6 ± 8.9 | 68.8 ± 9.0 | NS1 |
| Sex (male/female) | 15/9 | 15/9 | NS2 |
| Location of tumors (upper/middle/lower third) | 1/11/12 | 1/8/15 | NS2 |
| Helicobacter pylori infection (+/-/unknown) | 17/5/2 | 16/8/0 | NS2 |
| Initial ulcer size (mm2) | 1689.3 ± 835.7 | 633.5 ± 243.8 | < 0.0013 |
| Healing rate (%) | 94.6 ± 4.5 | 94.0 ± 6.6 | NS3 |
| Treatment period (2 wk/4 wk) | 7/17 | 13/11 | NS2 |
| Comorbidity | |||
| Diabetes (yes/no) | 4/20 | 5/19 | NS2 |
| Hyperlipidemia (yes/no) | 3/21 | 7/17 | NS2 |
| Hypertension (yes/no) | 10/14 | 3/21 | 0.0492 |
In previous studies, artificial ulcers that developed after ESD treatment healed within 8 wk, irrespective of size and location[11]. However, there is no consensus regarding the optimum duration of treatment following ESD. The most frequent complication that occurs after endoscopic therapy is bleeding and the rate of intraoperative bleeding is significantly higher with ESD than with endoscopic mucosal resection. Uedo et al[12] indicated that PPI therapy prevented delayed bleeding from the ulcer created by ESD more effectively than did histamine H2-receptor antagonist treatment. In this study, the ratio of ulcer healing was more than 90% in both groups at 4 wk after ESD treatment. In addition, the rate of intraoperative bleeding did not differ significantly between the groups.
Oh et al[13] reported that the degree of healing of ESD-induced ulcers was dependent on the initial ulcer size. Kobayashi et al[14] reported that a larger sized ulcer after ESD was the only significant predictor of delayed healing. In this study, the factor that influenced the area of healing during the healing stage at 4 wk after ESD was the initial ulcer size, the disease rate of hypertension and not the duration of PPI treatment. In addition, the ratios of H and S stages did not differ between the 2W and 4W groups. One patient showed re-bleeding but this occurred within 2 wk and, therefore, was not related to the duration of PPI treatment. The relation between hypertension and ulcer healing is unknown and further study is needed. Using the size of ESD as a deterministic factor for randomization might improve the analysis, but in-sub-group analysis of patients whose ulcers initially were sized within the average ± 1 SD showed no significant difference in the initial size and ulcer healing rate between the 2W and 4W groups (Table 3). PPIs are a very well tolerated drug class. Their high safety, efficacy and wide distribution lead to overuse, inappropriate dosage and excessive durations of treatment. Howell et al[15] reported that increasing levels of pharmacologic acid suppression are associated with increased risks of nosocomial Clostridium difficile (CD) infection and Barletta et al[16] reported that the duration of PPI therapy is significantly associated with CD infection and suggested consideration of restricting PPI use, given the short exposure time. However, more recent studies have offered conflicting viewpoints regarding the association between PPI use and increased risk of CD infection[17-19]. Freedberg et al[20] reported that receipt of PPIs concurrent with CD treatment was not associated with recurrence of CD infection among hospitalized adults with CD, and that increased age and increased comorbidities significantly predicted recurrence. In this study, the mean age of the patients who underwent ESD treatment was over 70 years, 13 patients (22%) being 80 years of age or more, and comorbidities (diabetes, hyperlipidemia, and hypertension) often were observed. In addition, PPIs are metabolized by the cytochrome P450 pathway, specifically CYP2C19 and CYP3A3. Clopidogrel, which is used to inhibit blood clots, requires biotransformation for conversion to its active form, mediated by the enzymes CYP2C19 and CYP3A3[21]. The Food and Drug Administration in the United States issued a recommendation against the combined use of clopidogrel and specific PPIs (potent CYP2C19 inhibitors). Therefore, it is not desirable to use PPIs blindly for a long period and we have to define the optimized period of PPI use, especially for patients with a past history of CD infection, pneumonia or use of clopidogrel.
In this study, all patients were treated with rebamipide for 4 wk. Rebamipide has been reported to induce healing of ulcers, especially artificial ulcers[14]. Nishizawa et al[22] reported that the administration of rebamipide could induce the expression of Sonic Hedgehog (Shh) and we also reported that the expression level of Shh in the gastric mucosa showed a good correlation with the speed of healing of artificial ulcers[23], that is, rebamipide could promote the healing of ulcers via Shh, which could influence some cellular and tissue functions, including cell proliferation and tissue repair[24,25]. The use of rebamipide might eliminate the distinction in healing rate between the 2W and 4W groups. But, in our previous report, we treated 45 artificial ulcers after ESD only by PPI (esomeprazole or omeprazole) for 4 wk and showed that the healing rate was 94.6% (95%CI: 90.7%-98.5%), which was not different from those of 2W or 4W groups in this study[23]. This result suggested us that the effect of adding rebamipide on healing ulcer was limited.
In conclusion, two-week treatment with PPI is as effective as four-week treatment for healing post ESD ulcers. In view of the results of this study, we could shorten the period of PPI treatment for artificial ulcers after ESD.
Endoscopic submucosal dissection (ESD) is a less invasive treatment for early gastric cancer than gastrectomy. Proton pump inhibitors (PPIs) are used widely to avoid delayed bleeding. PPIs have a strong effect in up-regulating gastric pH and promoting the healing of gastric ulcers, but various side effects of PPIs also have been reported, for example, pneumonia, intestinal infection, osteoporosis, microscopic colitis and drug interaction.
The pathophysiology of artificial ulcers which form after ESD might differ from peptic ulcers associated with Helicobacter pylori infection. However, there is no consensus regarding the optimal period of PPI treatment of patients with artificial ulcers after ESD treatment.
Two-week treatment with PPI is as effective as four-week treatment for healing post ESD ulcers.
We can shorten the period of PPI treatment for artificial ulcers after ESD. It can avoid the side effects of PPI.
Cytochrome P450 (CYP): By the CYP pathway, various drugs are metabolized. Most PPIs are metabolized by the cytochrome P450 pathway, specifically CYP2C19 and CYP3A3.
There was no study to evaluate the optimal period of PPI treatment for the artificial ulcer after ESD by a prospective, randomized trial. This study is the first report to show the possibility to shorten the period of PPI treatment.
P- Reviewer: Kamer E, Kurtoglu E, Nakajima N, Slomiany BL, Wang DR S- Editor: Ding Y L- Editor: A E- Editor: Zhang DN
| 1. | Ono H, Kondo H, Gotoda T, Shirao K, Yamaguchi H, Saito D, Hosokawa K, Shimoda T, Yoshida S. Endoscopic mucosal resection for treatment of early gastric cancer. Gut. 2001;48:225-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1134] [Cited by in RCA: 1149] [Article Influence: 47.9] [Reference Citation Analysis (4)] |
| 2. | Fujishiro M, Yahagi N, Nakamura M, Kakushima N, Kodashima S, Ono S, Kobayashi K, Hashimoto T, Yamamichi N, Tateishi A. Successful outcomes of a novel endoscopic treatment for GI tumors: endoscopic submucosal dissection with a mixture of high-molecular-weight hyaluronic acid, glycerin, and sugar. Gastrointest Endosc. 2006;63:243-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 200] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 3. | Yamamoto H, Kawata H, Sunada K, Satoh K, Kaneko Y, Ido K, Sugano K. Success rate of curative endoscopic mucosal resection with circumferential mucosal incision assisted by submucosal injection of sodium hyaluronate. Gastrointest Endosc. 2002;56:507-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Shiba M, Higuchi K, Kadouchi K, Montani A, Yamamori K, Okazaki H, Taguchi M, Wada T, Itani A, Watanabe T. Risk factors for bleeding after endoscopic mucosal resection. World J Gastroenterol. 2005;11:7335-7339. [PubMed] |
| 5. | Oda I, Gotoda T, Hamanaka H, Eguchi T, Saito Y, Matsuda T, Bhandari P, Emura F, Saito D, Ono H. Endoscopic submucosal dissection for early gastric cancer: technical feasibility, operation time and complications from a large consecutive series. Dig Endosc. 2005;17:54-58. [RCA] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 333] [Article Influence: 16.7] [Reference Citation Analysis (2)] |
| 6. | Johnson DA, Oldfield EC. Reported side effects and complications of long-term proton pump inhibitor use: dissecting the evidence. Clin Gastroenterol Hepatol. 2013;11:458-464; quiz e37-e38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 7. | Ho PM, Maddox TM, Wang L, Fihn SD, Jesse RL, Peterson ED, Rumsfeld JS. Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome. JAMA. 2009;301:937-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 739] [Cited by in RCA: 709] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 8. | Ray WA, Murray KT, Griffin MR, Chung CP, Smalley WE, Hall K, Daugherty JR, Kaltenbach LA, Stein CM. Outcomes with concurrent use of clopidogrel and proton-pump inhibitors: a cohort study. Ann Intern Med. 2010;152:337-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Hashimoto T, Adachi K. Changes in gastric mucosal blood flow during healing of EMR-induced ulcer-Comparison with peptic ulcer. Dig Endosc. 1997;9:127-131. [RCA] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Hashimoto T, Adachi K, Fukumoto S. [Changes of gastric mucosal blood flow during the healing process of artificial ulcer caused by endoscopic mucosal resection]. Gastroenterol Endosc. 1995;7:554-560. |
| 11. | Kakushima N, Yahagi N, Fujishiro M, Iguchi M, Oka M, Kobayashi K, Hashimoto T, Omata M. The healing process of gastric artificial ulcers after endosopic submucosal dissection. Digest Endosc. 2004;16:327-331. [RCA] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 12. | Uedo N, Takeuchi Y, Yamada T, Ishihara R, Ogiyama H, Yamamoto S, Kato M, Tatsumi K, Masuda E, Tamai C. Effect of a proton pump inhibitor or an H2-receptor antagonist on prevention of bleeding from ulcer after endoscopic submucosal dissection of early gastric cancer: a prospective randomized controlled trial. Am J Gastroenterol. 2007;102:1610-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 169] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 13. | Oh TH, Jung HY, Choi KD, Lee GH, Song HJ, Choi KS, Chung JW, Byeon JS, Myung SJ, Yang SK. Degree of healing and healing-associated factors of endoscopic submucosal dissection-induced ulcers after pantoprazole therapy for 4 weeks. Dig Dis Sci. 2009;54:1494-1499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Kobayashi M, Takeuchi M, Hashimoto S, Mizuno K, Sato Y, Narisawa R, Aoyagi Y. Contributing factors to gastric ulcer healing after endoscopic submucosal dissection including the promoting effect of rebamipide. Dig Dis Sci. 2012;57:119-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Howell MD, Novack V, Grgurich P, Soulliard D, Novack L, Pencina M, Talmor D. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med. 2010;170:784-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 312] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 16. | Barletta JF, El-Ibiary SY, Davis LE, Nguyen B, Raney CR. Proton Pump Inhibitors and the Risk for Hospital-Acquired Clostridium difficile Infection. Mayo Clin Proc. 2013;88:1085-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Khanna S, Aronson SL, Kammer PP, Baddour LM, Pardi DS. Gastric acid suppression and outcomes in Clostridium difficile infection: a population-based study. Mayo Clin Proc. 2012;87:636-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Leonard AD, Ho KM, Flexman J. Proton pump inhibitors and diarrhoea related to Clostridium difficile infection in hospitalised patients: a case-control study. Intern Med J. 2012;42:591-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | McCollum DL, Rodriguez JM. Detection, treatment, and prevention of Clostridium difficile infection. Clin Gastroenterol Hepatol. 2012;10:581-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Freedberg DE, Salmasian H, Friedman C, Abrams JA. Proton pump inhibitors and risk for recurrent Clostridium difficile infection among inpatients. Am J Gastroenterol. 2013;108:1794-1801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 21. | Kazui M, Nishiya Y, Ishizuka T, Hagihara K, Farid NA, Okazaki O, Ikeda T, Kurihara A. Identification of the human cytochrome P450 enzymes involved in the two oxidative steps in the bioactivation of clopidogrel to its pharmacologically active metabolite. Drug Metab Dispos. 2010;38:92-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 636] [Cited by in RCA: 593] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 22. | Nishizawa T, Suzuki H, Nakagawa I, Minegishi Y, Masaoka T, Iwasaki E, Hibi T. Rebamipide-promoted restoration of gastric mucosal sonic hedgehog expression after early Helicobacter pylori eradication. Digestion. 2009;79:259-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Tanaka T, Arai M, Minemura S, Oyamada A, Saito K, Jiang X, Tsuboi M, Sazuka S, Maruoka D, Matsumura T. Expression level of sonic hedgehog correlated with the speed of gastric mucosa regeneration in artificial gastric ulcers. J Gastroenterol Hepatol. 2014;29:736-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Echelard Y, Epstein DJ, St-Jacques B, Shen L, Mohler J, McMahon JA, McMahon AP. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell. 1993;75:1417-1430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1659] [Cited by in RCA: 1667] [Article Influence: 52.1] [Reference Citation Analysis (0)] |
| 25. | Chamoun Z, Mann RK, Nellen D, von Kessler DP, Bellotto M, Beachy PA, Basler K. Skinny hedgehog, an acyltransferase required for palmitoylation and activity of the hedgehog signal. Science. 2001;293:2080-2084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 322] [Article Influence: 13.4] [Reference Citation Analysis (0)] |