Published online Nov 21, 2014. doi: 10.3748/wjg.v20.i43.16227
Revised: January 6, 2014
Accepted: June 14, 2014
Published online: November 21, 2014
Processing time: 372 Days and 2.8 Hours
AIM: To investigate the mechanism and in vivo effects of MK-0626, a dipeptidyl peptidase-4 inhibitor, on hepatic steatosis using ob/ob mice.
METHODS: We analyzed obese (ob/ob) 8-wk-old male mice that had been randomly divided into two groups of ob/ob mice (n = 16 each) and were treated with 1.5 or 3 mg/kg MK-0626 and two control groups of untreated ob/ob mice and lean littermates (n = 16 each). All mice were fed a normal chow diet with or without MK-0626 for either four or eight weeks. Blood samples were collected, and total hepatectomy was performed.
RESULTS: The administration of dietary MK-0626 ameliorated the hepatic lipid accumulation in ob/ob mice treated with 3 mg/kg MK-0626 (3 MK), P < 0.05, vs untreated ob/ob mice (ob/ob). The MK-0626 treatment reduced the serum alanine aminotransferase levels (both treatment groups, P < 0.05 vs ob/ob) and glucoses/insulin levels/calculated HOMA scores (1.5 MK, P < 0.05 vs ob/ob; 3 MK, P < 0.01 vs ob/ob) and increased the serum adiponectin levels (3 MK, P < 0.05 vs ob/ob) in a dose-dependent manner. The MK-0626 treatment increased the mRNA expression of peroxisome proliferator-activated receptor α/microsomal triglyceride transfer protein (1.5 MK, P < 0.05 vs ob/ob; 3 MK, P < 0.01 vs ob/ob) but reduced the sterol regulatory element binding transcription factor-1c/fatty acid synthase/stearoyl-CoA desaturase-1 (both treatment groups, P < 0.01 vs ob/ob). The MK-0626 treatment increased the activity of AMP-activated protein kinase (AMPK) (both treatment groups, P < 0.01 vs ob/ob).
CONCLUSION: MK-0626 could attenuate hepatic steatosis through enhancing AMPK activity, inhibiting hepatic lipogenic gene expression, enhancing triglyceride secretion from liver and increasing serum adiponectin levels.
Core tip: Administration of MK-0626, a dipeptidyl peptidase-4 inhibitor, ameliorated hepatic steatosis; reduced serum alanine aminotransferase, glucose, and insulin levels; reduced HOMA scores; and increased serum adiponectin levels in ob/ob mice. MK-0626 treatment significantly increased the mRNA expression of peroxisome proliferator-activated receptor α and microsomal triglyceride transfer protein but significantly reduced sterol regulatory element binding transcription factor-1c, fatty acid synthase and stearoyl-CoA desaturase-1. AMP-activated protein kinase (AMPK) activity was significantly increased. These results suggest that MK-0626 could attenuate hepatic steatosis by enhancing AMPK activity, inhibiting hepatic lipogenic gene expression, enhancing triglyceride secretion from the liver and increasing serum adiponectin levels.
-
Citation: Ohyama T, Sato K, Yamazaki Y, Hashizume H, Horiguchi N, Kakizaki S, Mori M, Kusano M, Yamada M. MK-0626, a selective DPP-4 inhibitor, attenuates hepatic steatosis in
ob/ob mice. World J Gastroenterol 2014; 20(43): 16227-16235 - URL: https://www.wjgnet.com/1007-9327/full/v20/i43/16227.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i43.16227
Nonalcoholic fatty liver disease (NAFLD) is the hepatic manifestation of metabolic syndrome. Other comorbidities of metabolic syndrome include obesity, type 2 diabetes mellitus, hypertension and dyslipidemia coupled with insulin resistance, which is a central feature of metabolic syndrome. Thus, improving insulin sensitivity would decrease hepatic fat deposition and accordingly inhibit hepatocyte vulnerability to oxidative stress[1].
The incretin hormones glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) play a pivotal role in controlling blood glucose levels[2,3]. In response to meal ingestion, both hormones facilitate insulin synthesis and release[4-6] and, as a result, an increase in insulin sensitivity is observed. GLP-1 inhibits glucagon release, delays gastric emptying and increases satiety[4-6]. These incretin hormones are degraded and inactivated by dipeptidyl peptidase-4 (DPP-4)[7]. DPP-4 inhibitors prevent the degradation/inactivation of the biologically active form of GLP-1 and GIP, thereby augmenting the biological activity of GLP-1 and GIP [8], and have been approved for the treatment of type 2 diabetes.
Previous studies showed that inhibition of DPP-4 prevents hepatic steatosis in animal models[9-13], and a clinical pilot study with 30 NAFLD patients with type 2 diabetes mellitus showed that the DPP-4 inhibitor sitagliptin improved elevated liver enzymes[14]. However, the mechanisms by which the DPP-4 inhibitor prevents hepatic steatosis remain to be elucidated.
ob/ob mice have a naturally occurring spontaneous point mutation in the leptin gene that prevents the peptide from being produced[15] and are well-recognized as a naturally occurring model of hepatic steatosis and type 2 diabetes. The characteristics of the ob/ob mouse include several metabolic and neuroendocrine abnormalities such as obesity, hyperphagia, hyperinsulinemia, hyperlipidemia, hyperglycemia and insulin resistance. In addition, ob/ob mice have a decreased metabolic rate and body temperature. Because ob/ob mice have several characteristics that mimic metabolic syndrome in humans, these mice form one of the most widely studied mouse models of obesity and metabolic syndrome[16-18].
MK-0626 is a potent, orally active DPP-4 inhibitor (IC50 = 6.3 nmol/L) with excellent selectivity and oral bioavailability in preclinical species and in vivo efficacy in animal models[19]. The objectives of our study were to characterize the in vivo effects and mechanism of action of the α-amino amide DPP-4 inhibitor, MK-0626, on hepatic steatosis using ob/ob mice.
Obese (ob/ob) 6-wk-old male mice and their lean littermates were purchased from Charles Liver Co. Ltd. (Tokyo, Japan). All mice were housed in cages and maintained on a 12-h light/dark cycle with free access to food and water. The mice were acclimatized for 2 wk, during which time they were fed a normal chow diet (CLEA Rodent Diet CE-2) from CLEA Japan, Inc. (Tokyo, Japan). At 8 wk of age, they were placed on a normal chow diet (D12450B) from Research Diets (Tokyo, Japan) as a transition to MK-0626 supplemented D12450B chow. Mice were randomly divided into two groups of ob/ob mice (n = 16 each) and were fed either a normal chow diet or a normal chow diet supplemented with MK-0626 (1.5 mg/kg) or MK-0626 (3 mg/kg). In addition, two control groups (n = 16 each) of untreated ob/ob mice and lean littermates were fed a normal chow diet. After the mice were switched to D12450B, body weight and food intake were monitored weekly. All mice were fed an experimental diet for either four or eight weeks. At the completion of the study, fasting blood samples were drawn to analyze glucose and insulin levels and the homeostatic model assessment (HOMA). Further sera were drawn to measure serum active GLP-1 concentrations and biochemical parameters such as alanine aminotransferase (ALT). Total hepatectomy was performed at the time of euthanasia, and liver samples were divided for histopathology and other analyses. For protein or RNA analysis, tissues were frozen in liquid nitrogen and stored at -80 °C until needed. To achieve statistical power for the study, 64 mice were used for the experiment, and 16 mice were included in each treatment arm. All mouse procedures were performed in accordance with the guidelines for animal care and use established by the Gunma University School of Medicine.
Total liver lipids were extracted from liver homogenate using methanol and chloroform, and they were then reacted with a vanillin-phosphoric acid reagent[20]. The hepatic lipid content was measured enzymatically with triglyceride GPO-Trinder and Infinity cholesterol reagents (Sigma-Aldrich, St. Louis, MO).
Serum glucose and serum ALT were measured with an auto-analyzer. Adiponectin concentration was measured using a Quantikine ELISA Kit (RD Systems, Minneapolis, MN, United States). Insulin concentration was measured using a Mouse Ultrasensitive Insulin ELISA (ALPCO Diagnosis, Salem, NH). HOMA was calculated using serum glucose and insulin concentrations. Serum active GLP-1 was measured using a GLP-1 (Active) ELISA KIT (Shibayagi, Gunma, Japan). For histological examination, paraffin-embedded liver tissue specimens were stained with Hematoxylin-Eosin and Oil Red O. Quantitative analysis of Oil Red O-stained areas was performed using the NIH image software program in 10 microscopic fields at a 400-fold magnification.
Total RNA was isolated with the ISOGEN RNA extraction reagent (Nippon Gene, Toyama, Japan), quantified by spectrophotometry and reverse-transcribed to cDNA. Then, the cDNA was used for polymerase chain reaction (PCR). It was amplified using iQ-SYBR Green Supermix with specific oligonucleotide primers for target sequences or glyceraldehyde-3-phosphate dehydrogenase (for normalization). Taqman Universal PCR Master Mix and the Taqman Gene Expression Assay (Applied Biosystems Japan Ltd., Tokyo, Japan) were used to analyze the genes of interest, which included peroxisome proliferator-activated receptor (PPAR)α, sterol regulatory element binding transcription factor (SREBP)-1c, stearoyl-CoA desaturase (SCD)-1, fatty acid synthase (FAS), microsomal triglyceride transfer protein (MTP) activity and carnitine palmitoyltransferase (CPT)-1. The specific oligonucleotide primers are shown in Table 1.
| Gene name | Accession | Forward | Reverse |
| PPARα | Mm00440939 | ||
| SREBP-1c | Mm00550338 | ||
| SCD-1 | GTTGGCTCCATCCATTGC | AACCATGGGAAGCCAAGTTT | |
| FAS | AACCTCAGCAACACATCT | CGTTTACAAAGGGCATGCA | |
| MTP | Mm00435015 | ||
| CPT1 | CCCTGGGCATGATTGCAA | AAGAGGACGCCACTCACGAT |
The protein extracted from the liver tissue was run on a sodium dodecyl sulfate-polyacrylamide electrophoresis gel and then transferred to a polyvinylidene difluoride (PVDF) membrane (GE Healthcare United Kingdom Ltd., Buckinghamshire, England). After the membranes were blocked in PVDF Blocking Reagent from Can Get Signal (TOYOBO, Osaka, Japan) for one hour at room temperature, they were probed with primary antibodies to phospho-AMP-activated protein kinase (AMPK) (1:1000) and AMPK (1:5000).
Statistical analysis was performed using SPSS v.16.0 (IBM Corp., Armonk, NY, United States). Data were analyzed by one-way analysis of variance. Values are expressed as the mean ± SD. A minimum of three independent experiments were performed, unless indicated otherwise. P values of less than 0.05 were considered statistically significant.
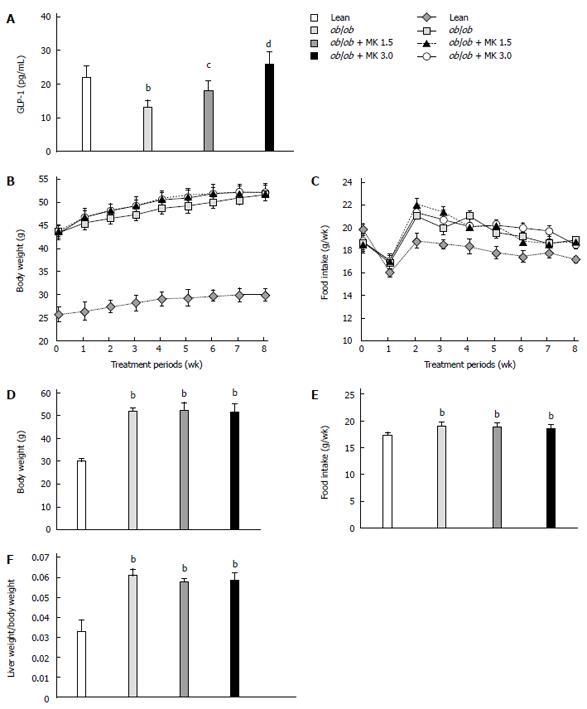
Serum active GLP-1 concentrations in ob/ob mice were significantly lower than those in lean mice (Figure 1A). MK-0626 treatment of ob/ob mice increased serum active GLP-1 concentrations in a dose-dependent manner (Figure 1A).
Treatment with MK-0626 for 4 wk in ob/ob mice reduced serum ALT, glucose, and insulin levels and the calculated HOMA scores and increased serum adiponectin levels compared to MK-0626-untreated ob/ob mice in a dose-dependent manner. However, the effect of 1.5 mg/kg MK-0626 on serum adiponectin levels did not reach statistical significance (Table 2).
| Laboratory findings | Lean | ob/ob | ob/ob + MK-0626 (1.5 mg/kg) | ob/ob + MK-0626 (3 mg/kg) |
| Glucose (mg/dL) | 141 ± 43.8 | 349 ± 15.7 | 288 ± 17.6a | 247 ± 42.5b |
| Insulin (μU/mL) | 1.83 ± 0.61 | 11.54 ± 0.59 | 8.42 ± 1.03a | 7.83 ± 0.44b |
| HOMA score | 0.63 ± 0.06 | 9.96 ± 2.32 | 5.98 ± 0.52a | 4.78 ± 0.166b |
| ALT (U/L) | 43.3 ± 10.3 | 590 ± 109.5 | 278 ± 70.5a | 272.5 ± 131.5a |
| Adiponectin (μg/mL) | 31.15 ± 2.07 | 34.57 ± 1.25 | 37.86 ± 3.40 | 39.90 ± 1.28a |
There was no significant change in body weight in either the low or high dose MK-0626 treatment groups compared with MK-0626-untreated ob/ob mice, a trend that was sustained throughout the treatment period (Figure 1B). In addition, the food intake of ob/ob mice was not significantly altered by MK-0626 treatment, and this trend was sustained over the entire treatment period (Figure 1C). Body weight and food intake of the mice at the end of treatment are shown in Figure 1D and E, respectively. Body weight and food intake of the ob/ob mice significantly increased throughout the experimental period compared to their lean littermates regardless of MK-0626 treatment, except for food intake at week 1 and 7 of therapy (Figure 1B and C). MK-0626 treatment did not significantly change the ratio of liver weight to body weight at the end of treatment (Figure 1F).
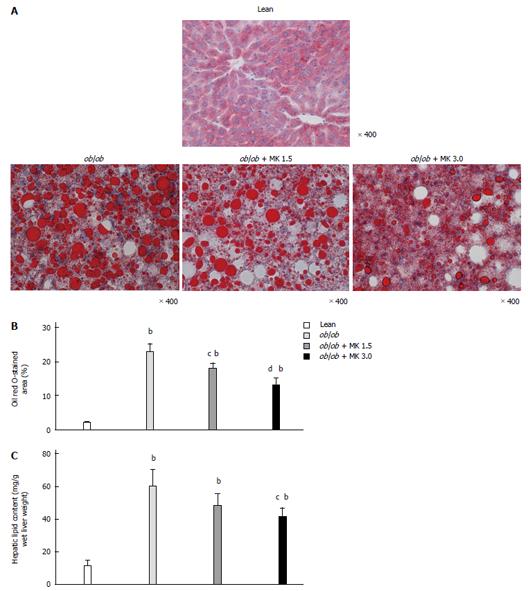
MK-0626-treated ob/ob mice had a significant reduction in Oil Red O-stained areas in the liver, and the reduction occurred in a dose-dependent manner (Figure 2A, B). Correspondingly, the hepatic lipid content was significantly reduced in MK-0626-treated ob/ob mice in a dose-dependent manner (Figure 2C).
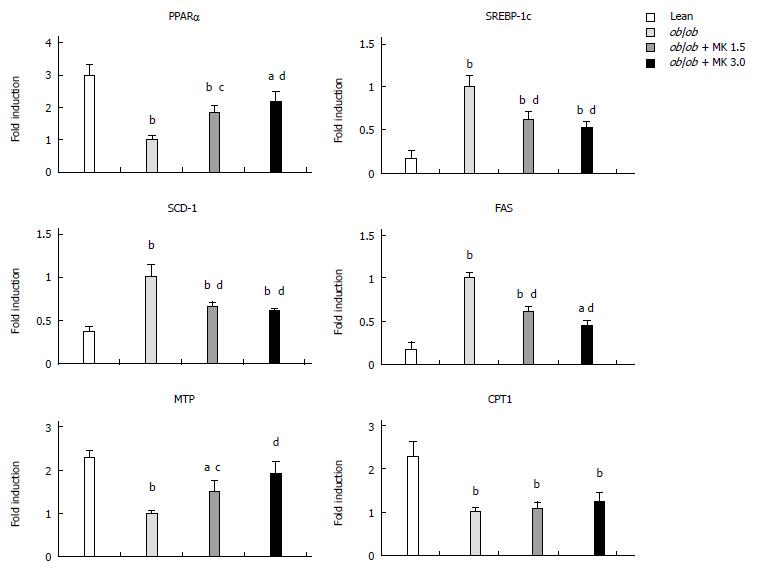
MK-0626 treatment increased PPARαand MTP mRNA expression and decreased the mRNA expression of SREBP-1c, SCD-1 and FAS
MK-0626 treatment significantly increased hepatic PPARα mRNA, a key element involved in β-oxidation of free fatty acids, compared with the MK-0626-untreated ob/ob control mice (Figure 3). MK-0626 treatment significantly reduced hepatic mRNA expression levels of SREBP-1c, FAS and SCD-1, key regulators of de novo hepatic lipogenesis, compared with MK-0626-untreated ob/ob control mice (Figure 3). In addition, MK-0626 treatment significantly increased MTP mRNA, a key factor responsible for intracellular lipid transport in the intestine and liver, compared with MK-0626-untreated ob/ob mice (Figure 3). However, MK-0626 treatment did not significantly change hepatic mRNA levels of CPT1, a key factor involved in lipolysis, compared with MK-0626-untreated ob/ob control mice (Figure 3).
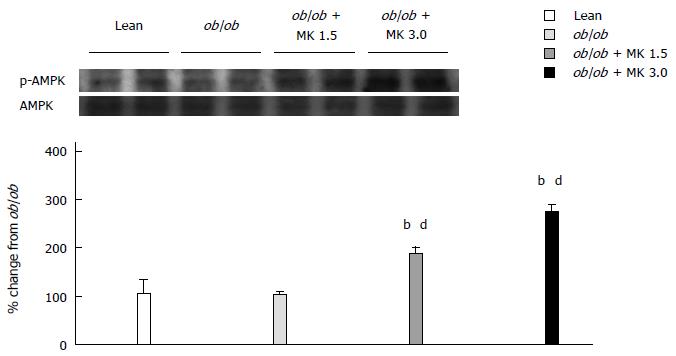
In a dose-dependent manner, MK-0626 treatment increased activation of AMPK, which is a sensor of cellular energy status and acts as a regulator of hepatic lipogenesis, in whole liver obtained from ob/ob mice compared with MK-0626-untreated ob/ob mice (Figure 4).
The major finding of our study was that MK-0626, a selective α-amino amide DPP-4 inhibitor, attenuated liver steatosis in experimental obese and diabetic mice. This conclusion is based on the result that MK-0626 decreased the degree of Oil red O-staining and hepatic lipid content in whole livers of ob/ob mice. However, the mechanism of this hypolipidemic effect was not due to a decrease in food intake and/or body weight because MK-0626 treatment did not significantly alter food intake and body weight in ob/ob mice. As for weight effects, a GLP-1 receptor agonist generally causes a weight loss of 1-4 kg in human studies[21-23] and a measurable weight loss in animal studies[10,24]. Conversely, DPP-4 inhibitors are weight neutral in patients with type 2 diabetes[25-27] and in animal studies[10,11,28], although a contradictory human study[29] exists. Our study showed that treatment with MK-0626 did not change body weight, which is consistent with the majority of prior reports. As for food intake, a DPP-4 inhibitor does not cause a decrease in food intake in animal studies[10,28], which is consistent with our study. Thus, the effect of MK-0626 on fatty liver was not due to a change in weight loss or food intake.
A possible mechanism of MK-0626 action on fatty livers is through enhancement of AMPK activity and the resultant inhibition of hepatic lipogenic gene expression. This hypothetical mechanism is supported by prior studies that demonstrated that high fat diet fed DPP4-deficient rats[9] reduce hepatic fat content and that wild-type and β-cell-specific glucokinase haploinsufficient (Gck+/-) diabetic mice treated with des-fluoro-sitagliptin (DFS), a DDP-4 inhibitor[11] also reduce grade of hepatic steatosis. A DPP4-deficient rat model is very useful for evaluating the action of DPP-4 inhibition. Although we also used ob/ob mice, one of the genetically modified animals, our model of oral administration of DPP4 inhibitor was also important in view of reflecting a more physiological state. Thus, we were able to achieve a dose-dependent effect of the DPP4 inhibitor on hepatic steatosis, which was supported by a dose-dependent increase in serum active GLP-1 concentrations in ob/ob mice. In diet-induced fatty livers from wild-type and Gck+/- diabetic mice, DFS treatment increased the mRNA expression of PPARα, which is one possible mechanism of ameliorating fatty liver[11] and is consistent with our results. In our study, we elucidated the increase in mRNA expression of microsomal triglyceride transfer protein as a new possible mechanism of ameliorating fatty liver. Another possible mechanism of ameliorating fatty liver is an increase in serum adiponectin, which was supported by Souza-Mello et al[13]. Adiponectin reportedly enhances AMPK and the PPARα pathway in the liver and skeletal muscle[30], which correlates well with our results. Interestingly, our results were also supported by data that demonstrate that plasma DPP-4 activity negatively correlates with plasma adiponectin in healthy young people[31].
In our model, MK-0626 improved the HOMA score, which is a surrogate measure of insulin resistance in a fasting state and tends to represent hepatic insulin resistance[32], which is consistent with the therapeutic effect of a DPP-4 inhibitor (P32/98) in the Vancouver diabetic fatty Zucker Rat[33] and a fructose-rich diet in normal rats[10] or sitagliptin in high-fat diet-induced obese rats[12]. However, normoglycemic DPP4-deficient rats did not show improved hepatic insulin sensitivity in vivo and maintained constant high levels of active GLP-1, implying that active GLP-1 has a direct effect on fat metabolism in a DPP4-deficient rat model[9]. Thus, dietary MK-0626 may improve insulin sensitivity when coupled with the direct effect of serum active GLP-1. A DPP-4 inhibitor may also be effective for nonalcoholic steatohepatitis in which insulin resistance plays a central role. However, there is a contradictory report that the HOMA-IR was not significantly changed with sitagliptin treatment in patients with type 2 diabetes[27].
In summary, we demonstrated that dietary MK-0626, a DPP-4 inhibitor, could attenuate hepatic steatosis by enhancing AMPK activity, inducing inhibition of hepatic lipogenic gene expression, enhancing triglyceride secretion from the liver and increasing serum adiponectin levels. Thus, some novel mechanisms of a DPP-4 inhibitor on hepatic steatosis were discovered in our study. Moreover, our study design was original and persuasive because of the following reasons: (1) we evaluated the dose response effect of a DPP-4 inhibitor on hepatic steatosis; (2) we used a naturally occurring model of hepatic steatosis and type 2 diabetes; (3) we used two distinct control groups; and (4) we used a sufficient number of mice in each group to achieve statistical power of the data outcomes. Because DPP-4 inhibitor medications are widely used in clinical practice and are characterized by good safety and tolerability profiles, these inhibitors may provide an effective treatment strategy for patients with hepatic steatosis induced by type II diabetes. Thus, the effects of a DPP-4 inhibitor on hepatic steatosis should be further evaluated in a large, prospective human study in the future.
The Nature Publishing Group Language Editing service provided writing assistance.
Nonalcoholic fatty liver disease is the hepatic manifestation of metabolic syndrome. Other comorbidities of metabolic syndrome include obesity, type 2 diabetes mellitus, hypertension and dyslipidemia coupled with insulin resistance, which is a central feature of metabolic syndrome. Thus, improving insulin sensitivity would decrease hepatic fat deposition and accordingly inhibit hepatocyte vulnerability to oxidative stress.
Administration of MK-0626, a dipeptidyl peptidase-4 inhibitor, ameliorated hepatic steatosis; reduced serum alanine aminotransferase, glucose, and insulin levels; reduced homeostatic model assessment scores; and increased serum adiponectin levels in ob/ob mice.
MK-0626 could attenuate hepatic steatosis through enhancing AMP-activated protein kinase activity, inhibiting hepatic lipogenic gene expression, enhancing triglyceride secretion from liver and increasing serum adiponectin levels.
The major finding of this experimental study was that MK-0626 attenuated liver steatosis. Interestingly authors demonstrated that this effect is not due to a decrease in food intake and/or body weight. They suggested that possible mechanisms are the inhibition of hepatic lipogenic gene expression, the enhancement of triglyceride secretion from the liver and the increase of serum adiponectin levels.
P- Reviewer: Di Minno MND, Navarro-Jarabo JM S- Editor: Gou SX L- Editor: A E- Editor: Liu XM
| 1. | Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842-845. [PubMed] |
| 2. | Kieffer TJ, Habener JF. The glucagon-like peptides. Endocr Rev. 1999;20:876-913. [PubMed] |
| 3. | Meier JJ, Nauck MA. Clinical endocrinology and metabolism. Glucose-dependent insulinotropic polypeptide/gastric inhibitory polypeptide. Best Pract Res Clin Endocrinol Metab. 2004;18:587-606. [PubMed] |
| 4. | Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696-1705. [PubMed] |
| 5. | Drucker DJ. Biological actions and therapeutic potential of the glucagon-like peptides. Gastroenterology. 2002;122:531-544. [PubMed] |
| 6. | Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153-165. [PubMed] |
| 7. | Mentlein R, Gallwitz B, Schmidt WE. Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-1(7-36)amide, peptide histidine methionine and is responsible for their degradation in human serum. Eur J Biochem. 1993;214:829-835. [PubMed] |
| 8. | Weber AE. Dipeptidyl peptidase IV inhibitors for the treatment of diabetes. J Med Chem. 2004;47:4135-4141. [PubMed] |
| 9. | Ben-Shlomo S, Zvibel I, Shnell M, Shlomai A, Chepurko E, Halpern Z, Barzilai N, Oren R, Fishman S. Glucagon-like peptide-1 reduces hepatic lipogenesis via activation of AMP-activated protein kinase. J Hepatol. 2011;54:1214-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 250] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 10. | Maiztegui B, Borelli MI, Madrid VG, Del Zotto H, Raschia MA, Francini F, Massa ML, Flores LE, Rebolledo OR, Gagliardino JJ. Sitagliptin prevents the development of metabolic and hormonal disturbances, increased β-cell apoptosis and liver steatosis induced by a fructose-rich diet in normal rats. Clin Sci (Lond). 2011;120:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Shirakawa J, Fujii H, Ohnuma K, Sato K, Ito Y, Kaji M, Sakamoto E, Koganei M, Sasaki H, Nagashima Y. Diet-induced adipose tissue inflammation and liver steatosis are prevented by DPP-4 inhibition in diabetic mice. Diabetes. 2011;60:1246-1257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 203] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 12. | Akaslan SB, Degertekin CK, Yilmaz G, Cakir N, Arslan M, Toruner FB. Effects of sitagliptin on nonalcoholic fatty liver disease in diet-induced obese rats. Metab Syndr Relat Disord. 2013;11:243-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Souza-Mello V, Gregório BM, Cardoso-de-Lemos FS, de Carvalho L, Aguila MB, Mandarim-de-Lacerda CA. Comparative effects of telmisartan, sitagliptin and metformin alone or in combination on obesity, insulin resistance, and liver and pancreas remodelling in C57BL/6 mice fed on a very high-fat diet. Clin Sci (Lond). 2010;119:239-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 111] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 14. | Iwasaki T, Yoneda M, Inamori M, Shirakawa J, Higurashi T, Maeda S, Terauchi Y, Nakajima A. Sitagliptin as a novel treatment agent for non-alcoholic Fatty liver disease patients with type 2 diabetes mellitus. Hepatogastroenterology. 2011;58:2103-2105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 15. | Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995;1:1155-1161. [PubMed] |
| 16. | Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995;269:546-549. [PubMed] |
| 17. | Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543-546. [PubMed] |
| 18. | Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540-543. [PubMed] |
| 19. | Edmondson SD, Mastracchio A, Mathvink RJ, He J, Harper B, Park YJ, Beconi M, Di Salvo J, Eiermann GJ, He H. (2S,3S)-3-Amino-4-(3,3-difluoropyrrolidin-1-yl)-N,N-dimethyl-4-oxo-2-(4-[1,2,4]triazolo[1,5-a]-pyridin-6-ylphenyl)butanamide: a selective alpha-amino amide dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. J Med Chem. 2006;49:3614-3627. [PubMed] |
| 20. | Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497-509. [PubMed] |
| 21. | Garber A, Henry R, Ratner R, Garcia-Hernandez PA, Rodriguez-Pattzi H, Olvera-Alvarez I, Hale PM, Zdravkovic M, Bode B. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373:473-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Nelson P, Poon T, Guan X, Schnabel C, Wintle M, Fineman M. The incretin mimetic exenatide as a monotherapy in patients with type 2 diabetes. Diabetes Technol Ther. 2007;9:317-326. [PubMed] |
| 23. | Blonde L, Klein EJ, Han J, Zhang B, Mac SM, Poon TH, Taylor KL, Trautmann ME, Kim DD, Kendall DM. Interim analysis of the effects of exenatide treatment on A1C, weight and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes. Diabetes Obes Metab. 2006;8:436-447. [PubMed] |
| 24. | Ding X, Saxena NK, Lin S, Gupta NA, Anania FA. Exendin-4, a glucagon-like protein-1 (GLP-1) receptor agonist, reverses hepatic steatosis in ob/ob mice. Hepatology. 2006;43:173-181. [PubMed] |
| 25. | Scott R, Wu M, Sanchez M, Stein P. Efficacy and tolerability of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy over 12 weeks in patients with type 2 diabetes. Int J Clin Pract. 2007;61:171-180. [PubMed] |
| 26. | Hanefeld M, Herman GA, Wu M, Mickel C, Sanchez M, Stein PP. Once-daily sitagliptin, a dipeptidyl peptidase-4 inhibitor, for the treatment of patients with type 2 diabetes. Curr Med Res Opin. 2007;23:1329-1339. [PubMed] |
| 27. | Rosenstock J, Sankoh S, List JF. Glucose-lowering activity of the dipeptidyl peptidase-4 inhibitor saxagliptin in drug-naive patients with type 2 diabetes. Diabetes Obes Metab. 2008;10:376-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Furuta Y, Horiguchi M, Sugaru E, Ono-Kishino M, Otani M, Sakai M, Masui Y, Tsuchida A, Sato Y, Takubo K. Chronic administration of DSP-7238, a novel, potent, specific and substrate-selective DPP IV inhibitor, improves glycaemic control and beta-cell damage in diabetic mice. Diabetes Obes Metab. 2010;12:421-430. [PubMed] |
| 29. | Yanai H, Adachi H, Hamasaki H, Masui Y, Yoshikawa R, Moriyama S, Mishima S, Sako A. Effects of 6-month sitagliptin treatment on glucose and lipid metabolism, blood pressure, body weight and renal function in type 2 diabetic patients: a chart-based analysis. J Clin Med Res. 2012;4:251-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 30. | Shehzad A, Iqbal W, Shehzad O, Lee YS. Adiponectin: regulation of its production and its role in human diseases. Hormones (Athens). 2012;11:8-20. [PubMed] |
| 31. | Kirino Y, Sei M, Kawazoe K, Minakuchi K, Sato Y. Plasma dipeptidyl peptidase 4 activity correlates with body mass index and the plasma adiponectin concentration in healthy young people. Endocr J. 2012;59:949-953. [PubMed] |