Published online Nov 14, 2014. doi: 10.3748/wjg.v20.i42.15467
Revised: March 24, 2014
Accepted: May 19, 2014
Published online: November 14, 2014
Processing time: 374 Days and 18.7 Hours
Angiogenesis is an essential process for organ growth and repair. Thus, an imbalance in this process can lead to several diseases including malignancy. Angiogenesis is a critical step in vascular remodeling, tissue damage and wound healing besides being required for invasive tumor growth and metastasis. Because angiogenesis sets an important point in the control of tumor progression, its inhibition is considered a valuable therapeutic approach for tumor treatment. Chronic liver disease including hepatitis C virus (HCV) infection is one of the main cause for the development of hepatic angiogenesis and thereby plays a critical role in the modulation of hepatic angiogenesis that finally leads to hepatocellular carcinoma progression and invasion. Thus, understanding of the molecular mechanisms of HCV-mediated hepatic angiogenesis will help design a therapeutic protocol for the intervention of HCV-mediated angiogenesis and subsequently its outcome. In this review, we will focus on the molecular mechanisms of HCV-mediated hepatic angiogenesis and the related signaling pathways that can be target for current and under development therapeutic approaches.
Core tip: This editorial elaborate the molecular mechanisms of hepatitis C virus (HCV)-mediated angiogenesis and its mechanisms, and the potential of angiogenic pathways as target for hepatocellular carcinoma therapy. We summarized the current knowledge of HCV-mediated angiogenesis and the possible therapeutic strategies.
- Citation: Hassan M, Selimovic D, El-Khattouti A, Soell M, Ghozlan H, Haikel Y, Abdelkader O, Megahed M. Hepatitis C virus-mediated angiogenesis: Molecular mechanisms and therapeutic strategies. World J Gastroenterol 2014; 20(42): 15467-15475
- URL: https://www.wjgnet.com/1007-9327/full/v20/i42/15467.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i42.15467
Hepatocellular carcinoma (HCC) is considered one of the most common cancers worldwide. Therefore, the limited treatment options and poor prognosis of HCC patients emphasize the importance of the development of a new therapeutic strategy. Chronic liver diseases including hepatitis C virus (HCV) infection are the major risk factors for developing HCC[1,2]. Although the molecular mechanisms that link HCV infections to the development and progression of HCC are not entirely characterized, increasing evidence indicates the involvement of hepatic angiogenesis in the modulation of HCV viral proteins-induced HCC malignancy[3-5]. Therefore, targeting the angiogenic signaling pathways is thought to be a relevant therapeutic strategy for tumor treatment. Accordingly, understanding the mechanistic role of HCV infection in the modulation of the imbalance of hepatic angiogenesis may help to develop novel therapeutic options for HCC treatment.
Angiogenesis is a dynamic, hypoxia stimulated and growth factor-dependent process that is responsible for the formation of new vascular structures from preexisting vessels[6,7]. Angiogenesis occurs in several organs in response to a pathophysiological alteration, and thereby is one of the most thoroughly studied pathophysiological phenomena. Besides its role in promotion of the etiopathogenesis of several diseases, angiogenesis is considered a potential therapeutic target for tumor treatment[8,9].
Hypoxia and inflammation are the main inducers of angiogenesis in liver and other organs[10-13]. Under hypoxia conditions angiogenesis is regulated through a mechanism mediated by hypoxia inducing factor (HIF)[14,15], where as its induction during the course of inflammation is regulated through a mechanism mediated by angiogenic cytokines and growth factors[11,16]. Thus, the formation of new functional vessels from preexisting vessels is mediated by tightly regulated mechanism, in which HIF plays a central role[17,18].
Although neo-angiogenesis is common for most chronic inflammatory and fibrogenic disorders, the processes of hepatic angiogenesis differ from homologous processes in other organs or tissues. This may be due to the unique phenotypic profile as well as to the functional role of both activated hepatic stellate cells and other liver myofibroblasts[19,20].
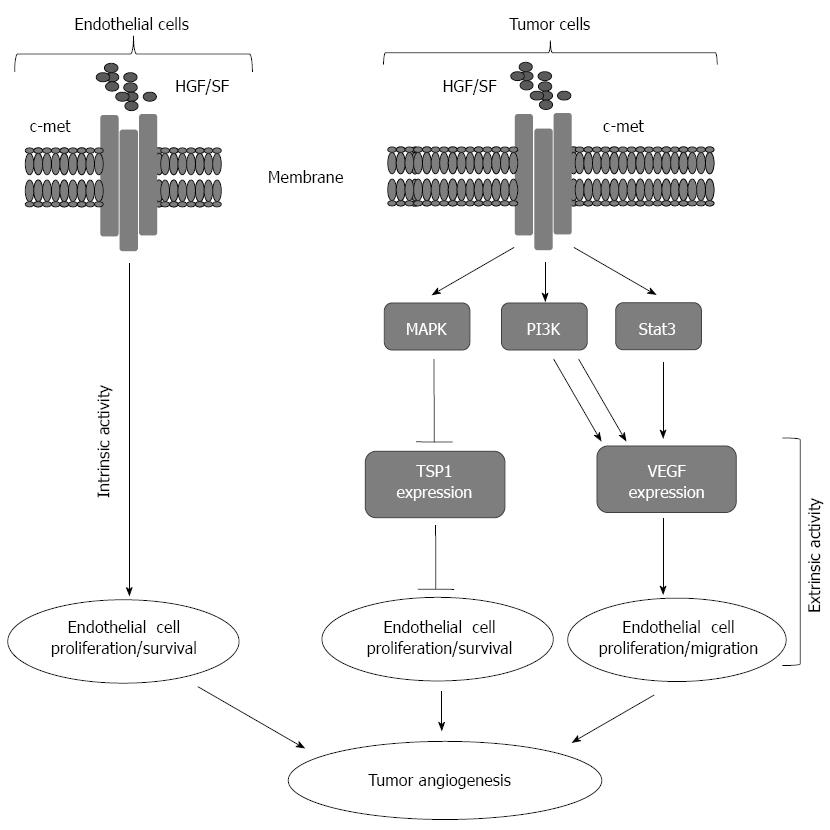
The regulation of angiogenesis is mediated by a mechanism regulated through the balance between the angiogenic growth factors and their inhibitors. These angiogenic growth factors can be released from different cell types including endothelial cells (ECs), monocytes, platelets, and smooth muscle as well as tumor cells[21]. Under normal physiological condition, the inhibitors of neovascularization is in excess in solid organs, and thereby can overcome the reservoir of growth factors that are essential for the initiation of the angiogenic process, a mechanism for the inhibition of neovascularization in solid organs[22-24]. Whereas, in tumors, the release of growth factors are in excess. Accordingly, the excess of the resleased growth factors has the ability to overcome the inhibitor of angiogenesis, and thereby contributes to the promotion of tumor progression. Thus, the initiation of the angiogenic process is an important mechanism for tumor development and progression. A model for the regulation of angiogenesis by hepatocyte growth factor via mechanism mediated by either vascular endothelial growth factor (VEGF) or thrombospondin 1 is shown (Figure 1)
The role of HCV infection in the regulation of hepatic angiogenesis is reported in several studies[5,25]. Also, the microvessel density in liver biopsies of patients with HCV chronic infection is significantly high when compared to those of patients with hepatitis B virus (HBV) infection[26]. Accordingly, in vitro analysis of HCV positive sera were found to stimulate the migration and the proliferation of human ECs[27]. These enhanced migration and proliferation of ECs are attributed to the HCV-induced production of VEGF[27]. There are two different types of microvascular structures in the liver including the large vessels that are mainly covered by a continuous endothelium, and the sinusoids that are lined by a fenestrated endothelium[28]. Sinusoidal capillarization identified by CD34-positive ECs that mainly reported in most HCCs[29,30]. Moreover, CD34-positive ECs have also been observed in the sinusoid of both higher-grade and lower-grade dysplastic nodules[31,32], as well as in HCV-associated HCC[33]. Also, the elevation of CD34 in response to the stimulation of ECs together with the detection of CD34 in liver biopsies of HCV infected patients provide evidence for the mechanistic role of HCV infection in the regulation of hepatic angiogenesis[5]. Although the direct relation between HCV infection and angiogenesis has been reported in vitro and in vivo, little is known about the molecular mechanisms, which are responsible for the modulation of HCV-promoted hepatic angiogenesis.
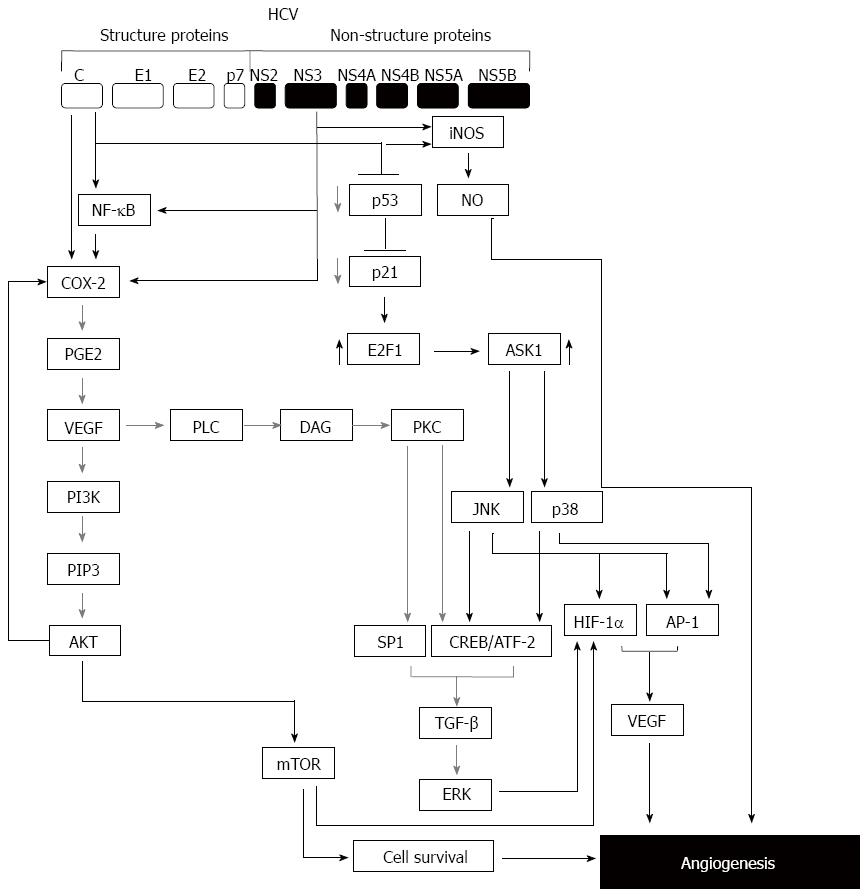
Accordingly, the infection of the liver derived cell line Huh7 with HCV subgenomic replicon was found to stabilize HIF-1α under normoxic conditions[34], an evidence for the involvement of HCV viral proteins in the regulation of HIF-1α, an essential factor for the regulation of angiogenesis. Further analysis of HCV proteins (structural or non-structural proteins) using several molecular biological techniques in combination with inhibitory experiments demonstrated that the oxidative stress, signal transducer and activator of transcription 3, nuclear transcription factor NF-κB, mitogen activated protein kinase (MAPK), phosphatidylinositol 3-kinases (PI3-kinases) play an essential role in the stabilization of HIF-1α[5,25]. Also, the role of HCV nonstructural proteins NS3 and NS4A-induced reactive oxygen species[35,36] seems to be essential for the stabilization of HIF-1α, that in turn, leads to the upregulation of VEGF and other angiogenic factors[5,37]. Also, the elevation of VEGF secretion in patient’s sera, in subgenomic replicon, and in HCV core-expressing Huh7 reveals an important role for HCV infection in the promotion of hepatic angiogenesis[5]. Moreover, HCV-induced VEGF, and subsequently the activation of endothelial have been reported to be regulated via mechanism mediated by multiple pathways including c-Jun-N-terminal kinase, p38 and extracellular regulated kinase (ERK)[5].
Although the several studies dealing with mechanistic role of HCV infection in the context of angiogenesis are limited, the induction of several angiogenic factors by HCV proteins has been demonstrated[5,38,39]. For example, elevation of Ang-2 in the sera of HCV-infected patients[39,40], as well as the upregulation of MMP-2 in response to expression of HCV viral proteins[41-43]. Furthermore, the enhancement of MMP-9 by HCV core protein is also reported[42,44-46]. Moreover, the overexpression of cyclooxygenase (COX)-2 in response to the expression of HCV core or NS5A in hepatocytes has been demonstrated in several studies[47-49]. The mechanism, by which HCV induces COX-2 has been investigated, and thought to be regulated via a mechanism mediated by HCV-induced oxidative stress[50].
The findings mentioned above are supported by a set of clinical investigations. For example, patients with HCV chronic infection revealed significant elevation of intrahepatic COX-2, MMP-2 and MMP-9[44]. These intrahepatic COX-2, MMP-2, and 9 along with VEGF and Ang-2 are thought to play an important role in the stimulation of angiogenesis in the context of HCV-associated HCC. A proposed model for the possible mechanisms demonstrating the pathways, which are involved in the modulation of HCV-induced hepatic angiogenesis is outlined in Figure 2.
The inhibition of angiogenesis is thought to be a relevant therapeutic strategy for HCC treatment. Despite of there has been poor efficacy with treatment using single-agents as anti-angiogenic approaches in advanced solid cancers[51,52], many molecular-targeted drugs have been proofed for their reliability in HCC treatment[53,54]. Although the multi-tyrosine kinase inhibitor sorafenib demonstrated an overall survival benefit for patients with HCC, the efficacy of anti-angiogenic agents, including sorafenib in HCC is limited[55]. This may due to that most of anti-angiogenic agents including sorafenib, can only target newly formed blood vessels rather than the matured one. As a consequence the vascular remodeling can substitute the eliminated newly formed vessels[56,57]. Apart from their ability to block the cell cycle of tumor cells, most anti-angiogenic agents fail to induce tumor death, a further limitation for their anti-tumor efficacy. Hence, the design of an anti-angiogenic approach for HCC treatment must be taken into account that targeting a unique signaling pathway by a small-molecule inhibitor is not sufficient to abrogate or even to block tumor development and progression. Thus, the combination of inhibitors of different angiogenic pathways may be more efficient.
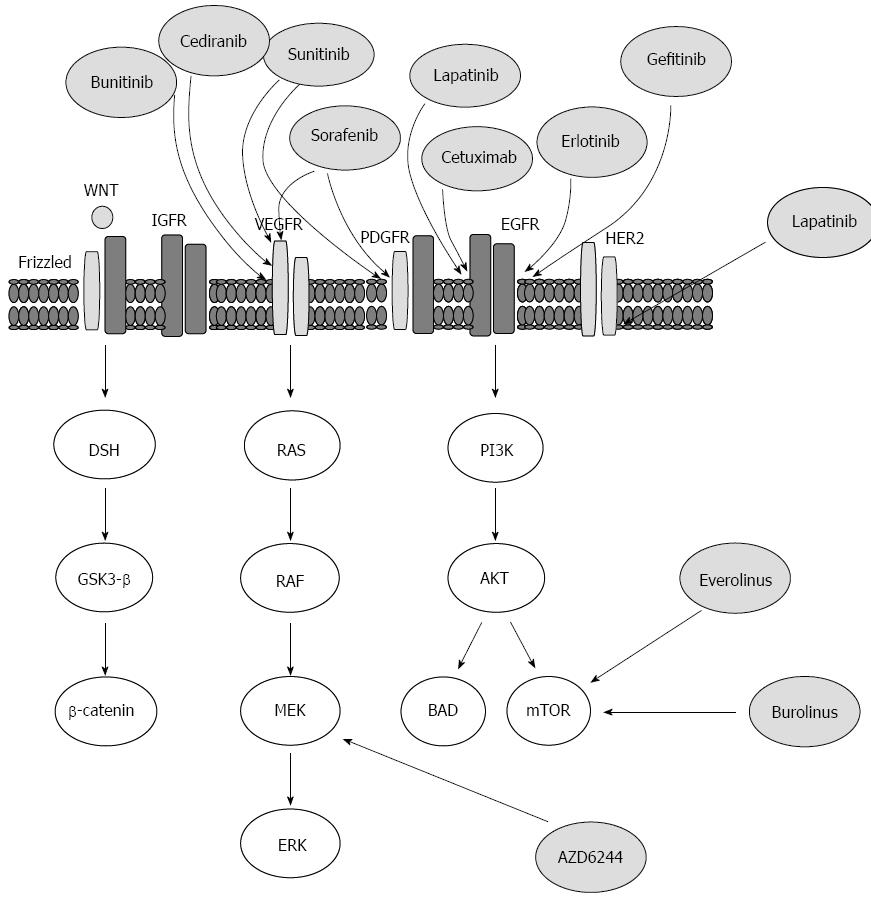
Tumor angiogenesis has received more attention as a potential target for therapeutic intervention. Although many of the research studies have focused on the inhibition of vascular endothelial growth factor receptor (VEGFR) or its ligand, VEGF[58,59], the VEGF/VEGFR axis, an important mediator of tumor angiogenesis, is only one of several angiogenic pathways that are essential for initiation and progression of angiogenesis[60-62]. Thus, recent evidence suggests that Src may be a mediator for the expression of multiple pro-angiogenic molecules[63,64]. Src is membrane-associated non-receptor protein tyrosine kinases, and is overexpressed and/or aberrantly activated in a variety of human tumors[65], therefore targeting of this pathway may be a relevant strategy for HCC treatment. Although some studies focused mainly on the ability of Src family kinase inhibitor that is acting directly on tumor cells through a mechanism mediated by the reduction of pro-angiogenic factors[66], the anti-angiogenic effect of Src family kinase inhibitors was found to be more efficient in vivo[61,67,68]. Thus, the inhibition of Src family kinase activity by highly potent and selective small-molecule inhibitor(s) may be a relevant therapeutic strategy for the treatment of human solid tumors.
Moreover, the ERK/MAP kinase also known as RAF/MEK/ERK pathway is a ubiquitous signal transduction pathway that is involved in the regulation of crucial cellular functions such as angiogenesis is thought to be a promising target for anti-angiogenic agents[69-71]. The activation of this pathway through the overexpression or activation of its components contributes to the regulation of angiogenesis that, in turn, leads to tumor progression and metastasis[5,71]. The ERK/MAPK pathway is a downstream pathway of various growth factors such as insulin growth factor receptor, endothelial growth factor (EGFR), VEGFR, and platelet-derived growth factor receptor (PDGFR), consequently the ERK/MAPK pathway is thought to be a valid therapeutic target for the treatment of HCC[72-76].
Furthermore, constitutive activation of the PI3K/AKT/mTOR signaling pathway has been established as determinant of cell growth and survival in solid tumors including HCC[77]. The activation of PI3K/AKT/mTOR signaling pathway can be mediated by the enhanced activation of tyrosine kinases receptors such as those of IGF and EGF[78]. The expression of both EGF and IGF receptors is upregulated in HCC and cirrhotic liver[79]. Accordingly, PI3K/AKT/mTOR signaling pathway may be a potential target for the development of therapeutic approaches for HCC treatment. Also, WNT/β-catenin pathway is considered a promised therapeutic target of HCC treatment, based on its potential role in the regulation of major and early carcinogenic processes of HCC[80].
Ligands that bind to the EGFR, such as EGF, play a central role both in tumor angiogenesis and proliferation, via mechanism mediated by the activation of RAF/MEK/ERK and PI3/AKT/mTOR pathways[81]. Thus, based on their efficacy in the treatment of most solid tumors, targeting of EGF/EGFR signaling pathway may be beneficial for HCC treatment[82]. As a result, variable therapeutic targets have been developed based on the reliability of this pathway as a relevant therapeutic target for tumor treatment. Thus, the current agents targeting EGFR in HCC includes erlotinib, lapatinip and gefitinib, as well as the monoclonal antibody cetuximab[83].
Based on the fact that the activation of IGF signaling pathway induced potent proliferative effects in hepatocytes and thereby promotes the development and progression of HCC, the targeting of this signaling pathway offers a relevant therapeutic intervention for HCC treatment. Thus, the inactivation of IGF-1R can induce growth inhibition, apoptosis or cell cycle arrest[84,85]. Also, the blockade of IGF-1R consequently leads to inhibition of its downstream signaling pathways in solid tumors[86]. Therefore, the development of a small-molecule inhibitor for IGF-1R may be relevant for HCC treatment. The targeted therapies currently available or those under the development for HCC treatment together with their possible molecular targets are outlined (Figure 3).
Hepatic angiogenesis sets an important point in the control of HCC progression, its inhibition is considered a valuable therapeutic approach for HCC treatment. In recent years, several studies focused on the investigation of cellular signaling mechanisms underlying HCC development, progression and invasion. In addition to the genetic alterations, chronic liver diseases including HCV infection clearly has a major role in the development and progression of HCC. Because chronic HCV infection is implicated in the modulation of abnormalities in several critical molecular signaling pathways, the attention of clinicians and researchers has focused on the mechanistic role of HCV infection in the regulation of HCC-associated signaling pathways. These pathways include both extra and intracellular-mediated mechanisms, which among them are the MAPK, PI3K/mTOR, WNT/β-catenin and IGF, and growth factor associated angiogenic signaling. Although a direct link between HCV infection and angiogenesis has been suggested in several studies, it is not clear, which factors actually drive the angiogenesis during the course of HCV infection. Further analysis is needed to address, in detail, the molecular mechanisms of HCV-induced hepatic angiogenesis. The investigation of these mechanisms may help to improve current therapies and in the design of an efficient alternative approach for HCC treatment. Thus, targeting signaling pathways that are directly involved in the regulation of hepatic angiogenesis may be a powerful strategy for HCC treatment.
P- Reviewer: Douglas MW, Lee JC, Nischalke HD S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN
| 1. | Jeong SW, Jang JY, Chung RT. Hepatitis C virus and hepatocarcinogenesis. Clin Mol Hepatol. 2012;18:347-356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 2. | Selimovic D, El-Khattouti A, Ghozlan H, Haikel Y, Abdelkader O, Hassan M. Hepatitis C virus-related hepatocellular carcinoma: An insight into molecular mechanisms and therapeutic strategies. World J Hepatol. 2012;4:342-355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Honda M, Nakamura M, Tateno M, Sakai A, Shimakami T, Shirasaki T, Yamashita T, Arai K, Yamashita T, Sakai Y. Differential interferon signaling in liver lobule and portal area cells under treatment for chronic hepatitis C. J Hepatol. 2010;53:817-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Pereira Tde A, Witek RP, Syn WK, Choi SS, Bradrick S, Karaca GF, Agboola KM, Jung Y, Omenetti A, Moylan CA. Viral factors induce Hedgehog pathway activation in humans with viral hepatitis, cirrhosis, and hepatocellular carcinoma. Lab Invest. 2010;90:1690-1703. [PubMed] |
| 5. | Hassan M, Selimovic D, Ghozlan H, Abdel-kader O. Hepatitis C virus core protein triggers hepatic angiogenesis by a mechanism including multiple pathways. Hepatology. 2009;49:1469-1482. [PubMed] |
| 6. | Paternostro C, David E, Novo E, Parola M. Hypoxia, angiogenesis and liver fibrogenesis in the progression of chronic liver diseases. World J Gastroenterol. 2010;16:281-288. [PubMed] |
| 7. | Valfrè di Bonzo L, Novo E, Cannito S, Busletta C, Paternostro C, Povero D, Parola M. Angiogenesis and liver fibrogenesis. Histol Histopathol. 2009;24:1323-1341. [PubMed] |
| 8. | Syed F, Sherris D, Paus R, Varmeh S, Singh S, Pandolfi PP, Bayat A. Keloid disease can be inhibited by antagonizing excessive mTOR signaling with a novel dual TORC1/2 inhibitor. Am J Pathol. 2012;181:1642-1658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Kundu SK, Nestor M. Targeted therapy in head and neck cancer. Tumour Biol. 2012;33:707-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 10. | Tanaka T, Nangaku M. Angiogenesis and hypoxia in the kidney. Nat Rev Nephrol. 2013;9:211-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 11. | Wu Y, Antony S, Meitzler JL, Doroshow JH. Molecular mechanisms underlying chronic inflammation-associated cancers. Cancer Lett. 2014;345:164-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 205] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 12. | Cullberg KB, Olholm J, Paulsen SK, Foldager CB, Lind M, Richelsen B, Pedersen SB. Resveratrol has inhibitory effects on the hypoxia-induced inflammation and angiogenesis in human adipose tissue in vitro. Eur J Pharm Sci. 2013;49:251-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Choi KS, Song H, Kim EH, Choi JH, Hong H, Han YM, Hahm KB. Inhibition of Hydrogen Sulfide-induced Angiogenesis and Inflammation in Vascular Endothelial Cells: Potential Mechanisms of Gastric Cancer Prevention by Korean Red Ginseng. J Ginseng Res. 2012;36:135-145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Cannito S, Paternostro C, Busletta C, Bocca C, Colombatto S, Miglietta A, Novo E, Parola M. Hypoxia, hypoxia-inducible factors and fibrogenesis in chronic liver diseases. Histol Histopathol. 2014;29:33-44. [PubMed] |
| 15. | Badr S, Salem A, Yuosif AH, Awadallah H, Awed N, Bakr A. Hypoxia inducible factor-1alpha and microvessel density as angiogenic factors in bilharzial and non-bilharzial bladder cancer. Clin Lab. 2013;59:805-812. [PubMed] |
| 16. | Jaipersad AS, Lip GY, Silverman S, Shantsila E. The role of monocytes in angiogenesis and atherosclerosis. J Am Coll Cardiol. 2014;63:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 330] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 17. | Rhoads RP, Johnson RM, Rathbone CR, Liu X, Temm-Grove C, Sheehan SM, Hoying JB, Allen RE. Satellite cell-mediated angiogenesis in vitro coincides with a functional hypoxia-inducible factor pathway. Am J Physiol Cell Physiol. 2009;296:C1321-C1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 18. | Berger M, Bergers G, Arnold B, Hämmerling GJ, Ganss R. Regulator of G-protein signaling-5 induction in pericytes coincides with active vessel remodeling during neovascularization. Blood. 2005;105:1094-1101. [PubMed] |
| 19. | Sciacca L, Vigneri R, Tumminia A, Frasca F, Squatrito S, Frittitta L, Vigneri P. Clinical and molecular mechanisms favoring cancer initiation and progression in diabetic patients. Nutr Metab Cardiovasc Dis. 2013;23:808-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 20. | Langley RR, Ramirez KM, Tsan RZ, Van Arsdall M, Nilsson MB, Fidler IJ. Tissue-specific microvascular endothelial cell lines from H-2K(b)-tsA58 mice for studies of angiogenesis and metastasis. Cancer Res. 2003;63:2971-2976. [PubMed] |
| 21. | Tandle A, Blazer DG, Libutti SK. Antiangiogenic gene therapy of cancer: recent developments. J Transl Med. 2004;2:22. [PubMed] |
| 22. | Na HJ, Hwang JY, Lee KS, Choi YK, Choe J, Kim JY, Moon HE, Kim KW, Koh GY, Lee H. TRAIL negatively regulates VEGF-induced angiogenesis via caspase-8-mediated enzymatic and non-enzymatic functions. Angiogenesis. 2014;17:179-194. [PubMed] |
| 23. | Symeonidis N, Papakonstantinou E, Psarras K, Ballas K, Pavlidis T, Karakiulakis G, Sakantamis A. The effect of celecoxib administration on the healing and neovascularization of colonic anastomosis in rats. J Invest Surg. 2014;27:139-146. [PubMed] |
| 24. | Westenskow PD, Kurihara T, Aguilar E, Scheppke EL, Moreno SK, Wittgrove C, Marchetti V, Michael IP, Anand S, Nagy A. Ras pathway inhibition prevents neovascularization by repressing endothelial cell sprouting. J Clin Invest. 2013;123:4900-4908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 25. | Nasimuzzaman M, Waris G, Mikolon D, Stupack DG, Siddiqui A. Hepatitis C virus stabilizes hypoxia-inducible factor 1alpha and stimulates the synthesis of vascular endothelial growth factor. J Virol. 2007;81:10249-10257. [PubMed] |
| 26. | Mazzanti R, Messerini L, Monsacchi L, Buzzelli G, Zignego AL, Foschi M, Monti M, Laffi G, Morbidelli L, Fantappié O. Chronic viral hepatitis induced by hepatitis C but not hepatitis B virus infection correlates with increased liver angiogenesis. Hepatology. 1997;25:229-234. [PubMed] |
| 27. | Wilson GK, Brimacombe CL, Rowe IA, Reynolds GM, Fletcher NF, Stamataki Z, Bhogal RH, Simões ML, Ashcroft M, Afford SC. A dual role for hypoxia inducible factor-1α in the hepatitis C virus lifecycle and hepatoma migration. J Hepatol. 2012;56:803-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 28. | McCuskey RS, Reilly FD. Hepatic microvasculature: dynamic structure and its regulation. Semin Liver Dis. 1993;13:1-12. [PubMed] |
| 29. | Ruck P, Xiao JC, Kaiserling E. Immunoreactivity of sinusoids in hepatocellular carcinoma. An immunohistochemical study using lectin UEA-1 and antibodies against endothelial markers, including CD34. Arch Pathol Lab Med. 1995;119:173-178. [PubMed] |
| 30. | Cui S, Hano H, Sakata A, Harada T, Liu T, Takai S, Ushigome S. Enhanced CD34 expression of sinusoid-like vascular endothelial cells in hepatocellular carcinoma. Pathol Int. 1996;46:751-756. [PubMed] |
| 31. | Park YN, Yang CP, Fernandez GJ, Cubukcu O, Thung SN, Theise ND. Neoangiogenesis and sinusoidal “capillarization” in dysplastic nodules of the liver. Am J Surg Pathol. 1998;22:656-662. [PubMed] |
| 32. | Dhillon AP, Colombari R, Savage K, Scheuer PJ. An immunohistochemical study of the blood vessels within primary hepatocellular tumours. Liver. 1992;12:311-318. [PubMed] |
| 33. | Ohmori S, Shiraki K, Sugimoto K, Sakai T, Fujikawa K, Wagayama H, Takase K, Nakano T. High expression of CD34-positive sinusoidal endothelial cells is a risk factor for hepatocellular carcinoma in patients with HCV-associated chronic liver diseases. Hum Pathol. 2001;32:1363-1370. [PubMed] |
| 34. | Abe M, Koga H, Yoshida T, Masuda H, Iwamoto H, Sakata M, Hanada S, Nakamura T, Taniguchi E, Kawaguchi T. Hepatitis C virus core protein upregulates the expression of vascular endothelial growth factor via the nuclear factor-κB/hypoxia-inducible factor-1α axis under hypoxic conditions. Hepatol Res. 2012;42:591-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 35. | Novo E, Povero D, Busletta C, Paternostro C, di Bonzo LV, Cannito S, Compagnone A, Bandino A, Marra F, Colombatto S. The biphasic nature of hypoxia-induced directional migration of activated human hepatic stellate cells. J Pathol. 2012;226:588-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 36. | Selimović D, Hassan M. Inhibition of hepatitis C virus (HCV) core protein- induced cell growth by non-structural protein 4A (NS4A) is mediated by mitochondrial dysregulation. Bosn J Basic Med Sci. 2008;8:4-11. [PubMed] |
| 37. | Mas VR, Maluf DG, Archer KJ, Yanek KC, Fisher RA. Angiogenesis soluble factors as hepatocellular carcinoma noninvasive markers for monitoring hepatitis C virus cirrhotic patients awaiting liver transplantation. Transplantation. 2007;84:1262-1271. [PubMed] |
| 38. | Vespasiani-Gentilucci U, Galati G, Mazzarelli C, D’Avola D, Spataro S, Gallo P, Rigon A, Pellicelli A, Dicuonzo G, Afeltra A. Angiogenic cytokines in patients undergoing antiviral treatment for chronic hepatitis C virus infection. J Interferon Cytokine Res. 2011;31:207-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 39. | Talaat RM. Soluble angiogenesis factors in sera of Egyptian patients with hepatitis C virus infection: correlation with disease severity. Viral Immunol. 2010;23:151-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 40. | Rodríguez-Muñoz Y, Martín-Vílchez S, López-Rodríguez R, Hernández-Bartolomé Á, García-Buey L, Borque MJ, Moreno-Otero R, Sanz-Cameno P. Preliminary evidence of sustained expression of angiopoietin-2 during monocyte differentiation in chronic hepatitis C. Liver Int. 2013;33:864-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 41. | Li Y, Chen J, Wu C, Wang L, Lu M, Chen X. Hepatitis B virus/hepatitis C virus upregulate angiopoietin-2 expression through mitogen-activated protein kinase pathway. Hepatol Res. 2010;40:1022-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 42. | Li Y, Zhang Q, Liu Y, Luo Z, Kang L, Qu J, Liu W, Xia X, Liu Y, Wu K. Hepatitis C virus activates Bcl-2 and MMP-2 expression through multiple cellular signaling pathways. J Virol. 2012;86:12531-12543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 43. | Akca G, Tunçbilek S, Sepici-Dinçel A. Association between matrix metalloproteinase (MMP)-2, MMP-9 and total antioxidant status of patients with asymptomatic hepatitis C virus infection. Lett Appl Microbiol. 2013;57:436-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 44. | Núñez O, Fernández-Martínez A, Majano PL, Apolinario A, Gómez-Gonzalo M, Benedicto I, López-Cabrera M, Boscá L, Clemente G, García-Monzón C. Increased intrahepatic cyclooxygenase 2, matrix metalloproteinase 2, and matrix metalloproteinase 9 expression is associated with progressive liver disease in chronic hepatitis C virus infection: role of viral core and NS5A proteins. Gut. 2004;53:1665-1672. [PubMed] |
| 45. | Gadd VL, Melino M, Roy S, Horsfall L, O’Rourke P, Williams MR, Irvine KM, Sweet MJ, Jonsson JR, Clouston AD. Portal, but not lobular, macrophages express matrix metalloproteinase-9: association with the ductular reaction and fibrosis in chronic hepatitis C. Liver Int. 2013;33:569-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 46. | Nanda KS, Brady JJ, Murray BF, Sullivan O, Fearon U, McKenna MJ, Hegarty JE, O’Farrelly C, Ryan EJ. Elevated circulating osteoprotegerin and reduced matrix-metalloprotease-9 in post-menopausal women with chronic Hepatitis C virus infection. Cytokine. 2012;60:328-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 47. | Jahan S, Khaliq S, Ijaz B, Ahmad W, Hassan S. Role of HCV Core gene of genotype 1a and 3a and host gene Cox-2 in HCV-induced pathogenesis. Virol J. 2011;8:155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 48. | Lu L, Wei L, Peng G, Mu Y, Wu K, Kang L, Yan X, Zhu Y, Wu J. NS3 protein of hepatitis C virus regulates cyclooxygenase-2 expression through multiple signaling pathways. Virology. 2008;371:61-70. [PubMed] |
| 49. | Manning DS, Sheehan KM, Byrne MF, Kay EW, Murray FE. Cyclooxygenase-2 expression in chronic hepatitis C and the effect of interferon alpha treatment. J Gastroenterol Hepatol. 2007;22:1633-1637. [PubMed] |
| 50. | Waris G, Siddiqui A. Hepatitis C virus stimulates the expression of cyclooxygenase-2 via oxidative stress: role of prostaglandin E2 in RNA replication. J Virol. 2005;79:9725-9734. [PubMed] |
| 51. | Rohrberg KS, Olesen RK, Pfeiffer P, Ladekarl M, Pappot H, Christensen IJ, Høyer-Hansen G, Sørensen M, Skov BG, Buysschaert I. Phase II trial of erlotinib and bevacizumab in patients with advanced upper gastrointestinal cancers. Acta Oncol. 2012;51:234-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 52. | Reiriz AB, Richter MF, Fernandes S, Cancela AI, Costa TD, Di Leone LP, Schwartsmann G. Phase II study of thalidomide in patients with metastatic malignant melanoma. Melanoma Res. 2004;14:527-531. [PubMed] |
| 53. | Shin JM, Jeong YJ, Cho HJ, Park KK, Chung IK, Lee IK, Kwak JY, Chang HW, Kim CH, Moon SK. Melittin suppresses HIF-1α/VEGF expression through inhibition of ERK and mTOR/p70S6K pathway in human cervical carcinoma cells. PLoS One. 2013;8:e69380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 54. | Tsimberidou AM, Fu S, Ng C, Lim JA, Wen S, Hong D, Wheler J, Bedikian AY, Eng C, Wallace M. A phase 1 study of hepatic arterial infusion of oxaliplatin in combination with systemic 5-fluorouracil, leucovorin, and bevacizumab in patients with advanced solid tumors metastatic to the liver. Cancer. 2010;116:4086-4094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 55. | Rizell M, Andersson M, Cahlin C, Hafström L, Olausson M, Lindnér P. Effects of the mTOR inhibitor sirolimus in patients with hepatocellular and cholangiocellular cancer. Int J Clin Oncol. 2008;13:66-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 105] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 56. | Lee S, Kim BK, Kim SU, Park Y, Chang S, Park JY, Kim do Y, Ahn SH, Chon CY, Han KH. Efficacy of sorafenib monotherapy versus sorafenib-based loco-regional treatments in advanced hepatocellular carcinoma. PLoS One. 2013;8:e77240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 57. | Whittaker S, Marais R, Zhu AX. The role of signaling pathways in the development and treatment of hepatocellular carcinoma. Oncogene. 2010;29:4989-5005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 608] [Cited by in RCA: 680] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 58. | Stollman TH, Ruers TJ, Oyen WJ, Boerman OC. New targeted probes for radioimaging of angiogenesis. Methods. 2009;48:188-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 59. | Huang KF, Yang HY, Xing YM, Lin JS, Diao Y. Recombinant human kallistatin inhibits angiogenesis by blocking VEGF signaling pathway. J Cell Biochem. 2014;115:575-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 60. | Fujita H, Miyadera K, Kato M, Fujioka Y, Ochiiwa H, Huang J, Ito K, Aoyagi Y, Takenaka T, Suzuki T. The novel VEGF receptor/MET-targeted kinase inhibitor TAS-115 has marked in vivo antitumor properties and a favorable tolerability profile. Mol Cancer Ther. 2013;12:2685-2696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 61. | Fraisl P. Crosstalk between oxygen- and nitric oxide-dependent signaling pathways in angiogenesis. Exp Cell Res. 2013;319:1331-1339. [PubMed] |
| 62. | Liu L, Gaboriaud N, Vougogianopoulou K, Tian Y, Wu J, Wen W, Skaltsounis L, Jove R. MLS-2384, a new 6-bromoindirubin derivative with dual JAK/Src kinase inhibitory activity, suppresses growth of diverse cancer cells. Cancer Biol Ther. 2014;15:178-184. [PubMed] |
| 63. | Aronis KN, Chamberland JP, Mantzoros CS. GLP-1 promotes angiogenesis in human endothelial cells in a dose-dependent manner, through the Akt, Src and PKC pathways. Metabolism. 2013;62:1279-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 64. | Li Q, Fu GB, Zheng JT, He J, Niu XB, Chen QD, Yin Y, Qian X, Xu Q, Wang M. NADPH oxidase subunit p22(phox)-mediated reactive oxygen species contribute to angiogenesis and tumor growth through AKT and ERK1/2 signaling pathways in prostate cancer. Biochim Biophys Acta. 2013;1833:3375-3385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 65. | Zgheib A, Lamy S, Annabi B. Epigallocatechin gallate targeting of membrane type 1 matrix metalloproteinase-mediated Src and Janus kinase/signal transducers and activators of transcription 3 signaling inhibits transcription of colony-stimulating factors 2 and 3 in mesenchymal stromal cells. J Biol Chem. 2013;288:13378-13386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 66. | Ma X, Shao Y, Zheng H, Li M, Li W, Xue L. Src42A modulates tumor invasion and cell death via Ben/dUev1a-mediated JNK activation in Drosophila. Cell Death Dis. 2013;4:e864. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 67. | Park SI, Shah AN, Zhang J, Gallick GE. Regulation of angiogenesis and vascular permeability by Src family kinases: opportunities for therapeutic treatment of solid tumors. Expert Opin Ther Targets. 2007;11:1207-1217. [PubMed] |
| 68. | Mao H, Kano G, Hudson SA, Brummet M, Zimmermann N, Zhu Z, Bochner BS. Mechanisms of Siglec-F-induced eosinophil apoptosis: a role for caspases but not for SHP-1, Src kinases, NADPH oxidase or reactive oxygen. PLoS One. 2013;8:e68143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 69. | Oneyama C, Hikita T, Nada S, Okada M. Functional dissection of transformation by c-Src and v-Src. Genes Cells. 2008;13:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 70. | Srinivasan R, Zabuawala T, Huang H, Zhang J, Gulati P, Fernandez S, Karlo JC, Landreth GE, Leone G, Ostrowski MC. Erk1 and Erk2 regulate endothelial cell proliferation and migration during mouse embryonic angiogenesis. PLoS One. 2009;4:e8283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 138] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 71. | Yang YH, Wang Y, Lam KS, Yau MH, Cheng KK, Zhang J, Zhu W, Wu D, Xu A. Suppression of the Raf/MEK/ERK signaling cascade and inhibition of angiogenesis by the carboxyl terminus of angiopoietin-like protein 4. Arterioscler Thromb Vasc Biol. 2008;28:835-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 92] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 72. | Liu L, Cao Y, Chen C, Zhang X, McNabola A, Wilkie D, Wilhelm S, Lynch M, Carter C. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006;66:11851-11858. [PubMed] |
| 73. | Cui XD, Lee MJ, Kim JH, Hao PP, Liu L, Yu GR, Kim DG. Activation of mammalian target of rapamycin complex 1 (mTORC1) and Raf/Pyk2 by growth factor-mediated Eph receptor 2 (EphA2) is required for cholangiocarcinoma growth and metastasis. Hepatology. 2013;57:2248-2260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 74. | Beei C, Iwamoto N, Inaba T, Shinkai Y, Kumagai Y. Activation of EGFR/MEK/ERK/AP-1 signaling mediated by 1,2-naphthoquinone, an atmospheric electrophile, in human pulmonary A549 cells. J Toxicol Sci. 2013;38:793-797. [PubMed] |
| 75. | Mendivil A, Zhou C, Cantrell LA, Gehrig PA, Malloy KM, Blok LJ, Burger CW, Bae-Jump VL. AMG 479, a novel IGF-1-R antibody, inhibits endometrial cancer cell proliferation through disruption of the PI3K/Akt and MAPK pathways. Reprod Sci. 2011;18:832-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 76. | Schnidar H, Eberl M, Klingler S, Mangelberger D, Kasper M, Hauser-Kronberger C, Regl G, Kroismayr R, Moriggl R, Sibilia M. Epidermal growth factor receptor signaling synergizes with Hedgehog/GLI in oncogenic transformation via activation of the MEK/ERK/JUN pathway. Cancer Res. 2009;69:1284-1292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 168] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 77. | Gedaly R, Angulo P, Hundley J, Daily MF, Chen C, Koch A, Evers BM. PI-103 and sorafenib inhibit hepatocellular carcinoma cell proliferation by blocking Ras/Raf/MAPK and PI3K/AKT/mTOR pathways. Anticancer Res. 2010;30:4951-4958. [PubMed] |
| 78. | Ríos-Moreno MJ, Jaramillo S, Díaz-Delgado M, Sánchez-León M, Trigo-Sánchez I, Padillo JP, Amérigo J, González-Cámpora R. Differential activation of MAPK and PI3K/AKT/mTOR pathways and IGF1R expression in gastrointestinal stromal tumors. Anticancer Res. 2011;31:3019-3025. [PubMed] |
| 79. | Bodzin AS, Wei Z, Hurtt R, Gu T, Doria C. Gefitinib resistance in HCC mahlavu cells: upregulation of CD133 expression, activation of IGF-1R signaling pathway, and enhancement of IGF-1R nuclear translocation. J Cell Physiol. 2012;227:2947-2952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 80. | Lionello M, Lovato A, Staffieri A, Blandamura S, Turato C, Giacomelli L, Staffieri C, Marioni G. The EGFR-mTOR pathway and laryngeal cancer angiogenesis. Eur Arch Otorhinolaryngol. 2014;271:757-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 81. | Park JY, Park WS, Nam SW, Kim SY, Lee SH, Yoo NJ, Lee JY, Park CK. Mutations of beta-catenin and AXIN I genes are a late event in human hepatocellular carcinogenesis. Liver Int. 2005;25:70-76. [PubMed] |
| 82. | Höpfner M, Sutter AP, Huether A, Schuppan D, Zeitz M, Scherübl H. Targeting the epidermal growth factor receptor by gefitinib for treatment of hepatocellular carcinoma. J Hepatol. 2004;41:1008-1016. [PubMed] |
| 83. | Steiner P, Joynes C, Bassi R, Wang S, Tonra JR, Hadari YR, Hicklin DJ. Tumor growth inhibition with cetuximab and chemotherapy in non-small cell lung cancer xenografts expressing wild-type and mutated epidermal growth factor receptor. Clin Cancer Res. 2007;13:1540-1551. [PubMed] |
| 84. | Zhao H, Desai V, Wang J, Epstein DM, Miglarese M, Buck E. Epithelial-mesenchymal transition predicts sensitivity to the dual IGF-1R/IR inhibitor OSI-906 in hepatocellular carcinoma cell lines. Mol Cancer Ther. 2012;11:503-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 85. | Yao WF, Liu JW, Sheng GL, Huang DS. Blockade of IGF-IR exerts anticancer effects in hepatocellular carcinoma. Mol Med Rep. 2011;4:719-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 86. | Höpfner M, Huether A, Sutter AP, Baradari V, Schuppan D, Scherübl H. Blockade of IGF-1 receptor tyrosine kinase has antineoplastic effects in hepatocellular carcinoma cells. Biochem Pharmacol. 2006;71:1435-1448. [PubMed] |