Published online Nov 7, 2014. doi: 10.3748/wjg.v20.i41.15299
Revised: June 8, 2014
Accepted: June 26, 2014
Published online: November 7, 2014
Processing time: 217 Days and 7.2 Hours
AIM: To evaluate the role of baicalin in ulcerative colitis (UC) with regard to the CD4+CD29+ T helper cell, its surface markers and serum inflammatory cytokines.
METHODS: Flow cytometry was used to detect the percentage of CD4+CD29+ cells in patients with UC. Real time polymerase chain reaction was used to detect expression of GATA-3, forkhead box P3, T-box expressed in T cells (T-bet), and retinoic acid-related orphan nuclear hormone receptor C (RORC). Western blotting was used to analyze expression of nuclear factor-κB (NF-κB) p65, phosphorylation of NF-κB (p-NF-κB) p65, STAT4, p-STAT4, STAT6 and p-STAT6. The concentrations of interferon-γ (IFN-γ), interleukin (IL)-4, IL-5, IL-6, IL-10 and TGF-β in serum were determined by ELISA assay.
RESULTS: The percentages of CD4+CD29+ T cells were lower in treatment with 40 and 20 μmol/L baicalin than in the treatment of no baicalin. Treatment with 40 or 20 μmol/L baicalin significantly upregulated expression of IL-4, TGF-β1 and IL-10, increased p-STAT6/STAT6 ratio, but downregulated expression of IFN-γ, IL-5, IL-6, RORC, Foxp3 and T-bet, and decreased ratios of T-bet/GATA-3, p-STAT4/STAT4 and p-NF-κB/NF-κB compared to the treatment of no baicalin.
CONCLUSION: The results indicate that baicalin regulates immune balance and relieves the ulcerative colitis-induced inflammation reaction by promoting proliferation of CD4+CD29+ cells and modulating immunosuppressive pathways.
Core tip: Ulcerative colitis is a kind of chronic and non-specific inflammatory bowel disease. Researchers believe that several distinguishing factors such as immune systems, genes and environment are likely necessary to result in ulcerative colitis (UC). The study here demonstrated that baicalin might be a potential immune inhibitor. It adjusted immune balance and relieved the inflammatory response caused by UC, probably by inhibiting the ratio of CD4+CD29+ cells and immunosuppressive pathways. Our results provide valuable information for further studies on the pathogenesis of UC and for the development of new drugs, and also provide a new view of studying T helper cell immune disorder-related diseases including UC.
- Citation: Yu FY, Huang SG, Zhang HY, Ye H, Chi HG, Zou Y, Lv RX, Zheng XB. Effects of baicalin in CD4 + CD29 + T cell subsets of ulcerative colitis patients. World J Gastroenterol 2014; 20(41): 15299-15309
- URL: https://www.wjgnet.com/1007-9327/full/v20/i41/15299.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i41.15299
Ulcerative colitis (UC) is a kind of chronic and non-specific inflammatory bowel disease (IBD) which is recurrent and debilitating. The pathogenesis of UC is not fully understood at present, but researchers believe that several distinguishing factors such as immune systems, genes and environment are likely necessary to result in UC[1]. Among immune systems, T cells (also called T lymphocytes) play a crucial role in developing UC[2]. It is known that the co-expression of CD29 and CD4 can be used as a marker for CD4+ subpopulations. CD4+CD29+ cells primarily assist B cells in producing antibodies and induce CD8+ cell-mediated dissolution of lymphocytes. The increase of CD4+CD29+ cells can result in a high activation of B cells and abnormal immune response. Hence, CD4+CD29+ cells can be used as an immunology index for monitoring UC. In 1986, Mosmann et al[3] classified murine CD4+ T cells into two groups, T helper type 1 (Th1) and T helper type 2 (Th2), based on the cytokines they produced and their related functional activities. Since then, relationships between Th1/Th2 imbalance and immune diseases have been widely studied. The abnormal Th1/Th2 balance is traditionally considered as one of the important immune factors that cause UC[4]. Much research has demonstrated that T-box expressed in T cells (T-bet)/GATA-3 and T helper type 17 (Th17)/regulatory or suppressor T cell (Treg) balance are critical to Th1/Th2 differentiation. T-bet and GATA3 are specifically expressed on Th1 and Th2 cells, respectively. They respectively regulate Th1 and Th2 cell growth, and both determine the switching of cells from Th0 to Th1/Th2 cells[2]. The fine Th17/Treg balance is crucial for maintenance of a steady state of intestinal immunity[5]. Retinoid-related orphan receptor gamma t (RORγt) has been identified as the lineage-specific transcription factor for Th17 cells. Treg cells can secrete cytokines such as interleukin (IL)-4, IL-10 and transforming growth factor beta (TGF-β). They are closely associated with onset of immune diseases. Forkhead box P3 (FOXP3) is a specific nucleic transcription factor for Treg cells and is important in the regulation of differentiation, development and function of Treg cells[6]. Recently, members of the signal transducer and activators of transcription (STAT) family have been reported to be involved in signal transduction of cytokines and cell growth factors, and to regulate human immune response, inflammatory reaction, cell growth and differentiation, playing an important role in the pathogenesis of IBD[7]. More information is needed to clarify the role of these immune factors in UC.
Currently, the treatments of Western medicine have some shortcomings such as disease recurring after stopping, long-term drug-taking secondary response and undesirable effects of some persistent cases. Thus, searching for a treatment with fewer side-effects is of importance for UC. Chinese medicine has significant benefits for treating ulcerative colitis, and has shown an outstanding value for development and broad prospects. Baicalin is a flavonoid isolated from Scutellaria baicalensis, a component of the digestive aid Huang Qin Tang (PHY906)[8], and is known to have an effect on multiple biological functions including the ability to modulate pro-inflammatory cytokines and inhibit aldose reductase activity[9]. Recently, researchers have paid more attention to its good therapeutic effect to UC. However, mechanisms involved in its effect are not fully understood.
In our previous study, CD4+CD29+ T cells were found to be significantly increased in UC patients and rats with heat-dampness[10]. In this study, we further examined the percentage of CD4+CD29+ T cells in an in vitro incubation of cells isolated from peripheral blood of patients with UC. Moreover, we investigated the effects of baicalin on cell proliferation of CD4+CD29+ cells and expression of T-bet/GATA-3 mRNA, T-bet/GATA-3 mRNA, nuclear factors (NF) and cytokines by adding different concentrations of baicalin in the in vitro incubation of cells. The study here will provide valuable information for better understanding the pathogenesis of UC and for developing new drugs.
The patients selected for the present study were outpatients and inpatients from the Gastroenterology Department of Nanfang Hospital, Southern Medical University (Guangzhou, China) and Hospital of Guangzhou University of Traditional Chinese Medicine (Guangzhou, China) during June 2010 to January 2011. All the samples used in this study were obtained with approval of the Ethics Committee at the corresponding hospital. The whole procedure of consent was approved and documented by the Ethics Committee. Three groups (UC, D-IBS, and control group) were involved in this study. The UC group consisted of thirty-three patients comprised of 18 men and 15 women with a median age of 39 year (range: 22-55 year), and they could be divided into 2 further groups (active: n = 18; inactive = 15) according to the modified Williams Disease Activity Index (DAI)[1]. The diagnosis of irritable bowel syndrome (IBS) patients was based on the Rome III diagnostic criteria[11]; thirty D-IBS patients were involved, including 16 men and 14 women aged 18-60 yr with an average age of 39 yr. Thirty healthy examinees including 15 males and 15 females with an average of 42.5 year (range: 23-62 year) were selected as controls.
The serum samples (2 mL) were obtained from fasting participants in the morning and peripheral blood mononuclear cells (PBMCs) were prepared by use of Ficoll-Hypaque (Miltenyi, Germany) density gradient centrifugation. The serum was diluted by equal volume of RPMI1640 (Gibco, United States). Lymphocyte separation liquid (2 mL) was filled into a 10-mL centrifuge tube. Then, the diluted anticoagulant blood was slowly added along the wall of tube, and centrifuged at 2500 r/min. After 20 min, the mononuclear cell layer was transferred to a sterile tube by a fresh sterile pipette (capillary tube), gently mixed with five volumes of RPMI1640 and centrifuged at 2000 r/min for 10 min, then washed with RPMI1640 twice. After the supernatant was discarded, the cells were resuspended in RPMI1640 containing 10% fetal bovine serum (Gibco, United States) for lymphocyte count. Then the cells suspensions were diluted to 1 × 106 cells/mL for later use.
PBMCs from the UC group were plated in a 96-well plate (1 × 105 cells per well) which was incubated with CD3 antibody overnight. PBMCs were cultured in RPMI1640 containing 10% fetal bovine serum at 37 °C in 5%CO2 and stimulated with antibody against CD28. PBMCs were treated with various concentration of baicalin (5 μmol/L, 10 μmol/L, 20 μmol/L and 40 μmol/L), and DMSO was the negative control (Table 1). Three replicates were designed for each treatment.
| No. | 1 | 2 | 3 | 4 | 5 | 6 |
| Treatment | Cell (PBMCs of UC group) | DMSO | 5 μmol/L baicalin | 10 μmol/L baicalin | 20 μmol/L baicalin | 40 μmol/L baicalin |
To detect the cell surface expression of a variety of molecules, isolated PBMCs from fresh blood were analyzed by flow cytometry (FACS) using standard staining methods. Briefly, the prepared cell suspension fluid (1 mL) was suspended in PBS (2 mL), centrifuged at 1500 r/min for 10 min, and rinsed twice with suspension fluid. The supernatant was discarded and cells were suspended with PBS to 100 μL, adding human CD4 antibody, CD29 antibody, and the other antibodies (about 1.25 μg, suggested by the manual) respectively, and incubated at 4 °C in the dark for 30 min. Then, the cell suspension was supplemented with 2 mL PBS, centrifuged at 1500 r/min for 10 min, and washed twice with suspension fluid followed by staining with flow cytometry staining buffer. Then, the percentage of CD4+ and CD29+ T cells was analyzed by the FACS Calibur (Becton Dickinson, Sunnyvale, CA) with appropriate software (CellQuest, BD Biosciences).
The percentage of viable cells was determined by MTS assay. Cells were collected at different time points (0, 24, 48 and 72 h), and then prepared as cell suspensions (1 × 105 cells/mL), and seeded into 96-well plates at a density of 1 × 104 per well (100 μL). The MTS reagent was added at a 1:10 ratio of MTS and culture fluid, and incubated at 37 °C for 4 h. The cell viability was determined by measuring optical density of each sample at 490 nm (OD490) using a microplate reader.
Total RNA was extracted in PBMCs by the Trizol method, and detected by gel electrophoresis. The RNA concentration and purity were determined by UV-visible spectroscopy (Eppendorf, Germany). The first-strand cDNA was synthesized following the instructions of the cDNA synthesis kit. Then it was amplified with specific primers (Table 2) by polymerase chain reaction (PCR). The PCR conditions included initial incubation at 50 °C for 2 min, at 95 °C for 2 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 32 s. For melting curve analysis, a melting temperature range of 60 °C-95 °C was used. The fluorescent signal was collected to obtain Ct (threshold cycle) value. Comparative real-time PCR was done in triplicate. Relative expression was calculated using the comparative Ct method.
| Gene name | Primer | |
| GATA3 | Forward | AGTTGGCCTAAGGTGGTTG |
| Reverse | CACGCTGGTAGCTCATACAC | |
| FOXP3 | Forward | CAGGATCTGAGGTCCCAACA |
| Reverse | TTTGGCAAGGCAGTGTGTGT | |
| T-bet | Forward | CTGCATATCGTTGAGGTGAA |
| Reverse | GTAGGCAGTCACGGCAATGA | |
| RORC | Forward | CAAGACTCATCGCCAAAGCA |
| Reverse | CAGTGCTGAAGAGCTCCTTG | |
| 18S | Forward | CCTGGATACCGCAGCTAGGA |
| Reverse | GCGGCGCAATACGAATGCCCC | |
The expression of nuclear factor-κB (NF-κB) p65, phosphorylation of NF-κB (p-NF-κB) p65, STAT4, p-STAT4, STAT6, and p-STAT6 in UC cells was analyzed by western blotting. Cells were collected and lysed in RIPA (Beytime, China). Equal amounts of proteins from each group were separated by 10% gradient SDS-polyacrylamide gels. The proteins were transferred onto a NC membrane, blocked with 5% fat-free milk powder for 1 h and incubated with primary antibodies of NF-κB p65, p-NF-κB p65, STAT4, p-STAT4, STAT6, and p-STAT6 overnight at 4 °C. The membrane was rinsed in Tris-buffered saline (TBST) three times, and respectively incubated with secondary antibodies at RT for 1 h. The membrane was washed in TBST again and incubated with substrate. The NC membrane was then rinsed in deionized water. After it was air-dried, the membrane was scanned and the molecular weight and optical density of the target band was analyzed by gel imaging system.
The ELISA assay was conducted according to the handbook provided by the ELISA kit (Jingmei BioTech Co. Ltd., Shenzhen, China). The optical density of each sample at 450 nm (OD450) was detected by an ELISA microplate reader and the concentrations of interferon-γ (IFN-γ), IL- 4, IL-5, IL-6, IL-10, and TGF-β levels in serum were determined. A standard curve was calculated using linear dilutions of the BSA supplied by the manufacturer.
All data are expressed as mean ± standard deviation (SD). SPSS13.0 was used to perform one-way analysis of variance (ANOVA) and a Q test for all intergroup comparisons. For all analyses, a probability less than an alpha value of 0.05 (P < 0.05) was considered to be statistically significant.
The percentage of CD4+ and CD4+CD29+ cells was significantly (P < 0.01) higher in the UC group than in the D-IBS or control group, but showed no significant (P > 0.05) difference between the IBS and control group (Table 3). In the UC group, CD4+ cell number was doubled and the percentage of CD4+CD29+ cells showed a fourfold increase compared with the D-IBS group and controls.
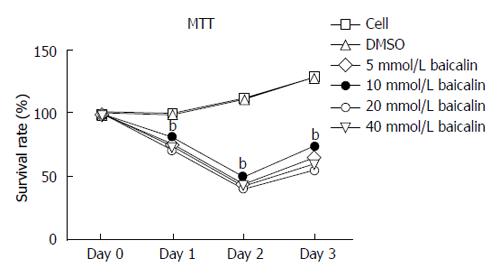
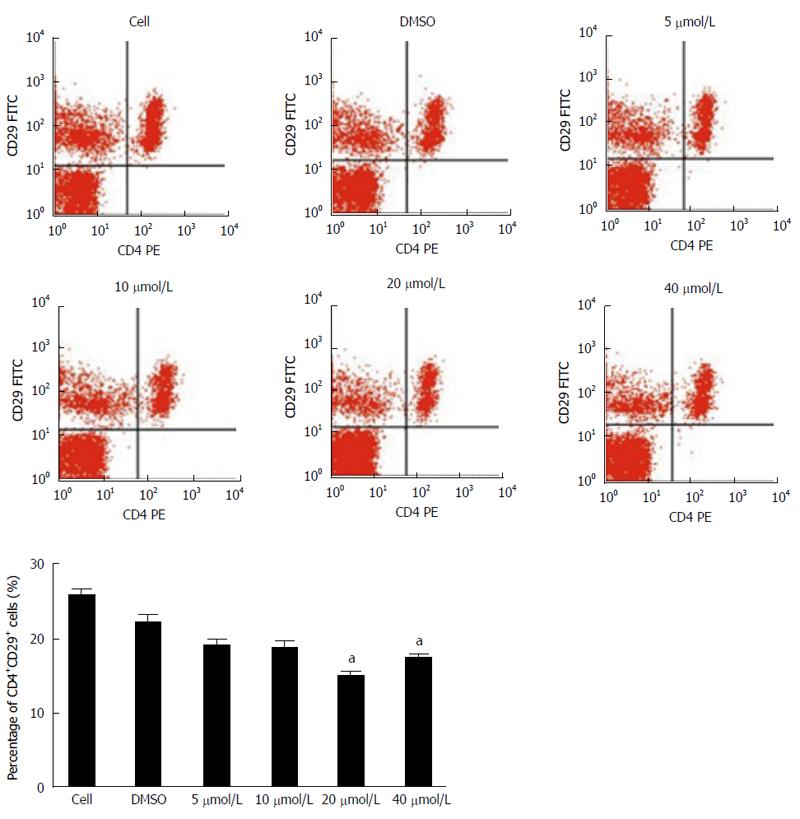
To investigate the effect of baicalin on the proliferation of CD4+CD29+ cells, cells were treated with 5 μmol/L, 10 μmol/L, 20 μmol/L, 40 μmol/L baicalin and detected at 0 h, 24 h, 48 h and 72 h by MTS assay. The results demonstrated that the proliferation of cells treated with different concentrations of baicalin was obviously repressed (P < 0.01) after 24 h compared to the control group (Figure 1). Furthermore, flow cytometry was performed 72 h after treatment, and we found that treatment with 20 μmol/L and 40 μmol/L baicalin significantly (P < 0.05) decreased the percentage of CD4+CD29+ cells (Figure 2).
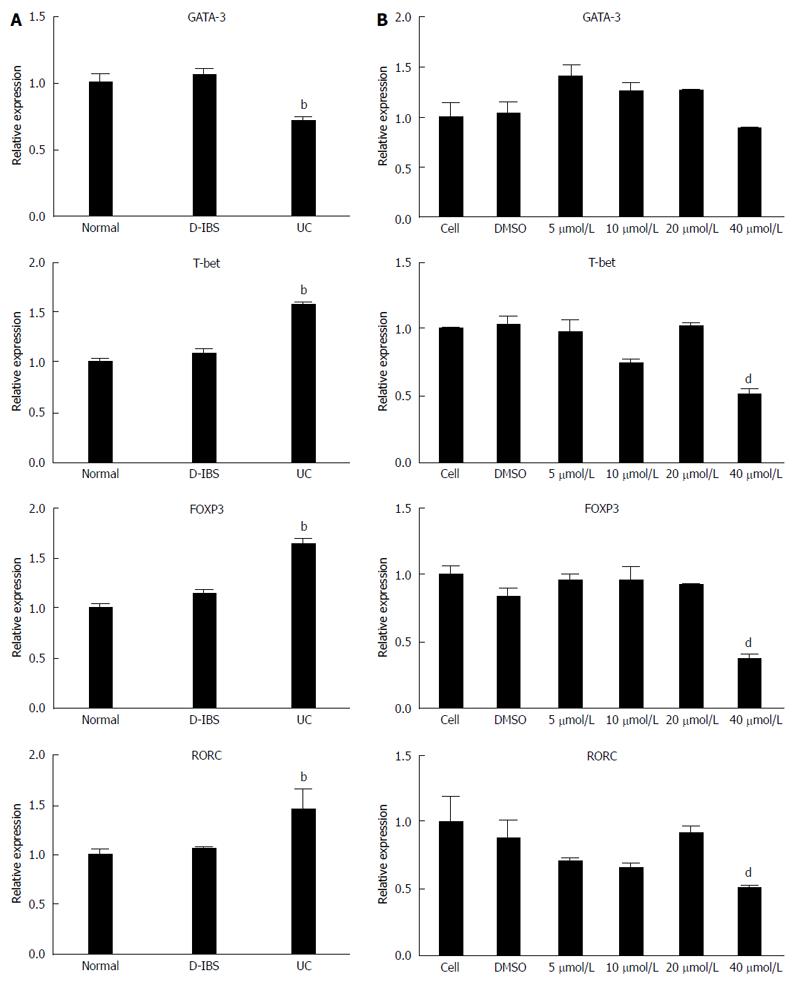
The expression level of GATA-3, FOXP3, T-bet and RORC in UC was detected by quantitative real-time PCR. The results indicated that GATA-3 was significantly (P < 0.05) decreased in UC while FOXP3, T-bet and RORC were increased compared to the D-IBS group and controls (Figure 3A). To test whether these proteins could be regulated by baicalin, we examined their mRNA expression in cells treated with baicalin. We found that GATA-3 mRNA level was obviously (P < 0.05) elevated in the 5 μmol/L, 10 μmol/L and 20 μmol/L groups (Figure 3B). Otherwise, FOXP3, T-bet and RORC mRNA levels were significantly (P < 0.05) reduced in the 40 μmol/L group.
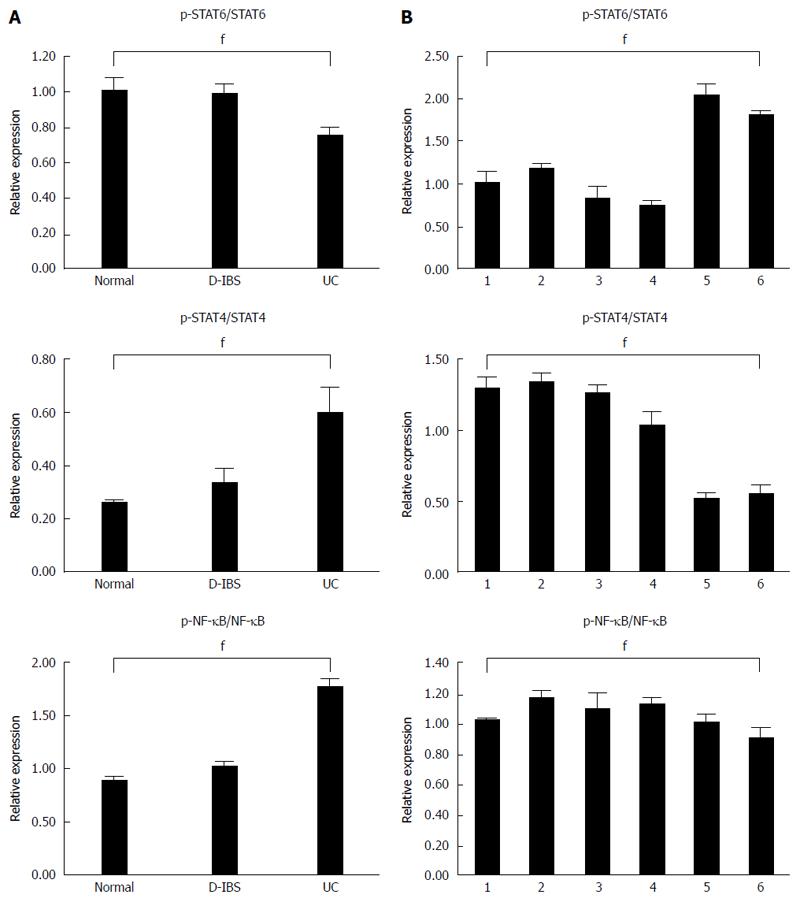
The expression level of proteins was detected using western blot assay (Figure 4A). Compared with the control and IBS groups, the expression of the p-STAT6/STAT6 ratio was significantly decreased (P < 0.05) while p-NK-κB/NK-κB and p-STAT4/STAT4 were significantly (P < 0.05) increased in the UC group (Figure 4B). However, no significant difference (P > 0.05) was displayed between the control and IBS groups. The p-STAT4/STAT4 and p-NK-κB/NK-κB were significantly (P < 0.05) increased in the treatments with 40 and 20 μmol/L baicalin when compared with other treatments, but p-STAT6/STAT6 decreased significantly (P < 0.05) (Figure 4C).
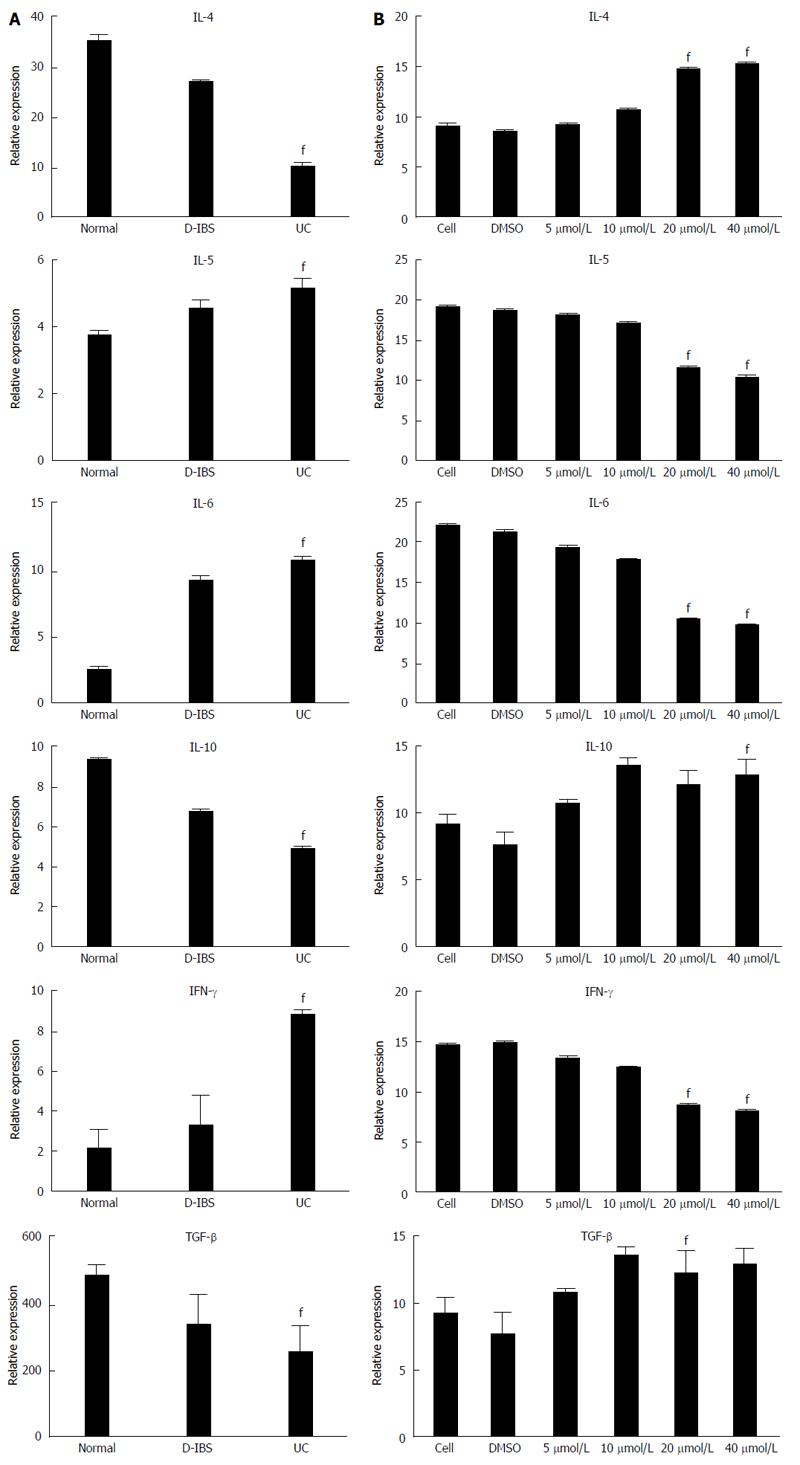
The UC group showed a significantly (P < 0.01) lower level of IL-4, IL-10 and TGF-β1 but significantly (P < 0.01) higher expression of IFN-γ, IL-6 and IL-5 compared with the control and IBS groups (Figure 5A). The concentrations of IL-6, IFN-γ and IL-5 were significantly (P < 0.05) lower in the treatment with 40 or 20 μmol/L baicalin than in the other treatments, but IL-4, IL-10 and TGF-β1 concentrations were higher (P < 0.05) (Figure 5B).
CD4+ T cells are important in the regulation of the T cell immune response to foreign antigens as well as self-antigens in the occurrence and development of UC[12-14]. CD4+CD29+ T cells are helper T cells which are involved in the onset of autoimmune diseases and can promote the production of B cell growth/differentiation factors in patients with hyperthyroidism[10]. The numbers of CD29+ T cells in peripheral blood of patients with gestational diabetes[14,15], allergic rhinitis[14], and acute hepatitis are higher than those in healthy persons[15]. However, little information is found on how baicalin regulates the activity of T cells via T cell subsets. Our previous study showed a significant increase in the percentage of CD4+CD29+ T cells in UC patients or rats with heat-dampness, indicating that CD4+CD29+ T cells might be involved in the onset of UC with heat-dampness. In this study, we confirmed that CD4+CD29+ T cells were significantly increased in the UC group than in the IBS and control groups in PBMCs isolated from peripheral blood of patients with UC. After cells were treated with baicalin, CD4+ and CD4+CD29+ cells significantly proliferated. The results indicated that baicalin, as an important component of Huang Qin Tang, might regulate immune function by promoting cell proliferation in the treatment of UC.
In recent years, studies have shown that Th17 cells are closely associated with Tregs in cell differentiation and both can be transformed to one another. They are independent in the immune response but sometimes show a close relationship. Disruption of the balance between Th17 and Treg cells results in diseases. It is reported that IL-23, its receptor IL-23R, and RORC play a key role in the differentiation and maintenance of Th17 cells and are closely related with the pathogenesis of UC[16-20]. It is also reported that the T-bet and GATA-3 balance is crucial to the regulation of Th1/Th2 differentiation. T-bet and GATA-3 can form a self-activated feed-back regulation circle. They formulate a dynamic regulation network of their own and other subpopulation-specific transcription factors[21,22]. T-bet can directly bind to GATA-3 and interfere with GATA-3 mediated Th2 response[23-25].
Our previous study demonstrated that Huang Qin Tang can regulate Th17/Treg balance and relieve the inflammatory reaction of rats with heat-dampness UC by regulating the expression of FOXP3, RORC, IL-17, IL-6, IL-10 and TGF-β, which might be one of the immune regulative mechanisms in the treatment of UC with heat-dampness by Hang Qin Tang. In this study, we further detected cytokines in blood by ELISA assay and found that the UC group had higher concentrations of IFN-γ, IL-6 and IL-5 but lower concentrations of IL-4, IL-10 and TGF-β1 than the IBS and control groups. Baicalin treatment, especially at doses of 20 and 40 μmol/L, resulted in a decrease of IFN-γ, IL-5 and IL-6 and an increase of IL-4, IL-10 and TGF-β1. The expressions of RORC and FOXP3 mRNA in PMBCs detected by PCR were higher (P < 0.05) in the UC group than in the control and IBS groups. When cells were treated with high concentrations of baicalin, the expression of RORC showed a significant decrease compared with FOXP3, which led to a significant lower ratio of RORC/FOXP3 and indirectly regulated the balance of Th17/Treg differentiation. qRT-PCR results indicated higher T-bet and lower GATA-3 expression in the UC group, so the T-bet/GATA-3 ratio was significantly higher compared with the control group. The high T-bet expression could promote the differentiation of Th1 cells and the production of cytokines. However, the low GATA-3 expression could not promote the shifting of cells from Th0 to Th2, and hence could result in high level of Th1 expression in the body, which was consistent with the results of Müller et al[26]. The treatment with 40 μmol/L baicalin displayed a significantly decrease in T-bet/GATA-3 ratio by highly decreasing T-bet. As a result, high concentrations of baicalin could reduce the ratio of T-bet/GATA-3, inhibit the secretion of cytokines (IFN-γ, IL-5, IL-6), but increase concentrations of IL-4, IL-10 and TGF-β1. Thus, we propose a novel view that baicalin might be a new therapy target by regulating the differentiation of Th0 into Th1/Th2 in the treatment of UC. It is also suggested that baicalin could correct Th1/Th2 imbalance by regulating expression of T-bet and GATA-3.
STAT6 and STAT4 belong to the STAT family and they respectively participate in the differentiation of Th cells into Thl and Th2[7,27,28]. STAT4 is a transcription factor that regulates Th1 differentiation and hence its activation can cause inflammation. A study showed that the expression of p-STAT4 is high in colonic mucosa tissue of patients with UC, CD and irritable bowel syndrome, but is highest in patients with UC. Other studies also showed that p-STAT4 expressed in UC tissues is significantly higher than in normal tissues. The p-STAT4 means that STAT4 is in an active state, indicating that STAT4 is playing a role in the pathogenesis of UC and the high expression of STAT4 could result in the inflammation of UC. STAT6 is one of the key transcription factors that mediate Th2, and the activated STAT6 has an anti-inflammation effect. It is reported that STAT6 in colonic mucosa tissues of patients with UC is not significantly different from that of the healthy person, but it can be greatly decreased after the Chinese medicine is used, and the clinical symptoms of UC can be improved by inhibiting STAT6[29]. We detected expression of STAT4, STAT6, p-STAT4, p-STAT6, and the related cytokines of PBMC in UC cells by western blotting and found that p-STAT4/STAT4 ratio was significantly higher but p-STAT6/STAT6 ratio was lower in the UC group than in the healthy control group and IBS group. In an in vitro culture of cells treated with baicalin, treatment with 40 and 20 μmol/L baicalin significantly decreased p-STAT4/STAT4 ratio but increased p-STAT6/STAT6 ratio compared with groups of medium only, DMSO, 5 μmol/L baicalin and 10 μmol/L baicalin.
Many researchers believed that immune dysfunction was a key factor that resulted in IBD. Neurath et al[29] firstly reported the existence of NF-κB activation in inflammatory intestine tissues in a TNBS-induced IBD animal model[30]. Schreiber et al[31] confirmed that NF-κB is inactive in normal human intestine tissues but is highly active in intestine tissues of patients with UC or CD, and it is important for the occurrence and development of UC[32,33]. Zhang et al[34] reported that baicalin, used for the treatment of rats with severe pancreatitis, can reduce concentrations of toxin and TNF-α in blood, relieve intestine mucosa damage, decrease production of NF-κB, inhibit inflammation reaction, and hence protect intestine mucosa. We here detected expression of NF-κB and p-NF-κB in PBMCs of patients with UC and found that the UC group had significantly increased p-NK-κB expression and p-NK-κB/NK-κB ratio but decreased NK-κB expression. In the in vitro culture of PBMCs treated with baicalin, 40 μmol/L baicalin significantly lowered the p-NK-κB/NK-κB ratio, indicating that baicalin could regulate immune balance and relieve inflammation response caused by UC by decreasing the p-NK-κB/NK-κB ratio.
This study demonstrated that baicalin might be a potential immune inhibitor. It adjusted immune balance and relieved the inflammatory response caused by UC, probably by inhibiting the ratio of CD4+CD29+ cells and immunosuppressive pathways[35]. Our results provide valuable information for further studies on the pathogenesis of UC and for the development of new drugs, and also provide a new view of studying T helper cell immune disorder-related diseases including UC.
Ulcerative colitis (UC) is a kind of chronic and non-specific inflammatory bowel disease (IBD) which is recurrent and debilitating. The pathogenesis of UC is not fully understood at present, but researchers believe that several distinguishing factors such as immune systems, genes and environment are likely necessary to result in UC. Among immune systems, T cells (also called T lymphocytes) play a crucial role in developing UC.
Baicalin is a flavonoid isolated from Scutellaria baicalensis in Huang Qin Tang (PHY906), and is known to have an effect on multiple biological functions including the ability to modulate pro-inflammatory cytokines and inhibit aldose reductase activity. Recently, researchers have paid more attention to its good therapeutic effect on UC.
In the authors’ previous study, CD4+CD29+ T cells are significantly increased in UC patients and rats with heat-dampness. In this study, the authors further examined the percentage of CD4+CD29+ T cells in an in vitro incubation of cells isolated from peripheral blood of patients with UC. Moreover, they investigated the effects of baicalin on proliferation of CD4+CD29+ cells and expression of T-box expressed in T cells (T-bet)/GATA-3 mRNA, T-bet/GATA-3 mRNA, nuclear factors (NF), and cytokines by adding different concentrations of baicalin to the in vitro incubation of cells. The study will provide valuable information for better understanding the pathogenesis of UC and for developing new drugs.
The study results suggest that baicalin regulates immune balance and relieves ulcerative colitis-induced inflammation reaction by promoting proliferation of CD4+CD29+ cells and modulating immunosuppressive pathways. This study provided valuable information for further studies on the pathogenesis of UC and for the development of new drugs.
Baicalin has an effect on multiple biological functions including the ability to modulate pro-inflammatory cytokines and inhibit aldose reductase activity. Baicalin regulates immune balance and relieves ulcerative colitis-induced inflammation reaction by promoting proliferation of CD4+CD29+ cells and modulating immunosuppressive pathways.
This is a good descriptive study in which the authors analyzed the preventive effect of baicalin, which regulates immune balance and relieves ulcerative colitis-induced inflammation reaction by promoting proliferation of CD4+CD29+ cells and modulating immunosuppressive pathways.
P- Reviewer: Roman RD, Sandborn W S- Editor: Qi Y L- Editor: Logan S E- Editor: Wang CH
| 1. | Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115:182-205. [PubMed] |
| 2. | Yamaji O, Nagaishi T, Totsuka T, Onizawa M, Suzuki M, Tsuge N, Hasegawa A, Okamoto R, Tsuchiya K, Nakamura T. The development of colitogenic CD4(+) T cells is regulated by IL-7 in collaboration with NK cell function in a murine model of colitis. J Immunol. 2012;188:2524-2536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. 1986. J Immunol. 2005;175:5-14. [PubMed] |
| 4. | Bai A, Lu N, Zeng H, Li Z, Zhou X, Chen J, Liu P, Peng Z, Guo Y. All-trans retinoic acid ameliorates trinitrobenzene sulfonic acid-induced colitis by shifting Th1 to Th2 profile. J Interferon Cytokine Res. 2010;30:399-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Xu J, Yang Y, Qiu G, Lal G, Yin N, Wu Z, Bromberg JS, Ding Y. Stat4 is critical for the balance between Th17 cells and regulatory T cells in colitis. J Immunol. 2011;186:6597-6606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Chen Z, Lin F, Gao Y, Li Z, Zhang J, Xing Y, Deng Z, Yao Z, Tsun A, Li B. FOXP3 and RORγt: transcriptional regulation of Treg and Th17. Int Immunopharmacol. 2011;11:536-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 121] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 7. | Mudter J, Weigmann B, Bartsch B, Kiesslich R, Strand D, Galle PR, Lehr HA, Schmidt J, Neurath MF. Activation pattern of signal transducers and activators of transcription (STAT) factors in inflammatory bowel diseases. Am J Gastroenterol. 2005;100:64-72. [PubMed] |
| 8. | Lai MY, Hsiu SL, Tsai SY, Hou YC, Chao PD. Comparison of metabolic pharmacokinetics of baicalin and baicalein in rats. J Pharm Pharmacol. 2003;55:205-209. [PubMed] |
| 9. | Shen YC, Chiou WF, Chou YC, Chen CF. Mechanisms in mediating the anti-inflammatory effects of baicalin and baicalein in human leukocytes. Eur J Pharmacol. 2003;465:171-181. [PubMed] |
| 10. | Chao K, Zhong BH, Zhang SH, Gong XR, Yao JY, Chen MH. [Imbalance of CD4(+) T cell subgroups in ulcerative colitis]. Zhonghua Yi Xue Za Zhi. 2011;91:1605-1608. [PubMed] |
| 11. | Cash BD, Chang L, Sabesin SM, Vitat P. Update on the management of adults with chronic idiopathic constipation. J Fam Pract. 2007;56:S13-S9; quiz S20. [PubMed] |
| 12. | Xu D, Liu H, Komai-Koma M, Campbell C, McSharry C, Alexander J, Liew FY. CD4+CD25+ regulatory T cells suppress differentiation and functions of Th1 and Th2 cells, Leishmania major infection, and colitis in mice. J Immunol. 2003;170:394-399. [PubMed] |
| 13. | Kanai T, Nemoto Y, Kamada N, Totsuka T, Hisamatsu T, Watanabe M, Hibi T. Homeostatic (IL-7) and effector (IL-17) cytokines as distinct but complementary target for an optimal therapeutic strategy in inflammatory bowel disease. Curr Opin Gastroenterol. 2009;25:306-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Mahmoud F, Arifhodzic N, Haines D, Novotney L. Levocetirizine modulates lymphocyte activation in patients with allergic rhinitis. J Pharmacol Sci. 2008;108:149-156. [PubMed] |
| 15. | Boykinova OB, Stoilova YD, Tsvetkova TZ, Baltadjiev IG. Epidemiological, immunological and clinical characteristics of acute hepatitis C. Folia Med (Plovdiv). 2009;51:61-69. [PubMed] |
| 16. | Christophi GP, Rong R, Holtzapple PG, Massa PT, Landas SK. Immune markers and differential signaling networks in ulcerative colitis and Crohn’s disease. Inflamm Bowel Dis. 2012;18:2342-2356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 17. | Kitani A, Xu L. Regulatory T cells and the induction of IL-17. Mucosal Immunol. 2008;1 Suppl 1:S43-S46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Ogino H, Nakamura K, Ihara E, Akiho H, Takayanagi R. CD4+CD25+ regulatory T cells suppress Th17-responses in an experimental colitis model. Dig Dis Sci. 2011;56:376-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 19. | Powell N, Walker AW, Stolarczyk E, Canavan JB, Gökmen MR, Marks E, Jackson I, Hashim A, Curtis MA, Jenner RG. The transcription factor T-bet regulates intestinal inflammation mediated by interleukin-7 receptor+ innate lymphoid cells. Immunity. 2012;37:674-684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 249] [Cited by in RCA: 279] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 20. | Montes M, Zhang X, Berthelot L, Laplaud DA, Brouard S, Jin J, Rogan S, Armao D, Jewells V, Soulillou JP. Oligoclonal myelin-reactive T-cell infiltrates derived from multiple sclerosis lesions are enriched in Th17 cells. Clin Immunol. 2009;130:133-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 21. | Ohtani K, Ohtsuka Y, Ikuse T, Baba Y, Yamakawa Y, Aoyagi Y, Fujii T, Kudo T, Nagata S, Shimizu T. Increased mucosal expression of GATA-3 and STAT-4 in pediatric ulcerative colitis. Pediatr Int. 2010;52:584-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Liu B, Tonkonogy SL, Sartor RB. Antigen-presenting cell production of IL-10 inhibits T-helper 1 and 17 cell responses and suppresses colitis in mice. Gastroenterology. 2011;141:653-662, 662.e1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 23. | Araki A, Nara H, Rahman M, Onoda T, Li J, Juliana FM, Jin L, Murata K, Takeda Y, Asao H. Role of interleukin-21 isoform in dextran sulfate sodium (DSS)-induced colitis. Cytokine. 2013;62:262-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Rengarajan J, Szabo SJ, Glimcher LH. Transcriptional regulation of Th1/Th2 polarization. Immunol Today. 2000;21:479-483. [PubMed] |
| 25. | Hwang ES, Szabo SJ, Schwartzberg PL, Glimcher LH. T helper cell fate specified by kinase-mediated interaction of T-bet with GATA-3. Science. 2005;307:430-433. [PubMed] |
| 26. | Müller B, Gimsa U, Mitchison NA, Radbruch A, Sieper J, Yin Z. Modulating the Th1/Th2 balance in inflammatory arthritis. Springer Semin Immunopathol. 1998;20:181-196. [PubMed] |
| 27. | Coskun M, Salem M, Pedersen J, Nielsen OH. Involvement of JAK/STAT signaling in the pathogenesis of inflammatory bowel disease. Pharmacol Res. 2013;76:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 257] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 28. | Seok Yang W, Lee J, Woong Kim T, Hye Kim J, Lee S, Hee Rhee M, Hong S, Youl Cho J. Src/NF-κB-targeted inhibition of LPS-induced macrophage activation and dextran sodium sulphate-induced colitis by Archidendron clypearia methanol extract. J Ethnopharmacol. 2012;142:287-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 29. | Neurath MF, Pettersson S. Predominant role of NF-kappa B p65 in the pathogenesis of chronic intestinal inflammation. Immunobiology. 1997;198:91-98. [PubMed] |
| 30. | Sakthivel KM, Guruvayoorappan C. Amentoflavone inhibits iNOS, COX-2 expression and modulates cytokine profile, NF-κB signal transduction pathways in rats with ulcerative colitis. Int Immunopharmacol. 2013;17:907-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 31. | Schreiber S, Nikolaus S, Hampe J. Activation of nuclear factor kappa B inflammatory bowel disease. Gut. 1998;42:477-484. [PubMed] |
| 32. | Ding Y, Liang Y, Deng B, Qiao A, Wu K, Xiao W, Gong W. Induction of TGF-β and IL-10 production in dendritic cells using astilbin to inhibit dextran sulfate sodium-induced colitis. Biochem Biophys Res Commun. 2014;446:529-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | Ke X, Zhou F, Gao Y, Xie B, Hu G, Fang W, Peng J, Chen Y, Sferra TJ. Qing Hua Chang Yin exerts therapeutic effects against ulcerative colitis through the inhibition of the TLR4/NF-κB pathway. Int J Mol Med. 2013;32:926-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 34. | Zhang XP, Zhang L, He JX, Zhang RP, Cheng QH, Zhou YF, Lu B. Experimental study of therapeutic efficacy of Baicalin in rats with severe acute pancreatitis. World J Gastroenterol. 2007;13:717-724. [PubMed] |
| 35. | Zhang ZQ, Liua W, Zhuang L, Wang J, Zhang S. Comparative pharmacokinetics of baicalin, wogonoside, baicalein and wogonin in plasma after oral administration of pure baicalin, radix scutellariae and scutellariae-paeoniae couple extracts in normal and ulcerative colitis rats. Iran J Pharm Res. 2013;12:399-409. [PubMed] |