Published online Nov 7, 2014. doi: 10.3748/wjg.v20.i41.15269
Revised: May 7, 2014
Accepted: June 12, 2014
Published online: November 7, 2014
Processing time: 236 Days and 18.9 Hours
Hepatocellular carcinoma is one of the most common cancers worldwide, and a leading cause of cancer-related death. Owing to unsatisfactory clinical outcomes under the current standard of care, there is a need to search for and identify novel and potent therapeutic targets to improve patient outcomes. Sorafenib is the first and only approved targeted therapy for the treatment of hepatocellular carcinoma. Besides functioning as a multiple tyrosine kinase, sorafenib also acts via a kinase-independent mechanism to target signal transducer and activator of transcription 3 (STAT3) signaling in hepatocellular carcinoma cells. STAT3 is a key regulator of inflammation, cell survival, and tumorigenesis of liver cells, and the high percentage of hepatocellular carcinoma cells with constitutively active STAT3 justifies targeting it for the development of novel therapeutics. Sorafenib inactivates STAT3 and STAT3-related signaling by inducing a conformational change in and releasing the autoinhibition of Src homology region 2 domain-containing phosphatase-1. This phosphatase negatively regulates STAT3 activity, which leads to the subsequent apoptosis of cancer cells. The novel anti-cancer property of sorafenib will be discussed in this review, not only adding information regarding its mechanism of action but also providing an innovative approach for the development of cancer therapeutics in the future.
Core tip: Hepatocellular carcinoma (HCC) is one of the major cancers worldwide, for which the only approved target therapy is sorafenib. In addition to its previously characterized kinase inhibition, sorafenib also acts via a kinase-independent mechanism to target signal transducer and activator of transcription 3 (STAT3) signaling in HCC cells. This review discusses these findings, adding to the knowledge concerning the mechanisms of action of sorafenib as well as exploring the potential use of STAT3 as a therapeutic target in future cancer drug development.
- Citation: Hung MH, Tai WT, Shiau CW, Chen KF. Downregulation of signal transducer and activator of transcription 3 by sorafenib: A novel mechanism for hepatocellular carcinoma therapy. World J Gastroenterol 2014; 20(41): 15269-15274
- URL: https://www.wjgnet.com/1007-9327/full/v20/i41/15269.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i41.15269
Hepatocellular carcinoma (HCC) is a leading primary malignancy of the liver, the fifth most common cancer and the third leading cause of cancer-related deaths worldwide[1,2]. A survey conducted by the World Health Organization found more than 700000 newly diagnosed cases of HCC in 2008, which corresponds to an age-adjusted incidence of 16 cases per 100000 inhabitants worldwide and up to 35.5 cases per 100000 male inhabitants of eastern Asia[3].
Treatment choices for HCC patients are made based on residual liver function and, as with other cancers, on the stage of disease as well as the patient’s general condition and comorbidities. For patients with early stage disease, percutaneous ablation, surgical resection, and liver transplantation offer the highest rates of complete response and, thus, the highest potentials for cure[4]. Unfortunately, because of a lack of associated signs and symptoms at the early stage, the majority of HCC patients are diagnosed with advanced disease and only 20%-30% are eligible for curative surgical resection[1]. Furthermore, nearly 90% of HCC develops in the background of chronic liver diseases that are either caused by chronic inflammation related to various etiologies, including hepatitis B or C infection and alcohol intake, or other hepatic toxin exposure, and even non-alcoholic fatty disease[2,5]. The complexity and heterogeneity of HCC tumorigenesis contributes to an intrinsic resistance of tumor cells to conventional chemotherapy and radiotherapy. Until recently, there were no effective treatments available for patients diagnosed with advanced stage HCC or whose disease deteriorated to an advanced stage after other treatments failed.
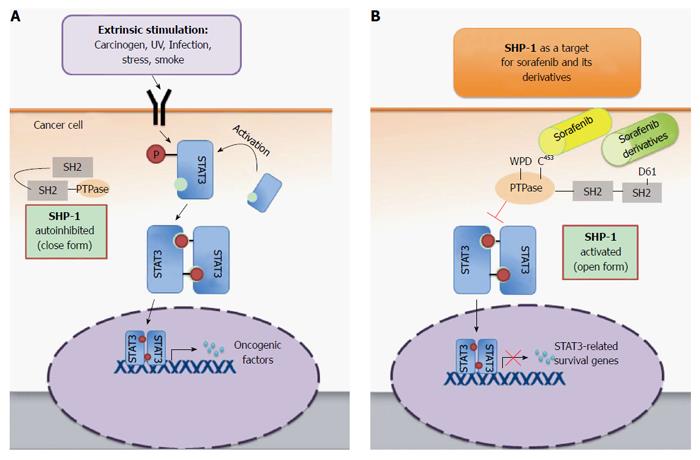
Although the first drug that demonstrated improvement in overall survival of patients with advanced HCC was sorafenib, an inhibitor of vascular endothelial growth factor receptor (VEGFR) tyrosine kinase activity[6,7], clinical trials testing several additional potent VEGFR inhibitors, such as sunitinib and brivanib, failed to show positive results[1,8,9]. The failures of these trials are thought to be multifactorial, including a lack of full understanding of the critical drivers of tumor progression[10]. In light of these results, it is essential to revisit the therapeutic mechanisms of sorafenib, focusing on the VEGFR-independent effects. This review outlines an important VEGFR-independent mechanism by which sorafenib induces apoptosis of HCC cells, via inhibition of signal transducer and activator 3 (STAT3) and its signaling pathway by increasing Src homology region 2 domain-containing phosphatase 1 (SHP-1) activity. This novel mechanism provides a promising foundation for further development of potent anti-cancer therapeutics.
The highly conserved STAT family proteins were first identified in 1994 as acute phase response factors associated with interleukin-6 (IL-6) stimulation[11]. STAT proteins, including STAT1-4, STAT5a, 5b and STAT6[12,13], are inactive and primarily located in the cytoplasm of non-stimulated cells. However, certain stimuli cause the activation and dimerization of STAT proteins by phosphorylation of specific tyrosine residues. The dimerized STATs are then translocated to the nucleus and enhance the transcription of genes[12] that govern various important cellular functions, such as cell differentiation, survival and immune response[14]. Among all the STAT proteins, STAT3 is particularly associated with oncogenesis[15,16].
STAT3 is activated by cytokines, growth factors, carcinogens, stress, infection and radiation[14,17,18], which cause phosphorylation of tyrosine 705. Various receptors that have tyrosine kinase activity can phosphorylate this residue, such as epidermal growth factor receptor, VEGFR and platelet-derived growth factor receptor (PDGFR), as well as other non-receptor tyrosine kinases, including Janus kinases (JAKs) and IL-6 receptors[19-21]. Additionally, the activity of STAT3 is also affected by phosphorylation of the serine residue at position 727 and acetylation of a lysine residue at position 685[22,23].
As tyrosine phosphorylation plays a major role in its activation, it is not surprising that tyrosine phosphatases negatively regulate STAT3. The protein tyrosine phosphatase superfamily, comprised of more than 100 members in humans, is classified into three subgroups: the classical protein tyrosine phosphatases (PTPs), dual-specificity phosphatases, and low molecular weight phosphatases[24]. PTPs are further divided into transmembrane tyrosine phosphatase CD45 and the non-transmembrane PTPs, including SHP-1, SHP-2, PTP-1B and T-cell protein tyrosine phosphatase. PTPs function is closely associated with JAKs, as the absence of SHP-1 predisposes cancer cells to constitutive activation of the JAK3/STAT3 pathway and tumorigenesis of anaplastic lymphoma kinase (ALK)+ large cell lymphoma[25]. Additionally, protein inhibitors of activated STAT (PIAS) and suppressors of cytokine signaling (SOCS) proteins are important negative regulators of STAT3-associated signals, and abnormal expression of these two protein families has been linked with many inflammatory diseases and cancers[14,26,27].
Constitutive STAT3 activity is observed in various cancer cell lines and tumor tissues, including breast, liver, lung, pancreas and prostate cancers, and melanoma[15,16,28]. He et al[29] found that nearly 60% of clinical HCC tumor samples showed nuclear phosphorylated-STAT3 staining, which correlated with the HCC aggressiveness. These observations suggest that STAT3 signaling is turned on in human HCC.
Hepatocarcinogenesis is a long and complex process involving chronic inflammation, oxidative stress, stimulation of cytokines (such as IL-6) and growth factors, loss of phosphatase function, or epigenetic modifications that silence SOCS proteins; these various changes can activate STAT3. Once activated, STAT3 may induce a series of signals that promote HCC proliferation (such as cyclin D1 and survivin), angiogenesis, and survival (such as Bcl-2 and Bcl-XL), and help tumors evade host immune surveillance[30-33]. Whereas activation of STAT3 by hepatitis C virus core protein or hepatitis B virus x protein promotes HCC growth[34,35], the downregulation of STAT3 reduces the growth of HCC in vitro and in vivo[36,37]. Furthermore, HCC development induced by diethylnitrosamine (DEN) in mice was prohibited when STAT3 signaling was reduced or conditionally knocked out[29,38,39]. Moreover, DEN-induced HCC was promoted and the expression of STAT3 was enhanced when negative regulators of STAT3 were deleted or IL-22 was overexpressed[40-42]. Collectively, these data demonstrate the role of STAT3 in promoting HCC tumorigenesis and indicate that it is a highly attractive target for treatment.
The efficacy of sorafenib (Nexavar) for the treatment of advanced HCC has been confirmed by two large, prospective, randomized, double-blind clinical trials[6,7]. First, in the Sorafenib Hepatocellular Carcinoma Assessment Randomized Protocol (SHARP) study that enrolled 602 patients with advanced HCC from Europe, North America, South America and Australia, a significant improvement in overall survival was observed for patients who received 400 mg sorafenib twice daily (10.7 mo vs 7.9 mo with placebo)[6]. Later in the Asia-Pacific trial, Cheng et al[7] validated the anti-cancer effects of sorafenib in 271 patients from the Asia-Pacific region, with significant improvements in overall survival and time-to-progression.
The safety profile of sorafenib was established by the results of the two previously mentioned studies and in the large-scale non-interventional phase IV Global Investigation of Therapeutic Decisions in Hepatocellular Carcinoma and of Its Treatment With Sorafenib (GIDEON) study[43], which followed 3202 sorafenib-treated unresectable HCC patients with Child-Pugh grade A and B liver functions. Although more than 80% of patients experience adverse events, only 9.3% of patients in the GIDEON study had grade 3-4 drug-related adverse events. The most frequent adverse events were diarrhea, fatigue and hand-foot skin reactions. Together, these data have established sorafenib as the standard of care for advanced HCC.
Sorafenib was originally developed to disrupt the Ras-Raf-MEK1/2-ERK1/2 signaling pathway by specifically targeting Raf-1 kinase[44]. Wilhelm et al[44] were the first to show that sorafenib inhibited the in vitro proliferation and transformation of pancreatic and colon cancer cell lines, and suppressed in vivo xenografted tumor growth. Furthermore, they demonstrated that the anti-cancer effects correlated with the inhibition of MEK and ERK phosphorylation. Several other investigators later found that sorafenib also affects other receptor tyrosine kinases, including VEGFR-2,3, PDGFR-β, Fms-like tyrosine kinase-3, and fibroblast growth factor receptor-1[45-47].
Our group was the first to report that STAT3 plays an important role in mediating the effects of sorafenib on tumor necrosis factor-related apoptosis-induced ligand (TRAIL)-sensitization[48]. Treatment of HCC cells with sorafenib and recombinant TRAIL (LBY 135) resulted in the downregulation of STAT3 and STAT3-related anti-apoptotic signaling (Mcl-1, cyclin D1, and survivin) and apoptosis of HCC cells. Furthermore, we generated a series of sorafenib derivatives devoid of kinase inhibition and found that they also inhibited STAT3 expression by increasing the activity of its negative regulator, SHP-1[49,50].
SHP-1, a member of the non-receptor family of PTPs, is comprised of two Src homology (SH) 2 domains that bind phosphotyrosine, a catalytic PTP domain, and a C-terminal tail. The activity of SHP-1 is closely related to its structural variability[51-53]. SHP-1 is autoinhibited when the N-terminal SH2 domain protrudes into the catalytic domain, blocking the active site of the catalytic pocket (closed form). The flexibility of the conserved WPD-loop, which contains an active site residue (Asp 421), also affects the catalytic activity of SHP-1[54]. Our group found that the anti-HCC effects of sorafenib are due to the formation of a critical salt bridge from the D61 residue in the N-SH2 domain, resulting in an “open” catalytic PTP domain and releasing the autoinhibition of SHP-1[49] (Figure 1). Furthermore, we found that several sorafenib derivatives, SC-1, SC-40 and SC-43, exhibit identical reactivation of SHP-1 and inhibition of STAT3 signaling in HCC cells. Excitingly, the anti-HCC potencies of these sorafenib derivatives were even more pronounced than those of sorafenib.
Various novel compounds have been developed to target STAT3 in different cancer types. Curcumin, a dihydroxyphenolic compound that downregulates JAK/STAT3 signaling by increasing the activity of PIAS3, has been tested in endothelial and ovarian cancer cells[27]. Cucurbitacin Q, a novel small molecule against STAT3 that does not inhibit JAK2, was shown to induce potent anti-cancer effects in lung and breast cancer cells with constitutively active STAT3[55].
Current data indicate that STAT3 is not only an important oncoprotein, but also a “druggable” target for various cancers, and reactivation of SHP-1 may be a feasible approach to target STAT3 and the STAT3-related signaling pathway. Sorafenib performs these functions in addition to its inhibition of multiple kinases. Knowledge of these kinase-independent properties of sorafenib provides an important foundation and suggests targeting of STAT3 and SHP-1 for future development of anti-cancer and HCC treatments.
P- Reviewer: Bloomston M, Takigawa N S- Editor: Qi Y L- Editor: A E- Editor: Liu XM
| 1. | Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3249] [Cited by in RCA: 3596] [Article Influence: 276.6] [Reference Citation Analysis (4)] |
| 2. | El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2881] [Cited by in RCA: 3088] [Article Influence: 220.6] [Reference Citation Analysis (0)] |
| 3. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11128] [Cited by in RCA: 11836] [Article Influence: 845.4] [Reference Citation Analysis (4)] |
| 4. | Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6573] [Article Influence: 469.5] [Reference Citation Analysis (1)] |
| 5. | Ding J, Wang H. Multiple interactive factors in hepatocarcinogenesis. Cancer Lett. 2014;346:17-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (1)] |
| 6. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10268] [Article Influence: 604.0] [Reference Citation Analysis (2)] |
| 7. | Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3854] [Cited by in RCA: 4651] [Article Influence: 273.6] [Reference Citation Analysis (0)] |
| 8. | Llovet JM, Decaens T, Raoul JL, Boucher E, Kudo M, Chang C, Kang YK, Assenat E, Lim HY, Boige V. Brivanib in patients with advanced hepatocellular carcinoma who were intolerant to sorafenib or for whom sorafenib failed: results from the randomized phase III BRISK-PS study. J Clin Oncol. 2013;31:3509-3516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 434] [Cited by in RCA: 485] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 9. | Cheng AL, Kang YK, Lin DY, Park JW, Kudo M, Qin S, Chung HC, Song X, Xu J, Poggi G. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J Clin Oncol. 2013;31:4067-4075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 593] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 10. | Llovet JM, Hernandez-Gea V. Hepatocellular carcinoma: reasons for phase III failure and novel perspectives on trial design. Clin Cancer Res. 2014;20:2072-2079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 323] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 11. | Akira S, Nishio Y, Inoue M, Wang XJ, Wei S, Matsusaka T, Yoshida K, Sudo T, Naruto M, Kishimoto T. Molecular cloning of APRF, a novel IFN-stimulated gene factor 3 p91-related transcription factor involved in the gp130-mediated signaling pathway. Cell. 1994;77:63-71. [PubMed] |
| 12. | Darnell JE, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415-1421. [PubMed] |
| 13. | Darnell JE. The JAK-STAT pathway: summary of initial studies and recent advances. Recent Prog Horm Res. 1996;51:391-403; discussion 403-404. [PubMed] |
| 14. | Kamran MZ, Patil P, Gude RP. Role of STAT3 in cancer metastasis and translational advances. Biomed Res Int. 2013;2013:421821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 224] [Cited by in RCA: 300] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 15. | Buettner R, Mora LB, Jove R. Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin Cancer Res. 2002;8:945-954. [PubMed] |
| 16. | Bowman T, Garcia R, Turkson J, Jove R. STATs in oncogenesis. Oncogene. 2000;19:2474-2488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1376] [Cited by in RCA: 1417] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 17. | Takeda K, Akira S. STAT family of transcription factors in cytokine-mediated biological responses. Cytokine Growth Factor Rev. 2000;11:199-207. [PubMed] |
| 18. | Hirano T, Ishihara K, Hibi M. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene. 2000;19:2548-2556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 889] [Cited by in RCA: 947] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 19. | Garcia R, Yu CL, Hudnall A, Catlett R, Nelson KL, Smithgall T, Fujita DJ, Ethier SP, Jove R. Constitutive activation of Stat3 in fibroblasts transformed by diverse oncoproteins and in breast carcinoma cells. Cell Growth Differ. 1997;8:1267-1276. [PubMed] |
| 20. | Leaman DW, Leung S, Li X, Stark GR. Regulation of STAT-dependent pathways by growth factors and cytokines. FASEB J. 1996;10:1578-1588. [PubMed] |
| 21. | Yu CL, Meyer DJ, Campbell GS, Larner AC, Carter-Su C, Schwartz J, Jove R. Enhanced DNA-binding activity of a Stat3-related protein in cells transformed by the Src oncoprotein. Science. 1995;269:81-83. [PubMed] |
| 22. | Chung J, Uchida E, Grammer TC, Blenis J. STAT3 serine phosphorylation by ERK-dependent and -independent pathways negatively modulates its tyrosine phosphorylation. Mol Cell Biol. 1997;17:6508-6516. [PubMed] |
| 23. | Yuan ZL, Guan YJ, Chatterjee D, Chin YE. Stat3 dimerization regulated by reversible acetylation of a single lysine residue. Science. 2005;307:269-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 610] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 24. | Chen W, Daines MO, Khurana Hershey GK. Turning off signal transducer and activator of transcription (STAT): the negative regulation of STAT signaling. J Allergy Clin Immunol. 2004;114:476-489; quiz 490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Han Y, Amin HM, Franko B, Frantz C, Shi X, Lai R. Loss of SHP1 enhances JAK3/STAT3 signaling and decreases proteosome degradation of JAK3 and NPM-ALK in ALK+ anaplastic large-cell lymphoma. Blood. 2006;108:2796-2803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 157] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 26. | Inagaki-Ohara K, Kondo T, Ito M, Yoshimura A. SOCS, inflammation, and cancer. JAKSTAT. 2013;2:e24053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 138] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 27. | Saydmohammed M, Joseph D, Syed V. Curcumin suppresses constitutive activation of STAT-3 by up-regulating protein inhibitor of activated STAT-3 (PIAS-3) in ovarian and endometrial cancer cells. J Cell Biochem. 2010;110:447-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 28. | Krasilnikov M, Ivanov VN, Dong J, Ronai Z. ERK and PI3K negatively regulate STAT-transcriptional activities in human melanoma cells: implications towards sensitization to apoptosis. Oncogene. 2003;22:4092-4101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 106] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 29. | He G, Yu GY, Temkin V, Ogata H, Kuntzen C, Sakurai T, Sieghart W, Peck-Radosavljevic M, Leffert HL, Karin M. Hepatocyte IKKbeta/NF-kappaB inhibits tumor promotion and progression by preventing oxidative stress-driven STAT3 activation. Cancer Cell. 2010;17:286-297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 375] [Cited by in RCA: 374] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 30. | Sinibaldi D, Wharton W, Turkson J, Bowman T, Pledger WJ, Jove R. Induction of p21WAF1/CIP1 and cyclin D1 expression by the Src oncoprotein in mouse fibroblasts: role of activated STAT3 signaling. Oncogene. 2000;19:5419-5427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 244] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 31. | Aoki Y, Feldman GM, Tosato G. Inhibition of STAT3 signaling induces apoptosis and decreases survivin expression in primary effusion lymphoma. Blood. 2003;101:1535-1542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 375] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 32. | Catlett-Falcone R, Landowski TH, Oshiro MM, Turkson J, Levitzki A, Savino R, Ciliberto G, Moscinski L, Fernández-Luna JL, Nuñez G. Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity. 1999;10:105-115. [PubMed] |
| 33. | Wang T, Niu G, Kortylewski M, Burdelya L, Shain K, Zhang S, Bhattacharya R, Gabrilovich D, Heller R, Coppola D. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat Med. 2004;10:48-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 828] [Cited by in RCA: 871] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 34. | Wang C, Yang W, Yan HX, Luo T, Zhang J, Tang L, Wu FQ, Zhang HL, Yu LX, Zheng LY. Hepatitis B virus X (HBx) induces tumorigenicity of hepatic progenitor cells in 3,5-diethoxycarbonyl-1,4-dihydrocollidine-treated HBx transgenic mice. Hepatology. 2012;55:108-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 125] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 35. | Machida K, Tsukamoto H, Liu JC, Han YP, Govindarajan S, Lai MM, Akira S, Ou JH. c-Jun mediates hepatitis C virus hepatocarcinogenesis through signal transducer and activator of transcription 3 and nitric oxide-dependent impairment of oxidative DNA repair. Hepatology. 2010;52:480-492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 36. | Zhang JF, He ML, Fu WM, Wang H, Chen LZ, Zhu X, Chen Y, Xie D, Lai P, Chen G. Primate-specific microRNA-637 inhibits tumorigenesis in hepatocellular carcinoma by disrupting signal transducer and activator of transcription 3 signaling. Hepatology. 2011;54:2137-2148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 37. | Avella DM, Li G, Schell TD, Liu D, Zhang SS, Lou X, Berg A, Kimchi ET, Tagaram HR, Yang Q. Regression of established hepatocellular carcinoma is induced by chemoimmunotherapy in an orthotopic murine model. Hepatology. 2012;55:141-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 38. | Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1362] [Cited by in RCA: 1488] [Article Influence: 82.7] [Reference Citation Analysis (0)] |
| 39. | Wang H, Lafdil F, Wang L, Park O, Yin S, Niu J, Miller AM, Sun Z, Gao B. Hepatoprotective versus oncogenic functions of STAT3 in liver tumorigenesis. Am J Pathol. 2011;179:714-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 40. | Park O, Wang H, Weng H, Feigenbaum L, Li H, Yin S, Ki SH, Yoo SH, Dooley S, Wang FS. In vivo consequences of liver-specific interleukin-22 expression in mice: Implications for human liver disease progression. Hepatology. 2011;54:252-261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 206] [Cited by in RCA: 215] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 41. | Riehle KJ, Campbell JS, McMahan RS, Johnson MM, Beyer RP, Bammler TK, Fausto N. Regulation of liver regeneration and hepatocarcinogenesis by suppressor of cytokine signaling 3. J Exp Med. 2008;205:91-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 130] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 42. | Bard-Chapeau EA, Li S, Ding J, Zhang SS, Zhu HH, Princen F, Fang DD, Han T, Bailly-Maitre B, Poli V. Ptpn11/Shp2 acts as a tumor suppressor in hepatocellular carcinogenesis. Cancer Cell. 2011;19:629-639. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 272] [Cited by in RCA: 278] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 43. | Lencioni R, Kudo M, Ye SL, Bronowicki JP, Chen XP, Dagher L, Furuse J, Geschwind JF, Ladrón de Guevara L, Papandreou C. First interim analysis of the GIDEON (Global Investigation of therapeutic decisions in hepatocellular carcinoma and of its treatment with sorafeNib) non-interventional study. Int J Clin Pract. 2012;66:675-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 44. | Wilhelm S, Chien DS. BAY 43-9006: preclinical data. Curr Pharm Des. 2002;8:2255-2257. [PubMed] |
| 45. | Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099-7109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2966] [Cited by in RCA: 3147] [Article Influence: 149.9] [Reference Citation Analysis (0)] |
| 46. | Liu L, Cao Y, Chen C, Zhang X, McNabola A, Wilkie D, Wilhelm S, Lynch M, Carter C. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006;66:11851-11858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1042] [Cited by in RCA: 1205] [Article Influence: 66.9] [Reference Citation Analysis (0)] |
| 47. | Auclair D, Miller D, Yatsula V, Pickett W, Carter C, Chang Y, Zhang X, Wilkie D, Burd A, Shi H. Antitumor activity of sorafenib in FLT3-driven leukemic cells. Leukemia. 2007;21:439-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 138] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 48. | Chen KF, Tai WT, Liu TH, Huang HP, Lin YC, Shiau CW, Li PK, Chen PJ, Cheng AL. Sorafenib overcomes TRAIL resistance of hepatocellular carcinoma cells through the inhibition of STAT3. Clin Cancer Res. 2010;16:5189-5199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 146] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 49. | Tai WT, Shiau CW, Chen PJ, Chu PY, Huang HP, Liu CY, Huang JW, Chen KF. Discovery of novel Src homology region 2 domain-containing phosphatase 1 agonists from sorafenib for the treatment of hepatocellular carcinoma. Hepatology. 2014;59:190-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 50. | Tai WT, Cheng AL, Shiau CW, Huang HP, Huang JW, Chen PJ, Chen KF. Signal transducer and activator of transcription 3 is a major kinase-independent target of sorafenib in hepatocellular carcinoma. J Hepatol. 2011;55:1041-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 139] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 51. | Yang J, Liang X, Niu T, Meng W, Zhao Z, Zhou GW. Crystal structure of the catalytic domain of protein-tyrosine phosphatase SHP-1. J Biol Chem. 1998;273:28199-28207. [PubMed] |
| 52. | Yang J, Liu L, He D, Song X, Liang X, Zhao ZJ, Zhou GW. Crystal structure of human protein-tyrosine phosphatase SHP-1. J Biol Chem. 2003;278:6516-6520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 137] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 53. | Wang W, Liu L, Song X, Mo Y, Komma C, Bellamy HD, Zhao ZJ, Zhou GW. Crystal structure of human protein tyrosine phosphatase SHP-1 in the open conformation. J Cell Biochem. 2011;112:2062-2071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 54. | Wu C, Sun M, Liu L, Zhou GW. The function of the protein tyrosine phosphatase SHP-1 in cancer. Gene. 2003;306:1-12. [PubMed] |
| 55. | Sun J, Blaskovich MA, Jove R, Livingston SK, Coppola D, Sebti SM. Cucurbitacin Q: a selective STAT3 activation inhibitor with potent antitumor activity. Oncogene. 2005;24:3236-3245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 199] [Article Influence: 10.0] [Reference Citation Analysis (0)] |