Published online Nov 7, 2014. doi: 10.3748/wjg.v20.i41.15098
Revised: April 29, 2014
Accepted: May 19, 2014
Published online: November 7, 2014
Processing time: 346 Days and 9.3 Hours
Endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) have recently been accepted as less invasive methods for treating patients with early esophageal cancers such as squamous cell carcinoma and dysplasia of Barrett’s esophagus. However, the large defects in the esophageal mucosa often cause severe esophageal strictures, which dramatically reduce the patient’s quality of life. Although preventive endoscopic balloon dilatation can reduce dysphagia and the frequency of dilatation, other approaches are necessary to prevent esophageal strictures after ESD. This review describes several strategies for preventing esophageal strictures after ESD, with a particular focus on anti-inflammatory and tissue engineering approaches. The local injection of triamcinolone acetonide and other systemic steroid therapies are frequently used to prevent esophageal strictures after ESD. Tissue engineering approaches for preventing esophageal strictures have recently been applied in basic research studies. Scaffolds with temporary stents have been applied in five cases, and this technique has been shown to be safe and is anticipated to prevent esophageal strictures. Fabricated autologous oral mucosal epithelial cell sheets to cover the defective mucosa similarly to how commercially available skin products fabricated from epidermal cells are used for skin defects or in cases of intractable ulcers. Fabricated autologous oral-mucosal-epithelial cell sheets have already been shown to be safe.
Core tip: Esophageal strictures after extensive endoscopic submucosal dissection (ESD) reduce quality of life. Endoscopic local injections and the oral administration of steroids are safe and effective for preventing esophageal strictures. In addition, several tissue engineering therapies have been used in attempts to overcome severe esophageal strictures. Cell-based tissue engineering therapy with fabricated autologous oral mucosal epithelial cell sheets has been used to prevent esophageal strictures after ESD in nine patients. This therapy has been shown to be safe and may be widely used in the future.
- Citation: Kobayashi S, Kanai N, Ohki T, Takagi R, Yamaguchi N, Isomoto H, Kasai Y, Hosoi T, Nakao K, Eguchi S, Yamamoto M, Yamato M, Okano T. Prevention of esophageal strictures after endoscopic submucosal dissection. World J Gastroenterol 2014; 20(41): 15098-15109
- URL: https://www.wjgnet.com/1007-9327/full/v20/i41/15098.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i41.15098
Endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) are accepted as less invasive treatments for early esophageal cancers, including squamous cell carcinoma and dysplasia of Barrett’s esophagus (BE)[1-17]. Additionally, radiofrequency ablation has been shown to be safe and effective for treating patients with dysplasia due to BE[18-24].
The occurrence of severe esophageal strictures after endoscopic treatments for large tumors remains an unsolved problem. However, the safety and efficacy of endoscopic treatments have been technically and oncologically established for a wide range of esophageal intramucosal neoplasms and BE. Risk factors for esophageal strictures after ESD include the cervical location, a tumor size greater than 3/4 of the esophageal circumference, and a longitudinal tumor diameter of more than 40 mm[9,25-27]. Furthermore, esophageal strictures after the treatment of BE are often observed at the resection site with at least 50% of the esophageal mucosal circumference[28].
Esophageal strictures cause dysphagia, and patients are required to receive repeated balloon dilatation procedures or temporary stents. These esophageal strictures decrease the patient’s quality of life, although the endoscopic treatment itself is less invasive than surgical therapy. Moreover, the use of endoscopic balloon dilatation (EBD) to treat esophageal strictures carries the risk of perforation. Takahashi et al[29] reported that esophageal perforations were observed in 7 of 76 patients who received EBD for the treatment of esophageal strictures. The occurrence of strictures after esophageal ESD is more common in EBD performed after esophageal strictures (92%) than in multiple sessions of EBD performed weekly before esophageal strictures (59%)[30]. Furthermore, the duration of EBD after esophageal stricture is generally shorter in patients who undergo multiple sessions of EBD before developing esophageal strictures (29 d) than in those who undergo EBD after esophageal strictures form (78 d). Although multiple sessions of EBD before esophageal strictures can prevent esophageal strictures after ESD, such frequent dilatation treatments are problematic because of their high invasiveness and cost. Therefore, less invasive approaches are desired.
Various approaches have been used to prevent esophageal strictures after ESD and can be generally categorized as either anti-inflammatory drugs or tissue engineering technologies.
Anti-inflammatory approaches for preventing esophageal strictures after ESD are based on the concept that subsequent strictures may be suppressed by inhibiting the infiltration of inflammatory cells, the hyperplasia of granulation, and the fibrosis of the remaining submucosal layer at the ulcer site. Anti-inflammatory treatments using steroids have recently been demonstrated to prevent esophageal strictures after ESD (Table 1). Basic research has also been conducted on several potential drugs that selectively inhibit fibrotic formation.
| Action | Administration | Advantages | Disadvantages and limitations | |
| Clinical study | ||||
| Corticosteroids | Steroidal | Oral intake | Strongly inhibits the infiltration of inflammatory cells, the hyperplasia associated with granulation, and the fibrosis of the remaining submucosal layer | General side effects (severe infection, peptic ulcer, hyperglycemia, psychiatric symptoms, and osteoporosis) |
| Delayed wound healing | ||||
| Triamcinolone acetonide | Steroidal | Local injection | Inhibits the infiltration of inflammatory cells, the hyperplasia associated with granulation, and the fibrosis of the remaining submucosal layer | Risk of ulcer formation due to accidental injection into the muscularis |
| Delayed wound healing | ||||
| Pre-clinical study | ||||
| MMC | Inhibition of DNA synthesis | Local injection | Inhibits the proliferation and activation of fibroblasts | An effect has not been shown for the prevention of esophageal strictures, although MMC improves recurrent dysphagia or restenosis after the dilatation of esophageal strictures |
| The risks of perforation and secondary malignancy | ||||
| N-acetylcysteine | Antioxidant molecule | Oral intake | Antifibrotic effect without the inhibition of wound healing | Insufficient effect in an animal model of severe esophageal stricture |
Endoscopic intralesional injections of steroids are applied based on the concept that inflammation and fibrosis after esophageal ESD are inhibited by the direct administration of steroids to the ulcer site. The initial reports regarding steroid therapy demonstrated that intralesional injections of triamcinolone acetonide after the dilatation of benign esophageal strictures reduces the frequency of esophageal strictures in the endoscopic field[31,32]. These results are similar to findings for the injection of triamcinolone acetonide to treat keloid scars[31,33,34]. Subsequently, triamcinolone acetonide has been used to prevent esophageal strictures after ESD (Table 2). Hashimoto et al[35] reported that the local injection of triamcinolone acetonide into the ulcer site prevents esophageal strictures after ESD. Twenty-one patients were treated with local injections of triamcinolone acetonide at 3, 7, and 10 d after ESD. The total dose of triamcinolone acetonide was 18 to 62 mg in each injection session. The stricture rate in the patients who were given the local injection (19%, 4/21) was lower than in the control patients (75%, 15/20) (P = 0.03). Furthermore, the frequencies of dilatation in the patients who were given a local injection (mean 1.7, range 0-15) were significantly lower than the frequency of control patients (mean 6.6, range 0-20) (P > 0.001). Hanaoka et al[36] also reported that local injection of triamcinolone prevented esophageal strictures after ESD using a single injection of triamcinolone acetonide immediately after ESD. The total dose of triamcinolone acetonide was 100 mg[36]. In Nagasaki University Hospital, 3/4-circumferential ESD cases are generally treated with this local injection therapy. Fifty mg of triamcinolone acetonide is endoscopically injected in submucosal layer 1 or 2 times for 3 wk and generally results in a satisfactory outcome. It is recommended that triamcinolone acetonide doses of approximately 18 to 100 mg are injected several times into the ulcer site in the early phase after ESD to prevent esophageal strictures. However, triamcinolone injection might be insufficient to prevent esophageal strictures for large mucosal defects because esophageal strictures can develop after the combinational treatments of dilatation and triamcinolone injection for benign esophageal strictures[31,32,37]. The patients with circumferential ESD were excluded in the clinical studies of local injections of triamcinolone[35,36]. There is also a high risk of ulcer formation due to endoscopic local injection when the muscularis is missed during the injection[38]. Additionally, insufficient fibrosis causes the ulcer site of the esophageal wall to be fragile and leads to perforation after balloon dilatation. Isomoto et al[27] developed a special injection needle for injecting ulcer sites after ESD. The needle, which is 25 gauge with a length of 1.8 mm, is finer and shorter than conventional injection needles, which are usually 23 or 25 gauge in diameter and 4 mm in length, thereby avoiding deep injections into the muscularis.
| Ref. | Resection size | The methodology of triamcinolone injection | Treatment outcomes | ||||||||
| Circumference1 | Length(mm) | Injection needle | Concentration (mg/mL) | Single dose (mL) | Number of punctures(/session) | Total amounts (mg) | Sessions | The rate of strictures | The number of EBD | Observation periods | |
| Hashimoto et al[35] | > 3/4 | 54 | 25 G, 4 mm | 10 | 0.2 | 9-31 | 18-62 | Three times2 | 19% | 1.7 (0-15) | 1 yr |
| (28-60) | (4/21) | ||||||||||
| Hanaoka et al[36] | > 3/4 | 58 ± 11 | 25 G | 5 | 0.5-1 | 20-40 | 100 | Single3 | 6.60% | 0 (0-2) | 2 mo |
| (3/30) | |||||||||||
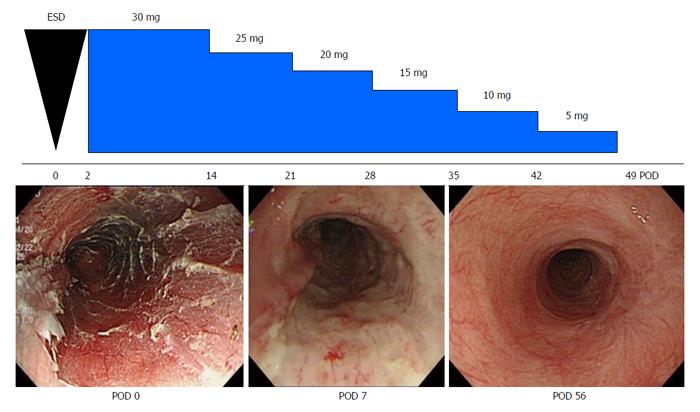
The efficacy of systemic steroid therapy for preventing esophageal strictures after ESD has been confirmed by Yamaguchi et al[39]. In their study, 22 patients who underwent multiple sessions of EBD before esophageal strictures were compared with 19 patients who underwent systemic steroid therapy. The stricture rate in the systemic steroid therapy group (5.3%, 1/19) was significantly lower than that in the multiple sessions of EBD before esophageal strictures group (31.8%, 7/22) (P < 0.0001). Furthermore, the frequency of dilatation in the systemic steroid therapy group (mean 1.7, range 0-7) was significantly lower than in the multiple sessions of EBD before esophageal strictures group (mean 15.6, range 0-48) (P < 0.0001). Sato et al[40] reported that systemic steroid therapy with EBD is also more effective than EBD alone in preventing esophageal strictures after circumferential ESD. In Nagasaki University Hospital, patients who have at least two of the following risk factors are treated with systemic steroid therapy after esophageal ESD: more than 3/4-circumferential ESD, a longitudinal tumor diameter greater than 40 mm, and cervical location. Beginning 2 d after ESD, 0.5 mg/kg prednisolone per day was administered orally for 2 wk. The dose of prednisolone was continuously reduced until the regenerative mucosa covered the artificial ulcer site of the esophageal ESD (Figure 1). However, complete healing of the esophageal mucosa generally requires 8-16 wk because steroids inhibit the activation of fibroblasts and inflammatory cells and reduce the proliferation and migration of epithelial cells. High doses of prednisolone have been associated with the possibility of developing adverse effects such as severe infections, peptic ulcers, hyperglycemia, psychiatric symptoms, and osteoporosis. However, no adverse events occurred in the clinical studies of systemic steroid therapy for preventing esophageal strictures after ESD[27,39]. Furthermore, the perforation risk of balloon dilatation after steroid therapy by either local injection or systemic administration may be higher than in multiple sessions of EBD treatment[38]. Although systemic administration of steroids prevents esophageal strictures after extensive ESD, systemic administration of steroids might not prevent esophageal strictures after a very long segmental circumferential ESD. This occurs because systemic steroid therapy does not prevent esophageal strictures after major injuries of the esophagus involving a long length of circumferential mucosal defect[41,42].
There are several studies regarding specifically preventing excessive fibrotic formation to avoid the various adverse effects of steroid therapies. The locoregional injection of mitomycin C into the esophageal stricture after ESD was shown to improve recurrent dysphagia or restenosis without serious complications in 5 patients[43]. Mitomycin C is also effective for the treatment of refractory esophageal strictures which include caustic, surgical, and peptic strictures[44]. Additionally, because mitomycin C injection has an anti-proliferative effect on fibroblasts it also prevents refractory esophageal strictures. Although the injection of mitomycin C is suggested for preventing esophageal strictures after ESD, it has poor reproducibility in an animal model[45]. Mitomycin C might cause several local adverse events such as delayed mucosal healing, ulcer formation, and perforation. In long-term studies, secondary malignancy should be examined because mitomycin induces DNA damage[46,47].
N-acetylcysteine is an antioxidant compound with antifibrotic effects that is also expected to prevent esophageal strictures. However, its efficacy is minimal in animal models of severe esophageal strictures after ESD[48]. N-acetylcysteine has been clearly shown to promote corneal wound healing better than steroid therapy. Thus, combination therapy involving N-acetylcysteine and other treatments may potentially be effective[49].
Tissue engineering approaches for preventing esophageal strictures after ESD are based on the concept that transplanted materials and tissues can repair and replace damaged tissues, maintain the physiological functions of these tissues, and enhance mucosal healing. This issue is especially important because mucosal defects cause severe inflammation at artificial ulcer sites. Tissue engineering approaches for preventing esophageal strictures after ESD are divided into two groups: scaffold-based therapy and cell-based therapy.
Temporary scaffolds made from biodegradable materials can support tissue and protect esophageal strictures. Saito et al[50] reported that biodegradable stents composed of poly-L-lactic acid prevented esophageal strictures after ESD in 2 patients. Additionally, Nieponice and Badylak reported that an extracellular matrix (ECM) biologic scaffold composed of porcine-derived small intestinal submucosa, together with a temporary metallic stent, is safe and efficacious for the prevention of esophageal strictures after endoscopic resection in animal models[51-53]. Biological scaffolds have been safely used to treat five high-grade BE patients undergoing endoscopic resection[54]. Long length (8 to 13 cm) circumferential resections were performed in these patients. The esophageal strictures after endoscopic resection and were improved by only a few sessions (0 to 9) of endoscopic dilatation even though the temporary stent support prevented strictures. Surprisingly, the small perforation site healed in 18 d by covering the perforation with a biological scaffold and stent. The scaffold provides an ECM, supports strictures, and promotes cell migration. However, it may be insufficient to cover an extensive mucosal defect after esophageal ESD. The acellular scaffold of the esophageal mucosa includes key proteins required for producing a basal membrane. Additionally, the acellular scaffold of the esophageal mucosa provides a suitable environment that facilitates cell adhesion and proliferation[55]. Consequently, the risk of local recurrence after scaffold transplantation may be higher than at normal ulcer sites because the scaffold itself is also a good environment for the engraftment of unwelcome malignant cells. The transplantation of biomaterials is also associated with the risk of developing a local infection, which affects the microenvironment of wound healing. This issue is especially important for esophageal mucosal healing because both the esophageal lumen and the oral cavity are constantly exposed to microorganisms. Scaffold-based therapies currently lack sufficient clinical evidence that prevent their use for esophageal strictures and have potential risks of adverse events such as a local recurrence and infection. In the future, the development of novel materials and the advancement of biological science will help solve these problems.
Cell-based therapies are expected to have trophic effects on the host as the transplanted cells release cytokines and growth factors and interact with other host cells. The direct injection of primary cells into the host organ has two major disadvantages, which are low viability and quick diffusion from the host sites after transplantation. Transplanted cells are difficult to engraft at the target site. As a result, there have been several in vitro attempts to engineer tissues with the ability to effectively engraft cells at the target site.
In our laboratory, epithelial cell sheets of oral mucosa without any scaffold are fabricated on temperature-responsive culture inserts that are grafted with poly-N-isopropylacrylamide (PIPPAm)[56]. At 37 °C, PIPPAm becomes hydrophobic, but below its lower critical solution temperature of 32 °C it is hydrophilic[57,58]. Cells attach and proliferate on the hydrophobic surface of the cell culture insert at 37 °C. However, due to the remarkable character of PIPPAm, the cells can detach themselves from the surface when the temperature is below 32 °C and PIPPAm becomes hydrophilic. This behavior enables the cell sheets to be harvested without the use of enzymes and allows the cells to preserve their cell-membrane proteins and cell-to-cell junctions. Subsequently, the fabricated epithelial cell sheets can be grafted to the host organ without any adhesive materials because the ECM is deposited on the basal layer of the epithelial cell sheets and the basal side can attach to the artificial ulcer site[59]. Furthermore, the transplantation of autologous oral epithelial cell sheets to an esophageal mucosal defect has been shown to promote re-epithelization of the esophageal mucosa in a canine model[60].
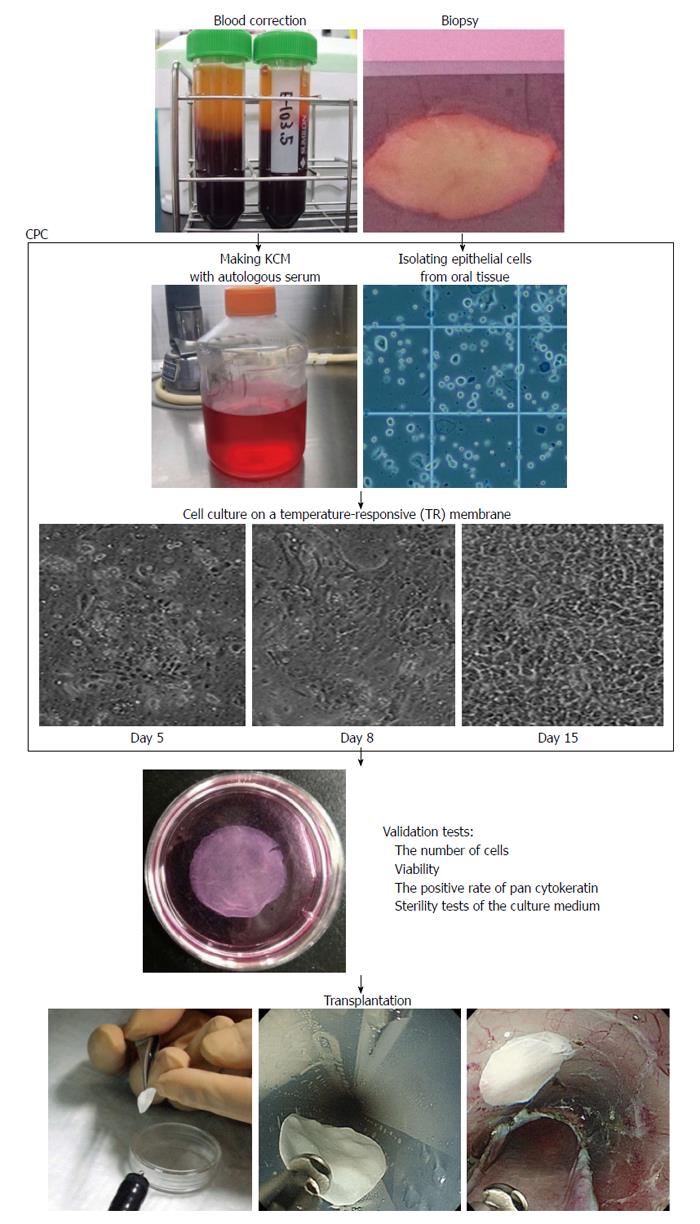
For clinical applications, oral mucosal epithelial cell sheets must be fabricated in a cell processing center (CPC) (Figure 2)[61,62]. CPCs have separate clean rooms that meet good manufacturing practice guidelines, and various other parameters (such as aerosol, temperature, and humidity) are monitored to validate the clean environment of the CPC. To prevent possible human errors and careless mistakes during the fabrication of oral mucosal epithelial cell sheets, standard operation procedures (SOPs) for culture methods are documented and a second person always checks the document while the first operator performs the procedure in the CPC. In addition, to ensure safety and avoid possible immune reactions and unknown infections animal-derived materials are eliminated as much as possible. In our clinical study, autologous serum is used instead of fetal bovine serum, which is commonly used for culturing cells[61]. 3T3 feeder cells are fibroblast cells obtained from mice. These cells are classified as a xenogeneic material by the Food and Drug Administration in the United States and have never been used for culture in our system. However, these feeder cells are known to promote the proliferation of various epithelial cells[63]. The fabricated oral mucosal epithelial cell sheets are required to pass all validation tests including a sterility test, a harvesting test, and a purity test prior to transplantation[64]. However, no tests for cell proliferation, migration, and attachment are included. In the future, noninvasive measurements of these test parameters will be required prior to clinical application.
Before transplantation of the autologous mucosal epithelial cell sheet into the artificial ulcer site after esophageal ESD, an esophageal endoscopic mucosal resection (EEMR) tube (Create Medic, Tokyo, Japan) is inserted into the patient’s esophagus. The endoscopic transplantation of the autologous oral mucosal epithelial cell sheet is immediately performed after esophageal ESD using a support membrane and endoscopic forceps. The transplanted autologous oral mucosal epithelial cell sheets adhere to the ulcer site within a few minutes without suturing or any adhesive materials because the cell sheets maintain adhesive proteins on their basal side and have cell-to-cell junctions. The fabricated autologous oral mucosal epithelial cell sheet is composed of a basal layer and an apical layer. The basal layer is gently attached to the ulcer site and this procedure requires careful handling. The transplant can be disturbed by spasm of the muscularis and by the entry balloon of the EEMR tube used to maintain the intraluminal pressure of the esophagus. Therefore, a device that facilitates transplantation of the autologous mucosal epithelial cell sheets into the ulcer site after esophageal ESD is needed.
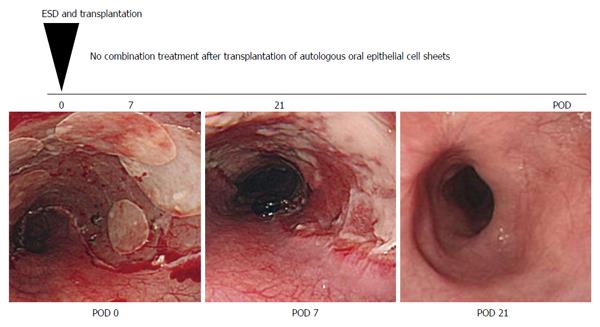
The safety of transplanting autologous oral mucosal epithelial cell sheets into artificial ulcer sites has been demonstrated in a phase I study[65]. The transplantation of oral mucosal epithelial cell sheets prevented esophageal strictures after ESD in 8 of 9 cases. In the eight successful cases, there was no dysphagia and strictures after esophageal ESD and no additional treatments for complications were required. Only one cases required balloon dilatation of the esophageal stricture after ESD. Additionally, mucosal healing was rapidly completed within 3 to 5 wk. This observation suggests that autologous oral epithelial cell sheets promote epithelial healing and result in the satisfactory prevention of esophageal strictures after ESD (Figure 3). In the future, a large study must be performed to confirm that autologous oral mucosal epithelial cell sheets are an effective material for the prevention of esophageal strictures after ESD.
Cell-based therapy using autologous oral mucosal epithelial cell sheets has several disadvantages compared with scaffold-based therapy and anti-inflammatory drug therapy. First, a CPC is necessary to culture the oral epithelial cells. Thus, the operating cost is high and is currently estimated to be at least 20000 to 30000 USD per case. Thus, fabricating oral mucosal epithelial cell sheets in a CPC in every hospital will be technically and financially difficult. One alternative is ready-made oral mucosal epithelial cell sheets that can be transported in a suitable environment from a production site to the hospital where the transplantation will be performed. Second, the fabrication of autologous oral mucosal epithelial cell sheets requires the patient’s own tissues and serum. Although autologous oral epithelial cell sheets have been successfully fabricated, it is difficult to maintain consistent fabrication quality and quantity because individual differences can affect cell proliferation and differentiation. Third, possible bacterial and fungal contamination during the culture of oral mucosal epithelial cell sheets is the most common factor leading to failure, which may be a disappointment to patients who have provided their own tissues. Fourth, multiple neoplasms in the oral and throat areas are sometimes observed in patients with esophageal cancer so harvesting tissues from the premalignant region in the oral mucosa must also be avoided because the transplantation of multipotent cancer cells may cause local recurrence[66,67]. Translational research studies aimed at improving these disadvantages are currently in progress in our laboratory.
Epidermal cells are expected to be suitable substitutes for oral mucosal epithelial cells. Epidermal cells have characteristics similar to esophageal and oral mucosal epithelial cells because these cells are classified as squamous cells. Furthermore, epidermal cells are frequently cultured for the production of cutaneous medical devices that are already used in clinics[68-72]. The risk of contamination with malignant cells is lower in the skin than in the oral mucosa, and the epidermis is composed of keratinocytes. Additionally, in a swine model, cell sheets made from autologous epidermal cells have been shown to prevent esophageal strictures after ESD as effectively as oral mucosal epithelial cell sheets[73].
The capability of fabricating cell sheets in serum-free medium is essential to reduce the variation between individual differences and to standardize cell culture procedures. Specifically, the addition of an IL-1 receptor antagonist has been found to be beneficial for fabricating cell sheets in serum-free medium[74].
Adipose-derived stem cells (ADSCs) are similar to bone marrow-derived stem cells. ADSCs have the following biological features: growth factor secretion, capacity to differentiate into multiple cell types, ability to suppress inflammatory cells, ability to promote angiogenesis and enhanced of wound healing[75-77]. Additionally, ADSCs can be obtained easily from adipose tissue. Cell therapies using ADSCs have been performed to repair bone defects, treat complex perianal fistulas in Crohn’s disease, and alleviate severe steroid-resistant graft-vs-host disease[78-80]. Cell-based therapy using ADSCs is also expected to prevent esophageal strictures after ESD. The local injection of autologous ADSCs after esophageal EMR has also been found to prevent esophageal strictures in a canine model[81]. This result is insufficient evidence for concluding that ADSCs prevent esophageal strictures after ESD because no evaluations of the surface markers, multipotentiality, and proliferation of the injected cells have been performed.
Tissue engineering approaches have great potential for treating various damaged tissues and organs. Currently, treatments using tissue engineering must include safety and quality controls and require careful observations after transplantation. Esophageal tissue engineering will be developed and become available in the near future because transplanted engineered tissues can be noninvasively observed by endoscopy and because unexpected complications such as local infections, immunological responses, and tumorigenesis are easily managed.
The replacement of mucosal structures with a basal membrane after esophageal ESD may be a novel tissue engineering therapy for the prevention of esophageal strictures after ESD. Therefore, sufficient numbers of cells and biomaterials in basal membranes are necessary to overcome severe esophageal strictures after ESD.
Tissue engineering approaches will also provide a treatment for refractory esophageal strictures. Patch esophagoplasty using biologic scaffolds for refractory esophageal strictures has already been performed in 4 patients[82]. Various treatments using tissue engineering for refractory esophageal strictures will be performed in the near future.
This review reports several strategies for preventing esophageal strictures after extensive ESD with a focus on anti-inflammatory, scaffold-based, and cell-based treatments. Anti-inflammatory treatments, which are mainly local and systemic steroid therapies, have shown positive outcomes in small comparative clinical studies. However, the clinical evidence of scaffold-based and cell-based treatments is still insufficient, and their efficacy needs to be confirmed in comparative studies because they are potentially new technologies for tissue engineering and novel treatment strategies for wound healing. To establish a truly minimally invasive treatment using endoscopic surgery, improvements to all of these methods are needed. Nonetheless, these three strategies will eventually become available as a combined therapy in the future.
The authors thank their colleagues. Toshiyuki Owaki provided particularly constructive comments regarding CPC management. Takanori Iwata provided constructive comments regarding the main structure of this article. The authors also acknowledge the cooperation with the Endoscopy Division of the National Cancer Center Hospital and the Endoscopy Division of the National Cancer Center Hospital East. Hiroyuki Morimoto provided particularly constructive comments regarding esophageal strictures after ESD. The authors appreciate the editor’s and reviewers’ valuable comments and suggestions for improving this article. Dr. Teruo Okano is a founder and director of the board of Cell Seed Inc., licensing technologies and patents from Tokyo Women's Medical University related to this submitted work. Dr. Teruo Okano and Dr. Masayuki Yamato are shareholders of CellSeed Inc. The last author Dr. Teruo Okano is a founder and director of the board of NanoCarrrier Co., Ltd.
P- Reviewer: Contini S, Heilmann RM, Kim BW, Xiao B, Yalniz M S- Editor: Wen LL L- Editor: A E- Editor: Ma S
| 1. | Inoue H, Tani M, Nagai K, Kawano T, Takeshita K, Endo M, Iwai T. Treatment of esophageal and gastric tumors. Endoscopy. 1999;31:47-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 86] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 2. | Fujita H, Sueyoshi S, Yamana H, Shinozaki K, Toh U, Tanaka Y, Mine T, Kubota M, Shirouzu K, Toyonaga A. Optimum treatment strategy for superficial esophageal cancer: endoscopic mucosal resection versus radical esophagectomy. World J Surg. 2001;25:424-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 116] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 3. | Shimizu Y, Tsukagoshi H, Fujita M, Hosokawa M, Kato M, Asaka M. Long-term outcome after endoscopic mucosal resection in patients with esophageal squamous cell carcinoma invading the muscularis mucosae or deeper. Gastrointest Endosc. 2002;56:387-390. [PubMed] |
| 4. | Katada C, Muto M, Momma K, Arima M, Tajiri H, Kanamaru C, Ooyanagi H, Endo H, Michida T, Hasuike N. Clinical outcome after endoscopic mucosal resection for esophageal squamous cell carcinoma invading the muscularis mucosae--a multicenter retrospective cohort study. Endoscopy. 2007;39:779-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 129] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 5. | Pech O, May A, Gossner L, Rabenstein T, Manner H, Huijsmans J, Vieth M, Stolte M, Berres M, Ell C. Curative endoscopic therapy in patients with early esophageal squamous-cell carcinoma or high-grade intraepithelial neoplasia. Endoscopy. 2007;39:30-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Ciocirlan M, Lapalus MG, Hervieu V, Souquet JC, Napoléon B, Scoazec JY, Lefort C, Saurin JC, Ponchon T. Endoscopic mucosal resection for squamous premalignant and early malignant lesions of the esophagus. Endoscopy. 2007;39:24-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 7. | Fujishiro M, Yahagi N, Kakushima N, Kodashima S, Muraki Y, Ono S, Yamamichi N, Tateishi A, Shimizu Y, Oka M. Endoscopic submucosal dissection of esophageal squamous cell neoplasms. Clin Gastroenterol Hepatol. 2006;4:688-694. [PubMed] |
| 8. | Takahashi H, Arimura Y, Masao H, Okahara S, Tanuma T, Kodaira J, Kagaya H, Shimizu Y, Hokari K, Tsukagoshi H. Endoscopic submucosal dissection is superior to conventional endoscopic resection as a curative treatment for early squamous cell carcinoma of the esophagus (with video). Gastrointest Endosc. 2010;72:255-264, 264.e1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 243] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 9. | Ono S, Fujishiro M, Niimi K, Goto O, Kodashima S, Yamamichi N, Omata M. Long-term outcomes of endoscopic submucosal dissection for superficial esophageal squamous cell neoplasms. Gastrointest Endosc. 2009;70:860-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 334] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 10. | Oyama T, Tomori A, Hotta K, Morita S, Kominato K, Tanaka M, Miyata Y. Endoscopic submucosal dissection of early esophageal cancer. Clin Gastroenterol Hepatol. 2005;3:S67-S70. [PubMed] |
| 11. | Hirasawa K, Kokawa A, Oka H, Yahara S, Sasaki T, Nozawa A, Tanaka K. Superficial adenocarcinoma of the esophagogastric junction: long-term results of endoscopic submucosal dissection. Gastrointest Endosc. 2010;72:960-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 12. | Ishii N, Horiki N, Itoh T, Uemura M, Maruyama M, Suzuki S, Uchida S, Izuka Y, Fukuda K, Fujita Y. Endoscopic submucosal dissection with a combination of small-caliber-tip transparent hood and flex knife is a safe and effective treatment for superficial esophageal neoplasias. Surg Endosc. 2010;24:335-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Kakushima N, Yahagi N, Fujishiro M, Kodashima S, Nakamura M, Omata M. Efficacy and safety of endoscopic submucosal dissection for tumors of the esophagogastric junction. Endoscopy. 2006;38:170-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 85] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Ishihara R, Iishi H, Uedo N, Takeuchi Y, Yamamoto S, Yamada T, Masuda E, Higashino K, Kato M, Narahara H. Comparison of EMR and endoscopic submucosal dissection for en bloc resection of early esophageal cancers in Japan. Gastrointest Endosc. 2008;68:1066-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 231] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 15. | Yoshinaga S, Gotoda T, Kusano C, Oda I, Nakamura K, Takayanagi R. Clinical impact of endoscopic submucosal dissection for superficial adenocarcinoma located at the esophagogastric junction. Gastrointest Endosc. 2008;67:202-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 94] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 16. | Repici A, Hassan C, Carlino A, Pagano N, Zullo A, Rando G, Strangio G, Romeo F, Nicita R, Rosati R. Endoscopic submucosal dissection in patients with early esophageal squamous cell carcinoma: results from a prospective Western series. Gastrointest Endosc. 2010;71:715-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 140] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 17. | Higuchi K, Tanabe S, Azuma M, Katada C, Sasaki T, Ishido K, Naruke A, Katada N, Koizumi W. A phase II study of endoscopic submucosal dissection for superficial esophageal neoplasms (KDOG 0901). Gastrointest Endosc. 2013;78:704-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 18. | Gondrie JJ, Pouw RE, Sondermeijer CM, Peters FP, Curvers WL, Rosmolen WD, Ten Kate F, Fockens P, Bergman JJ. Effective treatment of early Barrett’s neoplasia with stepwise circumferential and focal ablation using the HALO system. Endoscopy. 2008;40:370-379. [PubMed] |
| 19. | Ganz RA, Overholt BF, Sharma VK, Fleischer DE, Shaheen NJ, Lightdale CJ, Freeman SR, Pruitt RE, Urayama SM, Gress F. Circumferential ablation of Barrett’s esophagus that contains high-grade dysplasia: a U.S. Multicenter Registry. Gastrointest Endosc. 2008;68:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 174] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 20. | Fleischer DE, Overholt BF, Sharma VK, Reymunde A, Kimmey MB, Chuttani R, Chang KJ, Lightdale CJ, Santiago N, Pleskow DK. Endoscopic ablation of Barrett’s esophagus: a multicenter study with 2.5-year follow-up. Gastrointest Endosc. 2008;68:867-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 126] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 21. | Sharma VK, Kim HJ, Das A, Dean P, DePetris G, Fleischer DE. A prospective pilot trial of ablation of Barrett’s esophagus with low-grade dysplasia using stepwise circumferential and focal ablation (HALO system). Endoscopy. 2008;40:380-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Fleischer DE, Overholt BF, Sharma VK, Reymunde A, Kimmey MB, Chuttani R, Chang KJ, Muthasamy R, Lightdale CJ, Santiago N. Endoscopic radiofrequency ablation for Barrett’s esophagus: 5-year outcomes from a prospective multicenter trial. Endoscopy. 2010;42:781-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 153] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 23. | Shaheen NJ, Sharma P, Overholt BF, Wolfsen HC, Sampliner RE, Wang KK, Galanko JA, Bronner MP, Goldblum JR, Bennett AE. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N Engl J Med. 2009;360:2277-2288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1146] [Cited by in RCA: 971] [Article Influence: 60.7] [Reference Citation Analysis (0)] |
| 24. | van Vilsteren FG, Pouw RE, Seewald S, Alvarez Herrero L, Sondermeijer CM, Visser M, Ten Kate FJ, Yu Kim Teng KC, Soehendra N, Rösch T. Stepwise radical endoscopic resection versus radiofrequency ablation for Barrett’s oesophagus with high-grade dysplasia or early cancer: a multicentre randomised trial. Gut. 2011;60:765-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 217] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 25. | Katada C, Muto M, Manabe T, Boku N, Ohtsu A, Yoshida S. Esophageal stenosis after endoscopic mucosal resection of superficial esophageal lesions. Gastrointest Endosc. 2003;57:165-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 222] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 26. | Mizuta H, Nishimori I, Kuratani Y, Higashidani Y, Kohsaki T, Onishi S. Predictive factors for esophageal stenosis after endoscopic submucosal dissection for superficial esophageal cancer. Dis Esophagus. 2009;22:626-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 150] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 27. | Isomoto H, Yamaguchi N, Minami H, Nakao K. Management of complications associated with endoscopic submucosal dissection/ endoscopic mucosal resection for esophageal cancer. Dig Endosc. 2013;25 Suppl 1:29-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 28. | Lewis JJ, Rubenstein JH, Singal AG, Elmunzer BJ, Kwon RS, Piraka CR. Factors associated with esophageal stricture formation after endoscopic mucosal resection for neoplastic Barrett’s esophagus. Gastrointest Endosc. 2011;74:753-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 29. | Takahashi H, Arimura Y, Okahara S, Uchida S, Ishigaki S, Tsukagoshi H, Shinomura Y, Hosokawa M. Risk of perforation during dilation for esophageal strictures after endoscopic resection in patients with early squamous cell carcinoma. Endoscopy. 2011;43:184-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 30. | Ezoe Y, Muto M, Horimatsu T, Morita S, Miyamoto S, Mochizuki S, Minashi K, Yano T, Ohtsu A, Chiba T. Efficacy of preventive endoscopic balloon dilation for esophageal stricture after endoscopic resection. J Clin Gastroenterol. 2011;45:222-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 134] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 31. | Kochhar R, Makharia GK. Usefulness of intralesional triamcinolone in treatment of benign esophageal strictures. Gastrointest Endosc. 2002;56:829-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 51] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 32. | Kochhar R, Ray JD, Sriram PV, Kumar S, Singh K. Intralesional steroids augment the effects of endoscopic dilation in corrosive esophageal strictures. Gastrointest Endosc. 1999;49:509-513. [PubMed] |
| 33. | Ramage JI, Rumalla A, Baron TH, Pochron NL, Zinsmeister AR, Murray JA, Norton ID, Diehl N, Romero Y. A prospective, randomized, double-blind, placebo-controlled trial of endoscopic steroid injection therapy for recalcitrant esophageal peptic strictures. Am J Gastroenterol. 2005;100:2419-2425. [PubMed] |
| 34. | Hochman B, Locali RF, Matsuoka PK, Ferreira LM. Intralesional triamcinolone acetonide for keloid treatment: a systematic review. Aesthetic Plast Surg. 2008;32:705-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 35. | Hashimoto S, Kobayashi M, Takeuchi M, Sato Y, Narisawa R, Aoyagi Y. The efficacy of endoscopic triamcinolone injection for the prevention of esophageal stricture after endoscopic submucosal dissection. Gastrointest Endosc. 2011;74:1389-1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 227] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 36. | Hanaoka N, Ishihara R, Takeuchi Y, Uedo N, Higashino K, Ohta T, Kanzaki H, Hanafusa M, Nagai K, Matsui F. Intralesional steroid injection to prevent stricture after endoscopic submucosal dissection for esophageal cancer: a controlled prospective study. Endoscopy. 2012;44:1007-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 195] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 37. | Altintas E, Kacar S, Tunc B, Sezgin O, Parlak E, Altiparmak E, Saritas U, Sahin B. Intralesional steroid injection in benign esophageal strictures resistant to bougie dilation. J Gastroenterol Hepatol. 2004;19:1388-1391. [PubMed] |
| 38. | Yamashina T, Uedo N, Fujii M, Ishihara R, Mikamori M, Motoori M, Yano M, Iishi H. Delayed perforation after intralesional triamcinolone injection for esophageal stricture following endoscopic submucosal dissection. Endoscopy. 2013;45 Suppl 2 UCTN:E92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 39. | Yamaguchi N, Isomoto H, Nakayama T, Hayashi T, Nishiyama H, Ohnita K, Takeshima F, Shikuwa S, Kohno S, Nakao K. Usefulness of oral prednisolone in the treatment of esophageal stricture after endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. Gastrointest Endosc. 2011;73:1115-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 248] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 40. | Sato H, Inoue H, Kobayashi Y, Maselli R, Santi EG, Hayee B, Igarashi K, Yoshida A, Ikeda H, Onimaru M. Control of severe strictures after circumferential endoscopic submucosal dissection for esophageal carcinoma: oral steroid therapy with balloon dilation or balloon dilation alone. Gastrointest Endosc. 2013;78:250-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 41. | Pelclová D, Navrátil T. Do corticosteroids prevent oesophageal stricture after corrosive ingestion? Toxicol Rev. 2005;24:125-129. [PubMed] |
| 42. | Fulton JA, Hoffman RS. Steroids in second degree caustic burns of the esophagus: a systematic pooled analysis of fifty years of human data: 1956-2006. Clin Toxicol (Phila). 2007;45:402-408. [PubMed] |
| 43. | Machida H, Tominaga K, Minamino H, Sugimori S, Okazaki H, Yamagami H, Tanigawa T, Watanabe K, Watanabe T, Fujiwara Y. Locoregional mitomycin C injection for esophageal stricture after endoscopic submucosal dissection. Endoscopy. 2012;44:622-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 44. | Berger M, Ure B, Lacher M. Mitomycin C in the therapy of recurrent esophageal strictures: hype or hope? Eur J Pediatr Surg. 2012;22:109-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 45. | Wu Y, Schomisch SJ, Cipriano C, Chak A, Lash RH, Ponsky JL, Marks JM. Preliminary results of antiscarring therapy in the prevention of postendoscopic esophageal mucosectomy strictures. Surg Endosc. 2014;28:447-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 46. | Dusre L, Covey JM, Collins C, Sinha BK. DNA damage, cytotoxicity and free radical formation by mitomycin C in human cells. Chem Biol Interact. 1989;71:63-78. [PubMed] |
| 47. | Bakshi SR, Patel RK, Roy SK, Alladi P, Trivedi AH, Bhatavdekar JM, Patel DD, Shah PM, Rawal UM. Mitomycin C induced chromosomal aberrations in young cancer patients. Mutat Res. 1998;422:223-228. [PubMed] |
| 48. | Barret M, Batteux F, Beuvon F, Mangialavori L, Chryssostalis A, Pratico C, Chaussade S, Prat F. N-acetylcysteine for the prevention of stricture after circumferential endoscopic submucosal dissection of the esophagus: a randomized trial in a porcine model. Fibrogenesis Tissue Repair. 2012;5:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 49. | Sarchahi AA, Meimandi Parizi A, Eghtedari M, Keshavarz S. Effect of different treatment regimen with dexamethasone and acetylcysteine on corneal wound healing in rabbits. Iran J Med Sci. 2011;36:188-195. [PubMed] |
| 50. | Saito Y, Tanaka T, Andoh A, Minematsu H, Hata K, Tsujikawa T, Nitta N, Murata K, Fujiyama Y. Novel biodegradable stents for benign esophageal strictures following endoscopic submucosal dissection. Dig Dis Sci. 2008;53:330-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 108] [Article Influence: 6.4] [Reference Citation Analysis (1)] |
| 51. | Badylak SF, Vorp DA, Spievack AR, Simmons-Byrd A, Hanke J, Freytes DO, Thapa A, Gilbert TW, Nieponice A. Esophageal reconstruction with ECM and muscle tissue in a dog model. J Surg Res. 2005;128:87-97. [PubMed] |
| 52. | Nieponice A, McGrath K, Qureshi I, Beckman EJ, Luketich JD, Gilbert TW, Badylak SF. An extracellular matrix scaffold for esophageal stricture prevention after circumferential EMR. Gastrointest Endosc. 2009;69:289-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 120] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 53. | Badylak SF, Valentin JE, Ravindra AK, McCabe GP, Stewart-Akers AM. Macrophage phenotype as a determinant of biologic scaffold remodeling. Tissue Eng Part A. 2008;14:1835-1842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 554] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 54. | Badylak SF, Hoppo T, Nieponice A, Gilbert TW, Davison JM, Jobe BA. Esophageal preservation in five male patients after endoscopic inner-layer circumferential resection in the setting of superficial cancer: a regenerative medicine approach with a biologic scaffold. Tissue Eng Part A. 2011;17:1643-1650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 162] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 55. | Keane TJ, Londono R, Carey RM, Carruthers CA, Reing JE, Dearth CL, D’Amore A, Medberry CJ, Badylak SF. Preparation and characterization of a biologic scaffold from esophageal mucosa. Biomaterials. 2013;34:6729-6737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 56. | Yamato M, Utsumi M, Kushida A, Konno C, Kikuchi A, Okano T. Thermo-responsive culture dishes allow the intact harvest of multilayered keratinocyte sheets without dispase by reducing temperature. Tissue Eng. 2001;7:473-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 299] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 57. | Okano T, Yamada N, Sakai H, Sakurai Y. A novel recovery system for cultured cells using plasma-treated polystyrene dishes grafted with poly(N-isopropylacrylamide). J Biomed Mater Res. 1993;27:1243-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 844] [Cited by in RCA: 711] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 58. | Okano T, Yamada N, Okuhara M, Sakai H, Sakurai Y. Mechanism of cell detachment from temperature-modulated, hydrophilic-hydrophobic polymer surfaces. Biomaterials. 1995;16:297-303. [PubMed] |
| 59. | Kushida A, Yamato M, Konno C, Kikuchi A, Sakurai Y, Okano T. Decrease in culture temperature releases monolayer endothelial cell sheets together with deposited fibronectin matrix from temperature-responsive culture surfaces. J Biomed Mater Res. 1999;45:355-362. [PubMed] [DOI] [Full Text] |
| 60. | Ohki T, Yamato M, Murakami D, Takagi R, Yang J, Namiki H, Okano T, Takasaki K. Treatment of oesophageal ulcerations using endoscopic transplantation of tissue-engineered autologous oral mucosal epithelial cell sheets in a canine model. Gut. 2006;55:1704-1710. [PubMed] |
| 61. | Takagi R, Murakami D, Kondo M, Ohki T, Sasaki R, Mizutani M, Yamato M, Nishida K, Namiki H, Yamamoto M. Fabrication of human oral mucosal epithelial cell sheets for treatment of esophageal ulceration by endoscopic submucosal dissection. Gastrointest Endosc. 2010;72:1253-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 62. | Takagi R, Yamato M, Kanai N, Murakami D, Kondo M, Ishii T, Ohki T, Namiki H, Yamamoto M, Okano T. Cell sheet technology for regeneration of esophageal mucosa. World J Gastroenterol. 2012;18:5145-5150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 63. | Takagi R, Yamato M, Murakami D, Kondo M, Yang J, Ohki T, Nishida K, Kohno C, Okano T. Preparation of keratinocyte culture medium for the clinical applications of regenerative medicine. J Tissue Eng Regen Med. 2011;5:e63-e73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 64. | Takagi R, Yamato M, Murakami D, Kondo M, Ohki T, Sasaki R, Nishida K, Namiki H, Yamamoto M, Okano T. Fabrication and validation of autologous human oral mucosal epithelial cell sheets to prevent stenosis after esophageal endoscopic submucosal dissection. Pathobiology. 2011;78:311-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 65. | Ohki T, Yamato M, Ota M, Takagi R, Murakami D, Kondo M, Sasaki R, Namiki H, Okano T, Yamamoto M. Prevention of esophageal stricture after endoscopic submucosal dissection using tissue-engineered cell sheets. Gastroenterology. 2012;143:582-8.e1-582-8.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 355] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 66. | Poon RT, Law SY, Chu KM, Branicki FJ, Wong J. Multiple primary cancers in esophageal squamous cell carcinoma: incidence and implications. Ann Thorac Surg. 1998;65:1529-1534. [PubMed] |
| 67. | Watanabe A, Hosokawa M, Taniguchi M, Tsujie H, Sasaki S. Head and neck cancer associated with esophageal cancer. Auris Nasus Larynx. 2007;34:207-211. [PubMed] |
| 68. | Eaglstein WH, Iriondo M, Laszlo K. A composite skin substitute (graftskin) for surgical wounds. A clinical experience. Dermatol Surg. 1995;21:839-843. [PubMed] |
| 69. | Falanga V, Margolis D, Alvarez O, Auletta M, Maggiacomo F, Altman M, Jensen J, Sabolinski M, Hardin-Young J. Rapid healing of venous ulcers and lack of clinical rejection with an allogeneic cultured human skin equivalent. Human Skin Equivalent Investigators Group. Arch Dermatol. 1998;134:293-300. [PubMed] |
| 70. | Zaulyanov L, Kirsner RS. A review of a bi-layered living cell treatment (Apligraf) in the treatment of venous leg ulcers and diabetic foot ulcers. Clin Interv Aging. 2007;2:93-98. [PubMed] |
| 71. | Centanni JM, Straseski JA, Wicks A, Hank JA, Rasmussen CA, Lokuta MA, Schurr MJ, Foster KN, Faucher LD, Caruso DM. StrataGraft skin substitute is well-tolerated and is not acutely immunogenic in patients with traumatic wounds: results from a prospective, randomized, controlled dose escalation trial. Ann Surg. 2011;253:672-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 72. | Schurr MJ, Foster KN, Centanni JM, Comer AR, Wicks A, Gibson AL, Thomas-Virnig CL, Schlosser SJ, Faucher LD, Lokuta MA. Phase I/II clinical evaluation of StrataGraft: a consistent, pathogen-free human skin substitute. J Trauma. 2009;66:866-873; discussion 873-874. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 73. | Kanai N, Yamato M, Ohki T, Yamamoto M, Okano T. Fabricated autologous epidermal cell sheets for the prevention of esophageal stricture after circumferential ESD in a porcine model. Gastrointest Endosc. 2012;76:873-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 74. | Kondo M, Yamato M, Takagi R, Namiki H, Okano T. The regulation of epithelial cell proliferation and growth by IL-1 receptor antagonist. Biomaterials. 2013;34:121-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 75. | Huang SP, Hsu CC, Chang SC, Wang CH, Deng SC, Dai NT, Chen TM, Chan JY, Chen SG, Huang SM. Adipose-derived stem cells seeded on acellular dermal matrix grafts enhance wound healing in a murine model of a full-thickness defect. Ann Plast Surg. 2012;69:656-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 76. | Komatsu I, Yang J, Zhang Y, Levin LS, Erdmann D, Klitzman B, Hollenbeck ST. Interstitial engraftment of adipose-derived stem cells into an acellular dermal matrix results in improved inward angiogenesis and tissue incorporation. J Biomed Mater Res A. 2013;101:2939-2947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 77. | Guadalajara H, Herreros D, De-La-Quintana P, Trebol J, Garcia-Arranz M, Garcia-Olmo D. Long-term follow-up of patients undergoing adipose-derived adult stem cell administration to treat complex perianal fistulas. Int J Colorectal Dis. 2012;27:595-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 134] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 78. | Mesimäki K, Lindroos B, Törnwall J, Mauno J, Lindqvist C, Kontio R, Miettinen S, Suuronen R. Novel maxillary reconstruction with ectopic bone formation by GMP adipose stem cells. Int J Oral Maxillofac Surg. 2009;38:201-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 301] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 79. | de la Portilla F, Alba F, García-Olmo D, Herrerías JM, González FX, Galindo A. Expanded allogeneic adipose-derived stem cells (eASCs) for the treatment of complex perianal fistula in Crohn’s disease: results from a multicenter phase I/IIa clinical trial. Int J Colorectal Dis. 2013;28:313-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 259] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 80. | Fang B, Song Y, Liao L, Zhang Y, Zhao RC. Favorable response to human adipose tissue-derived mesenchymal stem cells in steroid-refractory acute graft-versus-host disease. Transplant Proc. 2007;39:3358-3362. [PubMed] |
| 81. | Honda M, Hori Y, Nakada A, Uji M, Nishizawa Y, Yamamoto K, Kobayashi T, Shimada H, Kida N, Sato T. Use of adipose tissue-derived stromal cells for prevention of esophageal stricture after circumferential EMR in a canine model. Gastrointest Endosc. 2011;73:777-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 82. | Nieponice A, Ciotola FF, Nachman F, Jobe BA, Hoppo T, Londono R, Badylak S, Badaloni AE. Patch esophagoplasty: esophageal reconstruction using biologic scaffolds. Ann Thorac Surg. 2014;97:283-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 83. | Yoshikawa T, Oyama T, Yagi N, Naito Y. Plvs vltre ESD Saranaruchosen. Japan: SGINDAN TO CHIRYO SHA, Inc 2011; 146-156. |