Published online Jan 28, 2014. doi: 10.3748/wjg.v20.i4.1095
Revised: October 28, 2013
Accepted: November 12, 2013
Published online: January 28, 2014
Processing time: 138 Days and 9.1 Hours
AIM: To investigate whether autologous dendritic cell (DC)-cytokine-induced killer (CIK) cell therapy is able to improve the therapeutic efficacy of chemotherapy in colon cancer.
METHODS: We conducted a systematic review of published papers from the sources of MEDLINE, the Cochrane Central Register of Controlled Trials, EMBASE, the Wanfang Database, the China Science and Technology Periodical Database and China Journal Net. Published data were extracted independently by two authors using predefined database templates. The quality of the data from individual papers was also assessed. The effects of chemotherapy were compared with those of chemotherapy in combination with DC-CIK immunotherapy. The pooled analysis was performed using the data from random or fixed-effect models.
RESULTS: Seven trials matched our inclusion criteria (n = 533). The overall analysis showed significant survival benefit [one-year overall survival (OS), P < 0.0001; two-year OS, P = 0.009; three-year OS, P = 0.002] in favor of DC-CIK immunotherapy combined with chemotherapy. Disease-free survival (DFS) rate was improved after the combination of DC-CIK immunotherapy and chemotherapy (one-year DFS, P < 0.0001; two-year DFS, P = 0.002; three-year DFS, P = 0.02). An improved overall response rate (P = 0.009) was also observed in patients who received DC-CIK therapy. Furthermore, the analysis of T-lymphocyte subsets in peripheral blood indicated that the number of CD4+ T cells significantly increased in the DC-CIK plus chemotherapy group (P < 0.05).
CONCLUSION: The combination of DC-CIK immunotherapy and chemotherapy was superior in prolonging the survival time and enhancing immunological responses.
Core tip: A growing body of knowledge on tumor immunosurveillance and loss thereof has contributed to the refinement of anti-tumor immunotherapy. The aim of our meta-analysis was to determine whether an association exists between dendritic cell (DC)-cytokine-induced killer (CIK) cell therapy combined with chemotherapy and chemotherapy alone. Our analysis demonstrates that DC-CIK therapy improved 1, 2 and 3-year overall survival, 1, 2 and 3-year disease-free survival, overall response rate and immune indices in colon cancer. In all, the combination of DC-CIK immunotherapy and chemotherapy was superior in prolonging the survival time and enhancing immunological responses, suggesting the possible application of this promising adjuvant immunotherapy in colon cancer.
- Citation: Wang ZX, Cao JX, Liu ZP, Cui YX, Li CY, Li D, Zhang XY, Liu JL, Li JL. Combination of chemotherapy and immunotherapy for colon cancer in China: A meta-analysis. World J Gastroenterol 2014; 20(4): 1095-1106
- URL: https://www.wjgnet.com/1007-9327/full/v20/i4/1095.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i4.1095
Colorectal cancer is the third most commonly diagnosed cancer in humans. As dietary habits have changed in recent years, the number of cases of colon cancer have been increasing faster in the Eastern world[1,2]. Although surgical resection is the first choice worldwide, colorectal cancer can also be treated effectively with chemotherapy, radiation therapy, or both to improve patient survival. However, 25% of patients that present with metastatic disease have a five-year survival of only 10%. A variety of therapeutic strategies for metastatic colon cancer have been evaluated over the last decade, however, most patients in advanced stages of the disease have little hope of longer survival. Therefore, an effective approach for the treatment of colorectal cancer patients with metastasis and post-operative cancer recurrence is critical. In recent years there has been great interest in cancer immunotherapy, which has the potential to control metastatic disease, prolong time to recurrence, and ultimately serve as a preventive measure.
Adoptive immunotherapy holds great promise in the scenario of potential new approaches for the treatment of solid tumors that are refractory to conventional therapies. Recently, the effectiveness of immunotherapy in the treatment of numerous forms of cancers has been studied. For instance, it was reported that adoptive cell transfer of ex vivo-activated autologous tumor-reactive Tc1 or Tc17 T cells can mediate effective anti-tumor immunity and tumor regression in melanoma[3]. Cytokine-induced killer (CIK) cell therapy is also able to induce complete clinical responses in renal cell carcinoma (RCC) patients, and was demonstrated to be safe and effective in these patients[4]. A systematic review and meta-analysis of randomized controlled trials in multiple myeloma (MM) indicate that autologous-allogeneic (auto-allo) hematopoietic cell transplantation (HCT) can induce higher complete remission rates. There is no improvement in overall survival (OS) with auto-allo HCT, however, this approach can achieve higher non-relapse mortality rates in patients with newly diagnosed MM[5]. Another study suggested that erythropoietin-producing hepatocellular carcinoma A2 (EphA2)-specific T-cell immunotherapy may be a promising approach for the treatment of EphA2-positive glioblastoma[6]. Furthermore, αβ and γδ T cell-based immunotherapy has been found to improve treatment efficacy in osteosarcoma, especially at the recurrent and metastatic stages[7]. A multi-center historical cohort study indicated that the effectiveness of immunotherapy in advanced lung cancer is limited, but may extend life span under certain conditions. Moreover, immunotherapy can maintain good quality of life in patients until near the time of death[8]. The bedside medication administration of Sipuleucel-T (Provenge, Dendreon) was the first-in class therapeutic autologous vaccine to be approved for the treatment of men with asymptomatic or minimally symptomatic castrate-resistant metastatic prostate cancer in the spring of 2010[9]. This product represents the culmination of basic immunological and prostate cancer investigations for decades, and 13 years of clinical trials[10]. Another immunotherapeutic drug, the cytotoxic T-lymphocyte antigen 4 antibody, Ipilimumab, has been demonstrated to improve OS in patients with metastatic melanoma in two phase III trials[11,12] and was approved in March of 2011. These important drug development milestones provide solid evidence that targeting of the immune system can lead to clinically relevant immune responses, thus extending the life span of cancer patients.
Challenging issues for all adoptive immunotherapy strategies include the obtainment of sufficient numbers of immune effectors, recognition of tumor targets and possible restriction to specific human leukocyte antigens-haplotypes. Such hurdles have also been encountered in immunotherapy trials for colon cancer. CIK cells are ex vivo-expanded T lymphocytes that share phenotypic and functional properties with both natural killer and T cells. It has been reported that secretory glycoprotein 90K-specific cytotoxic T lymphocytes (CTLs) generated by 90K-pulsed dendritic cells (DCs) are useful effector cells for immunotherapy in colon cancer[13]. Mucin 1 (CD227)-specific CTLs have also been shown to cause complete rejection of tumor cells, when in the therapeutic regimen, and tumor burden was significantly reduced[14]. CTLs specific for the tumor-associated antigen, CEP55, can efficiently recognize colon cancer stem-like cells or tumor-initiating cells which highly express the stem cell markers, SRY-box 2, POU class 5 homeobox 1, leucine-rich repeat-containing G protein-coupled receptor 5 and aldehyde dehydrogenase 1 family member A1 in vitro and in vivo[15]. DCs are rare leucocytes that are uniquely potent in their ability to capture, process and present antigens to T cells. They selectively migrate through tissues to reach lymph nodes and spleen where initiation of the immune response takes place. DC-based vaccinations are an attractive candidate adjunct therapy to treat colon cancer patients. It has been demonstrated that signal transducers and activators of transcription 3-depleted DC vaccination induces effective systemic anti-tumor effects through high antigen (Ag) -specific T cell responses accompanied by systemic T helper 1 immune responses in a murine colon cancer model[16]. DCs pulsed with carcinoembryonic antigen (CEA) peptide resulted in prolonged antigen-presentation and efficient T-cell activation, but not CEA mRNA for vaccination[17]. A phase I-II CEA-loaded DC vaccine trial in patients with colon cancer is ongoing (http://www.clinicaltrial.gov id: NCT 01219348). In the microenvironment of human colon adenocarcinoma, the supernatant mediates endothelial-like differentiation of induced DCs by extracellular signal-regulated protein kinases 1 and 2 signaling, which suggests that immunocytes are involved in the cancer microenvironment[18]. Based on findings in the tumor microenvironment and cancer stem cells in cancer therapy, immunotherapy is worth further investigation.
Over the past few years, advances in their understanding of the immune system, improved design of clinical trials, and improvement and compliance of manufacturing processes have provided opportunities to significantly improve efficacy and safety of immunotherapy. However, clinical studies on DC-CIK cells are still in their infancy and there is no clear consensus on how they may be best optimized. Therefore, we performed a systematic review and meta-analysis of clinical trials to assess the therapeutic efficacy of DC-CIK cells combined with chemotherapy in colon cancer.
Trials were identified by electronic search in the PubMed database (1976 onward), Embase (1966 onward), the Cochrane Central Registry of Controlled Trials (no date restriction), the Wanfang Database (no date restriction), the China Science and Technology Periodical Database (no date restriction), China Journal Net (no date restriction), reference lists of published trials and relevant review articles. The search strategy included the following medical subject headings: “colon cancer”, “cytokine-induced killer cells”, “dendritic cells”, “immunotherapy”, “colon rectal cancer” and free text search. No language limits were applied. The initial search was performed in June 2012 and updates were conducted in June 2013. Furthermore, we contacted drug manufacturers, asked experts in the field, and performed manual searches in reference lists, conference proceedings of the American Society of Clinical Oncology Annual Meetings and the European Cancer Conference. We also searched the http://www.ClinicalTrials.gov website for information on prospective and ongoing trials. No language restriction was applied. We excluded abstracts that were never subsequently published as full papers and studies on animals and cell lines.
Data extraction was independently conducted by two reviewers (Cao JX and Li CY) using a standardized approach. Disagreement was adjudicated by a third reviewer (Li D) after referring back to the original publications. We collected information including authors’ names, journal, year of publication, sample size per arm, regimen used, median or mean age of patients, sex, numbers of patients assessable for 1- and 3-year overall survival and numbers of patients assessable for 3-year disease-free survival and information pertaining to study design (whether the trial reported the mode of randomization, allocation concealment, description of withdrawals per arm, and blinding) for the trials included in the study.
OS was defined as the time from the initiation of treatment until death. The secondary objective was disease-free survival (DFS). Other endpoints were disease control rate [DCR = complete response (CR) + partial response (PR) + stable disease (SD)] and objective response rate (ORR = CR + PR), respectively. CR, PR, mixed response or SD were documented and extracted for analysis.
Statistical analysis was carried out by pair-wise comparison of the immunotherapy-containing arms of the identified trials with the respective non-immunotherapy arms. Treatment effects are reflected by odds ratios (OR) for OS, DFS, ORR and clinical benefit rate. To calculate the pooled OR, the number of OS, DFS, ORR and DCR in each arm were extracted from each study and combined using a method reported by Mantel and Haenszel. A pooled OR < 1 indicated lower recurrence or lower survival in the immunotherapy arm. To evaluate whether the results of the studies were homogeneous, we used Cochran’s Q test. This statistical test is a χ2 test with df equal to the number of studies minus one, and tests the null hypothesis that the difference between the study estimates of OR is due to chance. We also calculated the quantity, I2, which describes the percentage of variation across studies due to heterogeneity rather than chance. I2 values of 25%, 50%, and 75% were used as evidence of low, moderate, and high heterogeneity, respectively. SPSS 11.5 was also used to carry out the data analysis. The OR was calculated with a fixed-effect model when no statistically significant heterogeneity existed; otherwise, a random-effect model was employed. P-values < 0.05 were considered statistically significant. All reported P-values resulted from two-sided version tests of the respective tests.
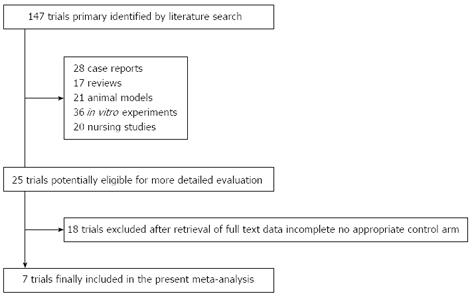
The electronic search yielded 147 references. After title and abstract review, 122 publications were excluded for various reasons (17 were review articles, 21 used animal models, 28 were case reports, 36 were in vitro experiments, 20 were nursing studies). The full texts of 25 articles were selected as potentially relevant and retrieved for more detailed assessment. We excluded a total of 18 studies as they did not include detailed patient clinical data or therapy response, and the phase I clinical study in Germany with CIK cells for colorectal cancer patients, which did not include a control arm[19]. The selection procedure is shown in Figure 1. As a result, 7 articles reporting clinical trials of DC-CIK cell-based therapy combined with chemotherapy were selected for meta-analysis.
After the selection process, 7 eligible trials with a total of 533 patients were included in the present analysis. All of the trials were fully published. The seven selected papers contained four randomized studies, two retrospective analyses and one study considering treatment programs to construct the control. These studies are listed in Table 1 and other related clinical data are also listed.
| Ref. | Age(yr) | Tumor characteristic (TNM) | Regimens(per arm) | Patients (male) | Culture conditions of CIK cells | Culture conditions of DC cells | CIK regimens |
| Zhang et al[24] | UK | II, III, IV | Chemo | 31 (UK) | IFNγ, CD3, IL1, IL-2 | GM-CSF,IL-4, TNFα | > 1.0 × 109/course |
| Chemo- | 32 (UK) | ||||||
| DC-CIK | Randomized | ||||||
| Zhu et al[25] | 58.3 (Mid) | II, III, IV | Chemo | 43 (27) | IFNγ, CD3, IL-2 | IFNγ, LPS | 1.1-8.0 × 1010/course |
| 59.2 (Mid) | Chemo- | 40 (24) | |||||
| DC-CIK | Treatment program | ||||||
| Ying et al[23] | UK | II, III | Chemo | 51 (25) | IFNγ, CD3, IL-1, IL-2 | GM-CSF,IL-4, TNFα | ≥ 1010/course |
| Chemo- | 51 (31) | ||||||
| DC-CIK | Retrospective analysis | ||||||
| Yuan et al[26] | UK | III, IV | Chemo | 21 (16) | IFNγ, CD3, IL-1,IL-2 | GM-CSF,IL-4, TNFα,IFNγ | ≥ 1010/course |
| Chemo- | 21 (15) | ||||||
| DC-CIK | Randomized | ||||||
| Cai et al[20] | 44.5 (Ave)46.7 (Ave) | II, III | Chemo | 40 (23) | IFNγ, CD3, IL-1,IL-2 | UK | |
| Chemo- | 40 (25) | ||||||
| CIK | Randomized | ||||||
| Wei et al[22] | 55.5 (Mid) 54 (Mid) | I, II, III | Chemo | 82 (41) | IFNγ, CD3, IL-1,IL-2 | GM-CSF,IL-4, TNFα,IFNγ | ≥ 1010/course |
| Chemo- | 41 (18) | ||||||
| DC-CIK | Retrospective analysis | ||||||
| Li et al[21] | 57.5 (Ave)54.5 (Ave) | II, III | Chemo | 20 (15) | IFNγ, CD3, IL-2 | GM-CSF,IL-4, IFNγ | UK |
| Chemo- | 20 (13) | ||||||
| DC-CIK | Randomized |
Most of the patients in these studies had a good performance status with an expected duration of survival > 3 mo and the median age of the patients was 54.5 years. In all seven trials, DC-CIK or CIK cell therapy combined with chemotherapy was evaluated in patients with colon cancer. Interferon-γ, CD3 monoclonal antibody and interleukin (IL) -2 were used in the CIK cell culture systems in all the trials analyzed. In addition, granulocyte-macrophage colony stimulating factor (GM-CSF), IL-4, and tumor necrosis factor-α were used in the DC cell culture system. Both CIK and DC-CIK cell therapy were included in this analysis. In one of the trials, only CIK cell therapy was used for colon cancer treatment[20], while the other six trials utilized both DC and CIK cell therapy[21-26]. The number of CIK cells transfused into patients in these studies was more than 1.0 × 109/course.
Information on patients in the two groups (DC-CIK cell therapy combined with chemotherapy and chemotherapy alone) in these trials, such as gender, chemotherapy category (FOLFOX or XELOX and other chemicals) and CIK cell dose were analyzed by χ2 test (data not shown). There was no statistically significant difference between the groups, with all P-values being > 0.05. The origins of the patient information from the articles in each group did not interfere with the results of the meta-analysis. However, the patient’s age (including all the unknown patients) did impact on the efficacy of DC-CIK cell therapy by χ2 test (data not shown). Furthermore, other clinical information from the trials such as tumor diameter and performance status were not analyzed due to insufficient data.
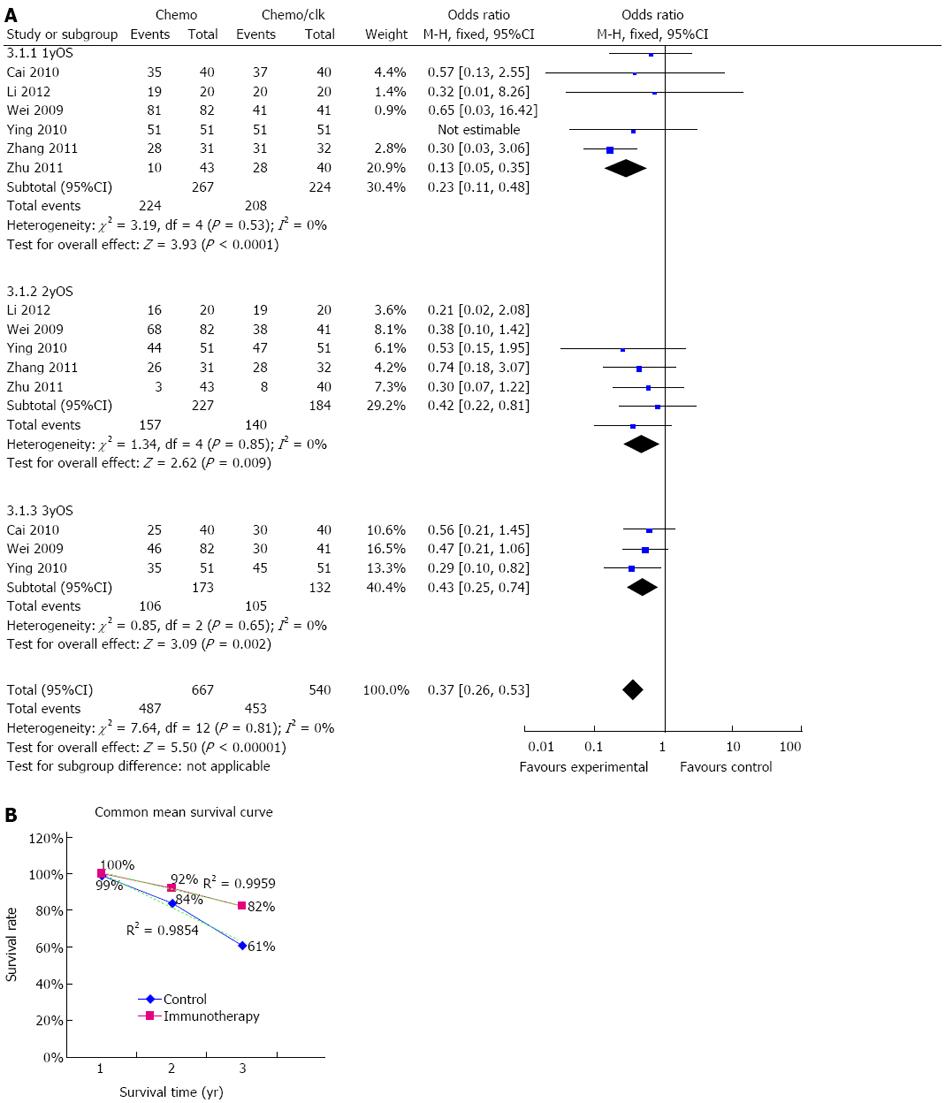
Information on 1-year survival was available in six trials[20-25]. These six trials included 491 patients (224 patients received immunotherapy combined with chemotherapy) in total. The 1-year overall survival rates were 93% (208/224) for colon cancer patients who received DC-CIK immunotherapy combined with chemotherapy. In comparison, 1-year overall survival rates were only 84% (224/267) in patients who did not receive DC-CIK immunotherapy. Each of the six trials showed longer survival for patients who received DC-CIK immunotherapy combined with chemotherapy. The estimated pooled OR for the six trials demonstrated a highly significant improvement in one-year survival for patients receiving DC-CIK immunotherapy combined with chemotherapy (OR 0.23; 95%CI: 0.11-0.48, P < 0.0001). The Cochran’s Q test resulted in a P value of 0.53 and the corresponding quantity, I2, was 0%, indicating that the degree of variability between the trials was consistent with what would be expected to occur by chance alone (Figure 2A).
Information on 2-year survival was available in four trials[21-25]. These studies included a collective total of 411 patients (184 patients received immunotherapy combined with chemotherapy) (Figure 2A). DC-CIK immunotherapy combined with chemotherapy resulted in 76% (140/184) of colon cancer patients achieving 2-year survival. In comparison, the 2-year overall survival for the control group was only 69% (157/227). The results of the pooled analysis showed that patients in the DC-CIK combined group had significantly improved two-year survival (OR = 0.42; 95%CI: 0.22-0.81, P = 0.009). There was no evidence of heterogeneity among individual studies (P = 0.85; I2 = 0%).
Information on 3-year survival was available in 3 trials[20,22,23], which included 305 patients (132 patients received immunotherapy combined with chemotherapy). DC-CIK immunotherapy combined with chemotherapy resulted in 80% (105/132) of colon cancer patients achieving 3-year overall survival, compared to 61% (106/173) in the chemotherapy only group. All three trials showed improved survival in the DC-CIK immunotherapy combined with chemotherapy patients. The estimated pooled OR for the three trials showed a highly significant improvement in three-year survival in patients receiving DC-CIK immunotherapy combined with chemotherapy (OR = 0.43; 95%CI: 0.25-0.74, P = 0.002) (Figure 2A). The Cochran’s Q test resulted in a P value of 0.65 and the corresponding quantity, I2, was 0%, indicating there was no evidence of heterogeneity among the individual studies.
Adequate information on the 1-year, 2-year and 3-year survival rate was only available in 2 trials (Wei et al[22] and Ying et al[23]), therefore, we simply summarized the data from these two trials to show the survival graph (Figure 2B). The data are shown on the figure and the dotted line represents the linear trend line (r2 = 0.9959 for the immunotherapy group and r2 = 0.9854 for the control group).
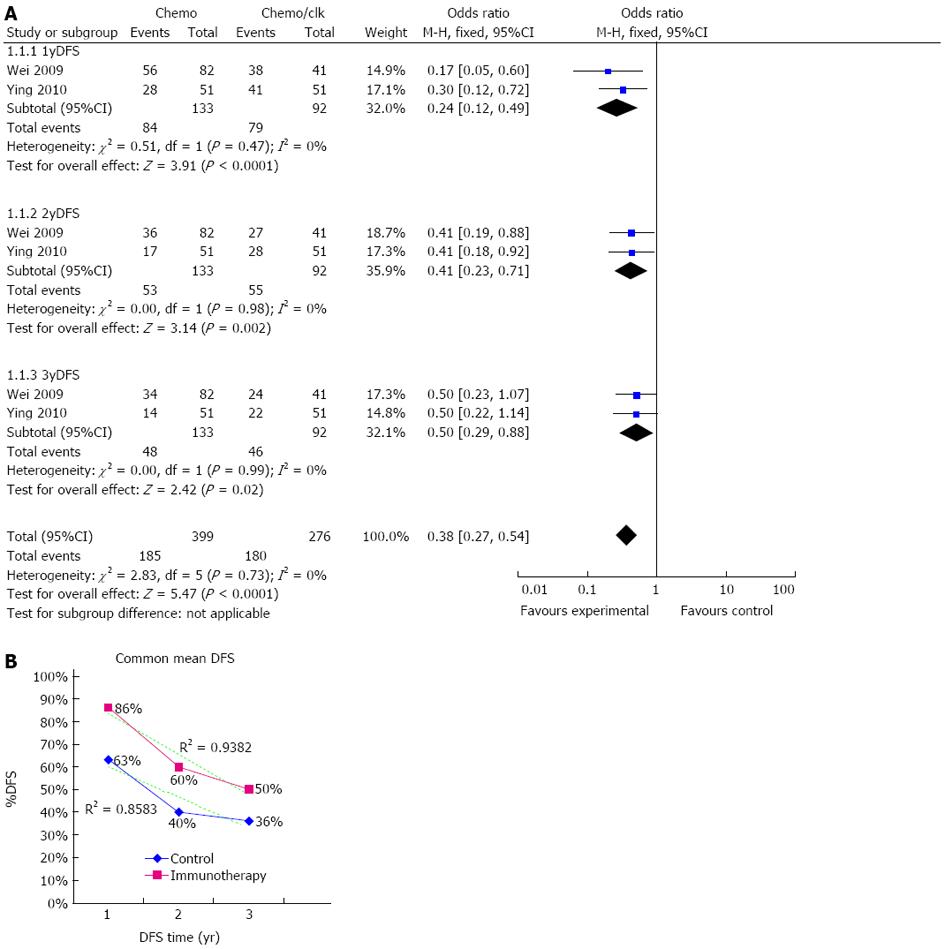
Information on 1-year DFS was available in two trials[22,23] and included 225 patients (92 patients received immunotherapy combined with chemotherapy) (Figure 3A). DC-CIK immunotherapy combined with chemotherapy led to 1-year DFS in 86% (79/92) of colon cancer patients. In contrast, 1-year DFS was only 63% (84/133) in patients who received only chemotherapy. Both trials showed longer DFS in DC-CIK immunotherapy combined with chemotherapy patients in comparison to chemotherapy only in the first year. The estimated pooled OR for the two trials showed a highly significant improvement in one-year DFS in patients receiving DC-CIK immunotherapy combined with chemotherapy (OR = 0.24; 95%CI: 0.120.49, P < 0.0001). The Cochran’s Q test resulted in a P value of 0.47 and corresponding quantity, I2, was 0%, indicating that the degree of variability between trials was consistent with what would be expected to occur by chance alone.
Information on 2-year DFS was available for two trials[22,23] and included 225 patients (92 patients received immunotherapy combined with chemotherapy). DC-CIK immunotherapy plus chemotherapy resulted in 60% (55/92) of colon cancer patients achieving 2-year DFS, compared to 40% (53/133) in the control group. The estimated pooled OR for the two trials showed a highly significant improvement in two-year DFS in patients receiving DC-CIK immunotherapy combined with chemotherapy (OR = 0.41; 95%CI: 0.23-0.71, P = 0.002). The Cochran’s Q test resulted in a P value of 0.98 and corresponding quantity, I2, was 0% (Figure 3A).
Information on 3-year DFS was available in two trials[22,23] and included 225 patients (92 patients received immunotherapy combined with chemotherapy). Immunotherapy plus chemotherapy resulted in 50% (46/92) of colon cancer patients achieving 3-year DFS. In contrast, chemotherapy alone resulted in 3-year DFS in only 36% (48/133) of control patients. The estimated pooled OR for the two trials showed a highly significant improvement in 3-year DFS in patients receiving DC-CIK immunotherapy combined with chemotherapy (OR = 0.50; 95%CI: 0.29-0.88, P = 0.02) (Figure 3A). The Cochran’s Q test resulted in a P value of 0.99 and the corresponding quantity, I2, was 0%, indicating that there was no evidence of heterogeneity among the individual studies.
Adequate information on 1-year, 2-year and 3-year DFS was only available in 2 trials (Wei et al[22] and Ying et al[23]), therefore, we simply summarized the data from these two trials to show the DFS graph (Figure 3B). The data are shown on the figure and the dashed line represents the linear trend line (r2 = 0.9382 for the immunotherapy group and r2 = 0.8583 for the control group).
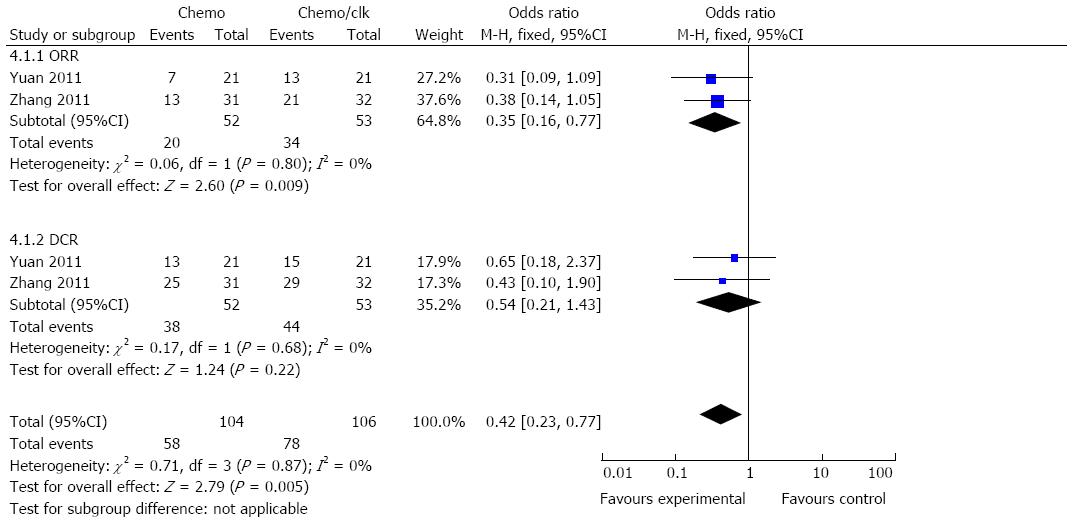
Analysis of the ORR also demonstrated favorable results for the DC-CIK therapy arm, with an OR of 0.35 (95%CI: 0.16-0.77, P = 0.009). However, the DCR for the chemotherapy combined with DC-CIK group did not significantly differ from the chemotherapy alone group (OR = 0.54; 95%CI: 0.21=1.43, P = 0.22) (Figure 4). The Cochran’s Q test resulted in a P value of 0.80 and 0.68, respectively, while the corresponding quantity, I2, was 0% for both groups, indicating that there was no evidence of heterogeneity among the individual studies.
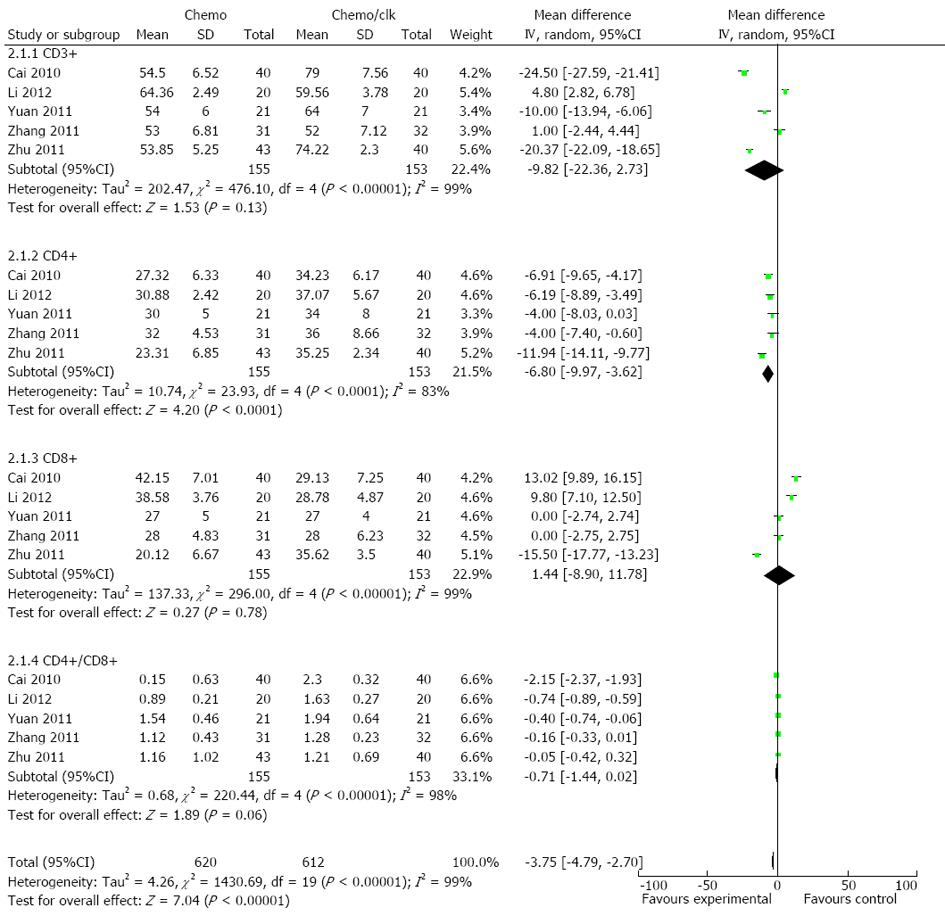
Analysis showed that the proportion of CD4+ cells was significantly increased in the DC-CIK group compared with corresponding baseline percentages before treatment, which was reflected by the pooled OR of -6.80 for CD4+ cells (95%CI: -9.97--3.62, P < 0.0001) which were significantly changed between the two groups (Figure 5), while -9.82 for CD3+ cells (95%CI: -22.36-2.73, P = 0.13), 1.44 for CD8+ cells (95%CI: -8.90-11.78, P = 0.78) and -0.71 for CD4+CD8+ cells (95%CI: -1.44-0.02, P = 0.06) did not differ. Overall, most of the selected T cell subsets were significantly increased after treatment with DC-CIK (P < 0.00001) (Figure 5).
Colon cancer is the third most common cancer in the world and more than half of patients diagnosed with colon cancer will eventually die as a result of cancer-associated complications. Therefore, there is an urgent need for improvements in adjuvant therapies, as well as improved treatment options for metastatic disease.
The first clinical trial of CIK therapy was reported in 1999 by Schmidt-Wolf, while the first clinical studies of DC-based vaccines were described in 1973 by Steinman and Cohn. Additional studies have demonstrated that CIK cells and DC vaccine therapies have anti-tumor effects. Despite the drawbacks associated with in vitro cell manipulation and upscaling, several approaches have been assessed in clinical cancer treatment. The use of DC vaccine, LAK3 cells, CTLs, CIK cells, and tumor infiltrating lymphocytes have been well studied, and additional trials are ongoing. Increasing information on the clinical anti-tumor activity of DC-CIK cells is available from autologous therapy trials and some systematic reviews yielded several findings. A meta-analysis of DC-based tumor vaccination in prostate and renal cell cancers[27], adoptive immunotherapy in postoperative hepatocellular carcinoma (HCC)[28] and CIK cell therapy in patients with HCC and solid carcinomas[29] confirmed that immunotherapy is a safe and feasible treatment option for cancer patients. However, there are also some limitations. For instance, cancers that escape host immune surveillance are generally more difficult to cure. Furthermore, it is often difficult to obtain the necessary numbers of cytotoxic cells that are required for effective tumor control.
A growing body of knowledge on tumor immunosurveillance and loss thereof has contributed to the refinement of anti-tumor immunotherapy. The aim of our meta-analysis was to determine whether an association exists between DC-CIK cell therapy combined with chemotherapy and chemotherapy alone in terms of 1, 2 and 3-year overall survival, 1, 2 and 3-year disease-free survival, ORR and immune indices in colon cancer. This study was also designed to elucidate whether DC-CIK cell therapy can enhance the therapeutic efficacy of chemotherapy in colon cancer.
First, our analysis showed that DC-CIK therapies were associated with significantly prolonged 1-year OS (OR = 0.23; 95%CI: 0.11-0.48, P < 0.0001), 2-year OS (OR = 0.42; 95%CI: 0.22-0.81, P = 0.009) and 3-year OS (OR = 0.43; 95%CI: 0.25-0.74, P = 0.002). There was no 5-year OS data in these studies. Our results, based on prospective studies showed that DC-CIK therapies combined with chemotherapy improved survival in colon cancer patients compared with chemotherapy alone. In contrast, DC-CIK therapies plus chemotherapy did not improve survival in HCC patients. In that analysis, 3-year or 5-year survival was not statistically different with CIK therapies[30]. In addition, different tumor stages (I to IV) were included in the trials and some trial data of 1-year, 2-year and 3-year survival rate were not adequately provided. Thus, when we collected the data, survival at 2 years (76%) included five trials, while survival at 3 years (80%) included three trials, which had an impact on the results.
Second, our analysis of DFS demonstrated that patients receiving DC-CIK cell therapy had better DFS compared with patients in the chemotherapy alone group, 1-year DFS (OR = 0.24; 95%CI: 0.12-0.49, P < 0.0001), 2-year DFS (OR = 0.41; 95%CI: 0.23-0.71, P = 0.002) and 3-year DFS (OR = 0.50; 95%CI: 0.29-0.88, P = 0.02). GOLFIG represents a chemo-immunotherapeutic regimen which includes the standard poly-chemotherapy FOLFOX (5-fluorouracil, FU, leucovorin and oxaliplatin) plus gemcitabine and an immunoadjuvant treatment with subcutaneous injections of GM-CSF and low-dose IL-2, which showed high response rates and disease control rates as well as prolonged time to progression in colon cancer patients[31-34]. It is known that DC-CIK immunotherapy promotes the secretion of many cytokine factors, including IL-2, thus our promising results, showing prolonged OS and DFS, provide evidence for the potential application of DC-CIK (adoptive cell therapy) combined with chemotherapy in colon cancer. Furthermore, there may be some interactions between different chemotherapy regimens and immunotherapy, therefore, we analyzed the different chemotherapy categories and found no significant impact on the results. Thus, immunotherapy plays a role in the treatment of colon cancer. It should be noted that the DFS analysis only included two trials for each endpoint, therefore, a larger number of trials are required to confirm these findings.
Third, the analysis of ORR demonstrated that the ORR increased significantly in the DC-CIK group with an OR of 0.35 (95%CI: 0.16-0.77, P = 0.009) compared with the non-DC-CIK group. However, the DCR did not significantly differ from the chemotherapy alone group (OR 0.54; 95%CI: 0.21-1.43, P = 0.22) (Figure 4). In an ongoing pilot study, sentinel node-derived lymphocytes were infused in colon cancer patients. Clinical responses were seen in stage IV colon cancer patients and complete remission (CR) was observed in four patients who received a significantly larger numbers of T cells than patients with SD and PR[35]. Therefore, in our selected studies, the difference in the number of transfused T cells in the different groups of patients, which would lead to the clinical response of DCR was not obvious.
Fourth, the human immune response against a tumor is mainly dependent on cellular immunity. The ratios of T-lymphocyte subsets in the peripheral blood are usually distorted in tumor patients. In the present analysis, the percentages of CD4+ T cells were significantly increased in the DC-CIK group compared with the chemotherapy group (P < 0.05). We demonstrated that DC-CIK cells were suitable for enhancing the anti-tumor activity in colon cancer patients. Furthermore, the tumor-specific responses generation were potent, long-lasting, and required T cells[36]. The therapeutic efficacy of immunotherapies generally correlates with the generation of strong antigen-specific T- and B-cell responses, and augmentation of such responses may increase the overall potency of immunotherapies. CD8+ cell (P = 0.78) percentages did not differ between the two groups after treatment. It is known that the mere presence of tumor-specific CD8+ T cells (cytotoxic T cells) in the peripheral blood is not correlated with improved clinical outcome. In contrast, several studies indicated a correlation between the number of tumor infiltrating CD8+ T lymphocytes (TILs) and an improved prognosis in colorectal cancer[37], but not in other cancers, such as HCC[38]. Thus, compared to chemotherapy alone, combining DC-CIK immunotherapy with chemotherapy has greater precision to seek and kill tumor cells and helps to increase the sensitivity of cancer cells to chemotherapy, thus providing hope for the treatment of colorectal cancer.
Our meta-analysis has limitations that affect interpretation of the results. First, all seven trials included in the analysis were conducted in China, and published only in the Chinese language. However, it should be noted that on the website http://www.immunitynet.com/coloncancer.asp, it has been demonstrated that there are many successful cases of patients with colon cancer treated with immunotherapy. A clinical trial performed by Guangxi Medical University was registered in http://clinicaltrials.gov/ (NCT01839539). Schmidt-Wolf et al[19] showed that seven colon cancer patients responded to CIK cell therapy and we excluded this Germany study from our selected trials. In addition, a successful case treated with CIK cells alone was reported by Sun Yatsen University Cancer Center and published in English[39]. Furthermore, the seven trials included a total of 533 patients, and none of the trials had more than 100 patients per arm. Thus, a larger sample size including more patients in all groups is needed. The follow-up period was also not sufficiently long. Some of the studies did not even report the follow-up time, tumor size, or background colon diseases. Moreover, patient information was limited in some cases.
The reliability of this systemic review might also be influenced by other factors. For example, not all of the included studies reported clinic random allocation concealment, thus, the meta-analysis may have distribution and implementation bias. We summarized the data from the published results, which will have introduced bias across the studies. Clinical studies with DC-CIK cells are still in their infancy and only involve a relatively small number of patients in most of these studies. The relatively robust and simple cell culture procedures to expand DC-CIK cells have enabled the approach of adoptive cellular immunotherapy to be widely studied. Based on the encouraging experimental and clinical evidence currently available, randomized clinical trials are justifiable and should be performed under stringent compliance with the CONSORT principles. This will involve a large number of patients in order to demonstrate statistical significance for a modest degree of outcome superiority. Such studies are urgently needed in order to provide unequivocal evidence of the clinical usefulness of immunotherapy.
Collectively, our analysis demonstrates that DC-CIK therapy can result in enhanced survival and improved clinical responses in colorectal patients. We also found that these DC-CIK-mediated improvements typically correspond with enhanced immune function. Thus, DC-CIK cells can enhance the therapeutic efficacy of chemotherapy in colon cancer patients. Hence, the efficacy of this therapy lies in its possible application as a promising adjuvant therapy for colon cancer. However, further development of this immunotherapy is needed.
Colorectal cancer is the third most commonly diagnosed cancer in humans. However, 25% of patients who present with metastatic disease have a five-year survival of only 10%. In recent years there has been great interest in cancer immunotherapy, which has the potential to control metastatic disease, prolong time to recurrence, and ultimately serve as a preventive measure. However, clinical studies on dendritic cell (DC)-cytokine-induced killer (CIK) cells are still in their infancy.
The bedside medication administration of Sipuleucel-T (Provenge, Dendreon) was the first-in class therapeutic autologous vaccine to be approved for the treatment of men with asymptomatic or minimally symptomatic castrate-resistant metastatic prostate cancer in the spring of 2010. A phase I-II carcinoembryonic antigen-loaded DC vaccine trial in patients with colon cancer is ongoing (http://www.clinicaltrial.gov id: NCT 01219348). Based on findings in the tumor microenvironment and cancer stem cells in cancer therapy, immunotherapy is worth further investigation.
Over the past few years, advances in our understanding of the immune system, improved design of cancer immunotherapy clinical trials and compliance of manufacturing processes have provided opportunities to significantly improve the efficacy and safety of treatment. However, clinical studies on DC-CIK cells have not achieved a clear consensus on how they may be best optimized. Therefore, the authors performed a systematic review and meta-analysis of clinical trials to assess the therapeutic efficacy of DC-CIK cells combined with chemotherapy in colon cancer. The pooled analysis was performed using the data from random or fixed-effect models. The overall analysis showed a significant one-year, two-year and three-year survival (overall survival) benefit with DC-CIK immunotherapy and chemotherapy. One-year, two-year and three-year disease-free survival rates were also improved after treatment with the combination of DC-CIK immunotherapy and chemotherapy. An improvement in overall response rate was also observed in patients who received additional DC-CIK cell therapy. Furthermore, the analysis of T-lymphocyte subsets in peripheral blood indicated that the number of CD4+ T cells significantly increased in the DC-CIK plus chemotherapy group, implying enhanced immunological responses for anti-tumor regulation. There were no alterations in the number of CD3+, CD8+ and CD4+CD8+ T cells following DC-CIK treatment, suggesting that T cell-mediated cytotoxicity was not aggravated. In all, the combination of DC-CIK immunotherapy and chemotherapy was superior in prolonging survival time and enhancing immunological responses.
The analysis demonstrated that the efficacy of DC-CIK therapy lies in its possible application as a promising adjuvant therapy for colon cancer.
DCs constitute a unique subset of extremely efficient antigen-presenting cells. They were first described in 1973 by Steinman and Cohn. Steinman received the 2011 Nobel Prize in Physiology or Medicine for the discovery of the dendritic cell and its role in adaptive immunity. CIK cells are non-major histocompatibility complex-restricted CD3+CD56+ T cells. They were first described as having a marked ability to proliferate and an increased superiority over lymphokine-activated killer cells in cytolytic activity against cancer by Schmidt Wolf et al. Adoptive immunotherapy is used in the treatment of cancer in which an individual’s own white blood cells are coupled with a naturally produced growth factor to enhance their cancer-fighting capacity and holds great promise in the scenario of potential new approaches for the treatment of solid tumors that are refractory to conventional therapies.
In this manuscript the authors investigated whether autologous DC-CIK therapy was able to improve the therapeutic efficacy of chemotherapy in colon cancer. They conducted a systematic review of published papers from several different sources. Their findings support that the combination of the DC-CIK immunotherapy and chemotherapy has superiority in prolonging the survival time and enhancing immunological responses. In recent years there has been great interest in cancer immunotherapy, which has the potential of controlling metastatic disease, prolonging time to recurrence, and ultimately serving as a preventive measure. The study is well performed and the manuscript is clear and convincing.
P- Reviewers: D'Orazi G, Padin-Iruegas ME, Yeh JY S- Editor: Gou SX L- Editor: Webster JR E- Editor: Liu XM
| 1. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9215] [Cited by in RCA: 9856] [Article Influence: 821.3] [Reference Citation Analysis (4)] |
| 2. | Yang VW, Lewis J, Wang TC, Rustgi AK. Colon cancer: an update and future directions. Gastroenterology. 2010;138:2027-2028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 54] [Reference Citation Analysis (0)] |
| 3. | Yu Y, Cho HI, Wang D, Kaosaard K, Anasetti C, Celis E, Yu XZ. Adoptive transfer of Tc1 or Tc17 cells elicits antitumor immunity against established melanoma through distinct mechanisms. J Immunol. 2013;190:1873-1881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Jäkel CE, Hauser S, Rogenhofer S, Müller SC, Brossart P, Schmidt-Wolf IG. Clinical studies applying cytokine-induced killer cells for the treatment of renal cell carcinoma. Clin Dev Immunol. 2012;2012:473245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Kharfan-Dabaja MA, Hamadani M, Reljic T, Nishihori T, Bensinger W, Djulbegovic B, Kumar A. Comparative efficacy of tandem autologous versus autologous followed by allogeneic hematopoietic cell transplantation in patients with newly diagnosed multiple myeloma: a systematic review and meta-analysis of randomized controlled trials. J Hematol Oncol. 2013;6:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Chow KK, Naik S, Kakarla S, Brawley VS, Shaffer DR, Yi Z, Rainusso N, Wu MF, Liu H, Kew Y. T cells redirected to EphA2 for the immunotherapy of glioblastoma. Mol Ther. 2013;21:629-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 200] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 7. | Li Z. Potential of human γδ T cells for immunotherapy of osteosarcoma. Mol Biol Rep. 2013;40:427-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Iwai K, Soejima K, Kudoh S, Umezato Y, Kaneko T, Yoshimori K, Tokuda H, Yamaguchi T, Mizoo A, Setoguchi Y. Extended survival observed in adoptive activated T lymphocyte immunotherapy for advanced lung cancer: results of a multicenter historical cohort study. Cancer Immunol Immunother. 2012;61:1781-1790. [PubMed] |
| 9. | Higano CS, Small EJ, Schellhammer P, Yasothan U, Gubernick S, Kirkpatrick P, Kantoff PW. Sipuleucel-T. Nat Rev Drug Discov. 2010;9:513-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 116] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 10. | Gardner TA, Elzey BD, Hahn NM. Sipuleucel-T (Provenge) autologous vaccine approved for treatment of men with asymptomatic or minimally symptomatic castrate-resistant metastatic prostate cancer. Hum Vaccin Immunother. 2012;8:534-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 11. | Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10799] [Cited by in RCA: 11786] [Article Influence: 785.7] [Reference Citation Analysis (0)] |
| 12. | Robert C, Thomas L, Bondarenko I, O’Day S, M D JW, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517-2526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3331] [Cited by in RCA: 3409] [Article Influence: 243.5] [Reference Citation Analysis (0)] |
| 13. | Lee JH, Park MS, Chung IJ. Induction of 90K-specific Cytotoxic T Lymphocytes for Colon Cancer Immunotherapy. Immune Netw. 2010;10:206-211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Mukherjee P, Pathangey LB, Bradley JB, Tinder TL, Basu GD, Akporiaye ET, Gendler SJ. MUC1-specific immune therapy generates a strong anti-tumor response in a MUC1-tolerant colon cancer model. Vaccine. 2007;25:1607-1618. [PubMed] |
| 15. | Inoda S, Hirohashi Y, Torigoe T, Morita R, Takahashi A, Asanuma H, Nakatsugawa M, Nishizawa S, Tamura Y, Tsuruma T. Cytotoxic T lymphocytes efficiently recognize human colon cancer stem-like cells. Am J Pathol. 2011;178:1805-1813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 16. | Iwata-Kajihara T, Sumimoto H, Kawamura N, Ueda R, Takahashi T, Mizuguchi H, Miyagishi M, Takeda K, Kawakami Y. Enhanced cancer immunotherapy using STAT3-depleted dendritic cells with high Th1-inducing ability and resistance to cancer cell-derived inhibitory factors. J Immunol. 2011;187:27-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | Lesterhuis WJ, De Vries IJ, Schreibelt G, Schuurhuis DH, Aarntzen EH, De Boer A, Scharenborg NM, Van De Rakt M, Hesselink EJ, Figdor CG. Immunogenicity of dendritic cells pulsed with CEA peptide or transfected with CEA mRNA for vaccination of colorectal cancer patients. Anticancer Res. 2010;30:5091-5097. [PubMed] |
| 18. | Lu J, Liu K, Zhao J, Zhao J, Ma J, Yang H, Huang Y, Qin Z, Bai R, Jiang L. VEGF-A not Ang2 mediates endothelial-like differentiation of immature DCs by ERK1/2 signaling in the microenvironment of human colon adenocarcinoma. Int J Oncol. 2011;38:1579-1588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Schmidt-Wolf IG, Finke S, Trojaneck B, Denkena A, Lefterova P, Schwella N, Heuft HG, Prange G, Korte M, Takeya M. Phase I clinical study applying autologous immunological effector cells transfected with the interleukin-2 gene in patients with metastatic renal cancer, colorectal cancer and lymphoma. Br J Cancer. 1999;81:1009-1016. [PubMed] |
| 20. | Cai XY, Xiong W, Li YF, Shen T. Clinical research of the treatment on patients of mid-low locally rectal cancer afteroperation with cytokine induced killers. Shiyong Aizheng Zazhi. 2010;25:37-39. [DOI] [Full Text] |
| 21. | Li S, Li Y, Liang J, Liu XL. The study of clinical application of DC-CIK combined with chemotherapy on colon cancer. Zhongguo Mianyixue Zazhi. 2012;9:835-839. [DOI] [Full Text] |
| 22. | Wei ZQ, Yang JW, Chen LC, Zheng QH, Ying MG. A retrospective analysis of postsurgical treatment for rectal cancer by chemotherapy and radiotherapy combined with dentritic cells. Fujian Yike Daxue Xuebao. 2009;43:483-487. [DOI] [Full Text] |
| 23. | Ying MG, Wei ZQ, Yang JW, Chen LC, Zheng QH. Retrospective analysis of pos-operative chemo-radiotherapy combined with DC-CIK in the treatment of patients with colorectal cancer. Shiyong Aizheng Zazhi. 2010;25:274-276. [DOI] [Full Text] |
| 24. | Zhang J, Geng J, Han ZX, Gao XY. Chemotherapy combined with CIK/DC cells in the treatment of advanced colorectal cancer: a clinical observation. Xuzhou Yixueyuan Xuebao. 2011;7:457-459. [DOI] [Full Text] |
| 25. | Zhu Y, Liu JQ, Zhang NZ, Chen FX, Chen L, Zhang S, Yang WaY, Zhou ZH, Xu YM. Clinical effects of treatment with comprehensive multipe autologous immune cells in patients with coclorectal carcinoma. Xuzhou Yixueyuan Xuebao. 2011;9:631-636. [DOI] [Full Text] |
| 26. | Yuan JH, Peng DW, Li JW, Wang MQ. Clinical research of dendritic cells combined with cytokine induced killer cells therapy for advanced colorectal cancer. Zhongguo Quanke Yixue. 2011;36:4139-4141. [DOI] [Full Text] |
| 27. | Draube A, Klein-González N, Mattheus S, Brillant C, Hellmich M, Engert A, von Bergwelt-Baildon M. Dendritic cell based tumor vaccination in prostate and renal cell cancer: a systematic review and meta-analysis. PLoS One. 2011;6:e18801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 163] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 28. | Xie F, Zhang X, Li H, Zheng T, Xu F, Shen R, Yan L, Yang J, He J. Adoptive immunotherapy in postoperative hepatocellular carcinoma: a systemic review. PLoS One. 2012;7:e42879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Ma Y, Zhang Z, Tang L, Xu YC, Xie ZM, Gu XF, Wang HX. Cytokine-induced killer cells in the treatment of patients with solid carcinomas: a systematic review and pooled analysis. Cytotherapy. 2012;14:483-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 30. | Ma Y, Xu YC, Tang L, Zhang Z, Wang J, Wang HX. Cytokine-induced killer (CIK) cell therapy for patients with hepatocellular carcinoma: efficacy and safety. Exp Hematol Oncol. 2012;1:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 31. | Correale P, Cusi MG, Tsang KY, Del Vecchio MT, Marsili S, Placa ML, Intrivici C, Aquino A, Micheli L, Nencini C. Chemo-immunotherapy of metastatic colorectal carcinoma with gemcitabine plus FOLFOX 4 followed by subcutaneous granulocyte macrophage colony-stimulating factor and interleukin-2 induces strong immunologic and antitumor activity in metastatic colon cancer patients. J Clin Oncol. 2005;23:8950-8958. [PubMed] |
| 32. | Correale P, Tagliaferri P, Fioravanti A, Del Vecchio MT, Remondo C, Montagnani F, Rotundo MS, Ginanneschi C, Martellucci I, Francini E. Immunity feedback and clinical outcome in colon cancer patients undergoing chemoimmunotherapy with gemcitabine + FOLFOX followed by subcutaneous granulocyte macrophage colony-stimulating factor and aldesleukin (GOLFIG-1 Trial). Clin Cancer Res. 2008;14:4192-4199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 33. | Correale P, Rotundo MS, Del Vecchio MT, Remondo C, Migali C, Ginanneschi C, Tsang KY, Licchetta A, Mannucci S, Loiacono L. Regulatory (FoxP3+) T-cell tumor infiltration is a favorable prognostic factor in advanced colon cancer patients undergoing chemo or chemoimmunotherapy. J Immunother. 2010;33:435-441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 168] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 34. | Ellebaek E, Andersen MH, Svane IM, Straten PT. Immunotherapy for metastatic colorectal cancer: present status and new options. Scand J Gastroenterol. 2012;47:315-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 35. | Karlsson M, Marits P, Dahl K, Dagöö T, Enerbäck S, Thörn M, Winqvist O. Pilot study of sentinel-node-based adoptive immunotherapy in advanced colorectal cancer. Ann Surg Oncol. 2010;17:1747-1757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 36. | Liu Z, Noh HS, Chen J, Kim JH, Falo LD, You Z. Potent tumor-specific protection ignited by adoptively transferred CD4+ T cells. J Immunol. 2008;181:4363-4370. [PubMed] |
| 37. | Koch M, Beckhove P, Op den Winkel J, Autenrieth D, Wagner P, Nummer D, Specht S, Antolovic D, Galindo L, Schmitz-Winnenthal FH. Tumor infiltrating T lymphocytes in colorectal cancer: Tumor-selective activation and cytotoxic activity in situ. Ann Surg. 2006;244:986-992; discussion 992-993. [PubMed] |
| 38. | Zhao M, Wu PH, Zeng YX, Xia JC, Zhang FJ, Xian LJ, Zhang YP, Zhou K, Fan WJ, Zhang L. [Cytokine-induced killer cell fusion to lower recurrence of hepatocellular carcinoma after transcatheter arterial chemoembolization sequentially combined with radiofrequency ablation: a randomized trial]. Zhonghua Yixue Zazhi. 2006;86:1823-1828. [PubMed] |
| 39. | Xia LP, Wu PH, Xia JC, Zhang B, Guan ZZ, Wan DS, Guo GF, Zeng YX. One patient with metastastic colorectal cancer successfully treated by combination of targeted agents after failure of chemotherapy. Chin J Cancer. 2010;29:1023-1028. [PubMed] |