Published online Jan 28, 2014. doi: 10.3748/wjg.v20.i4.1054
Revised: October 30, 2013
Accepted: December 5, 2013
Published online: January 28, 2014
Processing time: 148 Days and 15.5 Hours
AIM: To examine the effect of intra-gastric triacetin on both upper gastrointestinal motility and proximal gastric tone in conscious dogs.
METHODS: Three beagle dogs under sedation were surgically implanted with gastrocutaneous fistula in the gastric body and force transducers in the gastric antrum and duodenum. Beginning at week-2 after insertion, the animals were either fasted for 24 h or fed a liquid meal 2-3 h before the experiment. With the animals fully conscious, a polyethylene bag was inserted into the proximal stomach through the gastrocutaneous fistula, followed by 15 min of air inflation (minimal distending pressure of +2 mmHg) and then 20 mL of a low-, mid- or high-concentration triacetin solution (0.5%, 1.0% and 2.0%) or warm water (vehicle control). The proximal stomach receptive volume and gastric antral and duodenal contractions were measured over 10 min. The experiment was repeated twice per week over several months, with each animal receiving at least one infusion of the various triacetin solutions and the vehicle at different times. Intergroup differences were assessed by ANOVA and Bonferroni-Dunn post-hoc testing.
RESULTS: Intra-gastric infusion of mid- and high-concentration triacetin induced an increase in the proximal stomach receptive volume, and the average increase induced by the high-concentration at 0-4 min after infusion was significantly greater than that induced by the vehicle control (62.4 ± 9.8 vs 18.4 ± 4.7, P < 0.01). The mid- and high-concentration triacetin also produced a temporary inhibition of the gastric antral contractions at 2 min after infusions; however, only the fasted group showed triacetin-induced antral contractile inhibition that was significantly greater than that in the vehicle control group (P < 0.05). In addition, only the fasted group showed a high-concentration triacetin-induced increase in duodenal contractions at 9-10 min that was significantly different from that in the vehicle control group (P < 0.05).
CONCLUSION: Intra-gastric infusion of 1.0%-2.0% triacetin delays gastric emptying by increasing proximal stomach receptive volume, temporarily inhibiting gastric antral contractions and facilitating duodenal contractions.
Core tip: Intra-gastric infusion of short-chain triglycerides, such as triacetin, has been shown to delay gastric emptying in conscious dogs, but the influence on upper gastrointestinal motility is unknown. The current study examined time-dependent changes in motility following intra-gastric triacetin administration at various doses in conscious dogs and evaluated the effects of a fasted vs fed state. Compared to infusion of water (vehicle) alone, the 1.0% and 2.0% triacetin doses induced a significant increase in the proximal stomach receptive volume, a temporary inhibition of gastric antral contractions, and an increase in the duodenal contractions in fasted dogs.
- Citation: Oosaka K, Tokuda M, Furukawa N. Intra-gastric triacetin alters upper gastrointestinal motility in conscious dogs. World J Gastroenterol 2014; 20(4): 1054-1060
- URL: https://www.wjgnet.com/1007-9327/full/v20/i4/1054.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i4.1054
Triacetin is both the shortest-chain triglyceride (SCT), containing fatty acids with two carbons, and the only triglyceride that is soluble in water up to 6%. Its approval by the Food and Drug Administration as a safe human food ingredient has led to a series of studies examining its potential as a therapeutic agent for total parenteral nutrition[1-6]. While these studies have shown that triacetin can improve nitrogen balance[1] and protein metabolism[2], with a lack of toxicity[3], they have also shown minimal effects on mineral metabolism[4,5] due to the feature of water solubility. In an in vivo study by Lynch et al[6], wherein rats were fed diets containing triacetin to determine the effects on total adiposity, fat distribution and body composition, triacetin was shown to provide energy without accumulation in the body by decreasing adipocyte size. However, this field of research is relatively new and further investigations on the nutritive capacities and related mechanisms of triacetin are still in progress.
A number of other studies have examined the effects of long-chain triglycerides (LCTs) on gastrointestinal motility, demonstrating their effect of delaying gastric emptying and characterizing their feature of slow absorption. Specifically, it was shown that when digestive products of LCTs, such as mono- or diglycerides and long-chain fatty acids, are present in the duodenum and jejunum, the gastric emptying rate slows down[7,8], and that digestion and absorption of LCTs into the lymphatic system is dependent upon modification by bile salts. Moreover, Hunt et al[9] reported that gastric emptying is slower for 12- to 18-carbon fatty acids than for those composed of 2 to 10 carbons, suggesting that gastric emptying may be regulated in a manner that allows for optimal intestinal digestion and absorption of foodstuffs.
The processes of digestion and absorption of SCTs differ greatly from those of LCTs. SCTs do not require bile salts for digestion. Their passive diffusion from the gastrointestinal tract to the portal system has led to speculation that gastric emptying should not be delayed by SCTs. However, when triacetin was directly infused into the stomachs of conscious dogs, the gastric emptying rate was delayed remarkably[10]. Gastric emptying of a liquid is known to be facilitated by both the proximal gastric tone and antrum motility, both of which may also be influenced by the fasted/fed (postprandial) state. To determine whether triacetin can alter upper gastrointestinal motility during the observed delay of gastric emptying in conscious dogs a barostat and force transducers were applied to fasted and fed animals in the study described herein, and the effects of triacetin on upper gastrointestinal motility and the proximal gastric tone were comparatively analyzed.
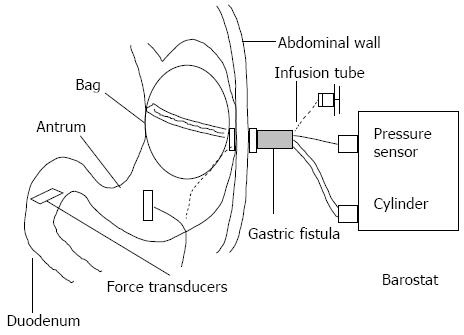
All animal care and experimental procedures were approved by the Animal Research Committee of the Kawasaki Medical School (Japan) and conducted in accordance with the Guide for the Care and Use of Laboratory Animals[11]. Three female beagle dogs (weight: 10-11 kg; age: 1-1.5 years) were anesthetized with medetomidine hydrochloride (20 μg/kg, subcutaneous) and pentobarbital sodium (25 mg/kg, intravenous). A midline laparotomy was performed, and a stainless steel gastrocutaneous fistula (inner diameter: 15 mm; length: 80 mm) was inserted into the middle corpus, with the placement ensuring exteriorization on the left side of the abdomen. Two force transducers were then sewn onto the wall of the gastric antrum and duodenum, following the direction of the circular muscle. The force transducer on the antrum was sutured at approximately 3 cm from the pyloric ring, while the force transducer on the duodenum was sutured at approximately 5 cm distal to the pyloric ring (Figure 1). The lead wires of the force transducers were positioned to ensure percutaneous exteriorization of the bilateral scapulae lower margins. The animals were allowed to recuperate for two weeks, with daily monitoring for procedure-related complications, before experimentation was initiated.
The fully conscious prepared animals were evaluated in either the fasting phase (after 24 h fasting) or in the postprandial phase (2-3 h after feeding of a high-calorie, high-protein liquid diet). For each phase, the experimental procedure was initiated by opening the gastrocutaneous fistula and aspirating any contents from the stomach; the mean aspirated volume from the postprandial stage was 29 ± 2.3 mL (n = 24), with a residual ratio of 11%-13%. A polyethylene bag (diameter: 12-13 cm; capacity: 0-1000 mL) fitted with a double-lumen polyvinyl tube was then inserted through the gastrocutaneous fistula into the proximal stomach and slowly inflated with 400-500 mL of air to ensure proper positioning. After complete deflation of the bag, a polyvinyl tube was introduced into the stomach through the gastrocutaneous fistula, to allow for infusion of a triacetin solution or vehicle control (water). The double-lumen tube and the infusion tube were fixed with adhesive vinyl tape. Thereafter, the conscious dogs were placed in a sling with their legs touching the ground so that they were supported upright without pressure on the abdomen. The double-lumen tube was then connected to a barostat (Isobar-3; G and J Electronics, Toronto, Canada) and the bag was gradually inflated from 2 to 5 mmHg in 1 mmHg stepwise 1 min increments. The minimal distending pressure (MDP) was determined by increasing the intra-gastric pressure to the point where volume variations were induced by respiratory motions. In this study, bag volumes of 30-100 mL were required for the respiratory motions to influence intra-gastric pressure in the dogs; therefore, the MDP was maintained at approximately 3 mmHg throughout the experiment.
This study used a barostat to examine the acute effects on fundic relaxation and gastric antral and duodenal contractions in conscious dogs in accordance with the methods of Furukawa et al[12]. Briefly, the intra-gastric bag was insufflated with air for 15 min, with the additional +2 mmHg bringing the MDP to 5 mmHg. After the initiation of insufflation for 5 min, 20 mL of pre-warmed (37 °C) 0.5%, 1% or 2% triacetin or water were infused directly into the stomach over a period of 30 s through an infusion tube in the gastrocutaneous fistula. At the same time as the infusions, the force transducers were used to continuously measure the gastric antral and duodenal contractions.
For each animal, the experimental procedures were performed twice per week over a period of several months. Each animal received at least one infusion of each of the triacetin solutions as well vehicle before completion of the experimental course. The total numbers of fasting and postprandial phase experiments were nearly identical for all three dogs.
During the 15 min gastric distension performed at a MDP of +2 mmHg, the intra-gastric bag volume was calculated as the gastric tone over 1 min intervals. According to Furukawa et al[12], the mean values of three intra-gastric bag volumes at 3, 4 and 5 min after initiation of the gastric distension were designated as the basal volume before intra-gastric infusion of either the triacetin solutions or water. To evaluate the effect of triacetin on the proximal stomach receptive volume at a constant distending pressure, the mean values of the 1 min interval after the infusion were compared with the mean values of the basal volume. In addition, the differences between the basal volume and the volume after the infusion were calculated at 1 min intervals, with the triacetin data for the periods between 0-10 min compared to those of water. The effect of triacetin on contractility was evaluated by plotting the relative values of gastrointestinal contractility size that were obtained at every minute interval and calculating the area under the curve (AUC). As per the method described by Furukawa et al[12], the average of the 1 min intervals of AUC from 3 to 5 min after the onset of distension was used as the control value. The changes in relative magnitudes of the contractions from the control values after the infusion were then compared between the triacetin and water administrations. These results were expressed as mean ± SE The significance of intergroup differences was assessed by ANOVA with Bonferroni-Dunn post-hoc testing.
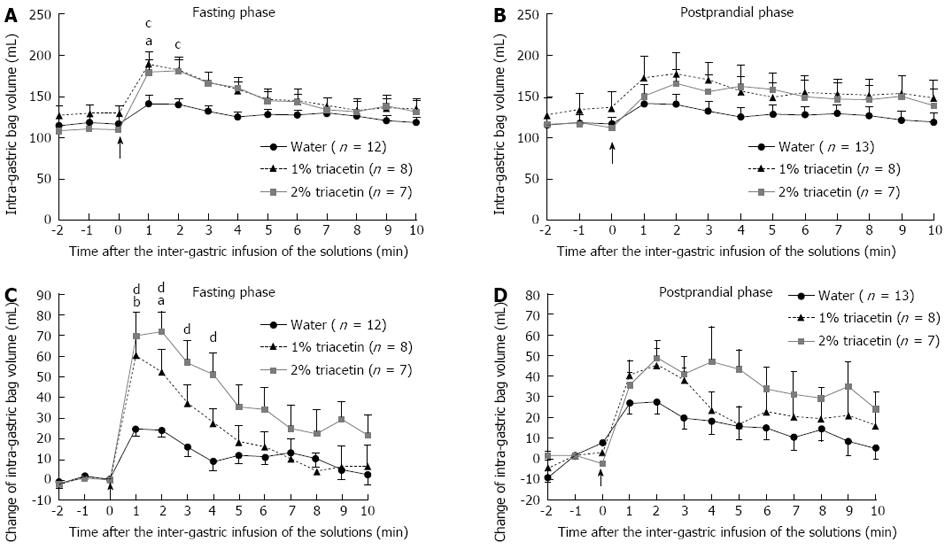
Fasting phase: A slight increase was observed in the intra-gastric bag volume immediately after the infusion of water, but was not significantly different from the pre-infusion volume (Figure 2A). Infusion of 0.5% triacetin did not produce a significant increase in the receptive volume, and only a slight distention of the intra-gastric bag was observed that was similar to that seen for the water infusion (data not shown). In contrast, infusion of 1.0% triacetin led to a rapid increase in the intra-gastric bag mean volume, from the mean basal volume of 129.3 ± 10.2 mL to a peak of 189.8 ± 14.5 mL at 1 min (n = 8). In addition, infusion of 2.0% triacetin led to a similar rapid increase, from the mean basal volume of 109.4 ± 7.2 mL to 179.1 ± 16.2 mL at 1 min (n = 7) and a peak of 181.2 ± 14.1 mL at 2 min (n = 7). These increases in the receptive volume induced by 1% and 2% triacetin infusions were significantly greater than the water infusion (Figure 2A).
Postprandial phase: In the postprandial phase, a slight increase in the receptive volume was observed that was similar to that seen during the fasting phase. Infusions of 1.0% and 2.0% triacetin led to rapid increases in the receptive volume, which were greater than those induced by the water infusion but the differences did not reach statistical significance (Figure 2B).
Fasting phase: At 0-2 min after infusion of 1.0% triacetin, the increase in the receptive volume (56.5 ± 10.1, n = 8) was significantly greater than that induced by the infusion of water alone (24.4 ± 3.7, n = 12). At 0-4 min after infusion of 2.0% triacetin, the increase in the receptive volume (62.4 ± 9.8, n = 7) was significantly greater than that induced by the infusion of water alone (18.4 ± 4.7, n = 12) (Figure 2C).
Postprandial phase: During the postprandial phase, the increases in the receptive volume induced by triacetin were also larger than those induced by water alone but the differences did not reach statistical significance (Figure 2D).
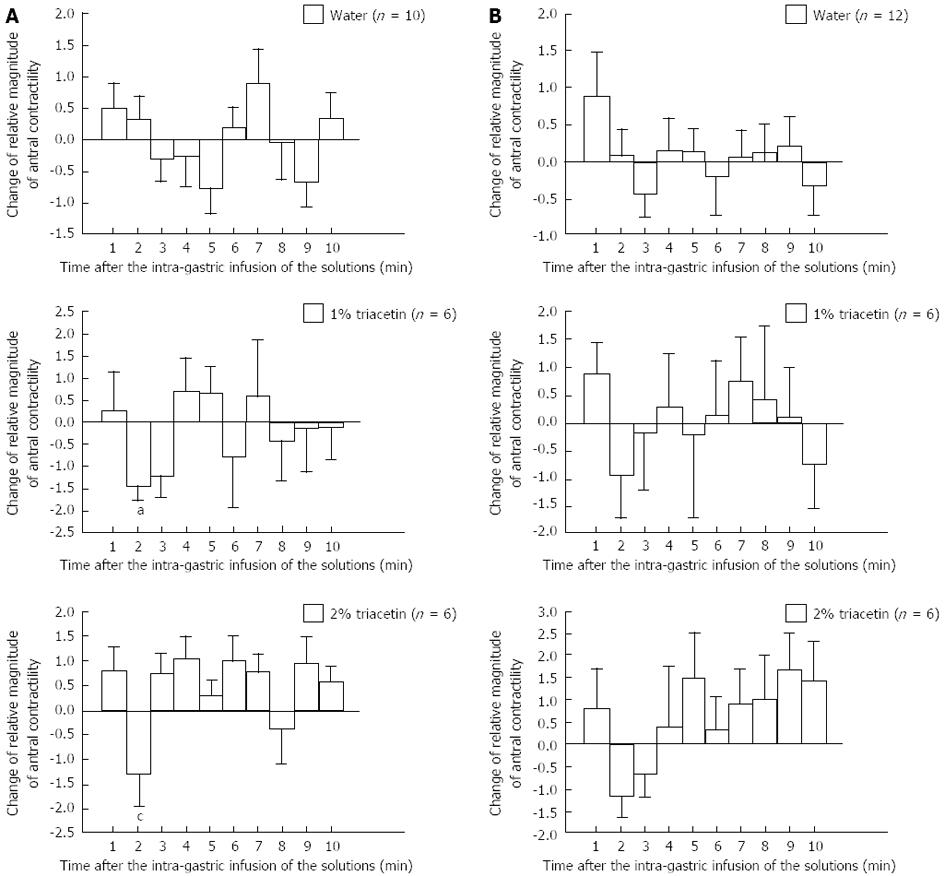
Infusion of water alone produced no obvious changes in the gastric antral contractions, in neither the fasting and postprandial phases (Figure 3). In contrast, both 1.0% and 2.0% triacetin induced a temporary inhibition of the gastric antral contractions at 2 min after the infusion. Only the triacetin-induced inhibition in the fasting phase was significantly different from the contractions observed with the water infusion. However, after the 2 min time point, there was a tendency for facilitation of the gastric antral contractions only for the 2.0% triacetin in both the fasting and postprandial phases but the differences from the water-related contractions did not reach statistical significance.
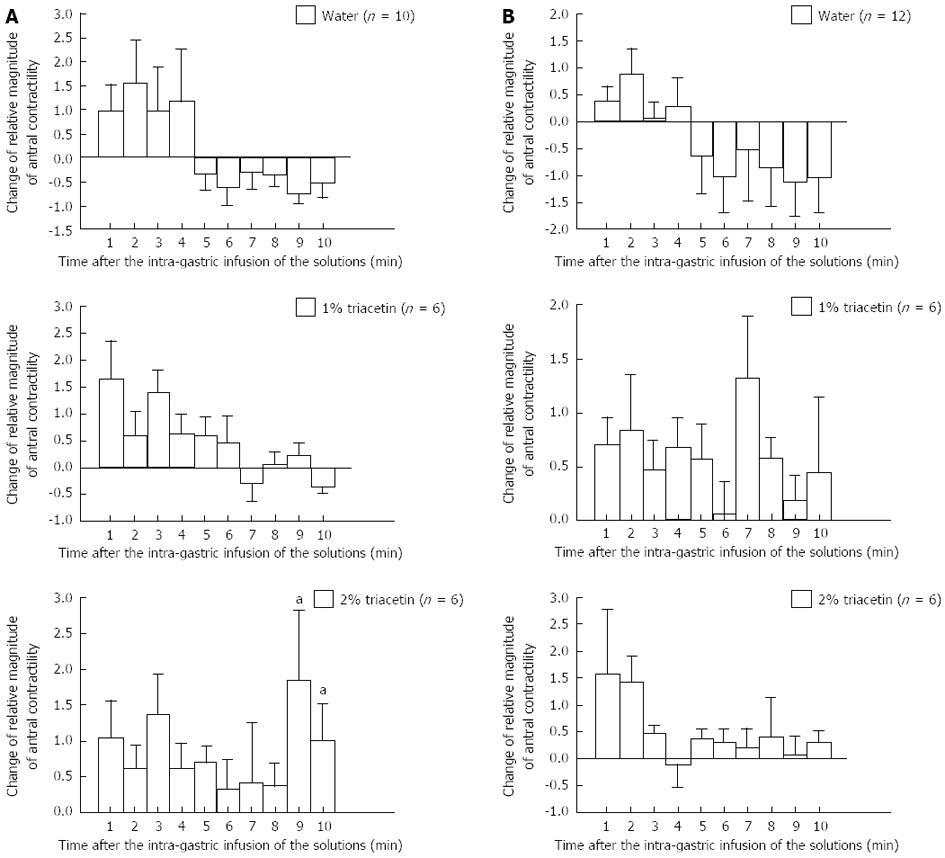
At 1-4 min after the water infusion, a temporary facilitation of the duodenal contractions was observed in both the fasting and postprandial phases (Figure 4). For all time points thereafter, water induced only inhibition of the duodenal contractions. In contrast, infusion of 1.0% and 2.0% triacetin inhibited the duodenal contractions for nearly the entire 10-min measurement period in both phases, with the exception of 2.0% triacetin inducing a significant increase (vs water alone) in duodenal contractions at 9-10 min after the infusion.
The current study demonstrated that direct infusion of triacetin into the stomach of conscious dogs alters upper gastrointestinal motility. Proximal gastric volume was also increased by the infusion, which reflects stimulation of gastric relaxation. Finally, the triacetin was shown to inhibit gastric antral contractions but induce duodenal contractions.
Induction of gastric relaxation of the proximal stomach involves two mechanisms. First, gastric relaxation is stimulated by intra-gastric filling (possibly by expansion of the gastric wall[13]), regardless of the substance involved (solids or liquids). This process is referred to as gastric accommodation and is responsible for an increased volume tolerance without perception but may also contribute to maintaining satiation signals at acceptable volume loads. Second, physical (e.g., osmotic pressure) and chemical (e.g., H+, lipids) parameters of the stomach and duodenum environments can enhance gastric relaxation via factors related to the nervous system or hormonal axes[14,15]. In the current study, triacetin did not appear to influence osmotic pressure, since the 0.5%, 1.0% and 2.0% triacetin was administered as hypotonic solutions. Moreover, the 20 mL volume that was infused into the stomach was not expected to cause expansion of the gastric wall. The triacetin infusion and subsequent absorption in the stomach and duodenum appeared to primarily affect upper gastrointestinal motility. However, the observation of duodenal facilitation occurring immediately after the infusion of triacetin suggests that the absorption-related process plays a smaller role than triacetin’s chemical stimulation of the gastric and duodenal mucosa to causes the gastric relaxation.
Gastric antrum and duodenum contractions have distinctive mechanisms and patterns. For example, LCT-mediated effects on contractility are region specific. Particular LCTs capable of inhibiting antral contractility[16] have also been shown to facilitate stimulation of contractions in segments of the upper duodenum[17]. In the current study, the triacetin infusion first led to inhibition of gastric antral contractions, which was followed by an increase at later time points of the infusion. Moreover, the infusion appeared to facilitate duodenal contractility. Since there was only a short period of inhibition of the antral contractions, it remains unclear whether this inhibition was responsible for causing the delay of gastric emptying. However, such modulations of the gastric antrum and duodenum are expected to induce the closing of the pylorus, which may be the ultimate cause of the delayed gastric emptying. With regard to the observed diphasic alterations (i.e., inhibition and facilitation) in the gastric antrum, the initial inhibition may be induced by stimulation of gastric antral mucosa, while the subsequent facilitation may be induced by stimulation of the duodenal mucosa.
LCTs are known to stimulate release of the hormone cholecystokinin (CCK), which acts to reduce proximal gastric tone[18] and inhibit gastric emptying[19] via activation of the CCK receptors on the vagal afferent nerves. In turn, the inhibitory vagal afferents in the brainstem are activated[20]. SCTs, on the other hand, produce very little to no effects on CCK release[21]. Therefore, the mechanism of effect for triacetin, a SCT, may differ from that of the LCTs.
In the current study, the effects of triacetin related to fasting and fed states were also examined. While the results were largely similar for both the fasted and postprandial phases, statistically significant differences were only observed in the fasted animals. It is possible that the up-regulated hormonal factors that occur during the postprandial phase may have interfered with the triacetin, masking the effects.
In conclusion, our current results indicate that triacetin rapidly induces a temporary relaxation in the proximal stomach that is followed by contraction in both the gastric antrum and duodenum. This mechanism of triacetin may differ from that of LCTs, but further investigations are needed to confirm this distinction.
Gastric emptying is adjusted very precisely by gastrointestinal motility, so it is very important to examine the change of gastrointestinal motility after administrated the dietary ingredient. The authors reported preliminarily that an infusion of triacetin, which is expected as a new nutritional ingredient, into the stomach delayed the gastric emptying rate in conscious dogs. However, it is still unclear whether triacetin can change upper gastrointestinal motility during a delay of gastric emptying.
Triacetin has been generally recognized by the Food and Drug Administration as being a safe human food ingredient. In the area of gastrointestinal motility with triacetin, the research hotspot is the change on the proximal gastric tone and the upper gastrointestinal motility.
In this study, to research the effect of triacetin on the gastric tone of the proximal stomach in conscious dogs, the authors used a polyethylene bag to measure the receptive volume of the proximal stomach at a constant pressure before and after the intra-gastric infusion of triacetin. Moreover, to determine the effect of triacetin on the upper gastrointestinal contractile activities, the authors used force transducers to measure the gastric antral and duodenal contractions. The animals were either fasted for 24 h or fed a liquid meal 2-3 h before the experiment. Triacetin rapidly induces a temporary relaxation in the proximal stomach that is followed by contraction in both the gastric antrum and duodenum. The effect of triacetin was largely similar for both the fasted and postprandial phases, statistically significant differences were only observed in fasted animals.
Intra-gastric infusion of 1.0%-2.0% triacetin delays gastric emptying by increasing proximal stomach receptive volume, temporarily inhibiting gastric antral contractions and facilitating duodenal contractions.
This manuscript reports novel information on the effect of short-chain triglycerides on gastric emptying in conscious dogs. The Authors demonstrate convincingly that Intra-gastric infusion of 1.0%-2.0% triacetin induced an increase of the receptive volume of the proximal stomach, and caused temporary inhibition of the gastric antral contractility, which are suggestive of a retardation of gastric emptying.
P- Reviewers: Francesco L, Izzo AA, Iwasaki Y S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH
| 1. | Bailey JW, Barker RL, Karlstad MD. Total parenteral nutrition with short- and long-chain triglycerides: triacetin improves nitrogen balance in rats. J Nutr. 1992;122:1823-1829. [PubMed] |
| 2. | Bailey JW, Miles JM, Haymond MW. Effect of parenteral administration of short-chain triglycerides on leucine metabolism. Am J Clin Nutr. 1993;58:912-916. [PubMed] |
| 3. | Bailey JW, Haymond MW, Miles JM. Triacetin: a potential parenteral nutrient. JPEN J Parenter Enteral Nutr. 1991;15:32-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Bleiberg B, Beers TR, Persson M, Miles JM. Metabolism of triacetin-derived acetate in dogs. Am J Clin Nutr. 1993;58:908-911. [PubMed] |
| 5. | Bailey JW, Heath H, Miles JM. Calcium, magnesium, and phosphorus metabolism in dogs given intravenous triacetin. Am J Clin Nutr. 1989;49:385-388. [PubMed] |
| 6. | Lynch JW, Bailey JW. Dietary intake of the short-chain triglyceride triacetin vs. long-chain triglycerides decreases adipocyte diameter and fat deposition in rats. J Nutr. 1995;125:1267-1273. [PubMed] |
| 7. | Hunt JN. Mechanisms and disorders of gastric emptying. Annu Rev Med. 1983;34:219-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Houghton LA, Mangnall YF, Read NW. Effect of incorporating fat into a liquid test meal on the relation between intragastric distribution and gastric emptying in human volunteers. Gut. 1990;31:1226-1229. [PubMed] |
| 9. | Hunt JN, Knox MT. A relation between the chain length of fatty acids and the slowing of gastric emptying. J Physiol. 1968;194:327-336. [PubMed] |
| 10. | Oosaka K. Effect of triacetin of gastric emptying. J Smooth Muscle Res (Jpn Section). 2005;9:67-7811. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | National Research Council (US) Committee for the update of the guide for the care and use of laboratory animals. Guide for the care and use of laboratory animals. 8th ed. Washington (DC): National Academies Press (US) 2011; 1-220. |
| 12. | Furukawa N, Manabe N, Kase Y, Hattori T, Imamura H, Kusunoki H, Haruma K. Intragastric infusion of rikkunshito (kampo) induces proximal stomach relaxation in conscious dogs. Auton Neurosci. 2013;179:14-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Distrutti E, Azpiroz F, Soldevilla A, Malagelada JR. Gastric wall tension determines perception of gastric distention. Gastroenterology. 1999;116:1035-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Jahnberg T, Abrahamsson H, Jansson G, Martinson J. Gastric relaxatory response to feeding before and after vagotomy. Scand J Gastroenterol. 1977;12:225-228. [PubMed] |
| 15. | Barbera R, Peracchi M, Brighenti F, Cesana B, Bianchi PA, Basilisco G. Sensations induced by medium and long chain triglycerides: role of gastric tone and hormones. Gut. 2000;46:32-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Heddle R, Dent J, Read NW, Houghton LA, Toouli J, Horowitz M, Maddern GJ, Downton J. Antropyloroduodenal motor responses to intraduodenal lipid infusion in healthy volunteers. Am J Physiol. 1988;254:G671-G679. [PubMed] |
| 17. | Norman WW. Gastric emptying. Gastrointestinal Physiology, 6th edn. St. Louis: Mosby, Inc 2001; 37-46. |
| 18. | Feinle C, D’Amato M, Read NW. Cholecystokinin-A receptors modulate gastric sensory and motor responses to gastric distension and duodenal lipid. Gastroenterology. 1996;110:1379-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 111] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Liddle RA, Morita ET, Conrad CK, Williams JA. Regulation of gastric emptying in humans by cholecystokinin. J Clin Invest. 1986;77:992-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 194] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 20. | Zarbin MA, Wamsley JK, Innis RB, Kuhar MJ. Cholecystokinin receptors: presence and axonal flow in the rat vagus nerve. Life Sci. 1981;29:697-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 187] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 21. | Ledeboer M, Masclee AA, Biemond I, Lamers CB. Effect of medium- and long-chain triglycerides on lower esophageal sphincter pressure: role of CCK. Am J Physiol. 1998;274:G1160-G1165. [PubMed] |