INTRODUCTION
Pancreatic adenocarcinoma is an aggressive disease with a high mortality rate. Despite intensive efforts, pancreatic cancer remains a formidable challenge for oncologists[1]. Investigation of the molecular and genetic bases of pancreatic as well as other cancers is very important for understanding tumor formation and growth as well as for development of anticancer strategies. Hypoxia has been recognized as one of the fundamentally important features of solid tumors and plays a critical role in various cellular and physiologic events, including cell proliferation, survival, angiogenesis, metabolism, as well as tumor growth, invasion and metastasis[2-6]. Moreover, hypoxia has the multifaceted role in the hallmarks of human cancers, including pancreatic cancer. Hypoxia-inducible factor 1 (HIF-1), represent key features in cell biochemistry, physiology and molecular biology.
Tumors are usually exposed to a hypoxic microenvironment due to their irregular growth and insufficient blood supply while pancreatic tumors have enhanced vascular supply[3,7,8]. Moreover, there is heterogeneity and genomic complexity between pancreatic tumors as well as hierarchy of cancer cells with different properties, including a subpopulation of cancer stem cells that are inherently resistant to traditional therapies[8]. Activation of genes that ameliorate or compensate for the oxygen deficit, especially of mRNAs involved in glycolysis and facilitate proliferation is important in adaptations to hypoxia[9-15]. A high rate of glycolytic flux, even in the presence of oxygen, is a central metabolic hallmark of neoplastic tumors. The high glucose metabolism of cancer cells is caused by a combination of hypoxia inducible transcription factors, activation of oncogenic proteins and the loss of tumor suppressor function. Over-expression of HIF-1α or HIF-2α and MYC, activation of RAS and loss of TP53 and/or other tumor suppressor functions each have been found to stimulate glycolysis in part by activating a family of regulatory bifunctional 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatases (PFKFB) and hexokinases[16-18].
PFKFB AS AN IMPORTANT FACTOR OF TUMOR GROWTH
Enzyme PFKFB controls of glycolysis through maintaining the cellular levels of fructose-2,6-bisphosphate, which is considered to be the major allosteric activator of 6-phosphofructo-1-kinase, a rate-limiting enzyme of glycolysis[19,20]. Thus, the PFKFB enzymes control glycolysis through fructose-2,6-bisphosphate level[20-23]. There are 4 different genes encode variable isoforms of this enzyme. Importantly, most cells and tissues express more than one isoform[23-25]. PFKFB enzyme also plays an important role in the Warburg effect and cancer growth[16,17,26-28]. Overexpression of PFKFB-3 as well as other variants of PFKFB is observed in various human cancers [29-33]. Moreover, enhanced expression of PFKFB as well as hexokinase 2 is an obligatory factor of activated tumor cell glycolysis and increased its proliferation[18,24,32-35].
The expression of different PFKFBs (PFKFB-1, PFKFB-2, PFKFB-3 and PFKFB-4) is induced by hypoxia in vivo in organ-specific manner[21]. At the same time, in vitro experiments clearly demonstrated that hypoxia affects the expression only two variants of PFKFB (3 and 4) mRNA in different cell lines[26,29,31-33]. In promoter region of PFKFB-4 and PFKFB-3 genes was identified HIF responsive element which bind transcription factor HIF and mediate hypoxic regulation, because deletion or point mutation of this HIF responsive element eliminates the hypoxic regulation both PFKFB-4 and PFKFB-3 genes[25,31,36,37]. Moreover, the phosphorylation - dephosphorylation of PFKFB isoenzymes is important for enhancing of glycolysis by hypoxia as well as by fructose-2,6-bisphosphate in monocytes upon activation[17,38,39]. There is also data supporting an important role for PFKFB-3 protein phosphorylation in the increased glycolysis, angiogenesis and tumor progression[40]. Thus, highly phosphorylated variant of PFKFB-3 was found in cancer cells as well as in other cells, including vascular endothelial cells[40].
Recently, a novel mechanism by which MK2, MAPK (mitogen-activated protein kinase)-activated protein kinase 2, a key component of the MAPK pathway, up-regulates glycolysis in response to stress in cancer cells was described[41]. By phosphorylating specific PFKFB3 residues, MK2 promotes both increased its gene transcription and allosteric activation.
It was also shown a significant increase of PFKFB-3 in the nuclei, which associates with enhanced cell proliferation through cyclin-dependent protein kinase[34]. Moreover, PFKFB-3 isoenzyme is degraded by the E3 ubiquitin ligase APC/C-CDH1, which also degrades cell-cycle proteins[42]. Thus, this ubiquitin ligase is linking glycolysis to cell proliferation mainly through PFKFB-3 enzyme, which promote glycolysis. It was shown that both aerobic glycolysis and proliferation are prevented by overexpression of this ubiquitin ligase and enhanced by its silencing. Furthermore, activation of glycolysis, as essential factor of cell proliferation, in the presence of active ubiquitin ligase APC/C-CDH1 does not change the rate of cell proliferation[42]. Recently was also shown that PTEN (phosphatase and tensin homolog) enhances interaction between PFKFB3 and E3 ligase APC/C-CDH1, and overexpression of CDH1 down-regulates the PFKFB3 protein level in wild-type, but not in PTEN-deficient cells[43]. Moreover, PTEN knockout cells were found to have high protein levels of PFKFB3 that has important consequences for cell proliferation.
There is data that ubiquitin ligase SKP1-CUL1-F(SCF)-beta-TrCP also participate in glycolysis regulation during the cell cycle through PFKFB because this enzyme or activation the glycolytic enzyme 6-phosphofructo-1-kinase is needed for glycolysis up-regulation[44]. Besides that, the induction of de novo lipid synthesis from glucose in prostate adenocarcinoma cells by androgen requires transcriptional up-regulation of PFKFB-2 and phosphorylation of PFKFB-2 generated by the PI3K/AKT signal pathway to supply the source for lipogenesis[45]. The increased glycolytic flux through the enhanced expression of PFKFB3 gene was also observed after interaction of adenosine with macrophage TLR4 receptor agonists[46]. Thus, the enzymes of PFKFB family participate in the regulation of glucose metabolism through glycolysis as well as in the control of the cell cycle, apoptosis, tumor growth, and invasiveness.
It is interesting to note that the transcriptional co-repressor myeloid translocation gene 16 (MTG16) is found in multiple transcription factor-containing complexes as a regulator of gene expression implicated in development and tumorigenesis. MTG16 can serve as a brake on glycolysis, a stimulator of mitochondrial respiration and an inhibitor of cell proliferation through suppression of PFKFB-3, PFKFB-4 and pyruvate dehydrogenase kinase isoenzyme 1 (PDK1)[47]. Furthermore, hypoxia-stimulated production of PFKFB3, PFKFB4 and PDK1 was inhibited by MTG16 expression.
Several alternative splice variants for PFKFB-3 were identified in normal and cancer cells which possibly are important for malignant tumor growth[35,48-51]. All these splice variants have similar N-terminus and catalytic both 6-phosphofructo-2-kinase and fructose-2,6-bisphosphatase domains, but differ in C-terminal regulatory region. It is possible that a variable C-terminus provide not only for differ regulatory properties and for a variable surviving of PFKFB-3 splice variants. Moreover, the expression of PFKFB-3 alternative splice variants in vivo differs in various organs and spectrum of these splice variants changes in rat model of diabetes in organ-specific manner[51].
Recent data[52-54] showed that PFKFB-4, which expression at mRNA and protein levels is strongly induced in the lung and breast cancers, has also pleiotropic functions. This variant of PFKFB together with other members of PFKFB family participates in the regulation of glycolysis and also promotes tumor growth and survival of cancer cells[30,33,52-56]. It was shown that PFKFB4 is required to balance glycolytic activity and antioxidant production to maintain cellular redox balance in prostate cancer cells[52]. Moreover, depletion of PFKFB4 inhibited tumor growth in a xenograft model, indicating that it is required under physiologic nutrient levels[52]. PFKFB4 mRNA expression was also found to be greater in metastatic prostate cancer compared with primary tumors[52]. Moreover, induction of apoptosis by sulforaphane in human hepatic cancer cells mediated by hypoxia inducible factor-1-dependent pathway through inhibition of PFKFB4[53]. Thus, PFKFB4, a glycolytic enzyme that shunts glucose into the pentose phosphate pathway for NADPH production, as a critical node for the survival of cancer cells[52-54,56].
Aerobic glycolysis links the high rate of glucose fermentation to cancer[57]. It was found that the regulatory glycolytic enzyme PFKFB4 is essential for prostate cancer cell survival by maintaining the balance between the use of glucose for energy generation and the synthesis of antioxidants. Cancer cells undergo several changes in their metabolism that contributes to the proliferation and survival of cancer cells. Blocking PFKFB4 induces reactive oxygen species and cancer cell death. Thus, targeting PFKFB4 may therefore present new therapeutic opportunities.
It is interesting to note that non-malignant gastric and colon tissues in contrast to lung and breast tissues have higher level of PFKFB-4 protein; at the same time, no significant differences in mRNA levels[30,32,33]. It is possible that there is some specific mechanism of PFKFB-4 protein stabilization as well as some additional functions of this enzyme in non-malignant gastric and colon tissues. These aspects of PFKFB biochemistry warrants further investigation.
Several alternative splice variants were identified for human, mouse and rat PFKFB-4[58-60]. Alternative splice variant with modified N-terminus was identified for PFKFB-4 in melanoma DB-1 cells[58]. Its expression was very high in these cells and is possibly related to melanoma growth. Other alternative splice variants of PFKFB-4 mRNA with modified C-terminus were founded in rat tissues[59]. One of them with a modified C-terminal part was observed only in the liver of rats treated by methyl tertial butyl ether, ecologically dangerous chemical compound[59]. It was not present in normal rat liver and lungs. Second alternative splice variant with deletion in fructose-2,6-bisphosphatase region is expressed in normal liver and lung tissues and its expression is affected by methyl tertial butyl ether[59]. Results of this investigation demonstrate the sensitivity of PFKFB-4 alternative splicing to the action of toxic chemical compounds, in particular methyl tretbutyl ether. Several unique alternative splice variants were identified in mouse brain and other tissues[60]. One of them has two ORFs (for 6-phosphofructo-2-kinase and fructose-2,6-bisphosphatase) as a result of insert after the 7th exon. Other alternative splice variants have inserts in kinase domain or a deletion in bisphosphatase domain[60]. Its functional significance is not elucidated as of yet.
At the same time, the analysis of PFKFB-3 and PFKFB-4 expression as well as its regulation by hypoxia in pancreatic and gastric cancer cells, which significantly differ from many other malignant cells, is needed for further advance our knowledge on the mechanisms of different tumors progression. Recently, it was shown that excess glucose induces hypoxia-inducible factor-1α in pancreatic cancer cells and stimulates glucose metabolism possibly through PFKFB as well as the migration of these cancer cells and that hypoxia strongly up-regulates the expression of PFKFB-4 and PFKFB-3[32,61].
PFKFB-4 AND PFKFB-3 GENE EXPRESSIONS IN PANCREATIC AND GASTRIC CANCER CELL LINES AND MOLECULAR MECHANISMS OF ITS REGULATION
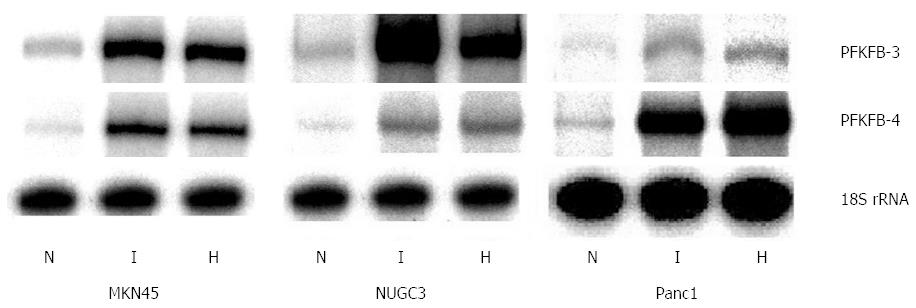
It was shown that different PFKFB genes are expressed in human pancreatic and gastric cancer cells and are up-regulated in hypoxic condition[32]. Hypoxia induces the expression of these genes through transcription factor HIF binding sites to hypoxia responsible element (HRE) of PFKFB-4 and PFKFB-3 genes, because deletion or point mutation in these HRE eliminates the hypoxic regulation of PFKFB-4 and PFKFB-3 genes[25,31,36,37]. As shown in Figure 1, the expression of PFKFB-4 mRNA is detectable at very low level in both MKN45 and NUGC3 gastric cancer cell lines growing under normal condition. The expression of PFKFB-3 mRNA in these cell lines was significantly higher as compared to the PFKFB-4 mRNA[32].
Figure 1 Effect of hypoxia (H) and hypoxia mimic dimethyloxalylglycine (I) on the expression of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3 and -4 mRNA in human gastric cancer cell lines MKN45 and NUGC3 and pancreatic cancer cell line Panc1.
Measured by ribonuclease protection assay, N: Control (normoxic) cells[32]. PFKFB: 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase.
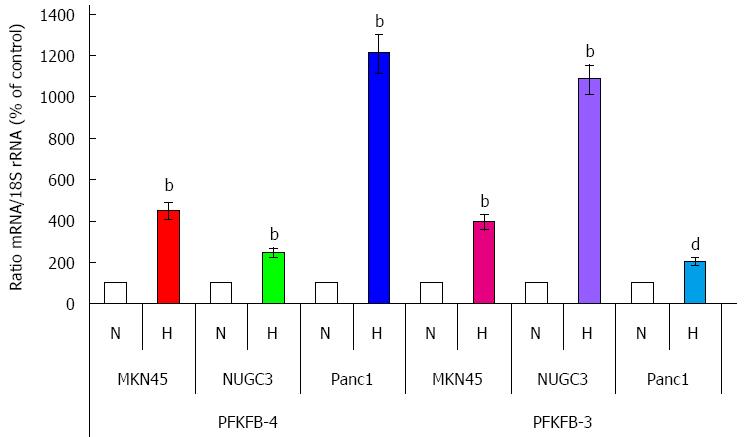
Exposure of MKN45 and NUGC3 gastric adenocarcinoma cells to hypoxia or dimethyloxalylglycine, which suppress prolyl hydroxylase enzymes, significantly enhanced the expression of PFKFB4 as well as PFKFB3 genes (P < 0.001; Figures 1 and 2). At the same time, the expression of PFKFB-3 mRNA in Panc1 pancreatic cancer cell line (P < 0.01) growing under normal condition was lower as compared to gastric cancer cell lines, but for PFKFB-4-slightly higher[32]. Moreover, the basal level of PFKFB-4 mRNA expression as well as its hypoxia responsiveness was more robust as compared to PFKFB-3 mRNA[32]. Thus, there is a difference in the basal level of these two variants of PFKFB mRNA between gastric and pancreatic cancer cells as well as in its sensitivity to hypoxia and dimethyloxalylglycine.
Figure 2 Quantification of ribonuclease protection assay of the effect of hypoxia (H) on the expression level of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-4 and -3 mRNAs in human gastric (MKN45 and NUGC3) and pancreatic (Panc1) cancer cell lines.
bP < 0.01 vs control cells; dP < 0.01 vs control cells[32]. N: Normoxic (control) cells. PFKFB: 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase.
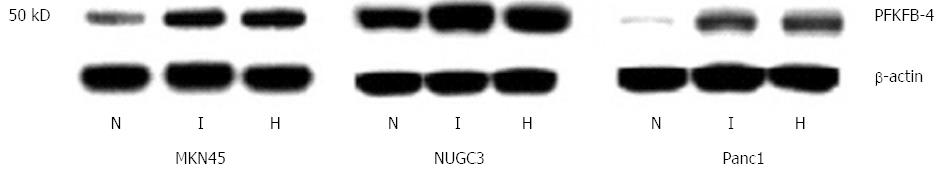
At the same time, the protein level of PFKFB-4 isoenzyme in non-treated gastric adenocarcinoma cells was much higher as compared to pancreatic cancer cells (Figure 3). Moreover, the protein level of PFKFB-4 in NUGC3 gastric cancer cells is significantly higher than in MKN45 cells. Hypoxia and dimethyloxalylglycine strongly enhances the expression of PFKFB-4 at protein level in pancreatic and gastric cancer cell lines. This increase of PFKFB-4 protein expression varies in different cancer cell lines possibly because these gastric and pancreatic cells have significantly different constitutive level of PFKFB-4 protein. Moreover, no strong correlation is present between mRNA and protein of PFKFB-4 in the pancreatic and gastric malignant cells in normoxic as well as in hypoxic condition[32]. This data agrees with results of previous investigations[31].
Figure 3 Western blot analysis of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-4 protein in human gastric (MKN45 and NUGC3) and pancreatic (Panc1) cancer cell lines: Effect of hypoxia (H) and dimethyloxalylglycine (I)[32].
PFKFB: 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase.
It is interesting to note that the level of PFKFB-4 mRNA and protein differs in different mammary gland adenocarcinoma cell lines both in normal condition and after hypoxic exposure[31]. Thus, the level of PFKFB-4 mRNA is more pronounced in the T47D malignant cell line as compared to MCF7 cells both in normal condition and after hypoxia. At the same time, the protein level of PFKFB-4 is much higher in the MCF7 cells vs T47D cell line in normal condition as well as after hypoxic exposure. Other cell lines (SKBR3 and MDA-MB-468) have similar level of PFKFB-4 mRNA expression both in normal condition and upon hypoxia. Unexpectedly, in MDA-MB-468 mammary gland adenocarcinoma cells PFKFB4 protein was detected at negligible level at the same experimental conditions, which were used for SKBR3 and other mammary gland adenocarcinoma cell lines[31]. In contrast, the level of PFKFB-4 mRNA is correlated with corresponding protein level both in SKBR3 and BT549 cell lines.
Really, the protein level of PFKFB-4, which in Panc1 pancreatic cancer cells is much lower as compared to gastric cancer cells and correlates with stronger induction of both PFKFB-4 mRNA and protein expressions upon hypoxia. At the same time, the hypoxia-induced PFKFB-4 protein level in Panc1 cells is in fact lower when compared to the level of this protein in NUGC3 cells. Moreover, the high PFKFB-4 protein level in NUGC3 gastric cancer cells is correlated with the lower induction of PFKFB-4 mRNA and protein expressions upon hypoxia; on the other hand, the level of PFKFB-4 protein in hypoxia-treated cells is very high as compared to the levels of PFKFB-4 protein in both Panc1 and MKN45 control or hypoxia-treated cells[32]. This difference between mRNA and protein levels of PFKFB-4 which in the different pancreatic and gastric cancer cells is possibly related to the mechanisms controlling PFKFB4 protein stability. However, the precise molecular mechanism for these discrepancies is complex and possibly includes PFKFB-4 enzyme posttranslational modification or its stability in a cell-specific manner and warranties further detailed investigation.
The induction of PFKFB-3 mRNA expression in the NUGC3 gastric cancer cell line by hypoxia and dimethyloxalylglycine is more pronounced as compared to PFKFB-4 mRNA expression. It is important to note that the expression PFKFB-3 mRNA in the Panc1 pancreatic cancer cells has the lowest hypoxia responsiveness as compared to both gastric cancer cell lines. We have previously shown that the hypoxic induction of PFKFB-3 mRNA expression in mammary gland cancer cells is more robust in MCF7 and T47D breast cancer cells (estrogen receptor-positive cell lines) as compared to SKBR-3 and MDA-MB-468 cells (estrogen receptor-negative cell lines)[31]. The dissimilar sensitivity of the PFKFB-3 gene expression to induction by hypoxia was also shown for other cell lines, like HeLa, Hep3B, RPE, and fibroblasts, while induction of Glut1 mRNA by hypoxia was similar in all these cell lines[25].
It is interesting to note that the induction of PFKFB-4 mRNA expression by hypoxia was simulated by dimethyloxalylglycine in different pancreatic and gastric as well as in many other cancer cell lines[25,27,31,32]. Dimethyloxalylglycine, a specific inhibitor of prolyl hydroxylases, is an oxoglutarate analog, which protects the HIF-1α protein from proteasomal degradation and significantly increases its level[59]. Suppression of prolyl hydroxylase enzymes can induce the level and functional activity of HIF-1α under normoxia and mimics hypoxic condition[62]. Induction of PFKFB-4 mRNA synthesis by hypoxia is mediated by the hypoxia responsive element located in the promoter region of this gene which is similar to the same elements, described in different hypoxia responsive genes, including PFKFB-3 gene[25,36,63,64].
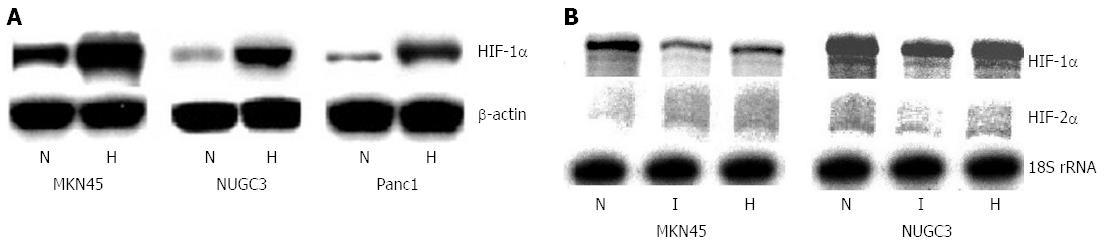
As shown in Figure 4A, hypoxia increases the expression level of PFKFB-3 and PFKFB-4 as well as VEGF and Glut1 genes in both gastric and pancreatic cancer cell lines and these changes are correlated with enhanced level of HIF-1α protein[32]. This data argues with HIF-1α-dependent mechanism of the induction of these genes under hypoxia. At the same time, the constitutive level of HIF-1α as well as HIF-2α mRNA expression and their induction by hypoxia (Figure 4B) is dissimilar in the MKN45 and NUGC3 gastric adenocarcinoma cells[32]. Thus, the level of HIF-1α mRNA expression is decreased in both gastric cancer cell lines treated by hypoxia or dimethyloxalylglycine, but no significant changes of HIF-2α mRNA expression were found in the NUGC3 gastric cancer cell line under hypoxia. However, the expression of HIF-2α mRNA in the MKN45 gastric cancer cells was slightly induced by dimethyloxalylglycine as well as hypoxia. A similar pattern of the expression of HIF-1α and HIF-2α mRNAs in the A549 lung adenocarcinoma cell line and many other cancer cell lines treated by hypoxia was shown[25,64-66].
Figure 4 Expression of hypoxia inducible factor-1α protein (Western blotting; A) and hypoxia inducible factor-1α and hypoxia inducible factor-2α mRNA (ribonuclease protection assay; B) in human gastric (MKN45 and NUGC3) and pancreatic (Panc1) cancer cell lines: effect of hypoxia (H) and dimethyloxalylglycine (I)[32].
HIF: Hypoxia inducible factor.
It is important to note that there is an inverse correlation between induction of HIF-1α mRNA and protein expressions upon hypoxic exposure. These observations suggest that the increase in HIF-1α protein expression was not reflected at the mRNA level. Moreover, the expression of HIF-1α mRNA is significantly decreased both under hypoxia and dimethyloxalylglycine action. It is possible that this discrepancy between HIF-1α mRNA and protein levels, which was found in different gastric, pancreatic and many other cancer cell lines, is related to the divergence in mechanisms which control the stability of HIF-1α mRNA and protein. Thus, the hypoxic induction of HIF-1α protein expression is a result of its stabilization, possibly mediated by specific prolyl hydroxylase enzymes, oxygen- and iron-dependent, which utilize oxoglutarate as a co-substrate[62,67,68]. At the same time, the decreased expression of HIF-1α mRNA both under hypoxia and dimethyloxalylglycine action is possibly mediated by suppression of transcription or by its increased degradation[65].
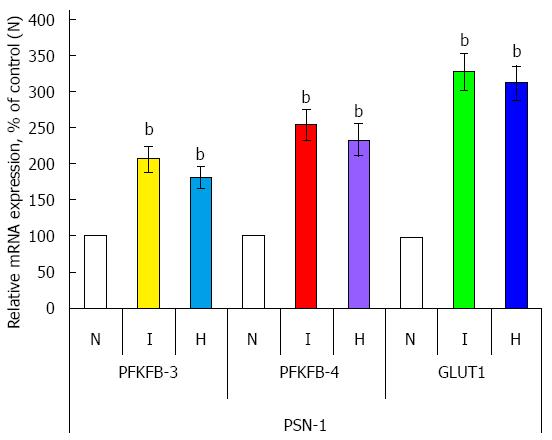
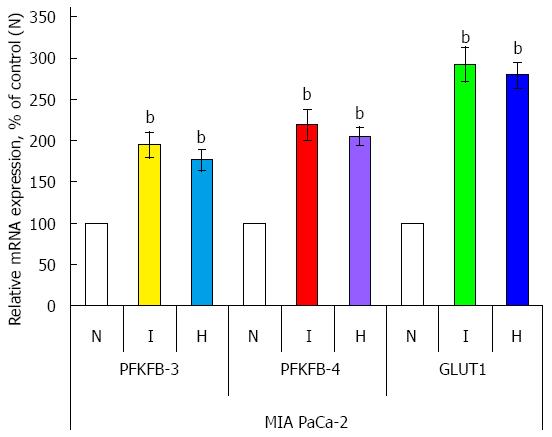
The expression mRNA level of PFKFB variant 3 and 4 was also investigated in two other pancreatic cancer cell lines: PSN-1 and MIA PaCa-2 (Figures 5 and 6). Hypoxia strongly induces (P < 0.001) the expression of both PFKFB4 and PFKFB3 genes in PSN-1 and MIA PaCa-2 cancer cells being more robust for PFKFB4 gene. Moreover, hypoxic induction of PFKFB4 and PFKFB3 gene expressions in both these pancreatic cancer cell lines correlates with a strong increase of GLUT1 gene expression (P < 0.001).
Figure 5 Effect of hypoxia (H) and hypoxia mimic dimethyloxalylglycine (I) on the expression level of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3, -4, and GLUT1 mRNAs (measured by qPCR) in human pancreatic (PSN-1) cancer cells.
n = 4; bP < 0.01 vs control cells. N: Normoxic (control) cells; PFKFB: 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase.
Figure 6 Effect of hypoxia (H) and hypoxia mimic dimethyloxalylglycine (I) on the expression level of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3, -4, and GLUT1 mRNAs (measured by qPCR) in human pancreatic (MIA PaCa-2) cancer cells.
n = 4, bP < 0.01 vs control cells. N: Normoxic (control) cells; PFKFB: 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase.
In conclusion, this chapter provides evidence that PFKFB-4 and PFKFB-3 mRNA are expressed in different cancer cell lines, including pancreatic and gastric adenocarcinoma cells, and strongly respond to hypoxia possibly through a HIF- dependent mechanism using active HIF-binding sites in PFKFB4 and PFKFB3 genes. At the same time, no clear correlation is existent between different variants of PFKFB mRNA expressions and its protein levels in different cancer cell lines both in normoxic and hypoxic conditions. Moreover, hypoxic induction of HIF-1α protein level correlates with a reduction of HIF-1α mRNA expression in these cell lines. Thus, there is an opposite correlation between hypoxic regulation of PFKFB-4 mRNA and protein levels in different hypoxia-treated adenocarcinoma cells in vitro[31,32,65]. It is possible that permanent degradation of HIF-1α protein in normoxic condition support high level expression of corresponding mRNA needed for synthesis of this protein. At the same time, stabilization of HIF-1α protein under hypoxia suppresses transcription of this gene or/and initiates the degradation of HIF-1α mRNA.
EXPRESSION OF DIFFERENT PFKFB IN HUMAN GASTRIC, COLON, LUNG, AND BREAST MALIGNANT TUMORS
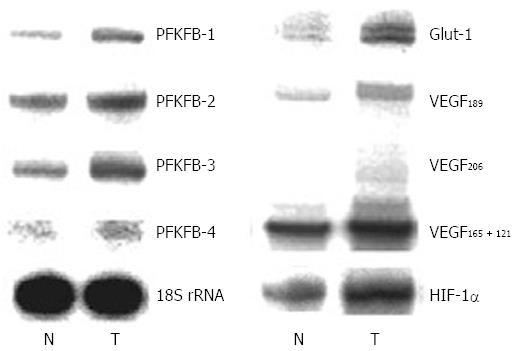
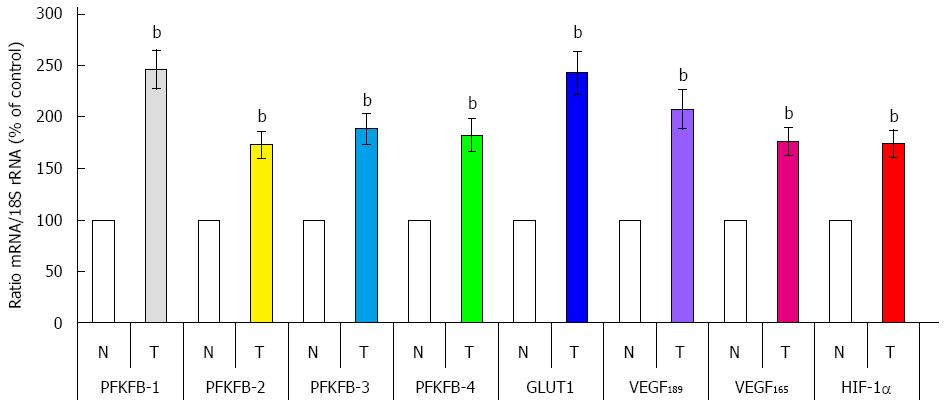
There is data that PFKFB-4 and PFKFB-3 mRNA and protein expressions are significantly increased (P < 0.001) in gastric cancers as compared to corresponding non-malignant tissue counterparts from the same patients (Figures 7 and 8)[32]. This increase in the expression of these PFKFB genes in gastric malignant tumors correlates with the up-regulation of HIF-1α and known HIF-dependent genes GLUT1 and VEGF (P < 0.001). Moreover, the expression of PFKFB-1 and PFKFB-2 mRNA is also increased in gastric cancer tissue. It is interesting to note that the expression level of different PFKFB mRNAs in non-malignant stomach tissue was highest for PFKFB-2, much less for PFKFB-3 and slight for PFKFB-4 and PFKFB-1[32].
Figure 7 Representative polyacrylamide gel autoradiograph employed in a typical ribonuclease protection assay of different 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase genes (PFKFB-1, PFKFB-2, PFKFB-3, and PFKFB-4), GLUT1, hypoxia inducible factor-1α, and different alternative splice variants of VEGF-A in gastric malignant tumors (T) and non-malignant tissue counterparts (N) from same patients.
The 18S rRNA expressions were used as control of RNA quantity used for analysis[32]. HIF: Hypoxia inducible factor; PFKFB: 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase.
Figure 8 Quantification of ribonuclease protection assay of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-1, 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-2, 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3, 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-4, GLUT1, hypoxia inducible factor-1α, and splice variants of VEGF-A mRNA in human gastric malignant tumors (T) and corresponding non-malignant tissue (N) from the same patients.
bP < 0.01 vs control cells[32]. HIF: Hypoxia inducible factor; PFKFB: 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase; VEGF: Vascular endothelial growth factor.
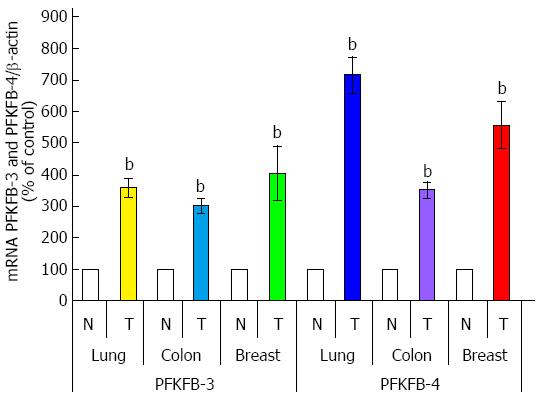
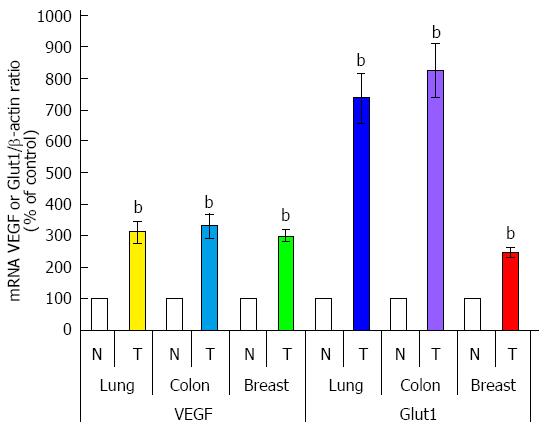
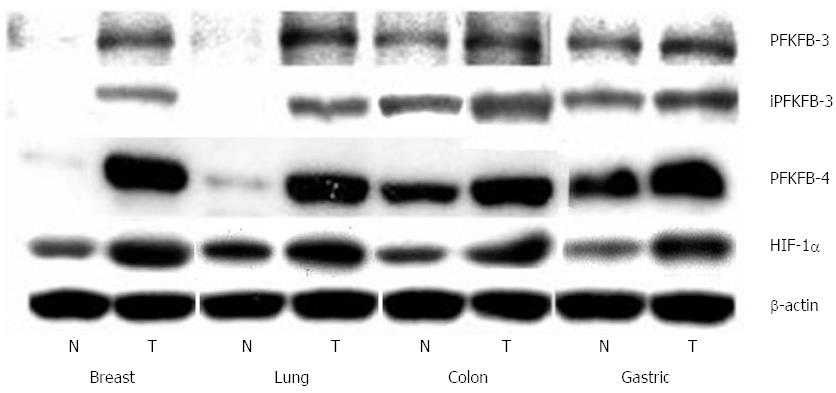
As shown in Figure 9, PFKFB-4 and PFKFB-3 mRNA are also overexpressed (P < 0.001) in lung, colon, and breast cancers as compared to corresponding non-malignant tissue counterparts from the same patients being more pronounced for PFKFB-4 in lung and breast tumors[30,33,69]. Up-regulation of PFKFB-2 mRNA is also shown for lung cancers[30]. Moreover, the level of GLUT1 and VEGF mRNA expressions is also significantly increased (P < 0.001) in all these cancer tissues, especially GLUT1 in lung and colon tumors (Figure 10)[30,33]. Western analysis of PFKFB-4 and PFKFB-3 proteins as well as HIF-1α protein clearly demonstrated its up-regulation in all analysed tumors with more pronounced changes in PFKFB-4 protein in lung and breast cancers (Figure 11)[30,32,33]. Thus, overexpression of PFKFB-3, PFKFB-4 and PFKFB-2 is observed in various human cancers through various mechanisms: by a combination of hypoxia inducible transcription factors (for PFKFB-4 and PFKFB-3), activation of oncogenic proteins and the loss of tumor suppressor function[29-33,37].
Figure 9 Quantification of ribonuclease protection assay of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3 and 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-4 mRNA expressions in lung, colon, and breast malignant tumors (T) and corresponding non-malignant tissue counterparts (C).
Values of PFKFB-3 and PFKFB-4 mRNA expressions were normalized to 18S rRNA; n = 15-20, bP < 0.01 vs non-malignant tissues[30,33]. PFKFB: 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase.
Figure 10 Quantification of ribonuclease protection assay of VEGF and Glut1 mRNA expressions in lung, colon, and breast malignant tumors (T) and corresponding non-malignant tissue counterparts (C).
Values of VEGF and Glut1 mRNA expressions were normalized to 18S rRNA; n = 15-20; bP < 0.01 vs non-malignant tissues[30,33]. PFKFB: 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase; VEGF: Vascular endothelial growth factor.
Figure 11 Representative Western blot analysis of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3, inducible 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3, 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-4, and hypoxia inducible factor-1α protein levels in breast, lung, colon, and stomach malignant tumors (T) and non-malignant (control) tissues counterparts (N) from same patients.
The actin was used to ensure equal loading of the sample[30,32,33]. HIF: Hypoxia inducible factor; PFKFB: 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase.
Recently, it was shown that amino acid activates AKT-dependent PFKFB2 phosphorylation at Ser-483 and that this activation was mediated by the PI3K and p38 signaling pathways[70]. Furthermore, AKT inactivation blocked PFKFB2 phosphorylation and fructose-2,6-bisphosphate production, thereby suggesting that the above signaling pathways converge at AKT kinase. Moreover, MACC1 (MUC1, mucin 1, cell surface associated) may affect tumor metabolism partly through expression and phosphorylation of PFKFB2[71].
At the same time, the protein level of both PFKFB-4 and PFKFB-3 is significantly different in non-malignant lung, breast, colon, and gastric tissues being more pronounced for colon and gastric tissues. It is interesting to note that the level of PFKFB-4 protein in all studied cancers (lung, breast, colon, and gastric) was also higher as compared to the PFKFB-3 isozyme (Figure 11). Thus, the main protein isoform of PFKFB enzyme family expressed in lung, breast, colon, and gastric malignant tumors is PFKFB-4.
Moreover, there is data that hypoxia is needed for tumor progression and initiates the endoplasmic reticulum stress for induction of neovascularization and apoptosis inhibition[72-76]. It is known that some PFKFB enzymes are components of the endoplasmic reticulum stress and participate in proliferation processes[77]. Recently, it was shown that PFKFB-3-driven glycolysis participates in vessel sprouting process which strongly depends upon endoplasmic reticulum stress[73,78,79]. Thus, endothelial cells relied on glycolysis rather than on oxidative phosphorylation for ATP production and loss of the glycolytic activator PFKFB3 in endothelial cells impaired vessel formation[78,80]. Moreover, the glycolytic activator PFKFB3 regulates stalk cell proliferation and renders endothelial cells more competitive to reach the tip[81].
The induction of different PFKFB as well as tumor angiogenesis and growth is realized not only through activation of transcription factor HIF[7,14,34,73,74,82-86]. For PFKFB3 it was shown that its transcription as well as allosteric activation is promoted by MAPK pathway[87]. Many growth factors may contribute to cancer progression, including pancreatic cancer, through induction of the expression of genes without hypoxia responsive elements[88]. Moreover, hypoxia-inducible mir-210 regulates normoxic gene expression involved in tumor initiation and growth[89]. Recently, it was shown that clathrin heavy chain promotes growth and angiogenesis of pancreatic adenocarcinoma, which is an aggressive disease with a high mortality rate, through the regulation of HIF-1α and VEGF signaling and that hypoxia-induced pancreatic cancer cells invasion is also mediated by transcription factor HIF[85,90]. One of the key functions of clathrin heavy chain protein is to bind with the HIF-1α protein, increasing the stability of this protein and facilitating its nuclear translocation, thereby regulating the expression of VEGF. Thus, suppression of clathrin heavy chain either by shRNA or by specific antibody inhibited pancreatic adenocarcinoma growth and angiogenesis[85].
A better understanding of the impact of PFKFB gene networks regulation on glycolysis as well as cell cycle control, apoptosis and cell survival promises to shed light on the emerging association between PFKFB-3, PFKFB-4, cell proliferation and cancer. These provide rationale for the development of agents that selectively inhibit the PFKFB3 and PFKFB-4 enzymes as antineoplastic agents. Recently was shown that inhibition of PFKFB-3 activity suppresses glycolytic flux and tumor growth by rapid induction of apoptosis in transformed cells[91,92]. It is possible, that the stimulation of glycolysis in cancer cells results by multimodal mechanism of stress stimuli affecting PFKFB3 transcriptional regulation and kinase activation by protein phosphorylation[41]. Moreover, the glycolytic enzyme PFKFB3 regulates autophagy and inhibition of PFKFB3 in tumor cells would induce autophagy as a pro-survival mechanism and inhibitors of autophagy could increase the anti-tumor effects of PFKFB3 inhibitors[93,94].
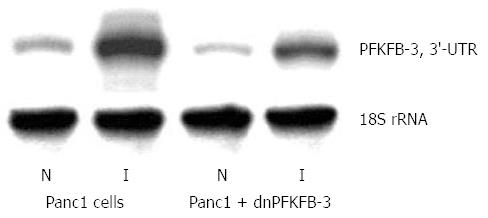
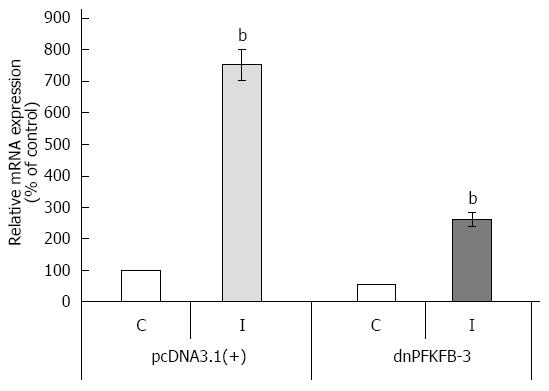
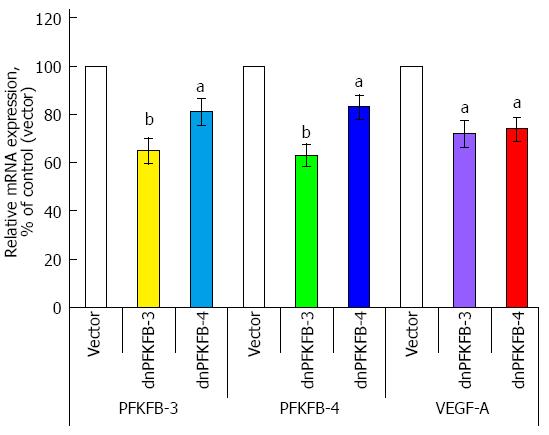
Previously, we have shown that suppression of PFKFB-3 and PFKFB-4 expression (P < 0.05-0.01) in pancreatic Panc1 and PSN-1 cancer cells by dominant/negative technology also decreases VEGF expression (P < 0.05) and proliferation rate (P < 0.05) of these cells (Figures 12, 13, 14)[95]. For this aim we introduced point mutation in ATP-binding domain of 6-phosphofructo-2-kinase part of PFKFB-3 as well as PFKFB-4 cDNA and cloned in pcDNA3.1 vector. Pancreatic Panc1 and PSN-1 cancer cells were stable transfected with dnPFKFB-3 and dnPFKFB-4 constructs and studied the expression of endogenous PFKFB-3, PFKFB-4, and VEGF-A mRNAs in these cells as well as its proliferation rate. It was shown that both dnPFKFB-3 and dnPFKFB-4 suppress the expression of endogenous PFKFB-3, PFKFB-4, and VEGF-A mRNAs as well as cell proliferation in pancreatic cancer cells[95]. Results of this investigation agree with the role of PFKFB3-driven glycolysis in vessel sprouting[78,80,81] and demonstrate possibility to apply the dominant-negative strategy for suppression of tumor cells glycolysis and proliferation through reduction of the expression of PFKFB-3 and PFKFB-4 enzymes.
Figure 12 Representative polyacrylamide gel autoradiograph employed in a typical ribonuclease protection assay of endogenous 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3 mRNA in pancreatic carcinoma cell line Panc1, stable transfected by pcDNA3.
1(+) vector (Panc1 cells) or by dominant/negative 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3 (Panc1 + dnPFKFB-3) in normoxic (N) condition and after treatment of Panc1 cells with dimethyloxalylglycine, inhibitor of prolyl hydroxylase (I; 1 mmol/L for 6 h). The 18S rRNA antisense probe was used as control of analyzed RNA quantity[89]. PFKFB: 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase.
Figure 13 Quantification of ribonuclease protection assay of endogenous 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3 mRNA expression in pancreatic carcinoma cell line Panc1, stable transfected by pcDNA3.
1(+) vector or dominant/negative 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3 in normoxic (control) condition (C) and after treatment of Panc1 cells with dimethyloxalylglycine, inhibitor of prolyl hydroxylase (I). n = 5; bP < 0.01 vs control[89]. dnPFKFB-3: Dominant/negative 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3.
Figure 14 Endogenous 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3, 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-4, and vascular endothelial growth factor mRNA expressions in pancreatic carcinoma cell line PSN-1, stable transfected with pcDNA3.
1(+) vector (Vector), dominant/negative 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3, and dominant/negative 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-4, measured by ribonuclease protection assay. n = 5; aP < 0.05 vs control; bP < 0.01 vs control[89]. PFKFB: 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase; VEGF: Vascular endothelial growth factor.
Thus, PFKFB-4 as well as PFKFB-3 participates in the regulation of glycolysis and promotes tumor growth and survival of cancer cells[34,52-54,91]. Moreover, targeting PFKFB3 by specific inhibitors is a perspective therapeutic strategy against cancer[91,92]. It was also shown that blocking of PFKFB4 induces reactive oxygen species and cancer cell death and that targeting PFKFB4 may also, therefore, present new therapeutic opportunities[52,53].
CONCLUSION
PFKFB-3 and PFKFB-4 play a significant role in the regulation of glycolysis and cancer growth by inducing cell proliferation and survival. The PFKFB-4 and PFKFB-3 genes have active HIF-responsible elements and its expression is increased in different malignant tumors and strongly induced in various cancer cell lines under hypoxia. The expression of PFKFB-4 and PFKFB-3 genes as well as its hypoxia responsibility was also shown for pancreatic (Panc1, PSN-1 and MIA Paca-2) and gastric (MKN45 and NUGC3) cancer cell lines. The highest constitutive expression level of PFKFB-4 protein was found in the NUGC3 adenocarcinoma cells and lowest in the Panc1 cells, with the maximum response to hypoxia in the pancreatic adenocarcinoma cells. Moreover, the hypoxia responsiveness of PFKFB-3 and PFKFB-4 mRNA expressions in pancreatic and gastric cancer cell lines is correlated with the increased level of HIF-1α protein and enhanced expression of VEGF and GLUT1 genes. At the same time, the basal expression level of HIF-1α as well as HIF-2α mRNA and their hypoxia responsiveness are variable in these cancer cells as well as in many other cancer cell lines. The overexpression of different PFKFB was also shown in gastric, colon, lung, and breast cancer tissues. It is interesting to note that the protein level of PFKFB-4 in colon and gastric malignant tumors as well as non-malignant tissue counterparts was greater as compared to the variant 3 of PFKFB. Both PFKFB-4 and PFKFB-3 isoenzymes are overexpressed in different malignant tumors and undergo changes in their metabolism that contribute to the proliferation and survival of cancer cells. A better understanding of the impact of PFKFB gene networks regulation on cell cycle control and glycolysis as well as nutrient balance at the molecular, cellular and system levels promises to shed light on the emerging association between PFKFB-3, PFKFB-4, cell proliferation and cancer. These provide rationale for the development of agents that selectively inhibit the PFKFB3 and PFKFB-4 enzymes as antineoplastic agents.