Published online Sep 28, 2014. doi: 10.3748/wjg.v20.i36.13195
Revised: April 29, 2014
Accepted: May 25, 2014
Published online: September 28, 2014
Processing time: 223 Days and 8.5 Hours
Cystic lymphangiomas of the adrenal gland are rare. A 79-year-old female presented in the emergency room with epigastric discomfort, and an immovable mass was palpated in her abdomen upon physical examination. Imaging studies revealed a large cystic lesion in the pancreatic tail. The radiologic impression ruled out the possibility of a mucinous cystic neoplasm, or a pseudocyst in the pancreas. The operative findings demonstrated that the cystic mass originated in the left adrenal gland. A laparoscopic excision of the cystic mass was performed, and immunohistochemistry confirmed that this mass was a lymphangioma of the adrenal gland. Several prior reports have suggested that lymphangioma can mimic renal or splenic cysts. However, lymphangioma cases mimicking pancreatic cysts are very rare.
Core tip: This paper reports the case of a large symptomatic adrenal cyst that was initially considered to be a mucinous neoplasm of the pancreas on radiologic imaging. To our knowledge, several prior reports have shown that lymphangioma can mimic renal or splenic cysts, but lymphangioma cases mimicking pancreatic tail cysts are rare.
- Citation: Jung HI, Ahn T, Son MW, Kim Z, Bae SH, Lee MS, Kim CH, Cho HD. Adrenal lymphangioma masquerading as a pancreatic tail cyst. World J Gastroenterol 2014; 20(36): 13195-13199
- URL: https://www.wjgnet.com/1007-9327/full/v20/i36/13195.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i36.13195
Cystic lymphangiomas of the adrenal gland are rare. Despite recent improvements in imaging, the origin of abdominal cysts remains ambiguous in some cases. In particular, left adrenal cystic lymphangiomas have been shown to mimic suprarenal cysts, pancreatic cysts and splenic cysts[1]. Because these lesions are clinically asymptomatic in the majority of cases, they are found incidentally during radiologic investigations or during surgery for other causes[2].
However, symptoms can occasionally occur in response to adrenal cystic lymphangiomas. The symptoms are typically related to the overall mass of the cyst and include a palpable mass, gastrointestinal discomfort and abdominal pain. Our case presented with a symptomatic mass, which was initially mistakenly thought to be a mucinous cystic neoplasm or a pseudocyst of the pancreatic tail. This case, which we report here, was eventually diagnosed as an adrenal cystic lymphangioma based on immunohistochemical results.
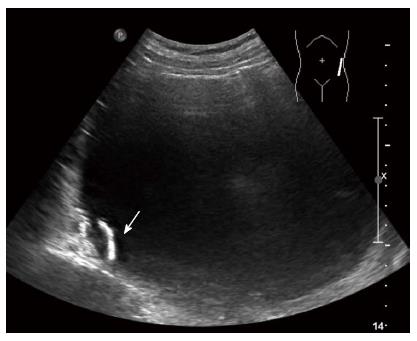
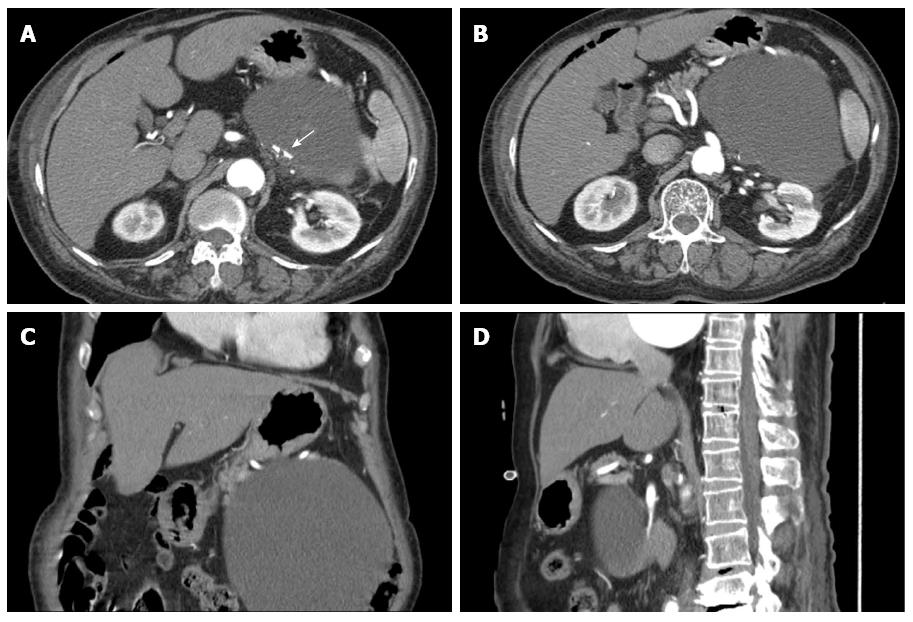
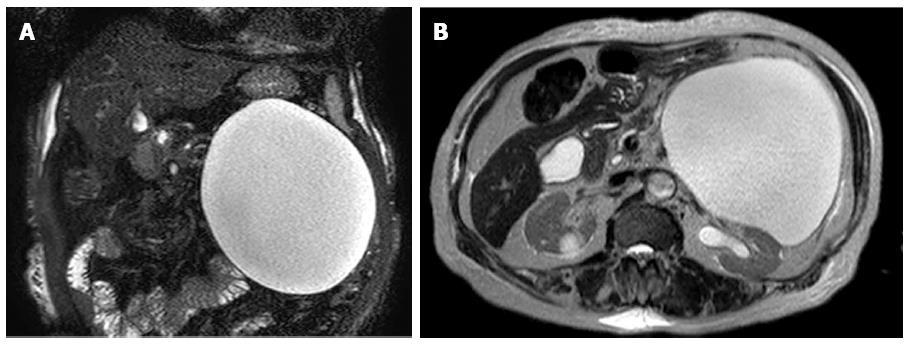
A 79-year-old female presented in the emergency room with epigastric discomfort, poor oral intake and general weakness over the previous 3 d. Her medical history included atrial fibrillation, variant angina and congestive heart failure for 6 years. She had no history of alcohol abuse, no prior operations and no familial history of pancreatic disease. On physical examination, she was febrile, looked unwell, and her tongue and skin appeared dehydrated. However, her other vital signs, including blood pressure, heart rate and respiration rate were stable. Her abdomen had mild tenderness and an immovable abdominal mass was found in the upper left quadrant. Initial laboratory findings identified mild leukocytosis (12340/mm3), a prolonged coagulation profile (prothrombin time 67.6%), proteinuria (+3), elevated bilirubin (1.8 mg/dL), elevated blood urea nitrogen/creatinine (39 mg/dL/1.6 mg/dL) and elevated creatinephosphokinase (5016 IU/L). However, the levels of aspartate transaminase, alanine transaminase, amylase and lipase were within the normal range. She was initially admitted to the medical department for acute kidney injury (AKI) with rhabdomyolysis. Her epigastric discomfort did not subside within several days. Because of this, an evaluation of the abdominal mass was performed during the management of AKI. Abdominal ultrasonography (US) revealed a large cystic mass with luminal calcification that measured 14 cm in diameter in the left retroperitoneum (Figure 1), and a computed tomography (CT) scan showed a large low attenuation lesion with calcification in the pancreatic tail measuring 16 cm (Figure 2). Once the radiologist ruled out a pancreatic mucinous cystic neoplasm and a pancreatic pseudocyst, we initially planned endoscopic retrograde cholangiography, which is a diagnostic and therapeutic tool. However, we performed magnetic resonance imaging (MRI) due to her overall condition. The MRI revealed a huge and a high attenuation lesion on the T2-weighted image, which was abutting the pancreatic tail (Figure 3). On the 14th day in the hospital, her laboratory parameters were within the normal range, but her abdominal discomfort remained.
Because of our clinical and radiological findings, we performed a laparoscopic excision of the cyst. Under general anesthesia, the left side of the patient was elevated approximately 30o (semilateral position). Four trocars including two 10 mm trocars and two 5 mm trocars (UnimaxMedical System Inc., Hain Tien, Taipei, Taiwan) were used. One 10 mm trocar was inserted into the supraumbilical incision, which was used for the camera port. The other 10 mm trocar was inserted above the umbilical port (at approximately 5 cm) and the 5 mm trocars were inserted into the left para-umbilical and the left subcostal area. Operative findings included the identification of a large cystic mass measuring approximately 20 cm adjacent to the pancreatic tail, transverse colon and mesentery. A harmonic scalpel (Ethicon Endosurgery, 5 mm, Cincinnati, OH, United States) and Ligasure (LigaSureAtlasTM 37 cm Hand Switching Laparoscopic Instrument, Covidien) were used to dissect this cystic mass. After the dissection was performed, we found that the cystic mass had originated in the left adrenal gland. Because the margin was clear,we excised the cystic mass and performed a left adrenalectomy. The specimen was placed into a retrieval bag (Lapbag, Sejong Medical Co., Ltd., Paju, South Korea), but the cystic tumor was too large for the bag, so we carefully aspirated the specimen contents inthe bag. The umbilical incision was elongated approximately 2 cm, and the specimen was removed through the umbilical incision. After 6 d, the patient was discharged without any complications. One year after the operation, the patient did not have any abdominal pain and was doing well without a recurrence.
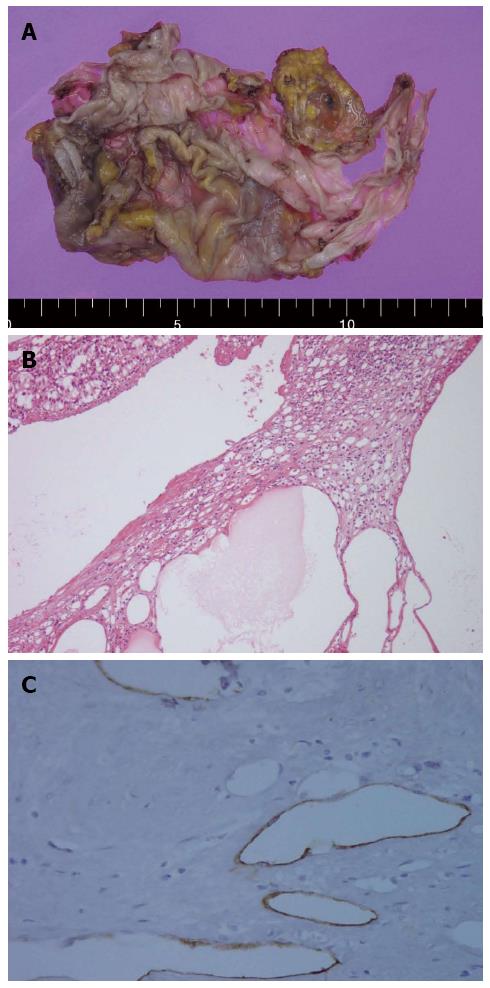
Pathologic reports showed that, the cyst measured 13.0 cm × 8.0 cm, and the adrenal gland was shown to contain smaller calcified cysts that had a thin membranous appearance (Figure 4A). Histological analysis revealed multiple cysts of variable sizes with thin walls and dilated spaces that contained proteinaceous material (Figure 4B). Immunohistochemical stains for CD34 and D2-40 were positive, but calretinin staining was negative (Figure 4C). The histopathological diagnosis was a cystic lymphangioma in the left adrenal gland.
Lymphangioma is the most common benign vascular malformation in childhood. Lymphangioma is primarily located in the neck or axilla (95%), but the other 5% of cases are found in the abdominal cavity, including the mesentery of the small intestine, the omentum, mesocolon and retroperitoneum. Lymphangioma can occur at all ages, but the peak incidence occurs between the third and sixth decade of life[3]. The male to female ratio is 1:2, and the majority of cases are unilateral. Bilateral cysts are reported in less than 10% of childhood cases[3]. Adrenal gland lymphangioma (AL) is very rare. It was first reported in autopsy cases in 1962[4]. Autopsy studies have reported that the incidence is between 0.064% and 0.18%[5], and the first AL was reported in 1965. To date, approximately 50 cases of AL diagnosed using immunohistochemistry have been reported in the literature. The largest study, which included nine cases of AL that had been verified by immunohistochemistry, was reported in 2011[6].
The etiology of AL is still controversial. Malformation, ectasia and the obstruction of lymphatic channels are currently the most favored potential mechanisms of AL pathogenesis[7]. Histologically, lymphangiomais classified into four types of malformation: cystic, capillary, cavernous, and vasculolymphatic. However, all four of these types are considered the same disease, and combinations of these different histological subtypes may exist in the same lesions[8]. Cystic lesions are also classified into four different types: endothelial cysts (45%), pseudocysts (39%), epithelial cysts (9%) and parasitic cysts (7%)[2]. Endothelial cysts can be further subdivided into angiomatous, lymphangiomatous and hamartomatous cysts. Most endothelial cysts are lymphangiomatous, and they are usually composed of multilocular, thin-walled cystic lesions. Typically, they are formed by irregular dilated spaces within endothelial lined cavities. The endothelial cells lining these cavities are immunoreactive for vascular cell markers such as CD31 and CD34. In addition, the D2-40 monoclonal antibody, which recognizes a transmembrane mucoprotein, only recognizes the lymphatic endothelium, thus suggesting that it is a specific marker for cells of lymphatic origin[6]. In our case, we observed significant CD31 and D2-40 immunoreactivity.
Most symptoms of adrenal cysts are related to their size and location. Frequently reported symptoms include gastrointestinal discomfort, abdominal pain and palpable masses. When cysts are ruptured with hemorrhages or get infected, acute abdominal pain can occur. However, most cysts are asymptomatic[7], and laboratory findings are not typically helpful. However, small cysts associated with pheochromocytoma, Cushing syndrome and virilization, though rare, can require laboratory evaluations. In our case, the patient complained of epigastric discomfort and a palpable mass, but the laboratory findings were not specific because of combined diseases including AKI and rhabdomyolysis.
The number of incidental discoveries of lymphangioma has increased recently because of improvements in imaging modalities (US, CT and MRI). US reveals well marginated, anechoic lesions, while CT scans demonstrate hypodense, non-enhancing masses. US and CT scans can reveal peripheral and curvilinear calcifications of the cystic wall in 15% of cases. Small adrenal cysts with a wall diameter of less than 3 mm and water attenuation in the CT scan are typically considered to be benign lesions. Uncomplicated adrenal cysts present as non-enhancing masses with smooth thin walls[5]. On MRI, simple cysts of AL are visible as low intensity signals on T1-weighted images and high intensity signals on T2-weighted images. In contrast, complex cysts, such as those characterized by hemorrhage or, infection, appear as high signal intensity signals on both T1- and T2-weighted images. In our case, we observed luminal calcification on the CT scan and high signal intensity on the MRI. For asymptomatic large cystic tumors, the radiologic diagnosis is very important in deciding whether to operate. However, the imaging findings are frequently ambiguous because of the adjacent organs. In our case, the cyst was located close to the pancreatic tail, and thus it appeared that the cyst originated in the tail of the pancreas. However, when we reviewed the imaging findings postoperatively, we realized that the cyst was located behind the splenic artery and vein, and the connection between the cystic tumor and the pancreas was ambiguous. Hence, other findings, such as the presence of a hypodense cystic mass or the peripheral calcification of the cystic wall, are important for differential diagnosis. These types of findings were also observed in our case.
In most cases, the clinical management of adrenal cysts depends on the findings of imaging studies. Several authors have recommended that uncomplicated cysts with smooth wall linings are managed by aspiration instead of surgical excision. However, the aspiration of cystic content has several limitations. One limitation is related to the potentially ambiguous histological type, and another is the high incidence of the re-accumulation of cystic fluid[9]. Nonfunctioning and small cysts of a benign nature can be treated conservatively. However, surgical excisions are recommended for cysts that are large, symptomatic, parasitic and considered likely to be malignant[10].
In this case, the cyst, which was large and symptomatic, was initially considered to be amucinous neoplasm of the pancreas based on radiologic findings. We are aware of several reports of lymphangioma mimicking a renal cyst or a splenic cyst[1,7], but cases in which lymphangiomas mimic mucinous neoplasm or pseudocysts of the pancreas are very rare.
A 79-year-old female presented with epigastric discomfort, poor oral intake and palpable mass on the abdomen.
An immovable mass was palpated in the upper left abdominal area.
The differential diagnosis was pancreatic tail mucinous neoplasm or pseudocyst.
Authors observed mild leukocytosis (12340/mm3), a prolonged coagulation profile (prothrombin time 67.6%), proteinuria (+3), elevated bilirubin (1.8 mg/dL), elevated blood urea nitrogen/creatinine (39 mg/dL/1.6 mg/dL) and elevated creatinephosphokinase (5016 IU/L). However, the levels of aspartate transaminase, alanine transaminase, amylase and lipase were within the normal range.
Computed tomography scans revealed a large low attenuation lesion measuring 16 cm in the pancreatic tail with calcification. The radiologist ruled out a pancreatic mucinous cystic neoplasm or pseudocyst.
The specimen had multiple thin walled cysts of variable size with dilated spaces containing proteinaceous material. Immunohistochemistry for CD34 and D2-40 was positive, but calretinin was negative. The histopathological diagnosis was cystic lymphangioma in the left adrenal gland.
A laparoscopic excision was performed, and the patient was discharged after 6 d without any complications.
To date, approximately 50 cases of adrenal gland lymphangioma diagnosed by immunohistochemistry have been reported in the literature.
The endothelial cells were immunopositive for vascular markers such as CD31 and CD34. In addition, the D2-40 monoclonal antibody, which stains a transmembrane mucoprotein and only exhibits immunoreactivity against the lymphatic endothelium, is a specific marker of lymphatic origin.
Several prior reports of lymphangioma mimicking renal or splenic cysts have been reported. However, this is one of the first cases of lymphangioma mimicking a pancreatic cyst.
The authors present a surgical resected case of adrenal cystic lymphangioma.
P- Reviewer: Aydemir G, Azhar R, Tsuji Y S- Editor: Nan J L- Editor: Cant MR E- Editor: Wang CH
| 1. | Schlittler LA, Dallagasperina VW, Carlotto JR, Vilarroel RU, Lazaretti NS. True adrenal cyst mimicking renal cancer in a young woman: a case report. Cases J. 2009;2:7351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Longo JM, Jafri SZ, Bis KB. Adrenal lymphangioma: a case report. Clin Imaging. 2000;24:104-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Khoda J, Hertzanu Y, Sebbag G, Lantsberg L, Barky Y. Adrenal cysts: diagnosis and therapeutic approach. Int Surg. 1993;78:239-242. [PubMed] |
| 4. | PLAUT A. Hemangiomas and related lesions of the adrenal gland. Virchows Arch Pathol Anat Physiol Klin Med. 1962;335:345-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Rozenblit A, Morehouse HT, Amis ES. Cystic adrenal lesions: CT features. Radiology. 1996;201:541-548. [PubMed] |
| 6. | Ellis CL, Banerjee P, Carney E, Sharma R, Netto GJ. Adrenal lymphangioma: clinicopathologic and immunohistochemical characteristics of a rare lesion. Hum Pathol. 2011;42:1013-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Chien HP, Chang YS, Hsu PS, Lin JD, Wu YC, Chang HL, Chuang CK, Tsuei KH, Hsueh C. Adrenal cystic lesions: a clinicopathological analysis of 25 cases with proposed histogenesis and review of the literature. Endocr Pathol. 2008;19:274-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Fauquenot-Nollen AM, Plaisier ML, Tjon A Tham RT. Combined thoracic and abdominal lymphangioma in an adult. JBR-BTR. 2002;85:130-131. [PubMed] |
| 9. | Tung GA, Pfister RC, Papanicolaou N, Yoder IC. Adrenal cysts: imaging and percutaneous aspiration. Radiology. 1989;173:107-110. [PubMed] |
| 10. | Kasperlik-Załuska AA, Otto M, Cichocki A, Rosłonowska E, Słowinska-Srzednicka J, Zgliczyński W, Jeske W, Papierska L, Tołłoczko T, Polański J. 1,161 patients with adrenal incidentalomas: indications for surgery. Langenbecks Arch Surg. 2008;393:121-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |