Published online Sep 28, 2014. doi: 10.3748/wjg.v20.i36.13178
Revised: May 29, 2014
Accepted: June 25, 2014
Published online: September 28, 2014
Processing time: 163 Days and 12.4 Hours
AIM: To compare magnetic imaging-assisted colonoscopy (MIC) with conventional colonoscopy (CC).
METHODS: Magnetic imaging technology provides a computer-generated image of the shape and position of the colonoscope onto a monitor to give visual guidance to the endoscopist. It is designed to improve colonoscopy performance and tolerability for patients by enabling visualization of loop formation and endoscope position. Recently, a new version of MIC technology was developed for which there are limited data.To evaluate this latest generation of MIC among experienced rather than inexperienced or trainee endoscopists, a prospective randomized trial was performed using only gastroenterologists with therapeutic endoscopy training. Consecutive patients undergoing elective outpatient colonoscopy were randomized to MIC or CC, with patients blinded to their group assignment. Endoscopic procedural metrics and quantities of conscious sedation medications were recorded during the procedures. The procedure was classified as “usual” or “difficult” by the endoscopist at the conclusion of each case based on the need for adjunctive maneuvers to facilitate endoscope advancement. After more than one hour post-procedure, patients completed a 10 cm visual analogue pain scale to reflect the degree of discomfort experienced during their colonoscopy. The primary outcome was patient comfort expressed by the visual analogue pain score. Secondary outcomes consisted of endoscopic procedural metrics as well as a sedation score derived from standardized dose increments of the conscious sedation medications.
RESULTS: Two hundred fifty-three patients were randomized and underwent MIC or CC between September 2011 and October 2012. The groups were similar in terms of the indications for colonoscopy and patient characteristics. There were no differences in cecal intubation rates (100% vs 99%), insertion distance-to-cecum (82 cm vs 83 cm), time-to-cecum (6.5 min vs 7.2 min), or polyp detection rate (47% vs 52%) between the MIC and CC groups. The primary outcome of mean pain score (1.0 vs 0.9 out of 10, P = 0.41) did not differ between MIC and CC groups, nor did the mean sedation score (8.2 vs 8.5, P = 0.34). Within the subgroup of cases considered more challenging or difficult, time-to-cecum was significantly faster with MIC compared to CC, 10.1 min vs 13.4 min respectively (P = 0.01). Sensitivity analyses confirmed a similar pattern of overall findings when each endoscopist was considered separately, demonstrating that the mean results for the entire group were not unduly influenced by outlier results from any one endoscopist.
CONCLUSION: Although the latest version of MIC resulted in faster times-to-cecum within a subgroup of more challenging cases, overall it was no better than CC in terms of patient comfort, sedation requirements and endoscopic procedural metrics, when performed in experienced hands.
Core tip: ScopeGuide is a magnetic imaging technology designed to improve colonoscopy performance and tolerability for patients by enabling visualization of loop formation and endoscope position. Previous studies have demonstrated a useful benefit of imaging-assisted colonoscopy in procedures performed by trainees. In this randomized, controlled trial we demonstrated that when colonoscopy is performed by experienced endoscopists, the use of ScopeGuide conferred no advantage over conventional colonoscopy in terms of patient comfort, sedation requirements, and endoscopic metrics such as cecal intubation rate or time-to-cecum. However, significantly faster times-to-cecum were achieved when using ScopeGuide in more challenging cases.
-
Citation: Teshima CW, Zepeda-Gómez S, AlShankiti SH, Sandha GS. Magnetic imaging-assisted colonoscopy
vs conventional colonoscopy: A randomized controlled trial. World J Gastroenterol 2014; 20(36): 13178-13184 - URL: https://www.wjgnet.com/1007-9327/full/v20/i36/13178.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i36.13178
Colonoscopy decreases the likelihood of developing colorectal cancer (CRC) and CRC-related mortality[1-5] and is the CRC screening modality of choice[6]. However, incomplete colonoscopy that fails to reach the cecum remains an important limitation, occurring in 10%-20% of cases[7,8]. Furthermore,a considerable proportion of the population remain averse to undergoing colonoscopy, particularly because of fears of procedure discomfort[9,10], decreasing the potential impact for overall CRC reduction. Thus, optimizing performance of colonoscopy and improving its tolerability for patients is important.
Colonoscopy is most successful at reaching the cecum and most comfortable for patients when the endoscope is kept in a straight position, achieved by minimizing loop formation and reducing loops once they have formed[11,12]. To help overcome these challenges, real-time magnetic imaging-assisted colonoscopy (MIC) was developed. MIC consists of electromagnetic generator coils embedded within the shaft of the endoscope that produce a magnetic field detected by a series of sensors external to the patient, which triangulate the coil position in three-dimensional (3D) space, giving rise to a computer-generated image of the shape of the endoscope on the monitor[13]. The initial studies using earlier versions of MIC demonstrated significant improvements in cecal intubation rates, time-to-cecum, duration of time spent managing loops, and success of straightening attempts when colonoscopy was performed by trainees but not by experienced endoscopists[14]. Consequently, magnetic imaging technology has been generally regarded as a learning tool. However, a recent meta-analysis suggested that MIC improves cecal intubation rates even among experienced endoscopists[15].
Recently, an updated version of MIC (ScopeGuide™, Olympus America, Center Valley, PA) was developed for incorporation into the latest colonoscopes, featuring a compact receiver dish mounted on a roll stand for convenient positioning during the procedure, integration of the 3D representation of the scope on the same screen as the endoscopic image, and an external hand-held coil used to identify the optimal location for abdominal pressure relative to the endoscope. It is hoped that the latest generation of ScopeGuide will be more user friendly, while facilitating a more comfortable patient experience and technically successful procedure, not just for trainees but also for experienced endoscopists.
The purpose of this prospective, randomized trial was to determine if real time visualization of the colonoscope using the latest generation ScopeGuide system is superior to conventional colonoscopy for improving patient experience in terms of reduced discomfort and decreased sedation requirements, and for improving endoscopic procedural outcomes, when performed by experienced endoscopists.
Consecutive, adult patients (18 years or older) referred for elective, outpatient colonoscopy at the University of Alberta Hospital (Edmonton, Canada) were considered for enrollment. Patients were excluded if they were admitted to hospital or if they had active, ongoing lower gastrointestinal bleeding, if they were undergoing colonoscopy without prior purgative bowel preparation or if they required anesthetist-administered propofol, if they had a history of previous colonic surgery, cardiac pacemaker or implantable cardioverter-defibrillator, or if the colonoscopy was to be performed by a trainee under staff supervision. Eligible patients who provided informed consent were then randomized to undergo conventional colonoscopy (CC) or MIC using the new ScopeGuide system, with patients, but not endoscopists, blinded to the randomization status. Simple, non-restricted randomization was performed using a computerized random-number generator immediately prior to the procedure. The study protocol was approved by the Health Research Ethics Board of the University of Alberta (effective 08/09/2011) and registered with Clinicaltrials.gov (registered 09/18/2011; NCT01438645).
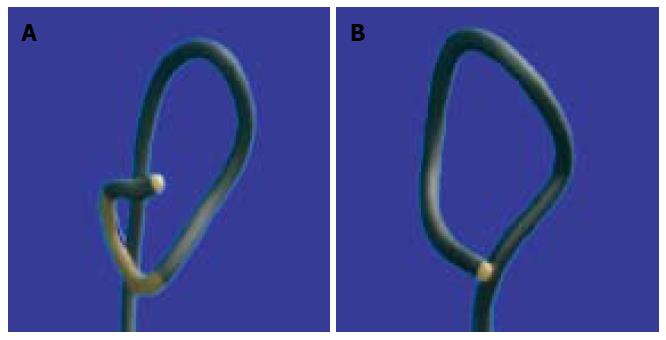
Colonoscopy was performed by one of three experienced endoscopists as clinically indicated. The control group underwent conventional colonoscopy using CF-H180AL variable-stiffness colonoscopes (Olympus America). The investigational group underwent magnetic imaging-assisted colonoscopy using Olympus CF-H180DL variable-stiffness colonoscopes, which differ only by the incorporation of the ScopeGuide system that generates a 3D image on the monitor depicting the shape of the colonoscope inside the patient’s body (Figure 1). Beyond the inclusion of ScopeGuide, the colonoscopy procedure did not differ between groups.
All patients received a purgative bowel preparation consisting of 4 L of a polyethylene glycol solution followed by an overnight fast (for morning procedures) or a 2 L/2 L split preparation (for afternoon procedures) according to the standard clinical practice at our center. Prior to the procedure, patients completed a visual analogue scale (VAS) reflecting their predictions for expected discomfort. The external ScopeGuide receiver dish was positioned for all patients to maintain patient blinding to randomization status. All procedures were then performed using conscious sedation consisting of a benzodiazepine and an opioid analgesic. Initially, all patients received standardized doses of midazolam 2 mg IV and fentanyl 25 mcg IV. Additional doses were then provided when the nurse or physician believed that the patient was becoming uncomfortable. Insufflation of the colon was accomplished using room air, and alternative methods such as CO2 were not permitted. In all cases, the endoscopist attempted to minimize the formation of loops within the colon and reduced any loops whenever possible according to standard clinical practice, using the additional guidance from the ScopeGuide image in the investigational group. The use of technical maneuvers to facilitate completion of the procedure were permitted, including external abdominal pressure, repositioning of the patient, or tightening of the variable-stiffness setting on the colonoscope. Any abnormalities or polyps detected during insertion were more closely inspected, biopsied or removed during subsequent colonoscope withdrawal. Any additional diagnostic or therapeutic applications were permitted as clinically indicated. During the case, the nurse documented all procedural data using a standardized reporting form. At the conclusion of the colonoscopy, the endoscopist rated the procedural difficulty as “usual” or “difficult” based on the need for adjunctive maneuvers described above, defined as any patient repositioning or the use of more than one instance of abdominal pressure. Patients completed another VAS reflecting their actual degree of discomfort experienced once they were completely awake after spending more than one hour in the recovery area.
The primary outcome measure was the patient experience during colonoscopy, defined by patient comfort as expressed by the mean pain score. The pain score was determined using the post-procedure visual analogue pain scale consisting of a 10 cm linear scale ranging from “0” at its extreme left representing “no pain” to “10” on its extreme right representing “unbearably severe pain.” The pain score was rated between 0.1 and 10.0, with a lower value representing a more comfortable procedure. The amount of sedation used during the procedure was then quantified by calculating a sedation score derived from the doses of the conscious sedation medications. Since the conscious sedation consisted of two different drugs, the doses of these drugs were converted into a single numerical score. By convention, typical dose increments of midazolam consist of 1 mg units whereas those of fentanyl consist of 25 mcg units, and each was assigned a numerical score of “1”. These were then added together to generate a unified sedation score for each patient. Additional secondary outcome measures consisted of endoscopic procedural outcomes such as time-to-cecum, cecal intubation rate, polyp detection rate, and insertion distance of the colonoscope to the cecal pole. As part of a secondary analysis to account for patient expectations regarding procedural discomfort that might modify their actual perception of pain during colonoscopy, the VAS was also measured pre-procedure, and the pain difference was determined by subtracting the pre-procedure from the post-procedure pain score. Finally, sensitivity analyses were performed to determine if the findings were consistent across all three endoscopists, and within the subgroup of “difficult” procedures.
The statistical software Stata 10.1 (Stata Corp, College Station, TX) was used to analyze the data. Means and ranges were used to summarize data for continuous variables and percentages were used for categorical variables. Student’s t-test was used to compare the primary outcome of the pain score between the two groups, as well as for other continuous data. The χ2 test was used to compare proportions for categorical data. A two-sided P≤ 0.05 with 80% power was considered statistically significant (after correction for multiple comparisons). Multivariate regression was used to perform the sensitivity analyses. A sample size calculation was performed to detect a difference of 0.5 (out of the 10 point VAS scale) in the mean pain scores between the CC and MIC groups. Based on a predicted pain score of 1.5 in the CC group and 1.0 in the MIC group, it was estimated that 126 patients (63 in each group) would be required to demonstrate a statistically significant difference.
Between September 2011 and October 2012, 253 patients participated in the study; mean age 58 years (range: 18-86 years); 52% male. The indications for colonoscopy and other patient characteristics are shown in Table 1. One hundred twenty-two patients (48%) underwent CC and 131 (52%) had MIC. Complete colonoscopy was accomplished in 121 cases (99%) in the CC group and 131 cases (100%) in the MIC group. Incomplete colonoscopy occurred in one case due to a failed bowel preparation that left formed stool obstructing the mid-transverse colon. The mean endoscope insertion distance was 83 cm (range: 53-130 cm) in the CC group and 82 cm (range: 49-150 cm) in the MIC group (P = 0.71), with a mean time-to-cecum of 7.2 min (range: 2-30 min) and 6.5 min (range: 1-28 min) respectively (P = 0.18). Polyps were detected in 52% of cases in the CC group and in 47% of cases in the MIC group (P = 0.42), with a mean of 1.7 (range: 1-7) and 1.9 (range: 1-8) polyps, respectively. No adverse events were recorded. See Table 2 for endoscopic procedural metrics.
| Characteristics | Conventional colonoscopy(n = 122) | Magnetic imaging colonoscopy(n = 131) | Total(n = 253) |
| Age (yr, range) | 58.2 (18-82) | 57.7 (19-86) | 57.9 (18-86) |
| Gender-male | 63 (51.6) | 68 (51.9) | 131 (51.8) |
| Previous colonoscopy | 77 (63.1) | 66 (50.4) | 143 (56.5) |
| Prior abdo or pelvic surgery | 19 (15.6) | 26 (19.9) | 45 (17.8) |
| Indication | |||
| Screening or polyp follow-up | 71 (58.2) | 79 (60.3) | 150 (59.3) |
| GI bleeding | 13 (10.7) | 23 (17.6) | 36 (14.2) |
| Anemia or FOBT+ | 7 (5.7) | 10 (7.6) | 17 (6.7) |
| Diarrhea | 5 (4.1) | 11 (8.4) | 16 (6.3) |
| IBD | 8 (6.6) | 4 (3.1) | 12 (4.7) |
| Other | 18 (14.8) | 4 (3.1) | 22 (8.7) |
| Endoscopic outcomes | Conventional colonoscopy(n = 122) | Magnetic imaging colonoscopy(n = 131) | Total(n = 253) | P value |
| Cecal intubation n (%) | 121 (99.2) | 131 (100) | 252 (99.6) | 0.30 |
| TI intubation n (%) | 42 (34.4) | 46 (35.1) | 88 (34.8) | 0.91 |
| Distance to cecum (cm) | 83 (53–130) | 82.4 (49–150) | 82.7 (49–150) | 0.71 |
| Time-to-cecum (min) | 7.2 (2-29.5) | 6.5 (1.2-28) | 6.9 (1.2-29.5) | 0.18 |
| Total procedure time (min) | 16.7 (8.1-36) | 15.7 (5.7-40) | 16.2 (5.7-40) | 0.19 |
| Polyp detection rate | 51.60% (0.43, 0.61) | 46.60% (0.38, 0.55) | 49.0% | 0.42 |
| Meanpolyps (range) | 1.7 (1-7) | 1.9 (1-8) | 1.8 (1-8) | 0.36 |
| Quality of bowel prep | ||||
| Excellent | 21.3% | 29.8% | 25.7% | |
| Acceptable | 49.2% | 43.5% | 46.3% | |
| Fair | 25.4% | 24.4% | 24.9% | |
| Poor | 4.1% | 2.3% | 3.2% | |
| Procedures self-rated as “difficult”n (%) | 25 (20.5) | 36 (27.5) | 61 (24.1) | 0.19 |
| Sedation, mean doses (range) | ||||
| Midazolam (mg) | 5.8 (3-15) | 5.5 (2-15) | 5.7 (2-15) | 0.31 |
| Fentanyl (mcg) | 86.3 (50-150) | 83.2 (50-150) | 84.7 (50-150) | 0.29 |
The outcomes regarding patient comfort and sedation are shown in Table 3. The primary endpoint of pain score (0.9 vs 1.0, P = 0.41) did not differ between the CC and MIC groups, nor did the secondary endpoints of sedation score (8.5 vs 8.2, P = 0.34) and pain difference (-1.3 vs -1.8, P = 0.14). A similar pattern was observed in the subgroup of 61 procedures (24%) rated as being “difficult” based on the requirement of adjunctive maneuvers (Table 4), in which there were no significant differences between the CC and MIC groups with respect to these patient comfort metrics. However, time-to-cecum was significantly shorter with MIC compared to CC among this subgroup of “difficult” cases, with mean times of 10.1 min (range: 3.8-28 min) and 13.4 min (range: 6.7-29.5 min), respectively (P = 0.01).
| Score | Conventional colonoscopy(n = 122) | Magnetic imaging colonoscopy(n = 131) | Total(n = 253) | P value |
| Pain score | 0.85 (0.1-8.4) | 1.03 (0.1-10) | 0.94 (0.1-10) | 0.41 |
| Pretest pain score | 2.2 (0.1-8.5) | 2.9 (0.1-9) | 2.5 (0.1-9) | 0.02 |
| Pain difference | -1.3 | -1.8 | -1.6 | 0.14 |
| Sedation score | 8.5 (4.5-17) | 8.2 (4-21) | 8.3 (4-21) | 0.34 |
| Colonoscopy procedures | Conventional colonoscopy(n = 25) | Magnetic imaging colonoscopy(n = 36) | Total(n = 61) | P value |
| Time-to-cecum (min) | 13.4 | 10.10 | 11.50 | 0.01 |
| (6.7-29.5) | (3.8-28) | (3.8-29.5) | ||
| Distance to cecum (cm) | 91.20 | 85.70 | 88 | 0.30 |
| (68-130) | (49-150) | (49-150) | ||
| Sedation score | 9.54 | 9.08 | 9.27 | 0.61 |
| (5-17) | (5-21) | (5-21) | ||
| Pain score | 1.48 | 1.15 | 1.28 | 0.53 |
| (0.1-8.4) | (0.1-7.9) | (0.1-8.4) | ||
| Pain difference | -1.05 | -1.50 | -1.32 | 0.54 |
| (-6.8-3.3) | (-7.8-4.9) | (-7.8-4.9) |
When sensitivity analyses were performed to determine if the same pattern of findings were observed for each endoscopist when considered separately, no differences emerged between the CC and MIC groups with respect to pain score, sedation score and endoscopic procedural metrics such as cecal intubation rate, time-to-cecum, insertion distance to cecum or polyp detection rate. This demonstrates that the overall pattern of findings was not unduly influenced by outlier results from any one endoscopist. Finally, regression analysis confirmed that no difference exists in the pain score between the CC and MIC groups after controlling for the sedation score.
Optimizing the technical performance of colonoscopy and its tolerability for patients is important. The development of magnetic imaging technology that enables real time visualization of the shape of the entire endoscope within the patient’s body is designed to help achieve that aim. In this randomized trial, MIC using the latest generation of ScopeGuide was compared to CC with regards to patient comfort based on a post-procedure VAS pain score and a sedation score derived from standard dose increments of conscious sedation medications, as well as endoscopic procedural metrics. However, MIC did not prove to be superior to CC for these patient experience outcomes, nor for any of the technical outcomes such as cecal intubation rate, time-to-cecum, endoscope insertion distance-to-cecum, or polyp detection rate. Thus, it appears that in general, MIC does not improve the technical performance of colonoscopy or the overall patient experience, when performed by experienced endoscopists. However, in a large subgroup (representing one-fourth of procedures) of difficult cases based upon the need for adjunctive maneuvers during the procedure, significantly faster times-to-cecum were achieved with MIC compared to CC, but with no differences in patient experience outcomes.
This study has several limitations that may affect the generalizability of the results. Firstly, the primary outcome of pain score contains inherent biases, chiefly that increased sedation could be used to overcome greater procedural discomfort, potentially creating an apparent, but false, difference in pain score modified by the amount of sedation used. The most effective means to compare patient tolerability between MIC and CC would have been to perform unsedated colonoscopy, using the VAS to compare patient comfort between the techniques. Such strategies have been used in studies that evaluated previous versions of MIC with conflicting results[16,17]. However, our local patient population is generally resistant to the concept of unsedated procedures and this was deemed to not be viable. In any event, neither the visual analogue pain score nor the sedation score differed between groups, nor was there any difference in pain score after controlling for the sedation score in a regression analysis, indicating that this potential for bias did not lead to confounding of our results. A second limitation was the performance of all procedures by individuals who have undergone advanced fellowship training in therapeutic endoscopy, for whom magnetic imaging assistance for routine colonoscopy was perhaps less likely to be of value. In hindsight, it may have been more informative to include all endoscopists performing colonoscopy at our center, which would have provided a more diverse range of levels of experience and expertise that may have led to the identification of individuals for whom MIC was truly beneficial. However, there is already data from a recent meta-analysis of 8 randomized trials comparing MIC to CC that demonstrate that inexperienced endoscopists benefit from the use of MIC[15]. What has remained controversial is whether MIC confers any advantage to experienced endoscopists in active clinical practice. In fact, this meta-analysis found, somewhat surprisingly, that even experienced endoscopists had improved rates of cecal intubation when using MIC. Thus, it was of greater interest for us to study the usefulness of MIC among experienced endoscopists, since this is the question for which uncertainty still remains. Finally, the classification of procedural difficulty was potentially prone to bias, since it was based on the subjective decision to utilize ancillary maneuvers such as patient position changes or the application of more than one instance of abdominal pressure, which then resulted in the procedure being regarded as “difficult”. Nevertheless, since the criteria defining the classification of “difficult” were quite liberal (one-fourth were considered as such), this subgroup actually represents a large cohort of likely only moderately difficult cases that are frequently encountered in clinical practice. In this context, the finding of faster times-to-cecum achieved with MIC in this subgroup becomes meaningful, and is at least hypothesis generating. What will be important is to determine the clinical features that accurately identify patients pre-procedure as being likely difficult colonoscopy cases that are also the ones in which MIC would prove beneficial, even for experienced endoscopists.
The main benefit derived from magnetic imaging assistance is the accurate identification and proper straightening of endoscope loops[18], as well as visualization of the endoscope position within the different regions of the colon, which conceivably should facilitate faster and more comfortable procedures, while enabling the accurate localization of polyps or other pathology. While our study suggests MIC is unnecessary in most cases when colonoscopy is performed in experienced hands, there is good reason to speculate that magnetic endoscope imaging may benefit less experienced endoscopists and trainees. The cecal intubation rate in this trial was 99.6%, whereas large database studies of real life clinical outcomes reveal rates of incomplete colonoscopy ranging from 13%-35%[7,8,19], demonstrating the likely need for additional tools to facilitate the performance of colonoscopy in non-expert settings. Whether MIC can help improve these rates of complete colonoscopy, and whether it would be cost effective to do so given the added expense of ScopeGuide, remains unclear. Further study is needed to examine the role of MIC in cases of previously incomplete colonoscopy. Regarding the likely benefit of magnetic imaging assistance during training,the initial study that evaluated the previous version of MIC demonstrated significant improvements in procedural metrics in cases performed by trainees[14] and similar findings were confirmed in the aforementioned meta-analysis[15]. More recently, a one-day training program at Stanford that used an older version of ScopeGuide as part of a simulator consisting of a soft plastic colon model mounted within a real-shaped body torso, led to significant improvements in subsequent colonoscopy performed on live patients without magnetic imaging assistance[20]. The trainees demonstrated improvements in their overall performance, as well as in cecal intubation rates, time-to-cecum and sedation requirements following the ScopeGuide training intervention. Thus, there is reason to speculate that magnetic endoscope imaging could become an essential training tool, forming the basis of a graduated process that transitions learners from computer simulation to unassisted colonoscopy. However, the group of non-trainee, non-expert endoscopists for whom MIC is likely beneficial remains undefined, and will also require further study.
In summary, the latest version of magnetic imaging-assisted colonoscopy (ScopeGuide) performed no better than conventional colonoscopy in terms of endoscopic procedural metrics and patient experience outcomes, when performed in experienced hands. However, within a subgroup of more challenging cases, MIC resulted in faster times-to-cecum. Further research is required to determine the usefulness of MIC in cases of previously incomplete colonoscopy, in defining the non-expert, non-trainee endoscopists for whom it is expected to be most useful, and in determining the clinical variables that may predict a more challenging case for which even expert endoscopists may find MIC helpful.
ScopeGuide-enabled colonoscopes and the ScopeGuide system used in this study were provided free-of-charge on temporary loan from Olympus America. However, Olympus had no role in the design or conduct of this study, had no access to, or control of, the data collected during or after the study, and had no role in the interpretation of the findings. Furthermore, the investigators were not provided any financial compensation for their roles in the study.
Colonoscopy is the most valuable test for the detection and prevention of colon cancer. However, despite its widespread use, colonoscopy is not always completed successfully. Furthermore, many people are nervous about undergoing colonoscopy because of concerns that it will be uncomfortable. Therefore, new technologies and methods to improve the performance of colonoscopy and to make it more comfortable for patients are important. Magnetic imaging-assisted colonoscopy has been developed with this aim in mind.
Magnetic imaging-assisted colonoscopy consists of electromagnetic generator coils embedded within the colonoscope that produce a magnetic field detected by special external sensors, that then give rise to a computer-generated image of the shape and position of the endoscope while it is inside the patient’s body. This provides important visual information that assists the endoscopist when navigating the colon. A new generation of this “ScopeGuide” technology has recently been developed but has not been previously evaluated.
The initial studies using earlier versions of this technology demonstrated its effectiveness in assisting trainees to perform colonoscopy, but this benefit was not seen among experienced endoscopists. However, a recent meta-analysis summarizing several studies showed that even experienced endoscopists had improved rates of complete colonoscopy when using the magnetic imaging guidance.
This study confirms that magnetic imaging colonoscopy offers no significant advantage compared to conventional colonoscopy with regards to patient comfort, sedation requirements, the success rate of complete colonoscopy or the time needed to navigate the entire colon, when the procedures are performed by experienced practitioners. However, magnetic imaging colonoscopy did enable faster times to reach the end of the colon (i.e., cecum) for challenging cases.
Cecum: Where the small intestine enters into the colon; this is the furthest point of insertion during colonoscopy. Magnetic imaging colonoscopy: Technology that creates cartoon image of the endoscope within the patient’s body that provides the endoscopist with visual cues regarding endoscope shape and position that aid in the navigation of the colon.Time-to-cecum: The time needed during colonoscopy to reach the cecum. Visual analogue scale: Tool that allows patients to report pain along a graphical bar rather than using a numerical scale.
This randomized controlled trial is well designed and there are no objections to the methodology or assessment of the results. The study shows that for challenging cases, magnetic imaging allows for faster times-to-cecum, but that overall, there is no advantage compared to conventional colonoscopy in terms of patient comfort, sedation requirements or endoscopic procedural metrics.
P- Reviewer: Lorenzo-Zuniga V, Mach TH, Ogura T S- Editor: Ding Y L- Editor: A E- Editor: Du P
| 1. | Winawer SJ, Zauber AG, Ho MN, O’Brien MJ, Gottlieb LS, Sternberg SS, Waye JD, Schapiro M, Bond JH, Panish JF. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977-1981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3107] [Cited by in RCA: 3127] [Article Influence: 97.7] [Reference Citation Analysis (1)] |
| 2. | Müller AD, Sonnenberg A. Protection by endoscopy against death from colorectal cancer. A case-control study among veterans. Arch Intern Med. 1995;155:1741-1748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 200] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 3. | Singh H, Turner D, Xue L, Targownik LE, Bernstein CN. Risk of developing colorectal cancer following a negative colonoscopy examination: evidence for a 10-year interval between colonoscopies. JAMA. 2006;295:2366-2373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 294] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 4. | Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L. Association of colonoscopy and death from colorectal cancer. Ann Intern Med. 2009;150:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 911] [Cited by in RCA: 920] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 5. | Zauber AG, Winawer SJ, O’Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, Shi W, Bond JH, Schapiro M, Panish JF. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1952] [Cited by in RCA: 2286] [Article Influence: 175.8] [Reference Citation Analysis (2)] |
| 6. | Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected]. Am J Gastroenterol. 2009;104:739-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 981] [Cited by in RCA: 1059] [Article Influence: 66.2] [Reference Citation Analysis (0)] |
| 7. | Shah HA, Paszat LF, Saskin R, Stukel TA, Rabeneck L. Factors associated with incomplete colonoscopy: a population-based study. Gastroenterology. 2007;132:2297-2303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 267] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 8. | Loffeld RJ, van der Putten AB. The completion rate of colonoscopy in normal daily practice: factors associated with failure. Digestion. 2009;80:267-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Ling BS, Moskowitz MA, Wachs D, Pearson B, Schroy PC. Attitudes toward colorectal cancer screening tests. J Gen Intern Med. 2001;16:822-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 105] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 10. | DeBourcy AC, Lichtenberger S, Felton S, Butterfield KT, Ahnen DJ, Denberg TD. Community-based preferences for stool cards versus colonoscopy in colorectal cancer screening. J Gen Intern Med. 2008;23:169-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 100] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 11. | Waye JD. The best way to painless colonoscopy. Endoscopy. 2002;34:489-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Rex DK. Achieving cecal intubation in the very difficult colon. Gastrointest Endosc. 2008;67:938-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Shah SG, Saunders BP, Brooker JC, Williams CB. Magnetic imaging of colonoscopy: an audit of looping, accuracy and ancillary maneuvers. Gastrointest Endosc. 2000;52:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 101] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Shah SG, Brooker JC, Williams CB, Thapar C, Saunders BP. Effect of magnetic endoscope imaging on colonoscopy performance: a randomised controlled trial. Lancet. 2000;356:1718-1722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 70] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Chen Y, Duan YT, Xie Q, Qin XP, Chen B, Xia L, Zhou Y, Li NN, Wu XT. Magnetic endoscopic imaging vs standard colonoscopy: meta-analysis of randomized controlled trials. World J Gastroenterol. 2013;19:7197-7204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Hoff G, Bretthauer M, Dahler S, Huppertz-Hauss G, Sauar J, Paulsen J, Seip B, Moritz V. Improvement in caecal intubation rate and pain reduction by using 3-dimensional magnetic imaging for unsedated colonoscopy: a randomized trial of patients referred for colonoscopy. Scand J Gastroenterol. 2007;42:885-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Shergill AK, McQuaid KR, Deleon A, McAnanama M, Shah JN. Randomized trial of standard versus magnetic endoscope imaging colonoscopes for unsedated colonoscopy. Gastrointest Endosc. 2012;75:1031-1036.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Shah SG, Brooker JC, Thapar C, Suzuki N, Williams CB, Saunders BP. Effect of magnetic endoscope imaging on patient tolerance and sedation requirements during colonoscopy: a randomized controlled trial. Gastrointest Endosc. 2002;55:832-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Singh H, Penfold RB, DeCoster C, Kaita L, Proulx C, Taylor G, Bernstein CN, Moffatt M. Colonoscopy and its complications across a Canadian regional health authority. Gastrointest Endosc. 2009;69:665-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 20. | Kaltenbach T, Leung C, Wu K, Yan K, Friedland S, Soetikno R. Use of the colonoscope training model with the colonoscope 3D imaging probe improved trainee colonoscopy performance: a pilot study. Dig Dis Sci. 2011;56:1496-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |