Published online Sep 28, 2014. doi: 10.3748/wjg.v20.i36.13035
Revised: March 12, 2014
Accepted: May 29, 2014
Published online: September 28, 2014
Processing time: 259 Days and 20.5 Hours
Minimally invasive surgery has become common in the surgical resection of gastrointestinal submucosal tumors (SMTs). The purpose of this article is to review recent trends in minimally invasive surgery for gastric SMTs. Although laparoscopic resection has been main stream of minimally invasive surgery for gastrointestinal SMTs, recent advances in endoscopic procedures now provide various treatment modalities for gastric SMTs. Moreover, investigators have developed several hybrid techniques that include the advantages of both laparoscopic and endoscopic procedure. In addition, several types of reduced port surgeries, modification of conventional laparoscopic procedures, have been recently applied to the surgical resection of SMTs. Meanwhile, robotic surgery for SMTs requires further evidence and improvement.
Core tip: Minimally invasive surgery has become common in surgical resection of gastrointestinal submucosal tumors (SMTs). Although laparoscopic resection has been the main stream of minimally invasive surgery for gastrointestinal SMTs, recent advance in endoscopic procedures also provide various modalities of treatment for gastric SMTs. Moreover, investigators developed several hybrid techniques, which include the advatages of the laparoscopic and endoscopic procedure. Additionally, reduced port surgeries, which is modified from the conventional laparoscopic procedures, have been recently applied to the surgical resection of SMTs. Meanwhile, the application of a robotic surgeries to the treatment of SMTs still request more evidence and improvement.
- Citation: Lee CM, Kim HH. Minimally invasive surgery for submucosal (subepithelial) tumors of the stomach. World J Gastroenterol 2014; 20(36): 13035-13043
- URL: https://www.wjgnet.com/1007-9327/full/v20/i36/13035.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i36.13035
Since Dubois et al[1] reported their experience with laparoscopic cholecystectomy in 1989, minimally invasive surgery has become common in all surgical fields. Surgical resection of gastrointestinal submucosal tumors (SMTs) is no exception. Laparoscopic partial gastrectomy is widely accepted for the treatment of gastric SMTs[2]. Moreover, with recent developments in endoscopic procedures, endoscopic resection and a hybrid approach that combines endoscopic and laparoscopic techniques have been applied to gastric SMTs. In addition, the laparoscopic approach is feasible for SMTs of the small bowel and other sections of the gastrointestinal tract, although this procedure is rarely performed due to the infrequency of these tumors[3-7].
The purpose of this article is to review recent trends in minimally invasive surgery for SMTs of the stomach.
Although the standard treatment for gastrointestinal SMTs is surgical resection[8], many reports have suggested that endoscopic resection can be applied to some gastric SMT cases. Even in cases of gastric gastrointestinal stromal tumors (GISTs), endoscopic treatment has been advocated in several reports. However, in these cases, tumor size must be small. Because malignant transformation has been reported in GIST, and R0 resection with a negative resection margin is generally required[9]. Moreover, since GISTs do not have a true capsule and often have a fragile consistency, the lesion is often incompletely removed[10-13].
Recent advances provide several modalities of endoscopic resection for gastric SMTs, including band ligation, snare polypectomy, unroofing, endoscopic submucosal dissection (ESD), endoscopic muscularis dissection (EMD), submucosal endoscopic tumor resection (SET), endoscopic submucosal tunnel dissection (ESTD), and endoscopic full-thickness resection (EFTR)[8,13-17].
Endoscopic band ligation[18-21], endoscopic snare polypectomy[22,23], and endoscopic unroofing[22,24] are indicated only for the treatment of small SMTs (diameter < 2 cm). In particular, the unroofing technique is usually applied for the diagnosis of SMTs to assess whether the tumor is a GIST or not[25-28]. However, for treatment purposes, the unroofing technique is applicable only to lipomas and cystic lymphangiomas, which may spontaneously resolve after this treatment. Although Hizawa et al[24] reported nine cases of cystic SMTs treated by this technique, some lipomas may require additional treatment (e.g., endoscopic mucosal resection)[29]. Therefore, more advanced techniques are indicated for wider lesions of unknown behavior.
Standard ESD procedures can be used safely for the resection of gastric SMTs in the submucosal layer[12,30,31]. This technique consists of a circumferential incision around the tumor as deep as the submucosal layer and subtumoral dissection of the submucosal layer[32]. However, it is challenging to resect tumors originating from the proper muscle layer. In these cases, including GISTs, subtumoral dissection should be as deep as the proper muscle layer; therefore, this procedure was named EMD[14]. As a result of the deeper dissection than in standard ESD, a rather high rate of gastric perforation has been reported[10-12,33-37]. Although a recent prospective study showed a relatively high success rate of 94.4%[13], this procedure should be performed by a well-experienced endoscopist.
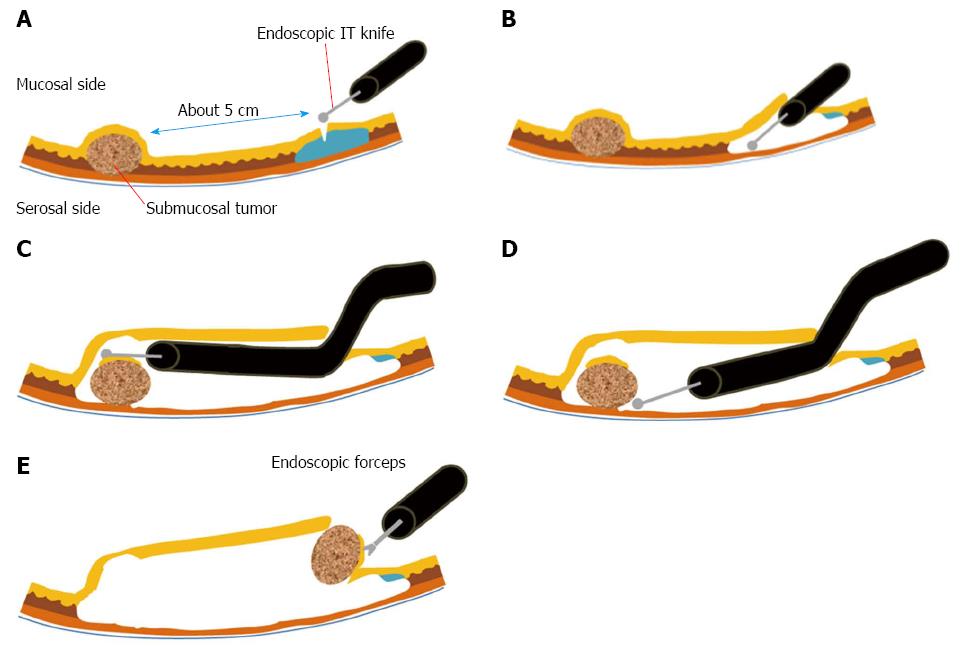
Since ESD and EMD, endoscopic resection has evolved continuously. In 2012, Inoue et al[15] reported SET, and Gong et al[16] reported ESTD. These are similar procedures, in which the dissection of the muscularis layer is the main technique. However, in these procedures, submucosal tunneling is begun far away from the lesion and is followed by muscular dissection under the lesion (Figure 1). Although tunnel dissection maintains the integrity of the mucosal layer over the tumor[13], there is still a risk of gastric perforation. Meanwhile, Zhou et al[17] reported EFTR, which involves full-thickness resection and closing of the defect with metallic clips endoscopically.
It is necessary to consider the long-term oncological consequences of iatrogenic gastric perforation when using this procedure. A case was reported in which an asymptomatic pelvic mass was noted 3 years after gastric perforation had occurred during endoscopic resection of a GIST[38].
For the treatment of SMTs, several studies[39-41] have reported that laparoscopic procedures have the following advantages over open surgery: less pain, less inflammation, less blood loss, earlier diet, and shorter hospital stay (Table 1). Nonetheless, several considerations are necessary when performing laparoscopic treatment of SMTs.
| Nishimura et al[39] (2007) | Karakousis et al[40] (2011) | De Vogelaere et al[41] (2013) | |||||||
| OR | LR | P value | OR | LR | P value | OR | LR | P value | |
| Number of patients | 28 | 39 | 40 | 40 | 16 | 37 | |||
| Operative time1 (min) | 115 | 136 | 0.06 | 89 (30-249) | 96 (48-200) | 0.320 | 155 ± 48.1 | 48.5 ± 16.0 | < 0.001 |
| EBL (mL) | 80 | 45 | 0.26 | 100 (5-400) | 25 (5-200) | 0.006 | |||
| Hospital stay (d) | 7 (4-25) | 4 (2-7) | 0.002 | 16.9 ± 10.6 | 8.2 ± 3.3 | 0.007 | |||
| Morbidity | 25% | 15% | 1% | 18.9% | 2.7% | 0.077 | |||
| Mortality | 0 | 0 | 0% | 0% | 6.3% | 0% | |||
Some SMTs have malignant potential, so there is a potential risk of intraperitoneal dissemination when manipulating the tumor during laparoscopic surgery[9]. In most SMTs with benign features, including leiomyomas, schwannomas, granular cell tumors, and lipomas, laparoscopic procedures are safe in terms of peritoneal dissemination because they seldom recur[8,42-46]. However, the possibility of GISTs should always be considered because some GISTs display malignant behavior. Although GISTs show acceptable survival and recurrence after laparoscopic resection[47-49], such favorable results cannot be expected if tumor rupture occurs. Therefore, many surgeons take the size of the tumor into consideration, with the assumption that larger tumors may require more manipulation, increasing the risk of rupture. For these reasons, laparoscopic surgery is only indicated for small GISTs (diameter ≤ 2 cm) according to the GIST Consensus Conference in 2004[50]. However, size cannot be the only factor used to determine the applicability of the laparoscopic procedure. Regardless of size, fragile or highly vascular tumors may require open surgery because of the increased risk of tumor rupture or bleeding. Therefore, it is important to manipulate a SMT without grasping it directly. This principle is widely accepted, and there are several techniques for handling the tumor without touching it. Yahchouchy-Chouillard et al[51] reported a “no-touch” technique that includes grasping the surrounding tissue, holding the threads sutured at the gastric wall around the tumor, and using a laparoscopic stapler or bag during laparoscopic resection. With this technique, the risk of tumor dissemination can be minimized. In addition, by extending the umbilical port incision, the resected tumor can be safely removed using the laparoscopic bag.
The tumor size limit, which was initially considered to be 2 cm, can be larger under certain conditions, on the basis of recent evidence. Otani et al[52] and Ryu et al[53] reported that laparoscopic resection of SMTs was feasible with tumors up to 5 cm. Moreover, some investigators have reported that laparoscopic resection of larger tumors (> 5 cm, but < 10 cm) is also possible[40,54-56]. It is likely that this size limitation will increase in the near future. Even in the current guidelines, no concrete indication of size is mentioned[57-59]. Compared to gastric SMTs, a size limit for laparoscopic surgery has not been established for SMTs in other sites (e.g., small bowel, colon, and rectum). However, since the intestinal tract is mainly a tubular structure, the “no-touch” technique can be applied easily by handling of segments near the segment involved.
The general principle of the treatment for SMTs is local resection with negative resection margin[2]. There is no exception to this principle, even with laparoscopic surgery; however, different surgical techniques are required depending on the location and configuration (i.e., endophytic or exophytic) of the tumor[2].
If exophytic tumors are located on the anterior wall of the stomach, the exogastric approach is the most widely used method[2,60,61]. In this situation, the tumor can be isolated by grasping the surrounding normal tissue with the laparoscopic instrument[9]. Then, the laparoscopic linear stapler is applied to the normal tissue to preserve the oncologic safety margin. However, this method could result in stenosis or deformity of the stomach by excessive gastric wall resection[2], so it is necessary to apply the linear stapler perpendicularly to the longitudinal axis of the stomach[9]. Another technique to prevent stenosis, the eversion method, was recently introduced[62,63]. If a large SMT is located on the anterior wall of the stomach, it is not easy to trace the tumor and avoid excessive resection of the gastric wall[62]. Firstly, a gastrotomy is made near the tumor site. Then, the tumor is everted out of the stomach, and a linear stapler is applied to the everted stalk of the tumor. The gastrotomy can be simultaneously closed with the linear stapler. Here, the direction of the linear stapler must be perpendicular to the longitudinal axis of the stomach.
For SMTs on the posterior wall of the stomach, the approaching method is selected based on whether the lesion is exophytic or endophytic. The exogastric approach, which was described earlier, can be used for an exophytic lesion if the tumor can be resected after mobilizing and rotating the stomach[63]. In the case of an endophytic lesion, the intragastric or transgastric approach is usually applied[64-67]. The intragastric approach involves resecting the tumor with the scope and instruments directly inserted into the gastric lumen[68-71]. With this procedure, minimal deformity and stenosis can be expected[2]. Balloon trocars are usually preferred for use in this procedure to prevent retraction of the trocars[64,72-74]. Recently, the single-incision intragastric approach has been introduced[75], whereby the stomach is brought out and opened through a single umbilical incision, which is kept open with a wound retractor. The intragastric resection is performed via a multi-channel port inserted through the gastrotomy. The transgastric approach, on the other hand, involves visualizing and resecting the tumor through an anterior gastrotomy[67,76]. The anterior gastrotomy should be closed with a linear stapler, perpendicularly to the longitudinal axis of the stomach, as for the exogastric approach or eversion method.
The management of SMTs at the esophagogastric junction is very challenging. If potentially malignant lesions are suspected, highly invasive procedures are often required[2]. Since the attempt to acquire an adequate resection margin can induce stenosis of this portion, radical procedures are safer than wedge resection or other local resection. Therefore, for such lesions, total gastrectomy is recommended in some institutions. Although laparoscopic proximal gastrectomy was reported in 1995[77], many surgeons have avoided this procedure because of serious complications, such as stricture and reflux esophagitis, related to esophagogastrostomy[2]. One potential solution for this problem is double tract reconstruction. Recently, Ahn et al[78] reported that laparoscopic proximal gastrectomy with double tract reconstruction results in less reflux and stricture. However, most SMTs, including some malignant lesions, do not require such an invasive procedure. Transgastric or intragastric resection can be a useful method for benign disease and even some malignant lesions. Enucleation is also a good option for certain benign lesions[69]. Also, this procedure could prevent stenosis of the esophagogastric junction.
Laparoscopic wedge resection of SMTs on the prepyloric antrum and lesser curvature are also technically demanding procedures[2]. There is a risk of stenosis of the gastric lumen because of the low distensibility of these areas, and a risk of vagus nerve injury, which could result in functional gastric outlet obstruction or delayed gastric emptying[2,9,72]. Therefore, many surgeons recommend full-thickness resection of the tumor following repair by hand sewing[2,79]. If deformity exists after resection of the tumor, distal gastrectomy can be considered.
Additionally, small bowel SMTs can be resected intracorporeally or extracorporeally depending on the surgeon’s preference. Either extracorporeal or intracorporeal resection can be performed through an umbilical port site even if the tumor is large[6,9], but surgeons should be careful to prevent iatrogenic spillage of tumors during the laparoscopic approach. If tumor invasion to an adjacent organ is suspected, open conversion should be seriously considered.
Recently, laparoscopic surgeries have been modified to reduce invasiveness and improve cosmesis. With this paradigm, reduced port surgeries, including single-incision laparoscopic surgery, are applied to the surgical resection of SMTs[80-84]. As a transitional procedure from conventional laparoscopic surgery, Hirano et al[80] reported their experience with reduced port surgery, which requires a 25 mm umbilical incision and a 2 mm extra incision. In this procedure, a mini-loop retractor was inserted through the extra port. In comparison, Sasaki et al[82] reported single-incision laparoscopic surgery for three SMT cases. However, they only used conventional laparoscopic instruments, and this procedure can only be used for small lesions at favorable locations (e.g., the anterior wall or greater curvature of the body). In contrast, Henckens et al[81] carried out single-incision laparoscopic surgery for a tumor located on the posterior wall by using double-bended instruments. The double-bended shape makes it possible to perform manipulations with minimal conflict between instruments passing through the single incision. The single-incision laparoscopic intragastric approach was developed as another way to address the technically demanding location of the tumor[75]. Applying the intragastric approach to single-incision laparoscopic surgery makes it easier to access high-lying gastric SMTs (i.e., SMTs of the fundus, cardia, and esophagogastric junction)[64,68,70,85]. Despite these advances, however, reduced port surgery is still technically demanding, and great care must be taken against unwanted injury. At present, it is necessary to develop more innovative instruments and procedures to overcome the ergonomic difficulties of reduced port surgeries.
Laparoscopic resection is the main stream of minimally invasive surgery for gastrointestinal SMTs, but it is difficult to localize small endophytic tumors by laparoscopy[86,87]. In that sense, the endoscopic approach has advantages such as easy localization of the tumors and less invasiveness. However, it also carries a risk of bleeding, perforation, and less radicality[88]. Therefore, to overcome these problems, investigators have developed several hybrid techniques that combine the laparoscopic method with endoscopic procedures[70,71,86-91].
At the beginning, ESD was performed with laparoscopic instruments using the intragastric approach[70,71,90,91]. If perforation occurred, it was repaired by the laparoscopic hand-sewing technique. The specimen was retrieved by endoscopy.
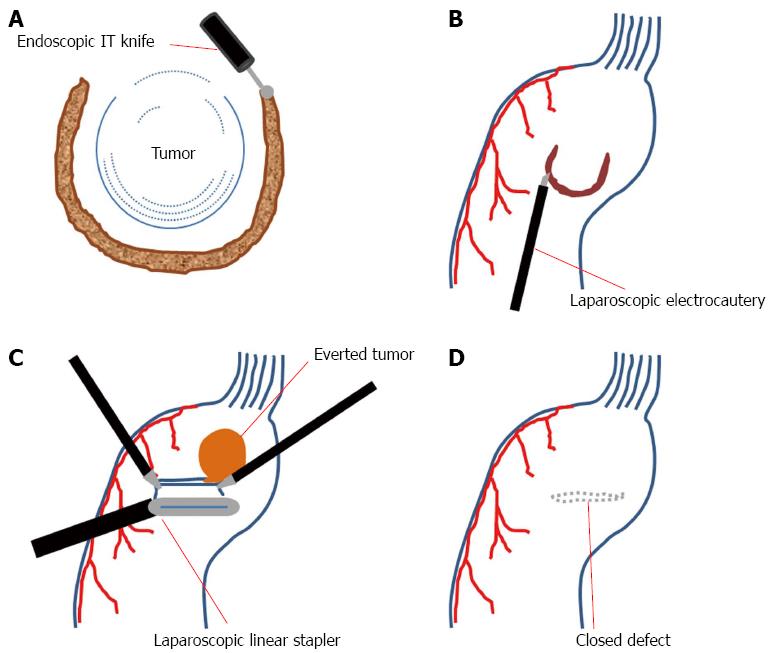
Laparoscopic endoscopic cooperative surgery (LECS) is a sequential procedure in which, after ESD, the seromuscular layer is partially dissected laparoscopically[86-89]. Laparoscopic linear staplers are then applied for resection of the tumor and closure of the gastric wall simultaneously, as in the eversion method (Figure 2)[88].
In laparoscopy-assisted endoscopic full-thickness resection (LAEFR), after full-layer dissection is performed by endoscopy, the gastric defect is repaired by a laparoscopic suture technique[92].
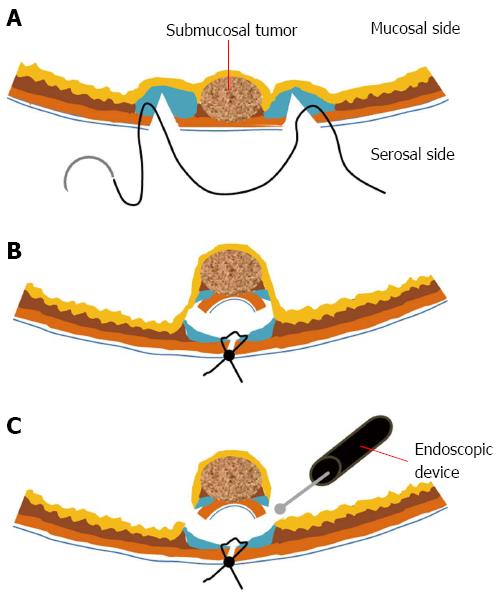
Recently, Goto et al[93] and Mitsui et al[94] reported non-exposed endoscopic wall-inversion surgery (NEWS) to prevent gastric luminal exposure, which can result in intra-abdominal contamination and peritoneal dissemination of the tumor. First, seromuscular dissection is performed laparoscopically, followed by closure of the dissection line by laparoscopic suturing. Consequently, the dissected seromuscular area is invaginated toward the inside. Finally, the invaginated tumor is resected by the ESD technique (Figure 3)[94].
Although all the cooperative procedures are a little different from each other, the common advantage is minimizing the loss of normal tissue by precise localization with endoscopy.
Although the role of robotic surgery using the da Vinci Surgical System (Intuitive Surgical Inc., Sunnyvale, CA) has been established for intra-abdominal procedures, few studies have evaluated the efficacy of robotic surgery for SMTs[95,96]. Moriyama et al[96] suggested that the benefit of robotic surgery is its ergonomic superiority, which permits more appropriate surgical margins. The articulated robotic arms are able to make even resection margins around the round shape of SMTs. If the tumor is located on the esophagogastric junction or the pylorus, an even resection margin can prevent stenosis of the gastric lumen. However, this advantage is not definitive[9], because experienced surgeons can make adequate and precise margins in the conventional laparoscopic setting[2]. In addition, several cooperative procedures can achieve the same benefit as robotic surgery with less cost.
Even regarding cosmesis, robotic surgery does not reduce the number of ports or the size of the trocar required. Moreover, if a stapling procedure is needed, another port must be established, because robotic stapling devices are not available yet[96].
To justify the application of a robotic surgical system to the treatment of SMTs, more evidence is needed and several problems, including the bulky size of the devices, the lack of a stapling instrument and the high cost, must be addressed.
With the development of minimally invasive surgery, including single-port surgery and hybrid techniques, the management of SMTs is advancing to preserve the volume and function of the stomach, even with malignant lesions.
P- Reviewer: Ahn JY, Jung SW, Wang LS, Yan SL S- Editor: Ma YJ L- Editor: A E- Editor: Ma S
| 1. | Dubois F, Berthelot G, Levard H. [Cholecystectomy by coelioscopy]. Presse Med. 1989;18:980-982. [PubMed] |
| 2. | Hwang SH, Park do J, Kim YH, Lee KH, Lee HS, Kim HH, Lee HJ, Yang HK, Lee KU. Laparoscopic surgery for submucosal tumors located at the esophagogastric junction and the prepylorus. Surg Endosc. 2009;23:1980-1987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Tsushimi T, Matsui N, Kurazumi H, Takemoto Y, Oka K, Seyama A, Morita T. Laparoscopic resection of an ileal lipoma: Report of a case. Surg Today. 2006;36:1007-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Lin MW, Chen KH, Lin HF, Chen HA, Wu JM, Huang SH. Laparoscopy-assisted resection of ileoileal intussusception caused by intestinal lipoma. J Laparoendosc Adv Surg Tech A. 2007;17:789-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Ako E, Morisaki T, Hasegawa T, Hirakawa T, Tachimori A, Nakazawa K, Yamagata S, Kanehara I, Nishimura S, Taenaka N. Laparoscopic resection of ileal lipoma diagnosed by multidetector-row computed tomography. Surg Laparosc Endosc Percutan Tech. 2010;20:e226-e229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Lucas LC, Fass R, Krouse RS. Laparoscopic resection of a small bowel lipoma with incidental intussusception. JSLS. 2010;14:615-618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Ferrara F, Duburque C, Quinchon JF, Gaudissart Q. Laparoscopic resection of small bowel lipoma causing obscure gastrointestinal bleeding. Updates Surg. 2012;64:153-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Ponsaing LG, Hansen MB. Therapeutic procedures for submucosal tumors in the gastrointestinal tract. World J Gastroenterol. 2007;13:3316-3322. [PubMed] |
| 9. | Kong SH, Yang HK. Surgical treatment of gastric gastrointestinal stromal tumor. J Gastric Cancer. 2013;13:3-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Jeong ID, Jung SW, Bang SJ, Shin JW, Park NH, Kim do H. Endoscopic enucleation for gastric subepithelial tumors originating in the muscularis propria layer. Surg Endosc. 2011;25:468-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 11. | Lee IL, Lin PY, Tung SY, Shen CH, Wei KL, Wu CS. Endoscopic submucosal dissection for the treatment of intraluminal gastric subepithelial tumors originating from the muscularis propria layer. Endoscopy. 2006;38:1024-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 152] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 12. | Białek A, Wiechowska-Kozłowska A, Pertkiewicz J, Polkowski M, Milkiewicz P, Karpińska K, Ławniczak M, Starzyńska T. Endoscopic submucosal dissection for treatment of gastric subepithelial tumors (with video). Gastrointest Endosc. 2012;75:276-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 131] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 13. | Li QL, Yao LQ, Zhou PH, Xu MD, Chen SY, Zhong YS, Zhang YQ, Chen WF, Ma LL, Qin WZ. Submucosal tumors of the esophagogastric junction originating from the muscularis propria layer: a large study of endoscopic submucosal dissection (with video). Gastrointest Endosc. 2012;75:1153-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 14. | Liu BR, Song JT, Qu B, Wen JF, Yin JB, Liu W. Endoscopic muscularis dissection for upper gastrointestinal subepithelial tumors originating from the muscularis propria. Surg Endosc. 2012;26:3141-3148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 15. | Inoue H, Ikeda H, Hosoya T, Onimaru M, Yoshida A, Eleftheriadis N, Maselli R, Kudo S. Submucosal endoscopic tumor resection for subepithelial tumors in the esophagus and cardia. Endoscopy. 2012;44:225-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 209] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 16. | Gong W, Xiong Y, Zhi F, Liu S, Wang A, Jiang B. Preliminary experience of endoscopic submucosal tunnel dissection for upper gastrointestinal submucosal tumors. Endoscopy. 2012;44:231-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 17. | Zhou PH, Yao LQ, Qin XY, Cai MY, Xu MD, Zhong YS, Chen WF, Zhang YQ, Qin WZ, Hu JW. Endoscopic full-thickness resection without laparoscopic assistance for gastric submucosal tumors originated from the muscularis propria. Surg Endosc. 2011;25:2926-2931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 245] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 18. | Chang KJ, Yoshinaka R, Nguyen P. Endoscopic ultrasound-assisted band ligation: a new technique for resection of submucosal tumors. Gastrointest Endosc. 1996;44:720-722. [PubMed] |
| 19. | Sun S, Jin Y, Chang G, Wang C, Li X, Wang Z. Endoscopic band ligation without electrosurgery: a new technique for excision of small upper-GI leiomyoma. Gastrointest Endosc. 2004;60:218-222. [PubMed] |
| 20. | Sun S, Ge N, Wang C, Wang M, Lü Q. Endoscopic band ligation of small gastric stromal tumors and follow-up by endoscopic ultrasonography. Surg Endosc. 2007;21:574-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | Jeong SH, Lee YJ, Park ST, Choi SK, Hong SC, Jung EJ, Joo YT, Jeong CY, Ha WS. Risk of recurrence after laparoscopy-assisted radical gastrectomy for gastric cancer performed by a single surgeon. Surg Endosc. 2011;25:872-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Shim CS, Jung IS. Endoscopic removal of submucosal tumors: preprocedure diagnosis, technical options, and results. Endoscopy. 2005;37:646-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Wei SC, Wong JM, Shieh MJ, Sun CT, Wang CY, Wang TH. Endoscopic resection of gastrointestinal submucosal tumors. Hepatogastroenterology. 1998;45:114-118. [PubMed] |
| 24. | Hizawa K, Matsumoto T, Kouzuki T, Suekane H, Esaki M, Fujishima M. Cystic submucosal tumors in the gastrointestinal tract: endosonographic findings and endoscopic removal. Endoscopy. 2000;32:712-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Lee CK, Chung IK, Lee SH, Lee SH, Lee TH, Park SH, Kim HS, Kim SJ, Cho HD. Endoscopic partial resection with the unroofing technique for reliable tissue diagnosis of upper GI subepithelial tumors originating from the muscularis propria on EUS (with video). Gastrointest Endosc. 2010;71:188-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 26. | Komanduri S, Keefer L, Jakate S. Diagnostic yield of a novel jumbo biopsy “unroofing” technique for tissue acquisition of gastric submucosal masses. Endoscopy. 2011;43:849-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 27. | Binmoeller KF, Shah JN, Bhat YM, Kane SD. Retract-ligate-unroof-biopsy: a novel approach to the diagnosis and therapy of large nonpedunculated stromal tumors (with video). Gastrointest Endosc. 2013;77:803-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Binmoeller KF, Shah JN, Bhat YM, Kane SD. Suck-ligate-unroof-biopsy by using a detachable 20-mm loop for the diagnosis and therapy of small subepithelial tumors (with video). Gastrointest Endosc. 2014;79:750-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Lee KJ, Kim GH, Park do Y, Shin NR, Lee BE, Ryu DY, Kim DU, Song GA. Endoscopic resection of gastrointestinal lipomas: a single-center experience. Surg Endosc. 2014;28:185-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 30. | Hoteya S, Iizuka T, Kikuchi D, Yahagi N. Endoscopic submucosal dissection for gastric submucosal tumor, endoscopic sub-tumoral dissection. Dig Endosc. 2009;21:266-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 31. | Probst A, Golger D, Arnholdt H, Messmann H. Endoscopic submucosal dissection of early cancers, flat adenomas, and submucosal tumors in the gastrointestinal tract. Clin Gastroenterol Hepatol. 2009;7:149-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 32. | Abe N, Takeuchi H, Ooki A, Nagao G, Masaki T, Mori T, Sugiyama M. Recent developments in gastric endoscopic submucosal dissection: towards the era of endoscopic resection of layers deeper than the submucosa. Dig Endosc. 2013;25 Suppl 1:64-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 33. | Huang LY, Cui J, Liu YX, Wu CR, Yi DL. Endoscopic therapy for gastric stromal tumors originating from the muscularis propria. World J Gastroenterol. 2012;18:3465-3471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Huang ZG, Zhang XS, Huang SL, Yuan XG. Endoscopy dissection of small stromal tumors emerged from the muscularis propria in the upper gastrointestinal tract: Preliminary study. World J Gastrointest Endosc. 2012;4:565-570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 35. | Bai J, Wang Y, Guo H, Zhang P, Ling X, Zhao X. Endoscopic resection of small gastrointestinal stromal tumors. Dig Dis Sci. 2010;55:1950-1954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 36. | Catalano F, Rodella L, Lombardo F, Silano M, Tomezzoli A, Fuini A, Di Cosmo MA, de Manzoni G, Trecca A. Endoscopic submucosal dissection in the treatment of gastric submucosal tumors: results from a retrospective cohort study. Gastric Cancer. 2013;16:563-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 37. | Hwang JC, Kim JH, Kim JH, Shin SJ, Cheong JY, Lee KM, Yoo BM, Lee KJ, Cho SW. Endoscopic resection for the treatment of gastric subepithelial tumors originated from the muscularis propria layer. Hepatogastroenterology. 2009;56:1281-1286. [PubMed] |
| 38. | Waterman AL, Grobmyer SR, Cance WG, Hochwald SN. Is endoscopic resection of gastric gastrointestinal stromal tumors safe? Am Surg. 2008;74:1186-1189. [PubMed] |
| 39. | Nishimura J, Nakajima K, Omori T, Takahashi T, Nishitani A, Ito T, Nishida T. Surgical strategy for gastric gastrointestinal stromal tumors: laparoscopic vs. open resection. Surg Endosc. 2007;21:875-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 175] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 40. | Karakousis GC, Singer S, Zheng J, Gonen M, Coit D, DeMatteo RP, Strong VE. Laparoscopic versus open gastric resections for primary gastrointestinal stromal tumors (GISTs): a size-matched comparison. Ann Surg Oncol. 2011;18:1599-1605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 131] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 41. | De Vogelaere K, Hoorens A, Haentjens P, Delvaux G. Laparoscopic versus open resection of gastrointestinal stromal tumors of the stomach. Surg Endosc. 2013;27:1546-1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 42. | Palazzo L, Landi B, Cellier C, Roseau G, Chaussade S, Couturier D, Barbier J. Endosonographic features of esophageal granular cell tumors. Endoscopy. 1997;29:850-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 53] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 43. | Nakachi A, Miyazato H, Oshiro T, Shimoji H, Shiraishi M, Muto Y. Granular cell tumor of the rectum: a case report and review of the literature. J Gastroenterol. 2000;35:631-634. [PubMed] |
| 44. | Fernandez MJ, Davis RP, Nora PF. Gastrointestinal lipomas. Arch Surg. 1983;118:1081-1083. [PubMed] |
| 45. | Agha FP, Dent TL, Fiddian-Green RG, Braunstein AH, Nostrant TT. Bleeding lipomas of the upper gastrointestinal tract. A diagnostic challenge. Am Surg. 1985;51:279-285. [PubMed] |
| 46. | Maderal F, Hunter F, Fuselier G, Gonzales-Rogue P, Torres O. Gastric lipomas--an update of clinical presentation, diagnosis, and treatment. Am J Gastroenterol. 1984;79:964-967. [PubMed] |
| 47. | Novitsky YW, Kercher KW, Sing RF, Heniford BT. Long-term outcomes of laparoscopic resection of gastric gastrointestinal stromal tumors. Ann Surg. 2006;243:738-45; discussion 745-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 292] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 48. | Sexton JA, Pierce RA, Halpin VJ, Eagon JC, Hawkins WG, Linehan DC, Brunt LM, Frisella MM, Matthews BD. Laparoscopic gastric resection for gastrointestinal stromal tumors. Surg Endosc. 2008;22:2583-2587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 81] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 49. | Kim KH, Kim MC, Jung GJ, Kim SJ, Jang JS, Kwon HC. Long term survival results for gastric GIST: is laparoscopic surgery for large gastric GIST feasible? World J Surg Oncol. 2012;10:230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 50. | Blay JY, Bonvalot S, Casali P, Choi H, Debiec-Richter M, Dei Tos AP, Emile JF, Gronchi A, Hogendoorn PC, Joensuu H. Consensus meeting for the management of gastrointestinal stromal tumors. Report of the GIST Consensus Conference of 20-21 March 2004, under the auspices of ESMO. Ann Oncol. 2005;16:566-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 500] [Cited by in RCA: 487] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 51. | Yahchouchy-Chouillard E, Etienne JC, Fagniez PL, Adam R, Fingerhut A. A new “no-touch” technique for the laparoscopic treatment of gastric stromal tumors. Surg Endosc. 2002;16:962-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 52. | Otani Y, Furukawa T, Yoshida M, Saikawa Y, Wada N, Ueda M, Kubota T, Mukai M, Kameyama K, Sugino Y. Operative indications for relatively small (2-5 cm) gastrointestinal stromal tumor of the stomach based on analysis of 60 operated cases. Surgery. 2006;139:484-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 165] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 53. | Ryu KJ, Jung SR, Choi JS, Jang YJ, Kim JH, Park SS, Park BJ, Park SH, Kim SJ, Mok YJ. Laparoscopic resection of small gastric submucosal tumors. Surg Endosc. 2011;25:271-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 54. | Sokolich J, Galanopoulos C, Dunn E, Linder JD, Jeyarajah DR. Expanding the indications for laparoscopic gastric resection for gastrointestinal stromal tumors. JSLS. 2009;13:165-169. [PubMed] |
| 55. | Lee JS, Kim JJ, Park SM. Totally laparoscopic resection for a large gastrointestinal stromal tumor of stomach. J Gastric Cancer. 2011;11:239-242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 56. | Thakkar DV, Wani SV, Shetty V, Patankar RV. Laparoscopic sleeve gastrectomy for a large gastrointestinal stromal tumor. Surg Laparosc Endosc Percutan Tech. 2012;22:e61-e62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 57. | von Mehren M, Benjamin RS, Bui MM, Casper ES, Conrad EU, DeLaney TF, Ganjoo KN, George S, Gonzalez R, Heslin MJ. Soft tissue sarcoma, version 2.2012: featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2012;10:951-960. [PubMed] |
| 58. | ESMO/European Sarcoma Network Working Group. Gastrointestinal stromal tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23 Suppl 7:vii49-vii55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 59. | Kang YK, Kang HJ, Kim KM, Sohn T, Choi D, Ryu MH, Kim WH, Yang HK. Clinical practice guideline for accurate diagnosis and effective treatment of gastrointestinal stromal tumor in Korea. Cancer Res Treat. 2012;44:85-96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 60. | Aogi K, Hirai T, Mukaida H, Toge T, Haruma K, Kajiyama G. Laparoscopic resection of submucosal gastric tumors. Surg Today. 1999;29:102-106. [PubMed] |
| 61. | Cugat E, Hoyuela C, Rodríguez-Santiago JM, Marco C. Laparoscopic ultrasound guidance for laparoscopic resection of benign gastric tumors. J Laparoendosc Adv Surg Tech A. 1999;9:63-67. [PubMed] |
| 62. | Sekimoto M, Tamura S, Hasuike Y, Yano M, Murata A, Inoue M, Shiozaki H, Monden M. A new technique for laparoscopic resection of a submucosal tumor on the posterior wall of the gastric fundus. Surg Endosc. 1999;13:71-74. [PubMed] |
| 63. | Kakeji Y, Nakanoko T, Yoshida R, Eto K, Kumashiro R, Ikeda K, Egashira A, Saeki H, Oki E, Morita M. Laparoscopic resection for gastrointestinal stromal tumors in the stomach. Surg Today. 2012;42:554-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 64. | Tagaya N, Mikami H, Kogure H, Kubota K, Hosoya Y, Nagai H. Laparoscopic intragastric stapled resection of gastric submucosal tumors located near the esophagogastric junction. Surg Endosc. 2002;16:177-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 73] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 65. | Tagaya N, Mikami H, Kubota K. Laparoscopic resection of gastrointestinal mesenchymal tumors located in the upper stomach. Surg Endosc. 2004;18:1469-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 66. | Hepworth CC, Menzies D, Motson RW. Minimally invasive surgery for posterior gastric stromal tumors. Surg Endosc. 2000;14:349-353. [PubMed] |
| 67. | Ibrahim IM, Silvestri F, Zingler B. Laparoscopic resection of posterior gastric leiomyoma. Surg Endosc. 1997;11:277-279. [PubMed] |
| 68. | Sekimoto M, Tamura S, Hasuike Y, Yano M, Murata A, Inoue M, Shiozaki H, Monden M. A new technique for laparoscopic resection of a submucosal tumor on the posterior wall of the gastric fundus. Surg Endosc. 1999;13:71-74. [PubMed] |
| 69. | Taniguchi E, Kamiike W, Yamanishi H, Ito T, Nezu R, Nishida T, Momiyama T, Ohashi S, Okada T, Matsuda H. Laparoscopic intragastric surgery for gastric leiomyoma. Surg Endosc. 1997;11:287-289. [PubMed] |
| 70. | Uchikoshi F, Ito T, Nishida T, Kitagawa T, Endo S, Matsuda H. Laparoscopic intragastric resection of gastric stromal tumor located at the esophago-cardiac junction. Surg Laparosc Endosc Percutan Tech. 2004;14:1-4. [PubMed] |
| 71. | Walsh RM, Ponsky J, Brody F, Matthews BD, Heniford BT. Combined endoscopic/laparoscopic intragastric resection of gastric stromal tumors. J Gastrointest Surg. 2003;7:386-392. [PubMed] |
| 72. | Privette A, McCahill L, Borrazzo E, Single RM, Zubarik R. Laparoscopic approaches to resection of suspected gastric gastrointestinal stromal tumors based on tumor location. Surg Endosc. 2008;22:487-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 97] [Article Influence: 5.4] [Reference Citation Analysis (1)] |
| 73. | Ludwig K, Wilhelm L, Scharlau U, Amtsberg G, Bernhardt J. Laparoscopic-endoscopic rendezvous resection of gastric tumors. Surg Endosc. 2002;16:1561-1565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 74. | Sahm M, Pross M, Lippert H. Intraluminal resection of gastric tumors using intragastric trocar technique. Surg Laparosc Endosc Percutan Tech. 2011;21:e169-e172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 75. | Na JU, Lee SI, Noh SM. The single incision laparoscopic intragastric wedge resection of gastric submucosal tumor. J Gastric Cancer. 2011;11:225-229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 76. | Watson DI, Game PA, Devitt PG. Laparoscopic resection of benign tumors of the posterior gastric wall. Surg Endosc. 1996;10:540-541. [PubMed] |
| 77. | Uyama I, Ogiwara H, Takahara T, Kikuchi K, Iida S. Laparoscopic and minilaparotomy proximal gastrectomy and esophagogastrostomy: technique and case report. Surg Laparosc Endosc. 1995;5:487-491. [PubMed] |
| 78. | Ahn SH, Jung do H, Son SY, Lee CM, Park do J, Kim HH. Laparoscopic double-tract proximal gastrectomy for proximal early gastric cancer. Gastric Cancer. 2014;17:562-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 130] [Article Influence: 13.0] [Reference Citation Analysis (1)] |
| 79. | Lee JH, Han HS, Kim YW, Min SK, Lee HK. Laparoscopic wedge resection with handsewn closure for gastroduodenal tumors. J Laparoendosc Adv Surg Tech A. 2003;13:349-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 80. | Hirano Y, Watanabe T, Uchida T, Yoshida S, Kato H, Hosokawa O. Laparoendoscopic single site partial resection of the stomach for gastrointestinal stromal tumor. Surg Laparosc Endosc Percutan Tech. 2010;20:262-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 81. | Henckens T, Van de Putte D, Van Renterghem K, Ceelen W, Pattyn P, Van Nieuwenhove Y. Laparoendoscopic single-site gastrectomy for a gastric GIST using double-bended instruments. J Laparoendosc Adv Surg Tech A. 2010;20:469-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 82. | Sasaki A, Koeda K, Nakajima J, Obuchi T, Baba S, Wakabayashi G. Single-incision laparoscopic gastric resection for submucosal tumors: report of three cases. Surg Today. 2011;41:133-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 83. | Takahashi T, Takeuchi H, Kawakubo H, Saikawa Y, Wada N, Kitagawa Y. Single-incision laparoscopic surgery for partial gastrectomy in patients with a gastric submucosal tumor. Am Surg. 2012;78:447-450. [PubMed] |
| 84. | Wu S, Chen Y, Tian Y, Jing K. Transumbilical single-incision laparoscopic multiple organ procedures: initial experience of 20 cases. J Laparoendosc Adv Surg Tech A. 2013;23:56-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 85. | Li VK, Hung WK, Chung CK, Ying MW, Lam BY, Kan DM, Chan MC. Laparoscopic intragastric approach for stromal tumours located at the posterior gastric wall. Asian J Surg. 2008;31:6-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 86. | Tsujimoto H, Yaguchi Y, Kumano I, Takahata R, Ono S, Hase K. Successful gastric submucosal tumor resection using laparoscopic and endoscopic cooperative surgery. World J Surg. 2012;36:327-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 87. | Kang WM, Yu JC, Ma ZQ, Zhao ZR, Meng QB, Ye X. Laparoscopic-endoscopic cooperative surgery for gastric submucosal tumors. World J Gastroenterol. 2013;19:5720-5726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 88. | Hiki N, Yamamoto Y, Fukunaga T, Yamaguchi T, Nunobe S, Tokunaga M, Miki A, Ohyama S, Seto Y. Laparoscopic and endoscopic cooperative surgery for gastrointestinal stromal tumor dissection. Surg Endosc. 2008;22:1729-1735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 340] [Article Influence: 18.9] [Reference Citation Analysis (2)] |
| 89. | Hiki N. [Feasible technique for laparoscopic wedge resection for gastric submucosal tumor-laparoscopy endoscopy cooperative surgery (LECS)]. Gan To Kagaku Ryoho. 2011;38:728-732. [PubMed] |
| 90. | Shim JH, Lee HH, Yoo HM, Jeon HM, Park CH, Kim JG, Song KY. Intragastric approach for submucosal tumors located near the Z-line: a hybrid laparoscopic and endoscopic technique. J Surg Oncol. 2011;104:312-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 91. | Rosen MJ, Heniford BT. Endoluminal gastric surgery: the modern era of minimally invasive surgery. Surg Clin North Am. 2005;85:989-1007, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 92. | Abe N, Takeuchi H, Yanagida O, Masaki T, Mori T, Sugiyama M, Atomi Y. Endoscopic full-thickness resection with laparoscopic assistance as hybrid NOTES for gastric submucosal tumor. Surg Endosc. 2009;23:1908-1913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 93. | Goto O, Mitsui T, Fujishiro M, Wada I, Shimizu N, Seto Y, Koike K. New method of endoscopic full-thickness resection: a pilot study of non-exposed endoscopic wall-inversion surgery in an ex vivo porcine model. Gastric Cancer. 2011;14:183-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 108] [Article Influence: 7.7] [Reference Citation Analysis (1)] |
| 94. | Mitsui T, Niimi K, Yamashita H, Goto O, Aikou S, Hatao F, Wada I, Shimizu N, Fujishiro M, Koike K. Non-exposed endoscopic wall-inversion surgery as a novel partial gastrectomy technique. Gastric Cancer. 2014;17:594-599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 95. | Buchs NC, Bucher P, Pugin F, Hagen ME, Morel P. Robot-assisted oncologic resection for large gastric gastrointestinal stromal tumor: a preliminary case series. J Laparoendosc Adv Surg Tech A. 2010;20:411-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 96. | Moriyama H, Ishikawa N, Kawaguchi M, Hirose K, Watanabe G. Robot-assisted laparoscopic resection for gastric gastrointestinal stromal tumor. Surg Laparosc Endosc Percutan Tech. 2012;22:e155-e156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |