Published online Sep 21, 2014. doi: 10.3748/wjg.v20.i35.12649
Revised: April 3, 2014
Accepted: May 12, 2014
Published online: September 21, 2014
AIM: To analyze the current available evidence of Kudo’s pit pattern classification for diagnosing colorectal neoplasms.
METHODS: A search was performed on Pubmed/Embase to identify studies reporting the outcomes of the pit pattern classification in colorectal polyps. Retrieved records were evaluated and selected by two independent investigators. The number of patients, polyps and diagnostic performance of Kudo’s pit pattern classification were retrieved from suitable studies. Pooled sensitivities and specificities were calculated using fixed or random effect models according to their heterogeneity. Publication bias was evaluated using funnel plot, Egger’s test, and Begg’s test. Sensitivity analysis was performed by omitting one study at a time and selecting a subgroup consisting of 11 magnifying chromoendoscopy studies.
RESULTS: 20 eligible studies were included in which a total of 5111 colorectal lesions in 3418 patients were identified for the differentiation of neoplastic and non-neoplastic polyps. Pit pattern classification in all the studies of mucosal patterns with magnification resulted in a pooled sensitivity of 89.0% (95%CI: 85.2-91.9) and pooled specificity of 85.7% (95%CI: 81.3-89.2) and the area under the SROC curve was 0.9354. There was significant publication bias (P = 0.038 and 0.006 for sensitivity and specificity using Egger’s test, P = 0.035 and 0.139 for sensitivity and specificity using Begg’s test, respectively). No single study significantly affected the pooled result, and the magnifying chromoendoscopy subgroup yielded a sensitivity of 92.7% (95%CI: 89.2-95.2) and specificity of 87.3% (95%CI: 81.6-91.4).
CONCLUSION: Kudo’s pit pattern classification is an accurate diagnostic method for the differentiation of neoplastic colorectal lesions. Publication bias is significant in the current available literature.
Core tip: According to the current available evidence, Kudo’s pit pattern classification is an accurate diagnostic method for the differentiation of neoplastic and non-neoplastic colorectal lesions. The risk of potential publication bias should be noted.
- Citation: Li M, Ali SM, Umm-a-OmarahGilani S, Liu J, Li YQ, Zuo XL. Kudo’s pit pattern classification for colorectal neoplasms: A meta-analysis. World J Gastroenterol 2014; 20(35): 12649-12656
- URL: https://www.wjgnet.com/1007-9327/full/v20/i35/12649.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i35.12649
Colorectal cancer (CRC) is a major disease worldwide and is responsible for numerous deaths. There is clear-cut evidence that the prognosis of patients with CRC is strictly dependent on early detection and therapy of premalignant and malignant lesions[1]. The majority of CRCs arise from neoplastic polyps which have been proved to be premalignant lesions[2,3]. Histologically, colorectal polyps are classified as neoplastic (adenoma, adenocarcinoma) or non-neoplastic (hyperplastic, inflammatory, hamartomatous, etc.). The ideal endoscopic management of colorectal polyps involves two steps. First, any mucosal lesions need to be detected. Second, the lesions require to be characterized based on mucosal surface architecture and vessel changes; which lead to an endoscopic judgment of whether the lesion is neoplastic or non-neoplastic. However, conventional white-light endoscopy is inadequate in the real-time characterization of detected lesions. Magnifying endoscopy was introduced for the diagnosis of gastrointestinal diseases with magnified observations. Endoscopists can visualize fine details of mucosal surface pattern and vascular architecture. In 1980, chromoendoscopy was introduced into the field of endoscopy which improved the identification of mucosal pit pattern[4]. Magnification endoscopy combined with narrow band imaging (NBI) is widely used in the area of advanced endoscopy.
Kudo et al[5] first highlighted the feasibility of applying the “pit patterns” to distinguish neoplastic and non-neoplastic polyps via magnifying endoscopy. Kudo et al[5] classified colorectal polyps according to their appearance, structure and staining patterns. Type I pits appear as roundish pits; Type II pits appear as stellar or papillary pits; Type III-s pits are small roundish, tubular pits (smaller than Type I) and Type III-L are roundish and tubular pits (larger than Type I); Type IV pits appear as branch-like or gyrus-like pits and Type V pits appear as non-structured pits. Type I and II are considered benign changes (e.g., normal, hyperplastic, inflammatory polyps), whereas pit pattern classes III-V are considered to show neoplastic and malignant changes.
Recently, several studies proposed that the pit pattern analysis of colorectal lesions by magnifying colonoscopy is a useful and objective tool for differentiating neoplastic from non-neoplastic lesions[6]. The aim of this study was to meta-analyze the data of existing magnifying endoscopy trials using Kudo’s pit pattern classification for colorectal polyp characterization.
An extensive English-language literature search of the PubMed/MEDLINE and Embase databases for human studies was performed to identify relevant publications on the performance of Kudo’s classification in diagnosing neoplastic colonic polyps. The following search terms were used: magnifying endoscopy, sensitivity, specificity, chromoendoscopy, colorectal polyp and Kudo’s classification. No initial date limit was used. The search was updated until the end of March 2014. To expand our search, we also used public search engines, such as Google, to identify potentially suitable references. In addition, we searched English abstracts of the non-English literature in PubMed. References from the extracted articles, reviews, and previous meta-analyses were also consulted to complete the data bank. Two authors (Ali SM and Umm-a-OmarahGilani S) contributed to the data search.
Before commencement of the literature search, the inclusion criteria were defined as follow: (1) papers with full-text or abstract written in the English language; and (2) papers providing sufficient data for authors to construct a 2 × 2 contingency table to calculate sensitivity and specificity. When two articles were reported from the same study, the more informative publication was selected. In studies comparing Kudo’s classification to other criteria, only the data from the arm using Kudo’s classification were included. Exclusion criteria included: case reports or case series, reviews, duplicate reports, insufficient data in the article, and criteria other than Kudo’s classification were used in the article.
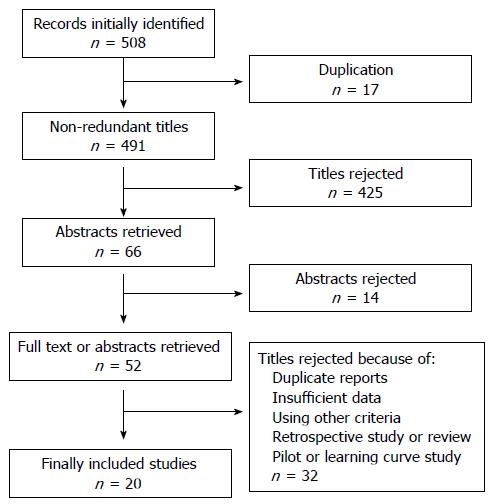
For manuscripts and abstracts which met our predefined eligibility criteria, two independent investigators (Ali SM and Liu J) independently selected data using a standard form from each study. Disagreements, if any, were resolved by a third reviewer (Li M). The flowchart of literature selection is shown in Figure 1.
For each included study, the following information was extracted: year of publication, country, type of endoscope, number of included patients, number of polyps examined, number of neoplastic polyps confirmed by histology, number of polyps truly positive and truly negative diagnosed according to Kudo’s classification. If one study had more than one arm using magnifying endoscopy in combination with a different imaging mode (i.e., staining and NBI), these arms were treated as individual studies.
For each study, the sensitivity and specificity of Kudo’s classification in comparison to histology were calculated from the original numbers given in the included studies by constructing 2 × 2 contingency tables. They were further calculated in the pooled results (with corresponding 95%CI). When significant heterogeneity was present, the random effects model (Dersimonian and Laird method) was used. Otherwise, the fixed effects model (Mantel and Haenszel method[7]) was used. Forest plots were constructed for visual presentation of the individual studies and the pooled results. A weighted symmetric summary receiver operating curve was plotted and the area under the curve (AUC) was calculated. An AUC of 1 indicated perfect performance and an AUC close to 0.5 indicated poor performance. The Cochran Q test and the inconsistency index (I2) were performed to evaluate the heterogeneity between studies[8]. Significant heterogeneity was deemed present if the P value was less than 0.1.
To evaluate publication bias, the funnel plot, Egger’s test, and Begg’s test were carried out. For the sensitivity analysis, we investigated the pooled sensitivity and specificity by removing one study each time. We also investigated a subgroup consisting of 11 magnifying chromoendoscopy studies.
Statistical analysis was performed using the Statistical Package for Social Science Software for Windows (SPSS 13.0 version), Meta-Disc Statistical Software Version 1.4, and Comprehensive Meta Analysis 2.
A flow chart describing the process of study selection is shown in Figure 1. A total of 507 titles were retrieved during the literature search, and 20 studies were finally included in the meta-analysis. These studies were all published as full-texts. The main characteristics of the studies eligible for the meta-analysis are shown in Table 1. The studies were conducted in Germany, Brazil, United States, United Kingdom, Netherlands, Japan, and China. All studies used Kudo’s classification as the diagnostic criteria, where Type I and Type II indicated non-neoplastic polyps, and Type III to V indicated neoplastic polyps. 10 studies used magnifying chromoendoscopy[9-18], and the other studies used chromoendoscopy[19,20] (n = 2), narrow band imaging (NBI)[21,22] (n = 2), NBI magnifying endoscopy[23,24] (n = 2), and i-SCAN[25] (n = 1). Three studies[26-28] used a mixture of imaging modalities.
| Ref. | Country | Type of study | Type of classification | Patients (n) | Polyps (n) | Type of endoscopy |
| Kiesslich et al[26] | Germany | Single center | Type I to Type V | 100 | 283 | Magnifying chromoendoscopy |
| Kiesslich et al[9] | Germany | Single center | Neoplastic/non-neoplastic | 84 | 118 | Magnifying chromoendoscopy |
| Liu et al[10] | China | Single center | Type I to Type V | 948 | 954 | Magnifying chromoendoscopy |
| Su et al[11] | China | Single center | Type I to Type V | 230 | 270 | Magnifying chromoendoscopy |
| Hurlstone et al[12] | United Kingdom | Single center | Neoplastic/non-neoplastic | 350 | 288 | Magnifying chromoendoscopy |
| Apel et al[19] | Germany | Single center | Neoplastic/non-neoplastic | 158 | 273 | Chromoendoscopy |
| Kato et al[13] | Japan | Single center | Neoplastic/non-neoplastic | 180 | 210 | Magnifying chromoendoscopy |
| Su et al[20] | China | Single center | Neoplastic/non-neoplastic | 79 | 110 | Chromoendoscopy |
| Chiu et al[14] | China | Single center | Neoplastic/non-neoplastic | 133 | 180 | Magnifying chromoendoscopy |
| Tischendorf et al[28] | Germany | Single center | Neoplastic/non-neoplastic | 52 | 100 | NBI magnifying endoscopy |
| Tischendorf et al[28] | Germany | Single center | Neoplastic/non-neoplastic | 47 | 100 | Magnifying chromoendoscopy |
| East et al[24] | United Kingdom | Single center | Type I to Type V | 62 | 116 | NBI magnifying endoscopy |
| Liu et al[15] | China | Single center | Neoplastic/non-neoplastic | 223 | 451 | Magnifying chromoendoscopy |
| Rogart et al[21] | United States | Single center | Neoplastic/non-neoplastic | 131 | 265 | NBI |
| van den Broek et al[23] | Netherlands | Single center | Neoplastic/non-neoplastic | 50 | 98 | NBI magnifying endoscopy |
| Togashi et al[16] | Japan | Single center | Neoplastic/non-neoplastic | 50 | 107 | Magnifying chromoendoscopy |
| dos Santos et al[17] | Brazil | Single center | Neoplastic/non-neoplastic | 72 | 137 | Magnifying chromoendoscopy |
| van den Broek et al[22] | Netherlands | Single center | Neoplastic/non-neoplastic | 48 | 153 | NBI |
| Chan et al[25] | United States | Single center | Neoplastic/non-neoplastic | 43 | 103 | i-SCAN |
| Dos Santos et al[18] | Brazil | Single center | Neoplastic/non-neoplastic | 69 | 120 | Magnifying chromoendoscopy |
| Schachschal et al[27] | Germany | Single center | Neoplastic/non-neoplastic | 309 | 675 | White light imaging, i-SCAN |
In total, 5111 colorectal lesions in 3418 patients were identified in the 20 eligible studies. 2817 of the 5111 lesions were confirmed to be neoplastic by biopsy and subsequent histopathology. Table 2 summarizes 4 of the 20 studies that reported the number of lesions in each pit pattern by pathological results. This subgroup included 1623 colorectal lesions. The pooled sensitivity was 90.4% (95%CI: 79.7-95.7) and the pooled specificity was 88.4% (95%CI: 82.9-92.3).
| Pit pattern | Hyperplasia/inflammation | Adenoma/cancer | Total |
| Type I | 108 | 5 | 113 |
| Type II | 419 | 79 | 498 |
| Type III-L | 74 | 668 | 742 |
| Type III-s | 14 | 53 | 67 |
| Type IV | 13 | 152 | 165 |
| Type V | 3 | 35 | 38 |
| Total | 631 | 992 | 1623 |
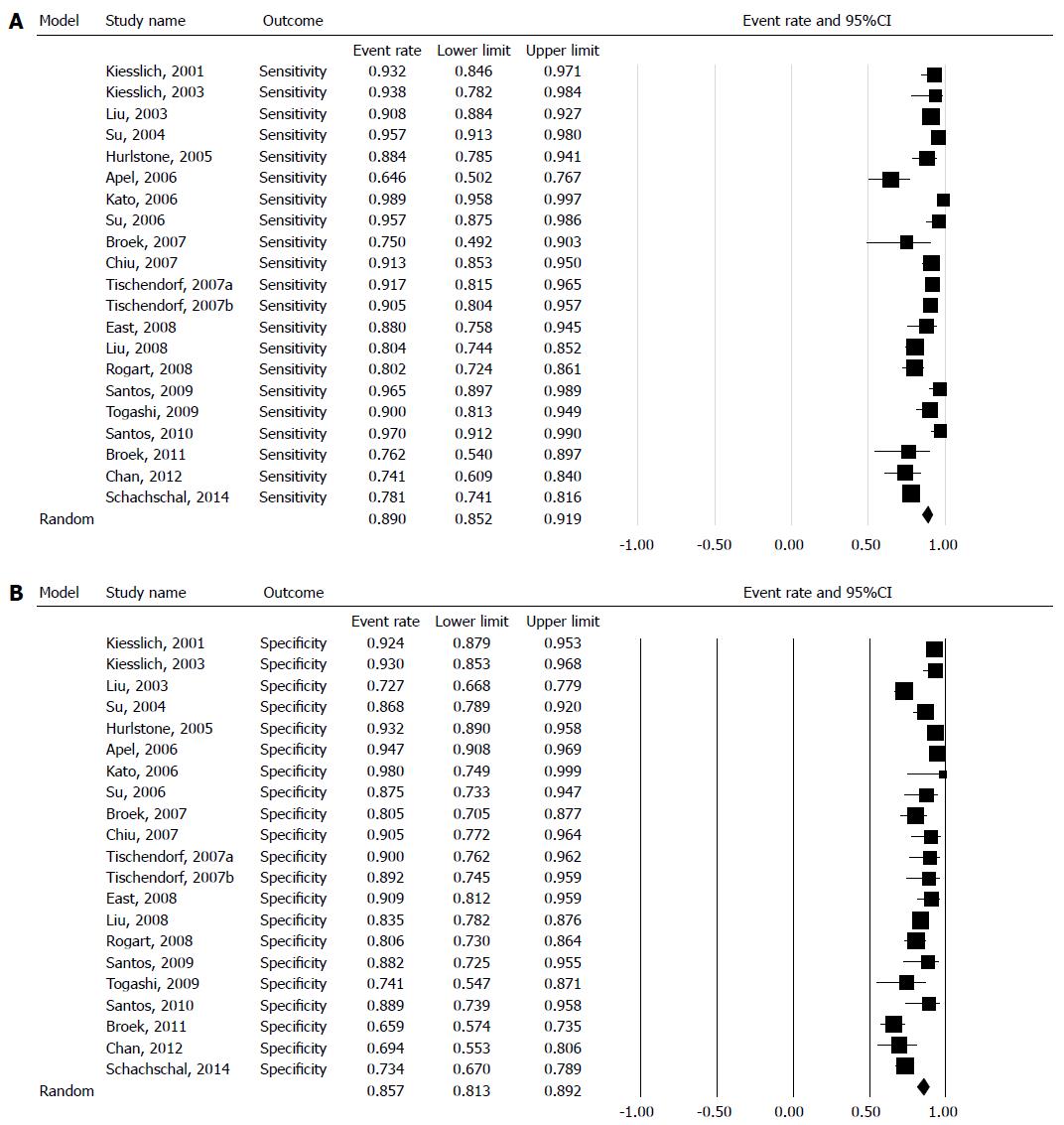
The sensitivity for each study is shown in the forest plot (Figure 2A). The inconsistency in sensitivity among the 20 studies was substantial (I2 = 84.3%), and was statistically significant (χ2 = 127, df = 20, P = 0.000). Thus, the pooled specificity was calculated using the random effects model. The pooled sensitivity was 89.0% (95%CI: 85.2-91.9).
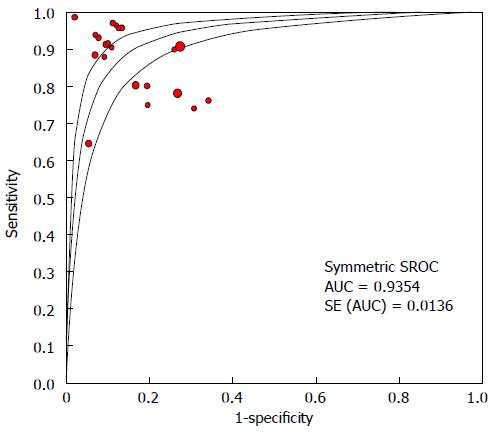
The specificity for each study is shown in the forest plot (Figure 2B). The inconsistency in specificity among the studies was substantial (I2 = 84.3%), and was statistically significant (χ2 = 127, df = 20, P = 0.0001). Thus, the pooled specificity was calculated using the random effects model. The pooled sensitivity was 85.7% (95%CI: 81.3-89.2). The SROC is displayed in Figure 3, and the AUC under the SROC was 0.9354 (95%CI: 0.9087-0.9621).
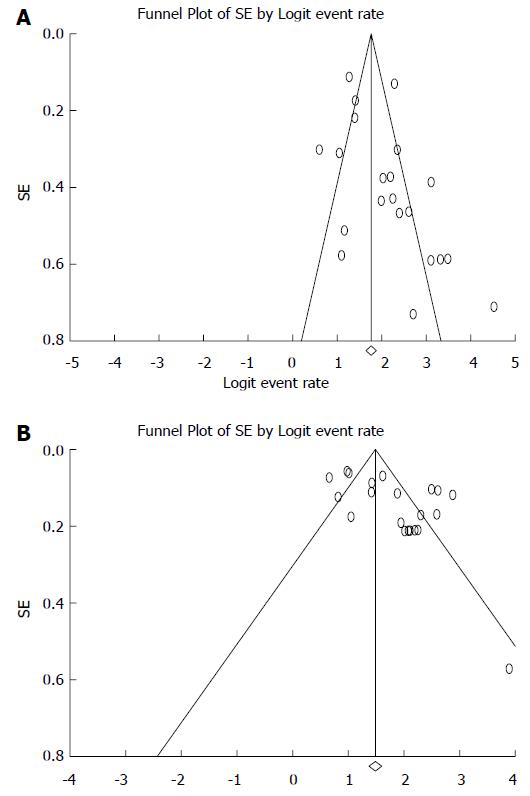
To investigate whether potential publication bias existed in the reviewed literature, the funnel plot was analyzed for sensitivity (Figure 4A) and specificity (Figure 4B). Both indices were distributed asymmetrically, indicating that publication bias may exist. Egger’s test indicated that publication bias was statistically significant (P = 0.038 and 0.006 for sensitivity and specificity) and Begg’s test was also significant (P = 0.035 and 0.139 for sensitivity and specificity).
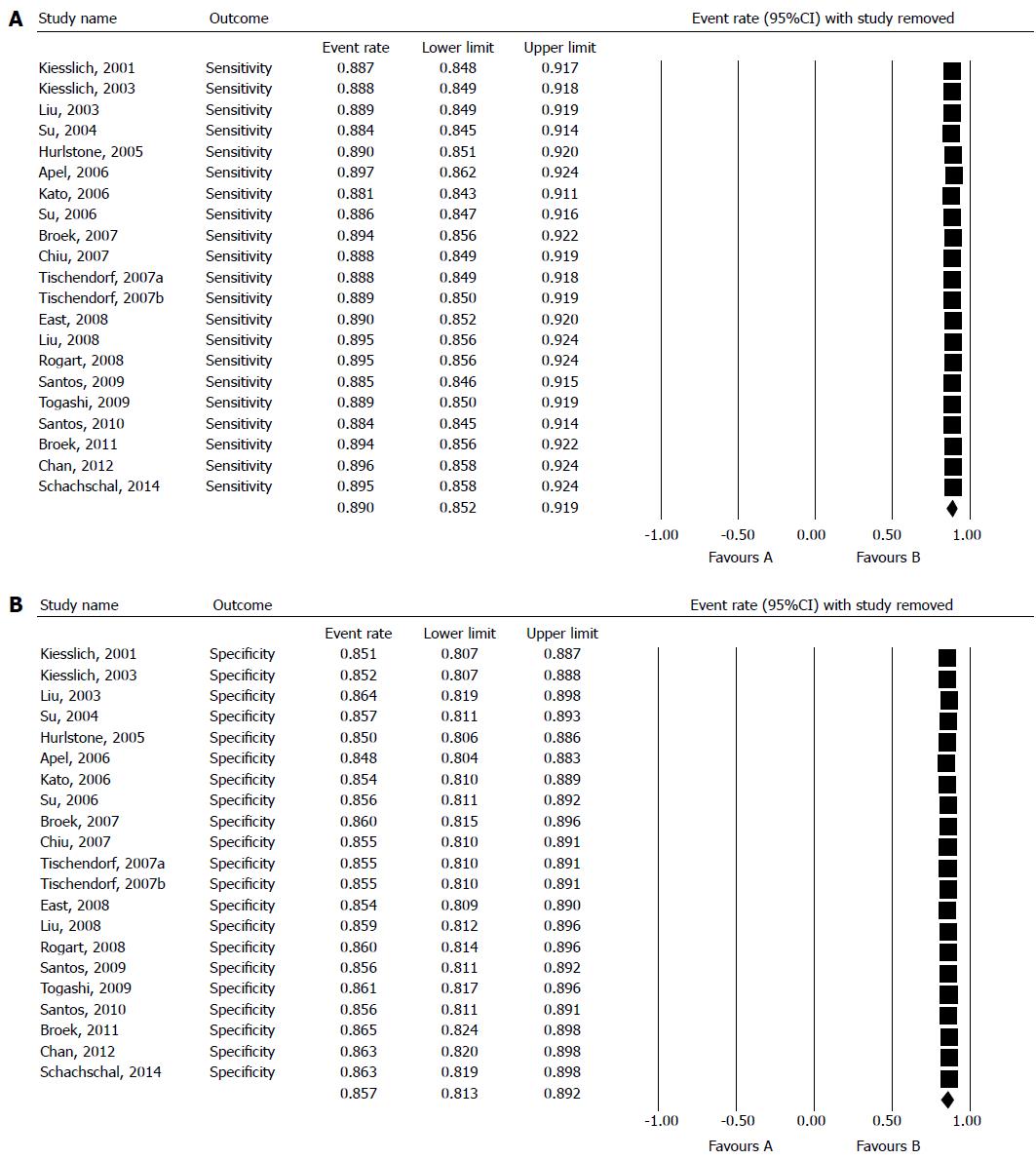
To investigate whether any single study significantly affected the pooled results, forest plots of sensitivity and specificity were plotted by removing one study at a time (Figure 5A, B). The 95%CI of pooled sensitivity and specificity was significantly affected by any single study. We also analyzed a subgroup which consisted of 11 studies using magnifying chromoendoscopy. The pooled sensitivity of these studies was 92.7% (95%CI: 89.2-95.2), and the pooled specificity was 87.3% (95%CI: 81.6-91.4).
Magnifying endoscopy is an extensively evaluated tool for detecting colorectal neoplasms and Kudo’s classification is the most frequently used criteria for colorectal neoplasm detection. To date, only a few trials have evaluated its efficiency in distinguishing neoplastic polyps from non-neoplastic polyps. In this study, the pooled sensitivity was 89.0% and specificity was 85.7%. The area under the SROC curve was 0.9354. This meta-analysis demonstrated that Kudo’s classification has substantial sensitivity and specificity for the diagnosis of colorectal neoplastic polyps.
The goal of colonoscopy is the early diagnosis of malignant and premalignant changes in the mucosa[29]. Magnifying endoscopy was invented in the 1990s to visualize the fine details of the mucosa[30]. Currently, there are two ways in which magnifying endoscopy is used in practice: Magnifying chromoendoscopy, where indigo carmine was originally used to delineate the mucosa pattern[17,30], and magnifying virtual chromoendoscopy, where a NBI is used to enhance the mucosa pattern[4]. Both modalities frequently use Kudo’s classification as the diagnostic criteria to diagnose neoplasia. The present study suggests that Type I (roundish pits) and Type II (stellar or papillary pits) represent hyperplastic or inflammation polyps and Type III to V represent neoplastic polyps with a high sensitivity and specificity. This provided the basis for real-time decisions during ongoing colonoscopy. During real-time diagnosis, endoscopists can use the recognize-and-discharge strategy thus preventing unnecessary biopsy and pathological costs.
Besides the Kudo’s pit pattern system, there are other endoscopic diagnostic criteria for the classification and staging of lesions: Sano’s capillary pattern which shows the relationship between capillary thickness and lesions, where invisible capillaries are more common in hyperplastic polyps, thin capillaries are more common in low-grade adenomas and thick capillaries are present in neoplastic lesions[16]. The vascular pattern intensity (VPI) is also used, where strong VPI indicates neoplasia and normal or weak VPI indicates non-neoplastic polyp. Combining the pit pattern and VPI may improve the diagnostic yield in the prediction of dysplasia[24].
The present study has several limitations: First, publication bias was statistically significant in the included studies. Thus, it remains a challenge to evaluate Kudo’s pit pattern classification in a comprehensive and unbiased manner. The diagnostic yield of Kudo’s pit pattern was not stratified by polyp size. A previous report documented that larger polyps were associated with a higher risk of neoplasia[31]. Furthermore, the learning curve, the extra time needed, and diagnostic performance in combination with other imaging modalities (such as the i-Scan system) require further investigation.
In conclusion, current evidence indicates that Kudo’s pit pattern classification is a highly accurate method for differentiating between non-neoplastic and neoplastic polyps. Publication bias is significant in the current available literature.
Magnifying endoscopy is a technique which permits the visualization of mucosal details that cannot be seen with standard endoscopy. The aim of this study was to meta-analyze the currently available evidence on Kudo’s pit pattern classification for diagnosing colorectal neoplasms.
The early detection and removal of precancerous lesions plays a critical role in the prevention of gastrointestinal cancer death. Magnifying endoscopy may benefit patients by supporting real-time decisions and saving unnecessary biopsies.
This study was a meta-analysis of the currently available evidence on Kudo’s pit pattern classification for colorectal neoplasms. Kudo’s pit pattern classification is an accurate diagnostic method for the differentiation of neoplastic and non-neoplastic colorectal lesions. Publication bias is significant in the current available literature.
This study suggested that Kudo’s pit pattern classification is accurate for supporting real-time decisions during ongoing endoscopy.
Kudo’s pit pattern: Kudo and colleagues classified colorectal polyps according to their appearance, structure and staining patterns. Type I pits appear as roundish pits; Type II pits appear as stellar or papillary pits; Type III-s pits are small roundish, tubular pits (smaller than Type I) and Type III-L are roundish and tubular pits (larger than Type I); Type IV pits appear as branch-like or gyrus-like pits and Type V pits appear as non-structured pits. Type I and II are considered benign changes (e.g., normal, hyperplastic, inflammatory polyps), whereas pit pattern classes III-V are considered neoplastic and malignant changes.
The authors conducted a meta-analysis for the diagnostic performance of a widely used criteria, Kodu’s pit pattern, in detecting colorectal neoplastic polyps. There is scientific merit to evaluate this diagnostic performance.
P- Reviewer: Guo QN S- Editor: Gou SX L- Editor: Webster JR E- Editor: Zhang DN
| 1. | Kahi CJ, Rex DK, Imperiale TF. Screening, surveillance, and primary prevention for colorectal cancer: a review of the recent literature. Gastroenterology. 2008;135:380-399. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 2. | Lambert R, Provenzale D, Ectors N, Vainio H, Dixon MF, Atkin W, Werner M, Franceschi S, Watanabe H, Tytgat GN. Early diagnosis and prevention of sporadic colorectal cancer. Endoscopy. 2001;33:1042-1064. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Bond JH. Clinical evidence for the adenoma-carcinoma sequence, and the management of patients with colorectal adenomas. Semin Gastrointest Dis. 2000;11:176-184. [PubMed] [Cited in This Article: ] |
| 4. | Li HY, Ge ZZ, Fujishiro M, Li XB. Current clinical applications of magnifying endoscopy with narrow band imaging in the stomach. Diagn Ther Endosc. 2012;2012:271914. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Kudo S, Tamura S, Nakajima T, Yamano H, Kusaka H, Watanabe H. Diagnosis of colorectal tumorous lesions by magnifying endoscopy. Gastrointest Endosc. 1996;44:8-14. [PubMed] [Cited in This Article: ] |
| 6. | Tung SY, Wu CS, Su MY. Magnifying colonoscopy in differentiating neoplastic from nonneoplastic colorectal lesions. Am J Gastroenterol. 2001;96:2628-2632. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 132] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 7. | Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719-748. [PubMed] [Cited in This Article: ] |
| 8. | Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539-1558. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21630] [Cited by in F6Publishing: 23017] [Article Influence: 1046.2] [Reference Citation Analysis (0)] |
| 9. | Kiesslich R, Fritsch J, Holtmann M, Koehler HH, Stolte M, Kanzler S, Nafe B, Jung M, Galle PR, Neurath MF. Methylene blue-aided chromoendoscopy for the detection of intraepithelial neoplasia and colon cancer in ulcerative colitis. Gastroenterology. 2003;124:880-888. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 616] [Cited by in F6Publishing: 538] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 10. | Liu HH, Kudo SE, Juch JP. Pit pattern analysis by magnifying chromoendoscopy for the diagnosis of colorectal polyps. J Formos Med Assoc. 2003;102:178-182. [PubMed] [Cited in This Article: ] |
| 11. | Su MY, Ho YP, Chen PC, Chiu CT, Wu CS, Hsu CM, Tung SY. Magnifying endoscopy with indigo carmine contrast for differential diagnosis of neoplastic and nonneoplastic colonic polyps. Dig Dis Sci. 2004;49:1123-1127. [PubMed] [Cited in This Article: ] |
| 12. | Hurlstone DP, Sanders DS, Lobo AJ, McAlindon ME, Cross SS. Indigo carmine-assisted high-magnification chromoscopic colonoscopy for the detection and characterisation of intraepithelial neoplasia in ulcerative colitis: a prospective evaluation. Endoscopy. 2005;37:1186-1192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in F6Publishing: 206] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 13. | Kato S, Fu KI, Sano Y, Fujii T, Saito Y, Matsuda T, Koba I, Yoshida S, Fujimori T. Magnifying colonoscopy as a non-biopsy technique for differential diagnosis of non-neoplastic and neoplastic lesions. World J Gastroenterol. 2006;12:1416-1420. [PubMed] [Cited in This Article: ] |
| 14. | Chiu HM, Chang CY, Chen CC, Lee YC, Wu MS, Lin JT, Shun CT, Wang HP. A prospective comparative study of narrow-band imaging, chromoendoscopy, and conventional colonoscopy in the diagnosis of colorectal neoplasia. Gut. 2007;56:373-379. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 230] [Cited by in F6Publishing: 251] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 15. | Liu YX, Huang LY, Bian XP, Cui J, Xu N, Wu CR. Fuji Intelligent Chromo Endoscopy and staining technique for the diagnosis of colon tumor. Chin Med J (Engl). 2008;121:977-982. [PubMed] [Cited in This Article: ] |
| 16. | Togashi K, Osawa H, Koinuma K, Hayashi Y, Miyata T, Sunada K, Nokubi M, Horie H, Yamamoto H. A comparison of conventional endoscopy, chromoendoscopy, and the optimal-band imaging system for the differentiation of neoplastic and non-neoplastic colonic polyps. Gastrointest Endosc. 2009;69:734-741. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | dos Santos CE, Lima JC, Lopes CV, Malaman D, Salomão AD, Garcia AC, Teixeira CR. Computerized virtual chromoendoscopy versus indigo carmine chromoendoscopy combined with magnification for diagnosis of small colorectal lesions: a randomized and prospective study. Eur J Gastroenterol Hepatol. 2010;22:1364-1371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Dos Santos CE, Malaman D, Lopes CV, Pereira-Lima JC, Parada AA. Digital chromoendoscopy for diagnosis of diminutive colorectal lesions. Diagn Ther Endosc. 2012;2012:279521. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Apel D, Jakobs R, Schilling D, Weickert U, Teichmann J, Bohrer MH, Riemann JF. Accuracy of high-resolution chromoendoscopy in prediction of histologic findings in diminutive lesions of the rectosigmoid. Gastrointest Endosc. 2006;63:824-828. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Su MY, Hsu CM, Ho YP, Chen PC, Lin CJ, Chiu CT. Comparative study of conventional colonoscopy, chromoendoscopy, and narrow-band imaging systems in differential diagnosis of neoplastic and nonneoplastic colonic polyps. Am J Gastroenterol. 2006;101:2711-2716. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 183] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 21. | Rogart JN, Jain D, Siddiqui UD, Oren T, Lim J, Jamidar P, Aslanian H. Narrow-band imaging without high magnification to differentiate polyps during real-time colonoscopy: improvement with experience. Gastrointest Endosc. 2008;68:1136-1145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 22. | van den Broek FJ, Fockens P, van Eeden S, Stokkers PC, Ponsioen CY, Reitsma JB, Dekker E. Narrow-band imaging versus high-definition endoscopy for the diagnosis of neoplasia in ulcerative colitis. Endoscopy. 2011;43:108-115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 120] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 23. | van den Broek FJ, Fockens P, van Eeden S, Reitsma JB, Hardwick JC, Stokkers PC, Dekker E. Endoscopic tri-modal imaging for surveillance in ulcerative colitis: randomised comparison of high-resolution endoscopy and autofluorescence imaging for neoplasia detection; and evaluation of narrow-band imaging for classification of lesions. Gut. 2008;57:1083-1089. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 201] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 24. | East JE, Suzuki N, Bassett P, Stavrinidis M, Thomas HJ, Guenther T, Tekkis PP, Saunders BP. Narrow band imaging with magnification for the characterization of small and diminutive colonic polyps: pit pattern and vascular pattern intensity. Endoscopy. 2008;40:811-817. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 25. | Chan JL, Lin L, Feiler M, Wolf AI, Cardona DM, Gellad ZF. Comparative effectiveness of i-SCAN™ and high-definition white light characterizing small colonic polyps. World J Gastroenterol. 2012;18:5905-5911. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Kiesslich R, von Bergh M, Hahn M, Hermann G, Jung M. Chromoendoscopy with indigocarmine improves the detection of adenomatous and nonadenomatous lesions in the colon. Endoscopy. 2001;33:1001-1006. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 171] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 27. | Schachschal G, Mayr M, Treszl A, Balzer K, Wegscheider K, Aschenbeck J, Aminalai A, Drossel R, Schröder A, Scheel M. Endoscopic versus histological characterisation of polyps during screening colonoscopy. Gut. 2014;63:458-465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 28. | Tischendorf JJ, Wasmuth HE, Koch A, Hecker H, Trautwein C, Winograd R. Value of magnifying chromoendoscopy and narrow band imaging (NBI) in classifying colorectal polyps: a prospective controlled study. Endoscopy. 2007;39:1092-1096. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 150] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 29. | Kiesslich R, Jung M, DiSario JA, Galle PR, Neurath MF. Perspectives of chromo and magnifying endoscopy: how, how much, when, and whom should we stain? J Clin Gastroenterol. 2004;38:7-13. [PubMed] [Cited in This Article: ] |
| 30. | Kudo S, Tamura S, Nakajima T, Yamano H, Kusaka H, Watanabe H. Diagnosis of colorectal tumorous lesions by magnifying endoscopy. Gastrointest Endosc. 1996;44:8-14. [PubMed] [Cited in This Article: ] |
| 31. | Li D, Jin C, McCulloch C, Kakar S, Berger BM, Imperiale TF, Terdiman JP. Association of large serrated polyps with synchronous advanced colorectal neoplasia. Am J Gastroenterol. 2009;104:695-702. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |