Published online Sep 21, 2014. doi: 10.3748/wjg.v20.i35.12501
Revised: April 18, 2014
Accepted: May 19, 2014
Published online: September 21, 2014
Processing time: 241 Days and 20.7 Hours
Because of the low prevalence of non-ampullary duodenal epithelial tumors (NADETs), standardized clinical management of sporadic superficial NADETs, including diagnosis, treatment, and follow-up, has not yet been established. Retrospective studies have revealed certain endoscopic findings suggestive of malignancy. Duodenal adenoma with high-grade dysplasia and mucosal cancer are candidates for local resection by endoscopic or minimally invasive surgery. The use of endoscopic treatment including endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD), for the treatment for superficial NADETs is increasing. EMR requires multiple sessions to achieve complete remission and repetitive endoscopy is needed after resection. ESD provides an excellent complete resection rate, however it remains a challenging method, considering the high risk of intraoperative or delayed perforation. Minimally invasive surgery such as wedge resection and pancreas-sparing duodenectomy are beneficial for superficial NADETs that are technically difficult to remove by endoscopic treatment. Pancreaticoduodenectomy remains a standard surgical procedure for treatment of duodenal cancer with submucosal invasion, which presents a risk of lymph node metastasis. Endoscopic or surgical treatment outcomes of superficial NADETs without submucosal invasion are satisfactory. Establishing an endoscopic diagnostic tool to differentiate superficial NADETs between adenoma and cancer as well as between mucosal and submucosal cancer is required to select the most appropriate treatment.
Core tip: Superficial non-ampullary duodenal epithelial tumors (NADETs) including adenoma and mucosal cancer are candidates for local resection regarding the nil risk of lymph node metastasis. Pancreaticoduodenectomy remains a standard surgical procedure for treatment of duodenal cancer with submucosal invasion. Preoperative diagnosis to differentiate superficial NADETs between adenoma and cancer as well as between mucosal and submucosal cancer is essential to select minimally invasive treatment such as endoscopic resection or minimally invasive surgery.
- Citation: Kakushima N, Kanemoto H, Tanaka M, Takizawa K, Ono H. Treatment for superficial non-ampullary duodenal epithelial tumors. World J Gastroenterol 2014; 20(35): 12501-12508
- URL: https://www.wjgnet.com/1007-9327/full/v20/i35/12501.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i35.12501
The prevalence of non-ampullary primary duodenal tumors is extremely low among autopsy studies (0.02%-0.5%)[1-4]. Benign tumors are generally predominant, comprising 60%-75% of autopsy series[5], and 90% of duodenal polyps identified by endoscopic biopsy[6], but they frequently do not produce symptoms. Primary non-ampullary duodenal cancer (NADC) accounts for only 0.5% of all gastrointestinal malignancies[7]. A Japanese study reported that the incidence of non-ampullary duodenal epithelial tumors (NADETs) among patients who underwent screening or diagnostic esophagogastroduodenoscopy (EGD) over a 21-year period was 0.098% (52/53134)[8]. A European study reported that the incidence of duodenal villous adenoma was 0.1%-0.4%[9,10] among patients who underwent diagnostic or screening EGD.
The number of case studies related to the treatment of sporadic superficial NADETs has recently increased, possibly because of the use of EGD during regular medical check-ups and the standardized method of imaging the second portion of the duodenum during EGD. However, information regarding the biological behavior, treatment indication, and prognosis of NADETs is still lacking. In this review, the present status regarding the clinical management of sporadic superficial NADETs will be discussed.
The morphology of superficial NADETs is similar to that of epithelial tumors of the colorectum; protruding or elevated lesion types predominate. Therefore, several previous studies have classified the morphological type of superficial NADETs based on the classification criteria used for colorectal tumors[11-13]. Macroscopic types based on endoscopic features include protruded pedunculated (Ip), protruded sessile (Is), semipedunculated type (Isp), superficial elevated type (IIa), flat type (IIb), and superficial shallow or depressed type (IIc)[14].
Both benign adenoma and cancer arise most frequently in the second portion of the duodenum, especially the periampullary area[15-17]. Almost 90% of endoscopically treated lesions are reportedly located in the first or second portion of the duodenum[11,12]. However, an accurate prognosis according to the location remains unknown for superficial NADETs, although tumors in the first or second portion are reportedly favorable factors for surgically treatable duodenal cancer[18].
There is no established definition of early NADC with respect to the depth of invasion and risk of lymph node metastasis. However, previous studies have followed the rules used for early colorectal[14] or gastric cancer[19], i.e., tumor invasion to the lamina propria or muscularis mucosa (T1a) or the submucosa (T1b) regardless of lymph node metastasis[11,12,20]. Based on a Japanese analysis of early NADCs that included pT1a or pT1b cancer, Nagatani et al[11] reported no incidence of lymph node metastasis among 40 cases of pT1a cancers, and Fujisawa et al[12] reported none among 166 cases of mucosal cancers. The incidence of lymph node metastasis among pT1b cancer is reported to range from 5.3% (2/37) to 5.4% (4/75)[11,12]. The depth of pT1b cancer invasion has not been described in these reported cases; therefore, at present, pT1b NADC should be considered at risk of lymph node metastasis regardless of the depth of submucosal invasion.
It is important to determine the type of sporadic superficial NADETs that should be removed to lower the risk of those that have a substantial possibility to progress to invasive cancer and to avoid overtreatment of those with less malignant potential[21,22]. Small bowel adenomas, including duodenal adenomas, are reported to have a higher percentage of villous tumors than colorectal adenomas[5]; thus, the possibility of harboring cancer is estimated to be higher[23,24]. Patients with familial adenomatous polyposis (FAP) are known to have a high prevalence of duodenal adenomas, and prospective follow-up studies have demonstrated that such adenomas can slowly progress to cancer[24-26]. Sporadic NADC may occur because of the adenoma carcinoma sequence, as in FAP patients[15,27], or de novo. However, the incidence of sporadic NADC is extremely rare; thus, endoscopic findings suggestive of cancer have not yet been established.
Macroscopically, larger-sized lesions, those with a component of depression (IIa + IIc type or IIc type) and those with a red and ill-glistened surface, are reported to have a higher tendency to have a cancerous component[28,29]. In contrast, there are also a few reports of large IIa type, so-called carpet-like adenomas that increase in size without cancerous components[8,28]. In addition to the macroscopic features, findings of magnifying endoscopy with[30-32] or without[33-35] narrow-band imaging can offer information to distinguish potentially malignant lesions. Heterogeneous patterns of irregular or disappeared mucosal structures with irregular vascular patterns have been correlated with high-grade dysplasia (HGD) or mucosal cancer[30,33,34].
The necessity of obtaining biopsy specimens from superficial NADETs before treatment remains controversial. Because of the thinness of the duodenal wall, the biopsy procedure itself may induce unintended fibrosis associated with the lesion, which may complicate subsequent endoscopic resection (ER). In contrast, a histological proof of malignancy may be preferable before employing a treatment modality with a substantial risk of complication, such as ER or surgery. Discrepancy of endoscopic biopsy histology and that of resected specimens is sometimes experienced in gastric epithelial lesions[36] and has also been reported in duodenal lesions[20,37-39]. Post-treatment diagnosis may lead to an upgrade in pathology. In a previous study, comparisons between biopsy and resected specimens of 56 superficial NADETs revealed the same diagnosis in 59% patients, an upgrade in 36% patients, and a downgrade in 5% patients. Among the lesions with an upgrade in pathology, most had an upgrade from HGD to adenocarcinoma[40]. In another study, T1a cancer was observed in 13.5% cases in which initial biopsies indicated simple adenomas[38]. Therefore, lesions with a biopsy diagnosis of HGD should be considered for local treatment, and ER may present an improved diagnostic tool for borderline lesions.
It is difficult to differentiate T1a from T1b NADC by barium studies or endoscopy[11]. Nagatani et al[11] studied the macroscopic features of early NADC with little possibility of submucosal invasion and indicated the following lesions suggestive of T1a cancer: Ip type, ≤ 20 mm; Is type, ≤ 5 mm; IIa type, ≤ 10 mm; and carpet-like IIa type of any size. Endoscopic ultrasonography has been reported to be a promising tool for diagnosing and staging duodenal villous tumors[41,42]. Non-lifting sign or lack of mobility when manipulated with a snare suggest submucosal invasion[43].
Endoscopic treatment should be limited to lesions confined to the mucosa; however, endoscopic techniques to remove superficial NADETs have not yet been standardized[44,45]. Although case reports of polypectomy[46,47] and strip biopsy[48-50] for protruded lesions have been reported, the results of endoscopic mucosal resection (EMR) for NADETs are based on several small case series[51,52]. EMR often requires multiple sessions (piecemeal resection), and complete remission has been reported in 55%-100% cases[37,38,43,53-55]. The local recurrence rate among these previous studies was 0%-37%, and recurrent lesions were endoscopically re-treated by a second EMR or endoscopic ablation[37,38,43,53-55]. Reportedly, successful eradication by endoscopic treatment is dictated by the extent of the luminal circumference of the tumor[56] and the possibility of en bloc resection[57]. Complications after EMR include bleeding (0%-33%) and perforation (3%)[38,58].
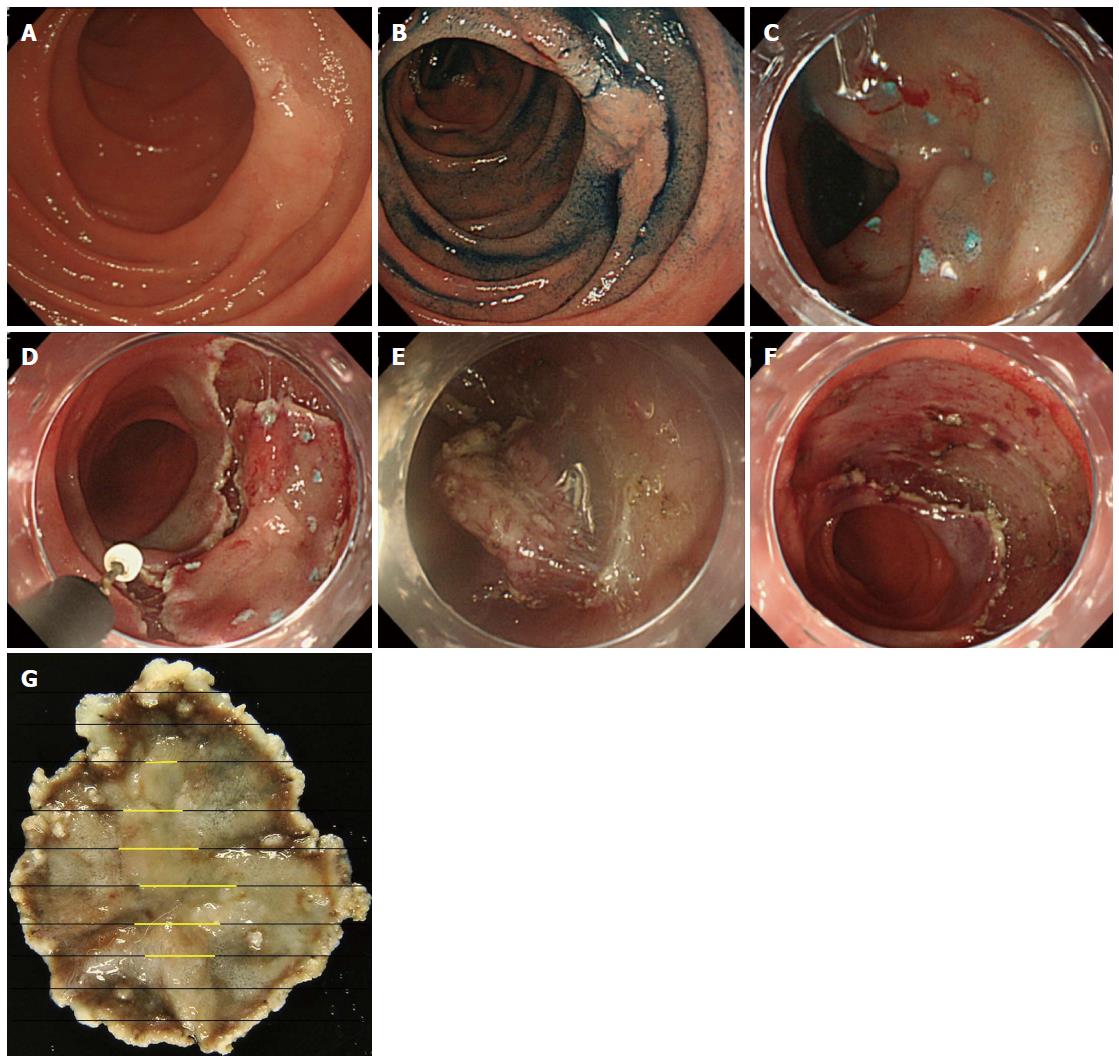
Compared with EMR, there are fewer reports of endoscopic submucosal dissection (ESD) (Figure 1) for superficial NADETs[59]. Nevertheless, ESD has been reported to have a superior complete resection rate (86%-100%), with no incidence of recurrence[20,39,40,60-63]. However, the complication rate of bleeding is 0%-7% and the perforation rate is higher at 6%-50%[20,39,40,60-63]. A retrospective multicenter survey of 421 duodenal EMR and ESD cases showed that both intraoperative and delayed perforation occurred more frequently among ESD cases[64]. In addition, there was no difference in the overall perforation rate between lesions located in the first or second portion of the duodenum; however, delayed perforation was mostly observed in the second portion[64] or distal to Vater’s ampulla[65]. Delayed perforation may occur even after successful ESD[61], and the high rate of delayed perforation may be because of the larger ulcer created by ESD, increased thermocoagulation effect induced by ESD, and presence of bile and pancreatic juices. To avoid the risk of delayed perforation, attempts have been made to completely close the mucosal defect after ESD with multiple endoclips[64,66] or with an over-the-scope clip[67], or by applying polyglycolic acid sheets[68,69]. Others have reported insertion of endoscopic nasobiliary drainage and nasopancreatic drainage tubes to avoid exposure of bile and pancreatic juices to the ESD ulcer[61].
Lienert et al[70] reported the use of argon plasma coagulation (APC) for the treatment of duodenal adenoma in a cohort of 15 patients. Eradication was successful in 87%, no patients developed cancer during the study period of 40 mo, and the local recurrence rate was 39%[70]. However, APC treatment is inadequate to obtain a histological assessment of the entire lesion; therefore, it is risky to solely rely on APC for the treatment of superficial NADETs. Nonetheless, APC may be used as an adjunct to EMR to reduce local recurrence[71] or for locally recurrent lesions after EMR.
An optimal surgical treatment of sporadic superficial NADETs has not yet been established. Pancreaticoduodenectomy (PD), also known as the Whipple procedure, is a radical surgery performed for advanced disease and cases of early stage NADC with the aim to avoid tumor recurrence[72-75]. Pancreatic fistula, anastomotic leakage, and pancreatitis are serious complications after PD. Although the morbidity and mortality rates following PD have decreased in recent years[76,77], the procedure still results in considerable intraoperative stress.
Transduodenal local excision[78,79], wedge resection (segmental, partial, and full-thickness)[78,80-83], and pancreas-sparing duodenectomy (PSD)[78,84,85] are organ-preserving surgical procedures suitable for patients with premalignant or early malignant duodenal lesions. In a previous study, the recurrence rate of villous duodenal tumors after transduodenal local excision was reported to be 32% at 5 years, and 24% recurrences were cancer[78]. However, the presence of cancer was not known before surgery, and the authors concluded that PD is appropriate for villous tumors with a malignant component[78]. Jurisić et al[82] reported local recurrence with nodal metastasis after local excision for cancer with submucosal invasion, suggesting the inadequacy of local excision for T1b NADC.
Wedge resection (segmental, partial, and full-thickness) has been associated with lower morbidity than PD[78,80,81,83,86,87]. Tanigawa et al[81] reported a good clinical course without recurrence after partial resection for T1a cancer. For early NADC located in the third and fourth portions of the duodenum, segmental resection is associated with negligible morbidity and mortality, while allowing for satisfactory clear margins during lymphadenectomy[83].
PSD was introduced as an alternative to PD for treatment of benign and premalignant duodenal disease and is reportedly a beneficial modality for treatment of patients with multiple lesions[78,84,85,88]. For PSD, patients should have a preoperative diagnosis of no lymphadenopathy and sampling of the lymph node surrounding the tumor before resection should be obtained for intraoperative pathological examination to detect lymph node metastasis[85]. Eisenberger et al[84] recommended analysis of intraoperative fresh frozen sections to exclude malignant disease and to consider conversion of the procedure from PSD to PD after intraoperative diagnosis of malignant disease in the fresh frozen sections. The incidence of local recurrence is low, suggesting that PSD is an acceptable procedure for treatment of selected duodenal neoplasms, such as superficial NADETs confined to the mucosa.
Because previous reports of surgical treatment for duodenal cancer included small numbers of patients with superficial NADETs, including adenoma or early NADC, little information is available regarding treatment success and treatment-related morbidity or mortality.
The most important prognostic factor is the depth of tumor invasion[80,89-93]. With regard to the risk of nodal metastasis, superficial NADETs, including adenoma and T1a cancer, are treatable by local resection; however, the ability to achieve a negative margin may be a prerequisite to successful local resection. For adenoma or T1a cancer, ER presents the same distant survival rates as surgery[94]. In contrast, T1b cancer should be resected with lymphadenectomy and PD appears to remain the treatment of choice. In a recent review of 67 patients, Barnes et al[95] reported a 5-year survival rate of 100% in stage I duodenal cancer. Therefore, preoperative diagnosis of the presence of submucosal invasion is important to select the appropriate surgical method.
Considering that EMR is often performed piecemeal, repetitive endoscopy after resection is necessary. Ahmad et al[37] suggested that 6 wk was a suitable interval to observe healing of the EMR site for lesions requiring multiple sessions to achieve and/or confirm complete remission. Others report that endoscopic surveillance every 3 mo is required initially. Subsequently, if no recurrence is observed, the surveillance should be reduced to every 6 mo or once a year[54,96]. Annual checkups should be performed for at least 2 years after complete removal[55]. For lesions with submucosal invasion, computed tomography and abdominal ultrasound should be performed to detect nodal or distant metastasis.
Recent studies of Western populations have suggested that patients with sporadic duodenal adenomas are at a higher risk for the development of colorectal neoplasia[97-100]. The odds ratio of colorectal neoplasia among patients with sporadic duodenal adenomas is reportedly 2.4-3.6, and the incidence of colorectal cancer was significantly higher[97,98]. Dietary risk factors correlated with cancer of the small intestine are similar to those correlated with colon cancer[101]. Therefore, patients with sporadic duodenal adenomas should undergo routine colonoscopy screening to increase the detection rate of colorectal neoplasia.
As with other malignancies, early detection is important for the treatment of sporadic superficial NADETs. Establishing an endoscopic diagnostic tool to differentiate the lesion between adenoma and cancer as well as between mucosal and submucosal cancer is required. For lesions confined to the mucosa, local resection is acceptable, and en bloc resection with negative margins is beneficial to lower the risk of local recurrence. The use of endoscopic treatment modalities, including EMR and ESD, for the treatment for superficial NADETs is increasing. However, ESD remains a challenging method, considering the high risk of intraoperative or delayed perforation. Therefore, primary closure of the endoscopically resected site, as a preventive measure, is preferable. The use of radical surgery for superficial NADETs has decreased with the introduction of minimally invasive surgery. Newer methods, such as the use of an over-the-scope clip[67-69] or combined surgery of endoscopy and laparoscopy[86,102], have been reported. Further studies are warranted to improve the current diagnostic and treatment methods to both preserve curability and improve the quality of life.
P- Reviewer: Chang JH, Chung WC, Jeon SE S- Editor: Ma N L- Editor: A E- Editor: Ma S
| 1. | Darling RC, Welch CE. Tumors of the small intestine. N Engl J Med. 1959;260:397-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 132] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Shukla SK, Elias EG. Primary neoplasms of the duodenum. Surg Gynecol Obstet. 1976;142:858-860. [PubMed] |
| 5. | Perzin KH, Bridge MF. Adenomas of the small intestine: a clinicopathologic review of 51 cases and a study of their relationship to carcinoma. Cancer. 1981;48:799-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Höchter W, Weingart J, Seib HJ, Ottenjann R. [Duodenal polyps. Incidence, histologic substrate and significance]. Dtsch Med Wochenschr. 1984;109:1183-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Alwmark A, Andersson A, Lasson A. Primary carcinoma of the duodenum. Ann Surg. 1980;191:13-18. [PubMed] |
| 8. | Yokoyama T, Saito D, Kondo H, Kido M, Hosokawa K, Shirao K, Yokota T, Yamaguchi H, Oguro Y, Ishikawa T. Endoscopic diagnosis of malignant lesions of the duodenum. Stomach Intestine. 1993;28:641-649. |
| 9. | Batra SK, Schuman BM, Reddy RR. The endoscopic variety of duodenal villous adenoma - an experience with ten cases. Endoscopy. 1983;15:89-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Jepsen JM, Persson M, Jakobsen NO, Christiansen T, Skoubo-Kristensen E, Funch-Jensen P, Kruse A, Thommesen P. Prospective study of prevalence and endoscopic and histopathologic characteristics of duodenal polyps in patients submitted to upper endoscopy. Scand J Gastroenterol. 1994;29:483-487. [PubMed] |
| 11. | Nagatani K, Takekoshi T, Baba Y, Kaku S, Koizumi K, Fujii A, Ogata E, Ohta H, Nishi M, Kato Y. Indications for endoscopic treatment of early duodenal cancer based on cases reported in the literature (in Japanese). Endosc Digest. 1993;5:969-976. |
| 12. | Fujisawa T, Tomofuji Y, Kuroda N, Hagino H, Sakamoto N, Sakashita M, Maeda M, Kouno T, Matsuno Y. A case of early duodenal cancer with tubulo-villous adenoma: report of a case and clinicopathological review of Japanese literature. Gastroenterol Endosc. 1995;37:2768-2775. |
| 13. | Oka S, Tanaka S, Nagata S, Hiyama T, Ito M, Kitadai Y, Yoshihara M, Haruma K, Chayama K. Clinicopathologic features and endoscopic resection of early primary nonampullary duodenal carcinoma. J Clin Gastroenterol. 2003;37:381-386. [PubMed] |
| 14. | Japanese Society for Cancer of the Colon and Rectum. Japanese classification of colorectal carcinoma. 8th ed. Tokyo: Kanehara Shuppan 2013; 9-10. |
| 15. | Sellner F. Investigations on the significance of the adenoma-carcinoma sequence in the small bowel. Cancer. 1990;66:702-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 16. | Shimizu N, Tanaka S, Morikawa J, Yorioka S, Fukuda S, Yamashita S, Bamba M, Hattori T, Hosoda S. [Early duodenal cancer of the bulb--report of a case]. Gan No Rinsho. 1989;35:100-106. [PubMed] |
| 17. | Schulten MF, Oyasu R, Beal JM. Villous adenoma of the duodenum. A case report and review of the literature. Am J Surg. 1976;132:90-96. [PubMed] |
| 18. | Sohn TA, Lillemoe KD, Cameron JL, Pitt HA, Kaufman HS, Hruban RH, Yeo CJ. Adenocarcinoma of the duodenum: factors influencing long-term survival. J Gastrointest Surg. 1998;2:79-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 112] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma. 14th ed. Tokyo: Kanehara Shuppan 2010; 7-8. |
| 20. | Takahashi T, Ando T, Kabeshima Y, Kawakubo H, Shito M, Sugiura H, Omori T. Borderline cases between benignancy and malignancy of the duodenum diagnosed successfully by endoscopic submucosal dissection. Scand J Gastroenterol. 2009;44:1377-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Witteman BJ, Janssens AR, Griffioen G, Lamers CB. Villous tumours of the duodenum. An analysis of the literature with emphasis on malignant transformation. Neth J Med. 1993;42:5-11. [PubMed] |
| 22. | Fujishiro M. Endoscopic treatment for nonampullary duodenal neoplasms (in Japanese). Endosc Digest. 2011;23:135-139. |
| 23. | Bail JP, Lozac’h P, Volant A, Charles JF. [Adenocarcinoma of the duodenum. Apropos of 2 cases: review of the literature]. J Chir (Paris). 1987;124:604-608. [PubMed] |
| 24. | Vasen HF, Möslein G, Alonso A, Aretz S, Bernstein I, Bertario L, Blanco I, Bülow S, Burn J, Capella G. Guidelines for the clinical management of familial adenomatous polyposis (FAP). Gut. 2008;57:704-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 471] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 25. | Spigelman AD, Talbot IC, Penna C, Nugent KP, Phillips RK, Costello C, DeCosse JJ. Evidence for adenoma-carcinoma sequence in the duodenum of patients with familial adenomatous polyposis. The Leeds Castle Polyposis Group (Upper Gastrointestinal Committee). J Clin Pathol. 1994;47:709-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 113] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Brosens LA, Keller JJ, Offerhaus GJ, Goggins M, Giardiello FM. Prevention and management of duodenal polyps in familial adenomatous polyposis. Gut. 2005;54:1034-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 100] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 27. | Maehata Y, Esaki M, Hirahashi M, Kitazono T, Matsumoto T. Duodenal adenomatosis in Japanese patients with familial adenomatous polyposis. Dig Endosc. 2014;26 Suppl 2:30-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Yamanaka T, Yamamichi N, Konishi F. Clinico-pathological study of duodenal adenoma (in Japanese with an English abstract). Gastroenterol Endosc. 1987;29:3070-3079. |
| 29. | Goda K, Kikuchi D, Yamamoto Y, Takimoto K, Kakushima N, Morita Y, Doyama H, Gotoda T, Maehata Y, Abe N. Endoscopic diagnosis of superficial non-ampullary duodenal epithelial tumors in Japan: Multicenter case series. Dig Endosc. 2014;26 Suppl 2:23-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 154] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 30. | Yoshimura N, Goda K, Tajiri H, Ikegami M, Nakayoshi T, Kaise M. Endoscopic features of nonampullary duodenal tumors with narrow-band imaging. Hepatogastroenterology. 2010;57:462-467. [PubMed] |
| 31. | Kikuchi D, Hoteya S, Iizuka T, Kimura R, Kaise M. Diagnostic algorithm of magnifying endoscopy with narrow band imaging for superficial non-ampullary duodenal epithelial tumors. Dig Endosc. 2014;26 Suppl 2:16-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 32. | Inatsuchi S, Maeda Y. Magnifying endoscopy with narrow band imaging for diagnosis of nonampullary duodenal adenomas and early cancers (in Japanese with an English abstract). Stomach Intestine. 2011;46:1604-1617. |
| 33. | Tanaka K, Toyoda H, Inoue H, Hamada Y, Aoki M, Kosaka R, Takamura M, Imoto I. Depressed-type early duodenal carcinoma (carcinoma in situ) observed by enhanced magnification endoscopy. Endoscopy. 2007;39 Suppl 1:E125-E126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 34. | Onozato Y, Kakizaki S, Ishihara H, Sohara N, Iizuka H, Okamura S, Mori M, Ogawa T, Itoh H. Magnifying endoscopic findings of early duodenal adenocarcinoma in relation to the pathological findings. Endoscopy. 2008;40 Suppl 2:E92-E93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 35. | Tanaka M, Usuda M, Okura Y, Fujimori T, Ito H, Nanasawa H, Orihara M, Iwamoto S, Makimoto S, Shinagawa K. Significance of magnifying endoscopy in diagnosis of duodenal elevated lesions (in Japanese with an English abstract). Stomach Intestine. 2003;38:1709-1720. |
| 36. | Takao M, Kakushima N, Takizawa K, Tanaka M, Yamaguchi Y, Matsubayashi H, Kusafuka K, Ono H. Discrepancies in histologic diagnoses of early gastric cancer between biopsy and endoscopic mucosal resection specimens. Gastric Cancer. 2012;15:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 37. | Ahmad NA, Kochman ML, Long WB, Furth EE, Ginsberg GG. Efficacy, safety, and clinical outcomes of endoscopic mucosal resection: a study of 101 cases. Gastrointest Endosc. 2002;55:390-396. [PubMed] |
| 38. | Lépilliez V, Chemaly M, Ponchon T, Napoleon B, Saurin JC. Endoscopic resection of sporadic duodenal adenomas: an efficient technique with a substantial risk of delayed bleeding. Endoscopy. 2008;40:806-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 39. | Endo M, Abiko Y, Oana S, Kudara N, Chiba T, Suzuki K, Koizuka H, Uesugi N, Sugai T. Usefulness of endoscopic treatment for duodenal adenoma. Dig Endosc. 2010;22:360-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 40. | Kakushima N, Ono H, Takao T, Kanemoto H, Sasaki K. Method and timing of resection of superficial non-ampullary duodenal epithelial tumors. Dig Endosc. 2014;26 Suppl 2:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 41. | Tio TL, Sie LH, Verbeek PC, Dé Wit LT, Tytgat GN. Endosonography in diagnosing and staging duodenal villous adenoma. Gut. 1992;33:567-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 42. | Perez A, Saltzman JR, Carr-Locke DL, Brooks DC, Osteen RT, Zinner MJ, Ashley SW, Whang EE. Benign nonampullary duodenal neoplasms. J Gastrointest Surg. 2003;7:536-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 43. | Alexander S, Bourke MJ, Williams SJ, Bailey A, Co J. EMR of large, sessile, sporadic nonampullary duodenal adenomas: technical aspects and long-term outcome (with videos). Gastrointest Endosc. 2009;69:66-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 120] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 44. | Yoshino J, Inui K, Wakabayashi T, Okushima K, Kobayashi T, Miyoshi Y, Nakamura Y, Takada M, Kado R. Endoscopic therapy for small tumors or tumorous lesions in the duodenum (in Japanese with an English abstract). Stomach Intestine. 2001;36:1541-1545. |
| 45. | Sato Y, Inoue H, Kaga M, Hayashi T, Wakamura K, Wada Y, Inui M, Sugaya S, Odata N, Satodate H. EMR/ESD for nonampullary duodenal tumors (in Japanese). Clin Gastroenterol. 2006;9:158-163. |
| 46. | Tringali M, Crotta S, Bodrato C, Lolli R, Cerrato C, Cerrato G. Early primary duodenal carcinoma successfully treated by endoscopic polypectomy. Endoscopy. 1994;26:709. [PubMed] |
| 47. | Friedrich-Rust M, Jaeger C, Gossner L, May A, Günter E, Stolte M, Ell C. [Early duodenal adenocarcinoma arising in gastric metaplasia treated by endoscopic resection]. Z Gastroenterol. 2006;44:323-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 48. | Obata S, Suenaga M, Araki K, Fujioka Y, Kimura K, Maeda K, Miura K, Mizutani J. Use of strip biopsy in a case of early duodenal cancer. Endoscopy. 1992;24:232-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 49. | Nishida K, Nojiri I, Kato M, Higashijima M, Takagi K, Akashi R. [A case of early duodenal cancer resected by endoscopic polypectomy in the elderly]. Nihon Ronen Igakkai Zasshi. 1993;30:705-708. [PubMed] |
| 50. | Sakaida I, Yoshida T, Okita K. Endoscopic resection of early duodenal cancer using a strip biopsy technique. Am J Gastroenterol. 2000;95:1600-1601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 51. | Kume K, Okubo Y, Ejiri Y, Abe H, Hakozaki H, Murata I, Yoshikawa I, Otsuki M. 1. Early duodenal carcinoma successfully treated by endoscopic mucosal resection. Digest Endosc. 2000;12:177-180. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 52. | Sanomura M, Tanaka S, Ito M, Chayama K. Depressed-type, early duodenal carcinoma (carcinoma in situ) treated by endoscopic mucosal resection. J Gastroenterol. 2003;38:813-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 53. | Hirasawa R, Iishi H, Tatsuta M, Ishiguro S. Clinicopathologic features and endoscopic resection of duodenal adenocarcinomas and adenomas with the submucosal saline injection technique. Gastrointest Endosc. 1997;46:507-513. [PubMed] |
| 54. | Apel D, Jakobs R, Spiethoff A, Riemann JF. Follow-up after endoscopic snare resection of duodenal adenomas. Endoscopy. 2005;37:444-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 82] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 55. | Abbass R, Rigaux J, Al-Kawas FH. Nonampullary duodenal polyps: characteristics and endoscopic management. Gastrointest Endosc. 2010;71:754-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 56. | Kedia P, Brensinger C, Ginsberg G. Endoscopic predictors of successful endoluminal eradication in sporadic duodenal adenomas and its acute complications. Gastrointest Endosc. 2010;72:1297-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 57. | Kim HK, Chung WC, Lee BI, Cho YS. Efficacy and long-term outcome of endoscopic treatment of sporadic nonampullary duodenal adenoma. Gut Liver. 2010;4:373-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 58. | Eswaran SL, Sanders M, Bernadino KP, Ansari A, Lawrence C, Stefan A, Mattia A, Howell DA. Success and complications of endoscopic removal of giant duodenal and ampullary polyps: a comparative series. Gastrointest Endosc. 2006;64:925-932. [PubMed] |
| 59. | Yamamoto Y, Yoshizawa N, Tomida H, Fujisaki J, Igarashi M. Therapeutic outcomes of endoscopic resection for superficial non-ampullary duodenal tumor. Dig Endosc. 2014;26 Suppl 2:50-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 60. | Takeuchi M, Kobayashi M, Shioji K, Togashi T, Hashimoto S, Sato Y, Narisawa R, Aoyagi Y. Prevention and management of complications in endoscopic resection for non-ampullary duodenal neoplasms (in Japanese with an English abstract). Endosc Digest. 2010;22:1561-158. |
| 61. | Jung JH, Choi KD, Ahn JY, Lee JH, Jung HY, Choi KS, Lee GH, Song HJ, Kim DH, Kim MY. Endoscopic submucosal dissection for sessile, nonampullary duodenal adenomas. Endoscopy. 2013;45:133-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 62. | Matsumoto S, Miyatani H, Yoshida Y. Endoscopic submucosal dissection for duodenal tumors: a single-center experience. Endoscopy. 2013;45:136-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 63. | Honda T, Yamamoto H, Osawa H, Yoshizawa M, Nakano H, Sunada K, Hanatsuka K, Sugano K. Endoscopic submucosal dissection for superficial duodenal neoplasms. Dig Endosc. 2009;21:270-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 115] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 64. | Ono H, Nonaka S, Uedo N, Kaise M, Oyama T, Doyama H, Kokawa A, Kaneko K, Kodashima S, Tanabe S. Clinical issues of duodenal EMR/ESD (in Japanese with an English abstract). Stomach Intestine. 2011;46:1669-1677. |
| 65. | Inoue T, Uedo N, Yamashina T, Yamamoto S, Hanaoka N, Takeuchi Y, Higashino K, Ishihara R, Iishi H, Tatsuta M. Delayed perforation: a hazardous complication of endoscopic resection for non-ampullary duodenal neoplasm. Dig Endosc. 2014;26:220-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 135] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 66. | Iizuka T, Kikuchi D, Hoteya S, Yamada A, Yamashita S, Fujimoto A, Nakamura M, Matsui A, Kuroki Y, Mitani T. How to manage duodenal tumors with EMR or ESD (in Japanese with an English abstract). Gastroenterol Endosc. 2011;53:87-94. |
| 67. | Mori H, Shintaro F, Kobara H, Nishiyama N, Rafiq K, Kobayashi M, Nakatsu T, Miichi N, Suzuki Y, Masaki T. Successful closing of duodenal ulcer after endoscopic submucosal dissection with over-the-scope clip to prevent delayed perforation. Dig Endosc. 2013;25:459-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 68. | Doyama H, Tominaga K, Yoshida N, Takemura K, Yamada S. Endoscopic tissue shielding with polyglycolic acid sheets, fibrin glue and clips to prevent delayed perforation after duodenal endoscopic resection. Dig Endosc. 2014;26 Suppl 2:41-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 69. | Takimoto K, Imai Y, Matsuyama K. Endoscopic tissue shielding method with polyglycolic acid sheets and fibrin glue to prevent delayed perforation after duodenal endoscopic submucosal dissection. Dig Endosc. 2014;26 Suppl 2:46-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 70. | Lienert A, Bagshaw PF. Treatment of duodenal adenomas with endoscopic argon plasma coagulation. ANZ J Surg. 2007;77:371-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 71. | Basford PJ, Bhandari P. Endoscopic management of nonampullary duodenal polyps. Therap Adv Gastroenterol. 2012;5:127-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 72. | Scott-Coombes DM, Williamson RC. Surgical treatment of primary duodenal carcinoma: a personal series. Br J Surg. 1994;81:1472-1474. [PubMed] |
| 73. | Solej M, D’Amico S, Brondino G, Ferronato M, Nano M. Primary duodenal adenocarcinoma. Tumori. 2008;94:779-786. [PubMed] |
| 74. | Han SL, Cheng J, Zhou HZ, Zeng QQ, Lan SH. The surgical treatment and outcome for primary duodenal adenocarcinoma. J Gastrointest Cancer. 2010;41:243-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 75. | Lang H, Nadalin S, Raab R, Jähne J. [Results of surgical therapy of primary adenocarcinoma of the duodenum]. Chirurg. 1999;70:571-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 76. | Cameron JL, Riall TS, Coleman J, Belcher KA. One thousand consecutive pancreaticoduodenectomies. Ann Surg. 2006;244:10-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 896] [Cited by in RCA: 968] [Article Influence: 50.9] [Reference Citation Analysis (34)] |
| 77. | Mukherjee S, Kocher HM, Hutchins RR, Bhattacharya S, Abraham AT. Impact of hospital volume on outcomes for pancreaticoduodenectomy: a single UK HPB centre experience. Eur J Surg Oncol. 2009;35:734-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 78. | Farnell MB, Sakorafas GH, Sarr MG, Rowland CM, Tsiotos GG, Farley DR, Nagorney DM. Villous tumors of the duodenum: reappraisal of local vs. extended resection. J Gastrointest Surg. 2000;4:13-21, discussion 22-23. [PubMed] |
| 79. | Haglund U, Fork FT, Genell S, Rehnberg O. Villous adenomas in the duodenum. Br J Surg. 1985;72:26-27. [PubMed] |
| 80. | Santoro E, Sacchi M, Scutari F, Carboni F, Graziano F. Primary adenocarcinoma of the duodenum: treatment and survival in 89 patients. Hepatogastroenterology. 1997;44:1157-1163. [PubMed] |
| 81. | Tanigawa K, Akino K, Kajiwara Y, Ozeki K, Kanematsu T, Shibata H, Yamashita S. A small depressed early cancer of the second portion of duodenum: report of a case and review of Japanese literature. Chin Med J (Engl). 1996;109:810-813. [PubMed] |
| 82. | Jurisić D, Doko M, Glavan E, Rosko D, Vidović D, Tomić K. Local recurrence of primary non-ampullary adenocarcinoma of duodenum after surgical treatment--a case report and a literature review. Coll Antropol. 2006;30:225-229. [PubMed] |
| 83. | Kaklamanos IG, Bathe OF, Franceschi D, Camarda C, Levi J, Livingstone AS. Extent of resection in the management of duodenal adenocarcinoma. Am J Surg. 2000;179:37-41. [PubMed] |
| 84. | Eisenberger CF, Knoefel WT, Peiper M, Yekebas EF, Hosch SB, Busch C, Izbicki JR. Pancreas-sparing duodenectomy in duodenal pathology: indications and results. Hepatogastroenterology. 2004;51:727-731. [PubMed] |
| 85. | Konishi M, Kinoshita T, Nakagohri T, Takahashi S, Gotohda N, Ryu M. Pancreas-sparing duodenectomy for duodenal neoplasms including malignancies. Hepatogastroenterology. 2007;54:753-757. [PubMed] |
| 86. | Abe N, Suzuki Y, Masaki T, Mori T, Sugiyama M. Surgical management of superficial non-ampullary duodenal tumors. Dig Endosc. 2014;26 Suppl 2:57-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 87. | Tanaka Y, Tokunaga K, Katsuragawa K, Yoshiura K, Naitoh M, Kino K, Tsuru M, Matsumoto K, Fukazawa T, Kuroiwa K. Early carcinoma of the distal second part of the duodenum treated by wedge resection. J Clin Gastroenterol. 1999;28:67-69. [PubMed] |
| 88. | Spalding DR, Isla AM, Thompson JN, Williamson RC. Pancreas-sparing distal duodenectomy for infrapapillary neoplasms. Ann R Coll Surg Engl. 2007;89:130-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 89. | Galandiuk S, Hermann RE, Jagelman DG, Fazio VW, Sivak MV. Villous tumors of the duodenum. Ann Surg. 1988;207:234-239. [PubMed] |
| 90. | Friedrich-Rust M, Ell C. Early-stage small-bowel adenocarcinoma: a review of local endoscopic therapy. Endoscopy. 2005;37:755-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 91. | Rose DM, Hochwald SN, Klimstra DS, Brennan MF. Primary duodenal adenocarcinoma: a ten-year experience with 79 patients. J Am Coll Surg. 1996;183:89-96. [PubMed] |
| 92. | Bakaeen FG, Murr MM, Sarr MG, Thompson GB, Farnell MB, Nagorney DM, Farley DR, van Heerden JA, Wiersema LM, Schleck CD. What prognostic factors are important in duodenal adenocarcinoma? Arch Surg. 2000;135:635-641; discussion 641-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 160] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 93. | Ryder NM, Ko CY, Hines OJ, Gloor B, Reber HA. Primary duodenal adenocarcinoma: a 40-year experience. Arch Surg. 2000;135:1070-1074; discussion 1074-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 67] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 94. | Seifert E, Schulte F, Stolte M. Adenoma and carcinoma of the duodenum and papilla of Vater: a clinicopathologic study. Am J Gastroenterol. 1992;87:37-42. [PubMed] |
| 95. | Barnes G, Romero L, Hess KR, Curley SA. Primary adenocarcinoma of the duodenum: management and survival in 67 patients. Ann Surg Oncol. 1994;1:73-78. [PubMed] |
| 96. | Kashida H, Inoue H, Yamamura F, Minami H, Sato Y, Kaga M, Kameda R, Kudo S. Treatment selection, prevention for complication and follow-up for duodenal tumors (in Japanese). Clin Gastroenterol. 2008;11:260-265. |
| 97. | Murray MA, Zimmerman MJ, Ee HC. Sporadic duodenal adenoma is associated with colorectal neoplasia. Gut. 2004;53:261-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 80] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 98. | Ramsoekh D, van Leerdam ME, Dekker E, Ouwendijk RT, van Dekken H, Kuipers EJ. Sporadic duodenal adenoma and the association with colorectal neoplasia: a case-control study. Am J Gastroenterol. 2008;103:1505-1509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 99. | Pequin P, Manfredi S, Quentin V, Heresbach D, Boyer J, Siproudhis L, Bretagne JF. Patients with sporadic duodenal adenoma are a high-risk group for advanced colorectal neoplasia: results of a case-control study. Aliment Pharmacol Ther. 2007;26:277-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 100. | Adler DG, Qureshi W, Davila R, Gan SI, Lichtenstein D, Rajan E, Shen B, Zuckerman MJ, Fanelli RD, Van Guilder T. The role of endoscopy in ampullary and duodenal adenomas. Gastrointest Endosc. 2006;64:849-854. [PubMed] |
| 101. | Negri E, Bosetti C, La Vecchia C, Fioretti F, Conti E, Franceschi S. Risk factors for adenocarcinoma of the small intestine. Int J Cancer. 1999;82:171-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 102. | Sakon M, Takata M, Seki H, Hayashi K, Munakata Y, Tateiwa N. A novel combined laparoscopic-endoscopic cooperative approach for duodenal lesions. J Laparoendosc Adv Surg Tech A. 2010;20:555-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |