Published online Sep 21, 2014. doi: 10.3748/wjg.v20.i35.12473
Revised: January 28, 2014
Accepted: April 1, 2014
Published online: September 21, 2014
Processing time: 278 Days and 10.5 Hours
The incidence of hepatocellular carcinoma (HCC) is increasing worldwide, largely due to hepatitis B virus (HBV), hepatitis C virus and liver cirrhosis. Chronic HBV infection is estimated to cause 55%-60% of the cases of HCC worldwide and over 70% in Asian countries. Liver resection is currently the mainstay of treatment due to the low surgical mortality, a wider treatment indication, and simplicity of post-treatment follow-up. There is an ever-increasing demand on surgeons to perform curative liver resection in HCC, with the hope of avoiding tumor recurrences. Hepatitis B-related-HCC has distinct clinicopathological features, which should be considered when treating the disease. The author presents a review of the recently evolving strategies and emerging therapies to improve HCC postresectional outcomes and focus on perioperative measures to improve patient outcome, with particular reference to the current status of adjuvant therapies in HCC patients after liver resection.
Core tip: Chronic hepatitis B virus infection is the main cause of hepatocellular carcinoma (HCC). Liver resection is currently the mainstay of treatment. However, the postresectional tumor recurrence rates remain high. There is an ever-increasing demand on surgeons to perform curative liver resection in HCC, with the hope of avoiding tumor recurrences. The author presents a review of the recently evolving strategies and emerging therapies to improve patient outcomes, including a policy of accurate preoperative staging, adequate resection, intensive postoperative follow-up, and early initiation of effective adjuvant therapy, with particular reference to the current status of adjuvant therapies in HCC patients after liver resection.
- Citation: Chau GY. Resection of hepatitis B virus-related hepatocellular carcinoma: Evolving strategies and emerging therapies to improve outcome. World J Gastroenterol 2014; 20(35): 12473-12484
- URL: https://www.wjgnet.com/1007-9327/full/v20/i35/12473.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i35.12473
Hepatocellular carcinoma (HCC) is a common malignancy worldwide, and its incidence continues to increase[1,2]. The main HCC risk factors include hepatitis B, hepatitis C, alcoholic liver disease, hemochromatosis and nonalcoholic steatohepatitis[3]. Chronic hepatitis B virus (HBV) infection is estimated to cause 55%-60% of HCC worldwide[4], and is the most important etiologic agent of HCC in Asia[5,6]. Surgical resection and liver transplantation are the only curative therapies for HCC. For HCC patients with solitary tumors and preserved liver function, hepatic resection is the treatment of choice and it has potentially curative intent. Hepatic resection is also the most available efficient treatment of HCC patients with tumors size > 5 cm, with nodules > 3, or with tumor macroscopic vascular invasion, who are less likely to benefit from liver transplantation or local ablative therapy[7,8]. Recent advances in surgical techniques[9], peri-operative care, and more accurate evaluation of the underlying liver function have increased the safety of liver resection even in patients with chronic liver disease and cirrhosis[10,11]. With appropriate patient selection, hepatectomy for HCC is currently a safe procedure that can be performed with less than 5% operative mortality[10,12,13], and a 5-year survival after resection that exceeds 50%[14]. Long term (> 10 years) disease-free survival is consistently seen in a part of the patient cohorts reported from many centers[15,16]. However, tumor recurrences and subsequent death remain common after resection[17,18]. Tumor recurrences usually occur due to occult intrahepatic metastasis from the primary tumor, and less frequently, from the new development of primary tumors from the underlying liver that is affected by chronic disease[19].
Surgical resection is the treatment of choice for HCC patients with a solitary tumor and no evidence of liver cirrhosis, irrespective of the tumor size. This disease pattern exists more frequently in patients with hepatitis B-related HCC. Up to 40% of patients with chronic hepatitis B virus infection can develop HCC without cirrhosis[20]. Compared with HCV-related HCC patients, HBV-related HCC patients tend to be younger, show a higher male/female ratio, and present higher serum albumin levels, lower serum ALT levels, higher white blood cell counts, higher serum α-fetoprotein levels, a larger tumor size, and a lower proportion of multiple tumors[21,22]. Almost equivalent tumor aggressiveness and peritumoral microenvironments were noted in subjects with isolated HBV or HCV infections[23-25]. These characteristics make HBV-related HCC more amenable for surgical resection.
Current studies show that a steady improvement in survival after HCC treatment occurred over the past decade[26,27]. The reasons for these improvements are likely multifactorial, and may include progress in disease screening, imaging studies, patient selection, surgical techniques, and patient care. From a clinical point of view, practical ways to improve long-term results of surgical resection for HCC include: accurate preoperative tumor staging, optimal surgical approach, early detection and treatment of recurrent tumors, and development of effective adjuvant therapies. This review summarizes evolving strategies and emerging therapies over the past decade to improve post-resectional outcome in HCC patients, with special reference to adjuvant therapies in HBV-related HCC patients after curative resection.
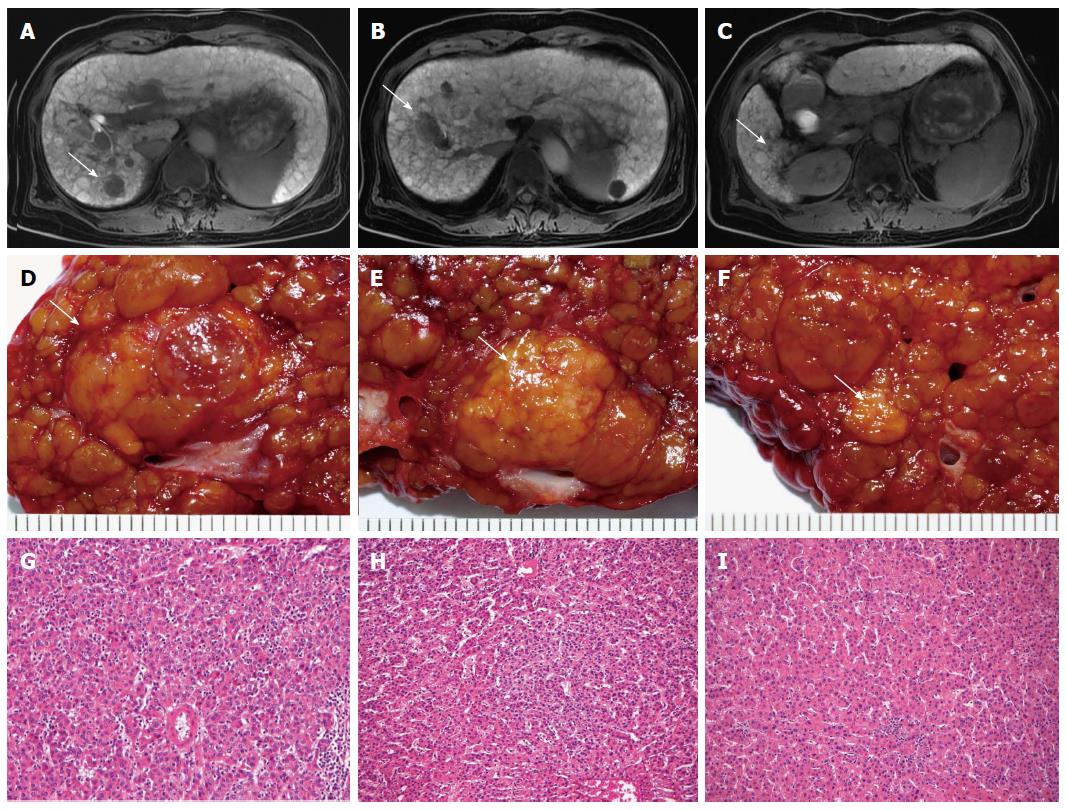
In HCC patients undergoing hepatic resection, preoperative tumor staging and characterization of the extent of disease (accurate identification of the number and locations of HCCs) are of upmost importance to correctly select appropriate candidates for surgery and to guide optimal surgical strategies, so as to increase the chance of a cure. Amidst reports of impaired survival due to potential tumor dissemination and tumor seeding, biopsy of suspected liver nodules was not recommended in these situations[28]. At present, HCC diagnostic confirmation and assessing the extent of disease depend mainly on contrast material-enhanced computed tomography (CT) and magnetic resonance (MR) imaging[29]. The American Association for the Study of Liver Diseases (AASLD) recommends that a diagnosis of HCC be made if a mass larger than 2 cm shows typical features of HCC (hypervascularity in the arterial phase and washout in the venous phase) at contrast material-enhanced CT or MR imaging, or if a 1-2 cm mass shows these features in both modalities[19]. In HCC patients, it is important to differentiate small HCC and premalignant from benign nodules, as surgical strategies may differ and have implications on the disease-free survival of patients. In the liver, small nodules of benign regenerative and dysplastic nodules, and early small HCC (< 2 cm) may co-exist and small HCC may mimic benign lesions[30]. Hyperattenuating and contrast-enhancing hepatic nodules small than 2 cm are frequently detected in the cirrhotic liver during arterial phase, and the majority of these nodules are benign[31]. In this setting, it is particularly important to characterize lesions less than 2 cm. Magnetic resonance imaging outperforms CT in these areas, and the pooled estimate of the sensitivity for detecting small HCC is 81% for MR imaging compared with 68% for CT[32,33]. The use of new hepatocyte-specific contrast agent, Gd-EthOxyBenzl-DTPA (Gd-EOB-DTPA; Bayer Health Care), in MR imaging is associated with both improved diagnostic accuracy and lesion discrimination[34]. A hypointense nodule against the background of enhancing liver parenchyma in the hepatobiliary phase of Gd-EOB-DTPA-enhanced MR imaging strongly suggests HCC (including well-differentiated HCC) or a high-grade dysplastic nodule with malignant potential (Figure 1). However, differentiation of these lesions is not currently possible[35]. A multicenter study reported by Hammerstingl et al[36] indicated that the frequency of correctly detected lesions was significantly higher with Gd-EOB-DTPA-enhanced MR imaging than with CT in clinical evaluation (10.44%). A change in planned surgical procedures after performing Gd-EOB-DTPA-enhanced MR imaging was documented in 19 of 131 patients (14.5%), and can be considered as an increase in the accuracy of a preoperative planning of liver resection. Incorporating a combination of ancillary features of liver lesions (lesion size, interval growth, signal intensity of T1- and T2-weighted imaging, the presence of intratumoral fat, and detection of fibrous capsule) enhances the diagnostic performance of Gd-EOB-DTPA-enhanced MR imaging. This technique is emerging as the best imaging method currently available for the preoperative staging of HCC patients with chronic liver disease, particularly in the detection of supplementary nodules between 1 and 2 cm without the hallmark vascular features of HCC on dynamic imaging.
Hepatocellular carcinoma prognosis after resection is associated with pretreatment tumor staging and tumor biology. However, the surgeon’s performance is also an important determinant of the long-term disease-free survival. In view of the fact that in HCC, vascular invasion with hematogenous spread and occult metastases is considered to be the major cause of tumor recurrences after hepatic resection[19,37], a rational surgical approach and an adequate resection should be practical ways to improve patients’ long-term survival[38].
Hepatic resections for HCC can be either anatomical resections (lobectomy, segmentectomy, or subsegmentectomy) or non-anatomical resections (wedge resection). In HCC, intrahepatic metastasis occurs mainly through the portal tract, and may be present before surgery. It has been proposed that the intrahepatic spread of hepatic tumors follows the pattern of step-by-step intrahepatic dissemination[39]. With small tumors and early metastases, satellite nodules usually lie in the same segment of the main tumor. The anatomical approach, which removes the entire portal unit of the tumor-bearing segment along with Glisson’s vessels en bloc, is particularly important for the purpose of reducing postoperative intrahepatic tumor recurrence rates. In 1996, the author reported techniques of hepatic segmentectomy for the curative resection of HCC[38]. Studies[40,41] indicated that overall and disease-free survival times were significantly longer, and local recurrence rates were lower after anatomical as compared to non-anatomical resection. Shindoh et al[42] indicated that anatomical resection decreases local recurrences and improves the surgical outcomes in solitary HCC measuring 2-5 cm in diameter. The 5-year recurrence rate was 56.7% in the anatomical resection group and 74.7% in the non-anatomical resection group. Recent study employing meta-regression analysis indicated that the 5-year overall survival and disease-free survival are significantly better with anatomical resection[43].
The surgical margin of the resected HCC represents the shortest distance between the section line and the tumor(s) margin. An adequate margin has been defined by Lee et al[44] as a margin equal to or exceeding 1.0 cm. The status of the surgical margin is an important prognostic factor in patients undergoing hepatic resection, because the value of the resection is diminished when the hepatic transection margin is involved by tumor and usually associated with disease recurrence. Histological examination of the resected margin is an important method for assessing whether residual disease existed in the adjacent liver tissue. A surgical margin equal to or exceeding 1.0 cm increases the chances of histological clearance of disease in the liver tissue and decreases the chances of local recurrences after resection, especially for tumors with locoregional disease extension. In 1997, the author reported that a narrow surgical margin (< 1 cm) significantly influences HCC recurrences after resection, and minor resection and centrally located tumors are factors related to a narrow surgical margin[45]. Recently, one randomized controlled trial reported that a surgical margin aiming at 2 cm significantly decreased HCC recurrence rates and increased the 5-year survival rate compared with a margin aiming at 1 cm[46]. However, two points should be emphasized in the discussion about the significance of surgical margins. Firstly, in author’s series[45] of 165 consecutive patients, despite attempts to keep a surgical margin of 1 cm or more in width in each case, only 52% of the patients had the tumors successfully resected with a surgical margin of 1.0 cm or more. These data are compatible with those of previously reported series, which showed that 20%-88% of the resection can achieve a 1.0 cm clearance of the surgical margin[47,48]. Secondly, in patients with a well-encapsulated solitary tumor and no evidence of macroscopic vascular invasion, a narrow surgical margin, even a null-margin, does not exclude the possibility of a cure after resection[49,50]. In the preoperative evaluation of HCC resections, a prediction of an obtainable narrow surgical margin should not be a contraindication for hepatectomy.
The extent of liver resection influences the outcome of patients with HCC. In HCC resection, the choice for the type of resection should certainly be based on the maintenance of an adequate postoperative liver reserve. In patients with preserved liver function, major hepatectomy can be considered. In patients with chronic liver disease and associated marginal liver function, less extensive resection is necessary. Surgeons who are less familiar with liver surgery may be inclined to perform less radical resections. This may represent a lost opportunity for some patients who, had they received an adequate and radical resection, may have had better long-term prognoses. To minimize the risk for disease local recurrences and maximize the overall survival probability, a surgical strategy that supports the preference for anatomical and adequate resection should be adopted when possible.
In the conventional surgical approach for right liver lobe resections, operative procedures include the complete mobilization of the right liver and the control of the right hepatic vein outside the liver before parenchymal transection[51]. In the resection of large right liver tumors, which is frequently encountered in HBV-related HCC, these procedures may induce tumor rupture and the forceful squeezing of the tumor may induce intra- or extra-hepatic spread[52,53]. The anterior approach, a technique originally proposed by Ozawa[54] in 1990, involves vascular inflow control, completion of parenchymal transection, and complete venous outflow control without initial right liver mobilization. Its beneficial effects on the patients’ operative and survival outcomes have been evaluated prospectively. A randomized study[55] from Hong Kong University showed that for 109 patients with HCC of > 5 cm in diameter (97% being hepatitis B virus related-HCC), resection using an anterior approach is associated with lower blood loss and longer overall survival. The survival benefit appeared more obvious in patients with stage II disease and patients with vascular invasion of the tumor. After accumulating adequate experience, a surgeon may perform the anterior approach safely and effectively. This is the formal practice for most right hepatectomies in large HCC cases in our center.
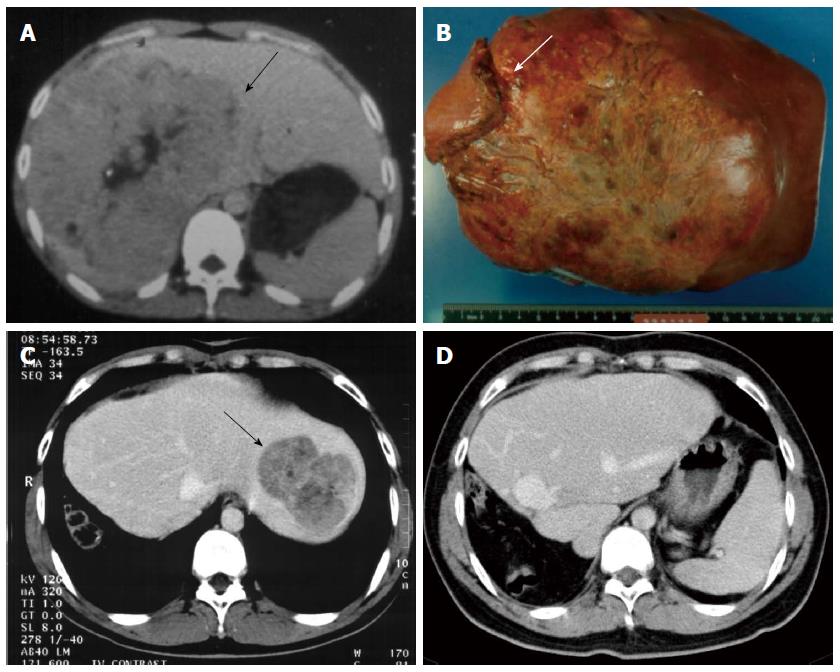
Tumor recurrence remains the major cause of death after curative resection for HCC. After hepatic resection, the majority of recurrences are due to residual tumor or intrahepatic tumor metastases and not due to metachronous tumors. Such recurrences tend to appear during the first 2 years of follow-up[15]. Author previous study[17] of 286 consecutive patients with recurrent HCC indicated that median survival times after tumor recurrences were 468 d; the overall 1-, 3-, 5-, and 10-year post-recurrence survival rates were 62%, 33%, 18%, and 9%, respectively. By multivariate analysis, repeated resectional treatment of recurrent tumors was an independent prognostic factor for improved post-recurrence survival rates, and some patients survived long-term after repeated resection, without recurrences or decompensation. Multiple studies[17,56,57] over the past decade support an aggressive approach for postoperative follow-up evaluation, and re-resection of recurrent tumors (either intrahepatic or extrahepatic lesions) remains the best treatment option to prolong patient survival and may also provide a chance for cure (Figure 2). Early detection of recurrent tumors is important. Continued imaging follow-up using contrast-enhanced CT or MR imaging is therefore necessary in the first two years after resection to detect recurrences. Gd-EOB-DTPA-enhanced MR imaging with hepatobiliary phase is especially useful for follow-up after HCC resection with respect to the assessment of new small lesions. For patients with high-risk of HCC recurrences (e.g., patients with macroscopic vascular invasion, multiple tumors, and positive resection margins), closer follow-up may have detected the recurrent tumors before they were no longer resection candidates.
Salvage liver transplantation is an evolving strategy to improve treatment results in patients with HCC and well-compensated cirrhosis. For patients with HCC who are within the Milan criteria and have cirrhosis, liver transplantation is the treatment of choice and shows favorable survival rates and low tumor recurrence rates[58]. However, due to the limited availability of donor livers and the prolonged waiting times, many patients were dropped out from the waiting lists as a result of tumor progression[59]. Recently, a strategy has been suggested for salvage transplantation using liver resection as the primary treatment for patients, followed by transplantation in the event of HCC recurrence or liver failure[60]. In 2008, Del Gaudio et al[61] from the University of Bologna presented their experience with the strategy of liver resection followed by salvage transplantation. They treated 227 cirrhotic patients with HCC who were transplant candidates, by performing 80 liver resections and 147 liver transplantations of 293 patients listed for transplantation. Among the 80 HCC patients eligible for transplantation undergoing liver resection, 39 (49%) developed HCC recurrences and 31% of them presented tumor recurrences outside the Milan criteria. Ten of the 39 patients underwent liver transplantation. The median time in this series between primary liver resection and secondary liver transplantation for HCC recurrence was 2.1 years. Comparable 5-year overall (62% vs 73%) survival rates were obtained for salvage liver transplantation and primary liver transplantation for HCC. The study concluded that liver transplantation after liver resection is safe and feasible with minimal operative mortality, similar operative morbidity, no increase risk of recurrence and similar long-term outcomes compared to primary liver transplantation. A recent meta-analysis[62] showed no statistically significant differences in the overall survival rates of salvage liver transplantation and primary liver transplantation. The main weak point of the idea of salvage transplantation is that the reported salvage transplantation rates have been low, ranging from 16.2% to 25%[63,64]. To solve this problem, careful follow-up with close image monitoring after liver resection is necessary in order to increase salvage liver transplantation rates, and bridge liver transplantation has subsequently been proposed[65]. With this strategy, HCC patients that had undergone liver resection with pathological parameters at higher risk of recurrence (i.e., microvascular invasion, satellite nodules or additional nodules) enter the waiting list for liver transplantation directly without waiting for evidence of HCC liver recurrence. Whether this policy is clinically effective and could further improve the long-term outcomes of resected patients remained to be elucidated in the future.
The aim of adjuvant therapy is to decrease the incidence or delay tumor recurrences, or to prevent new tumor formation in the liver remnant after hepatic resection. Many efforts to reduce recurrence risk after HCC resection have been made, including transarterial chemoembolization[66,67], systemic and loco-regional chemotherapy[68,69], I-131-lipiodol transarterial infusion[70], and immunotherapy[71]. Studies[72,73] evaluating the effects of adjuvant therapy on the prevention of postresectional HCC recurrences have shown divergent results, most of them have failed to achieve a significant survival benefit. In some studies, the overall survival at 5 years was even worse in the treatment arm[68,69]. At present, the role of adjuvant therapy after hepatic resection for HCC patients has not been well established. Because there is no definite effective adjuvant therapy for HCC patients, most centers only performing follow-up after hepatic resection. Recently, some light has been shed on this topic and a few encouraging reports have appeared. New treatment strategies and new effective drugs for the treatment of HCC are currently under active investigation as adjuvant therapy after curative resection for HCC. Prospective randomized controlled trials are underway to confirm the efficacy of the adjuvant protocols.
Hepatocellular carcinoma is a complex and heterogeneous disease with potentially diverse oncologic results after treatment. Adjuvant therapy after liver resection is particularly challenging because both factors, malignancy and underlying chronic liver disease, must be simultaneously considered[74]. Improved understanding of the biologic behavior of HCC and the development of suitable treatment strategies in well-selected subgroups of patients is the key to improve results of adjuvant therapy in this difficult disease.
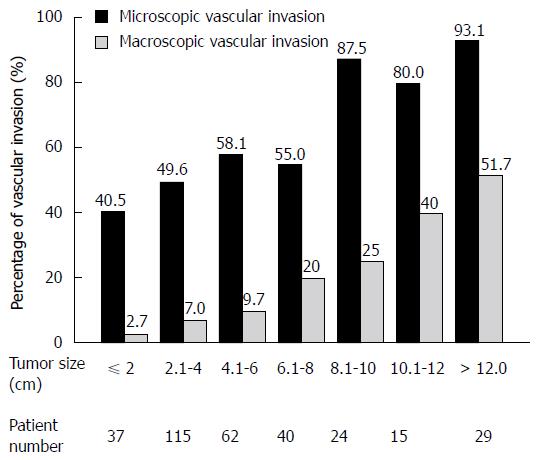
In HCC, tumor vascular invasion is an important biological phenomenon[75,76]. Tumor vascular invasion can be either macroscopic with microscopic or microscopic alone. Macroscopic vascular invasion is a well-established negative prognostic factor in HCC. In patients with resectable HCC, the presence of macroscopic tumor thrombus in portal veins indicates a poor prognosis[14,77,78]. When patients with HCC undergo liver transplantation, those with macroscopic or large-vessel tumor invasion invariably suffer tumor recurrence[79]. The role of microscopic vascular invasion, a relatively early-stage event of HCC vascular invasion, has been systematically evaluated[76]. Among 322 patients undergoing curative resection for HCC, 50 (15%) had macroscopic vascular invasion and 190 (59%) had microscopic invasion. Of 37 patients with tumor sizes of less than 2 cm, 15 (40.5%) had microscopic vascular invasion. The frequency of invasion increased with tumor size (Figure 3). Microscopic vascular invasion is an independent predictor of postresectional tumor recurrence and survival. Presence of microscopic vascular invasion is a common phenomenon in patients with resectable HCC. While macroscopic vascular invasion can be easily detected at imaging, microscopic vascular invasion is impossible to visualize before operation. Presence of microvascular invasion can only be confirmed by histological examination of resected specimen[37].
After HCC resection, the hepatic remnant is the most common site of tumor recurrence and is involved at diagnosis in 68%-91% of patients[47,80,81]. Because tumor vascular invasion is the most important factor related with recurrence after resection, a strategy of intraportal infusion chemotherapy was adopted to improve HCC patient postresectional outcomes. In 2006 the author reported a pilot study[82] of improved overall and disease-free survival for HCC patients with TNM stage I and stage II disease (based on the American Joint Committee on Cancer TNM staging system) after adjuvant intraportal infusional chemotherapy (IPIC). After hepatic resection, 28 HCC patients (IPIC group) underwent postresectional intraportal infusion of 5-fluorouracil (650 mg/m2), leukovorin (45 mg/m2), doxorubicin (10 mg/m2), and cisplatin (20 mg/m2) for 2 d. Treatment was repeated every 3 wk for 6 cycles. Patient outcomes were compared with those of 66 matched HCC patients (control group) who underwent hepatectomy without adjuvant therapy. The IPIC group patients received an average of 5.2 cycles of chemotherapy, starting 5-24 d after surgery. Five-year disease-free and overall survival rates for the IPIC group were 44.6% and 60.7%, respectively. Subgroup analysis of patients with stage I and II disease, showed that patients who received IPIC had a significantly lower recurrence risk, improved disease-free and overall survival rates compared with patients who received no adjuvant therapy. Patients with stage III disease did not benefit from IPIC. In the study, early intraportal chemotherapy after hepatic resection was not associated with a morbidity of severe hepatic dysfunction or hepatitis virus reactivation; this was because of the lower drug dosages used in this regimen compared with those used in systemic chemotherapy for patients with advanced HCC, because most of the patients had a good liver function reserve, and because therapy was given as a continuous infusion. Another interesting finding is that IPIC is effective only in patients with relatively early stage diseases (tumor-node-metastasis stage I or II disease). These results may be explained by the following hypothesis: In the study intraportal chemotherapy is designed to achieve high drug concentrations in portal venules. This treatment modality is expected to be most effective in eradicating residual microscopic disease in the liver remnant after HCC resection. It is likely that the clinical benefit of IPIC treatment limited to a subgroup of patients with relatively early-stage HCC, and this may explain findings that adjuvant IPIC improved survival for the patients with tumor-node-metastasis stage I and II disease. In patients with stage III disease, there is a high probability of postresectional residual disease due to intrahepatic and/or extrahepatic tumor metastases not detected by preoperative imagings. Intraportal infusional chemotherapy is difficult to achieve favorable effect on the complete eradication of residual disease in these patients[82].
When designing studies of postoperative adjuvant therapy aiming at preventing tumor recurrence in HCC patients, the distinction between recurrent disease after a curative operation, and residual disease after a palliative resection is crucial. In early stage HCCs, recurrent disease after a curative resection is most likely due to preoperative and intraoperative tumor micrometastases due to presence of microscopic vascular invasion. Given the fact that most chemotherapeutic agents for HCC exert modest efficacy in anticancer effect, and that appropriate dosing is critical to avoid drug-induced liver dysfunction, the target of adjuvant treatment in HCC patients should focus on the remaining micrometastases after a curative resection, which theoretically can be more easily eradicated with an early and low dose adjuvant protocol. In patients with advanced HCC, recurrence in the early postoperative period (i.e., within the first 3 mo) was frequently observed[83,84], presumably due to rapid progression of residual disease following a de facto incomplete resection. Tumor recurrence in the initiation period of adjuvant therapy results in early discontinuation of study drug treatment, and will preclude the observation of any potential benefit from the treatment protocol.
Novel targeted agents or anti-angiogenesis agents have recently been employed in the treatment of advanced HCC[85]. With its low liver toxicity, these agents have opened the way for possible long-term maintenance therapy for patients after HCC resection. Presently two randomized controlled adjuvant trials in patients with higher risks are undergoing. Sorafenib, a small-molecule multi-tyrosine-kinase inhibitor that impedes tumor growth and angiogenesis, has been shown to extend survival in patients with advanced HCC[86]. Efficacy of use of sorafenib as adjuvant setting for HCC patients is now under studied in a phase III randomized, double-blind placebo-controlled study. In this largest trial of adjuvant therapy for HCC, 1114 patients were randomized to receive either sorafenib treatment or placebo after curative resection or ablation (STORM trial: http://clinicaltrials.gov.com, NCT00692770). Primary endpoint of the trial is recurrence-free survival. The study initiated in August 2008. Final data collection for primary outcome measure is estimated by May 2014. The key selection criteria[87] for patient enrollment in the STORM trial include: (1) patient liver function must be Child-Pugh score 5-7 points. A Child-Pugh score of 7 points is allowed only in the absence of ascites; (2) no demonstrable residual tumors on the eligibility CT or MRI scan by independent radiological review conducted 3-7 wk after surgery, to ensure absence of residual disease before initiation of adjuvant treatment; (3) an Eastern Cooperative Oncology Group performance of 0; (4) for surgical resection, tumor(s) must be single lesion or 2-3 lesions, each ≤ 3 cm. Patients with single lesions < 2 cm without microscopic vascular invasion, or patients with tumor macroscopic vascular invasion was excluded; and (5) patients must be able to initiate adjuvant therapy within 6-12 wk after hepatectomy. It is conceivable that for the STORM trial the main targets of adjuvant treatment are tumor micrometastases due to presence of microscopic vascular invasion after a potentially curative resection.
In another clinical trial, 500 HCC patients are tested using PI-88 for adjuvant therapy after curative resection. PI-88 is a heparanase inhibitor which has shown to exert anti-angiogenic and anti-metastatic effects[88]. A phase II study showed the preliminary efficacy of PI-88 as an adjuvant therapy for patients after curative resection for HCC[89]. A prospective, randomized, double-blind, placebo controlled, phase III trial of PI-88 in the adjuvant treatment of subjects with hepatitis virus related HCC after surgical resection (PATRON trial)(ClinicalTrials.gov Identifier: NCT01402908) is now underway. In this study, only patients with either HBV-related and/or HCV-related HCC were enrolled, to avoid the heterogeneity of study patients with different etiologies for chronic liver disease and associated HCC. The primary endpoint of the study is disease-free survival. The study initiated in August 2011 and the estimated study completion date is December 2015.
Hopefully answers to the questions of the role and the feasibility of adjuvant therapy after HCC resection will be revealed when the results of these ongoing trials become available.
Another challenge in HBV-related HCC patients undergoing hepatic resection is the underlying hepatitis B infection. For patients with chronic HBV infection, antiviral therapy, both interferon and nucleoside/nucleotide analogs, have been shown to decrease the incidence of HCC[90,91]. Recently, accumulating evidence has shown that recurrence of HBV-related HCC is associated with high viral load at the time of resection[92,93]. In our institution a retrospective study[94] of 193 patients who underwent surgical resection for HBV-related HCC showed that 69% had HCC recurrences after a median follow-up of 58 mo; 71% of the recurrences occurred within the first 2 years after resection. Multivariate analysis showed that serum HBV DNA greater than 106 copies/mL is independently associated with tumor recurrences. Liver resection could reactivate HBV replication, especially in patients who did not receive any antiviral therapy[95]. In a retrospective analysis of 42 patients, Chan et al[96] showed that posthepatectomy antiviral treatment improved the prognosis of HBV-related HCC. In this study survival benefit was observed only in patients with early-stage tumors (TNM stages I and II tumors or HCCs without major vascular invasion). In a study reported by Wu et al[97] in 2012, using data from the Taiwan National Health Insurance Research Database, the authors identified 4569 HCC patients who received curative liver resections between 2003 and 2010 (including 518 patients from the treated cohort who had received an HBV nucleoside analogue-lamivudine, entecavir, or telbivudine-for at least 90 d, and 4051 patients from the untreated cohort).The authors found that 21% in the treated cohort and 44% in the untreated cohort had HCC recurrences. Overall mortality was lower in the treated cohort, with a 6-year cumulative mortality of 29% vs 42% in the untreated cohort. The results of this study seem to support the strategy of using antiviral treatment with nucleotide or nucleoside analogues in HBV-related HCC patients to decrease tumor recurrences after surgical resection. However, up to now, no single large randomized trial proved the benefit of antiviral treatment using nucleoside analogs as adjuvant therapy after curative treatment of HBV-related HCC. Clinical evidence is still not definite enough to answer the question of whether antiviral therapy after curative resection of hepatitis B-related HCC will prevent disease recurrences. Firstly, the study by Wu et al[97]. had some limitations concerning the details of study information. The authors failed to indicate that whether patient and tumor baseline characteristics (including tumor staging, liver function, performance status, and surgical performance), were comparable in the treated and untreated cohorts. These factors are critical to understand the contribution of antiviral treatment in predicting better patient outcomes, without confounding preselection of the untreated cohort based on different patient or tumor background characteristics. Secondly, the adjuvant therapy effects of antiviral treatment depend on preventing de novo primary tumors in the liver and on the progression of liver disease. Obviously administration of antiviral therapy after HCC surgery cannot prevent intrahepatic early recurrences, or recurrence outside of the liver, which account for at least 70% and 10% of HCC recurrences after resection, respectively[17,81]. Thirdly, the development of viral resistance to antiviral drugs may be a concern that should be avoided, particularly in subjects with potentially cured HCC[21]. Further studies including prospective, randomized controlled trials are necessary to establish the role for postoperative antiviral treatment in HBV-related HCC patients after surgical resection.
Hepatectomy is the best therapeutic choice for HBV-related HCC patients. Clinicians are searching for practical ways to improve patient outcome after resection. Evolving strategies and emerging therapies, including a policy of accurate preoperative staging, adequate resection, intensive follow-up, and early initiation of effective adjuvant therapy will continue to develop in the future.
The author thanks Yi-Chen Yeh, MD, Department of Pathology, Taipei Veterans General Hospital, and Ivy Yenwen Chau, BS, Poznan University of Medical Sciences Medical Faculty II, for their assistance in preparing the manuscript.
P- Reviewer: Aghakhani A, Chintana PY S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN
| 1. | Bosch FX, Ribes J, Díaz M, Cléries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5-S16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1799] [Cited by in RCA: 1816] [Article Influence: 86.5] [Reference Citation Analysis (0)] |
| 2. | Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907-1917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3241] [Cited by in RCA: 3282] [Article Influence: 149.2] [Reference Citation Analysis (0)] |
| 3. | But DY, Lai CL, Yuen MF. Natural history of hepatitis-related hepatocellular carcinoma. World J Gastroenterol. 2008;14:1652-1656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 145] [Cited by in RCA: 157] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 4. | Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol. 2008;48:335-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 909] [Cited by in RCA: 967] [Article Influence: 56.9] [Reference Citation Analysis (0)] |
| 5. | Lim SG, Mohammed R, Yuen MF, Kao JH. Prevention of hepatocellular carcinoma in hepatitis B virus infection. J Gastroenterol Hepatol. 2009;24:1352-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | Pollicino T, Saitta C, Raimondo G. Hepatocellular carcinoma: the point of view of the hepatitis B virus. Carcinogenesis. 2011;32:1122-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 7. | Pawlik TM, Poon RT, Abdalla EK, Ikai I, Nagorney DM, Belghiti J, Kianmanesh R, Ng IO, Curley SA, Yamaoka Y. Hepatectomy for hepatocellular carcinoma with major portal or hepatic vein invasion: results of a multicenter study. Surgery. 2005;137:403-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 189] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 8. | Andreou A, Vauthey JN, Cherqui D, Zimmitti G, Ribero D, Truty MJ, Wei SH, Curley SA, Laurent A, Poon RT. Improved long-term survival after major resection for hepatocellular carcinoma: a multicenter analysis based on a new definition of major hepatectomy. J Gastrointest Surg. 2013;17:66-77; discussion 77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 9. | Chau GY, Lui WY, King KL, Wu CW. Evaluation of effect of hemihepatic vascular occlusion and the Pringle maneuver during hepatic resection for patients with hepatocellular carcinoma and impaired liver function. World J Surg. 2005;29:1374-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Hsu KY, Chau GY, Lui WY, Tsay SH, King KL, Wu CW. Predicting morbidity and mortality after hepatic resection in patients with hepatocellular carcinoma: the role of Model for End-Stage Liver Disease score. World J Surg. 2009;33:2412-2419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Dahiya D, Wu TJ, Lee CF, Chan KM, Lee WC, Chen MF. Minor versus major hepatic resection for small hepatocellular carcinoma (HCC) in cirrhotic patients: a 20-year experience. Surgery. 2010;147:676-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 110] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 12. | Torzilli G, Makuuchi M, Inoue K, Takayama T, Sakamoto Y, Sugawara Y, Kubota K, Zucchi A. No-mortality liver resection for hepatocellular carcinoma in cirrhotic and noncirrhotic patients: is there a way? A prospective analysis of our approach. Arch Surg. 1999;134:984-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 352] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 13. | Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, Yeung C, Wong J. Hepatectomy for hepatocellular carcinoma: toward zero hospital deaths. Ann Surg. 1999;229:322-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 542] [Cited by in RCA: 559] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 14. | Lei HJ, Chau GY, Lui WY, Tsay SH, King KL, Loong CC, Wu CW. Prognostic value and clinical relevance of the 6th Edition 2002 American Joint Committee on Cancer staging system in patients with resectable hepatocellular carcinoma. J Am Coll Surg. 2006;203:426-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Chang CH, Chau GY, Lui WY, Tsay SH, King KL, Wu CW. Long-term results of hepatic resection for hepatocellular carcinoma originating from the noncirrhotic liver. Arch Surg. 2004;139:320-335; discussion 326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 85] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Fan ST, Mau Lo C, Poon RT, Yeung C, Leung Liu C, Yuen WK, Ming Lam C, Ng KK, Ching Chan S. Continuous improvement of survival outcomes of resection of hepatocellular carcinoma: a 20-year experience. Ann Surg. 2011;253:745-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 290] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 17. | Chen WT, Chau GY, Lui WY, Tsay SH, King KL, Loong CC, Wu CW. Recurrent hepatocellular carcinoma after hepatic resection: prognostic factors and long-term outcome. Eur J Surg Oncol. 2004;30:414-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 89] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Ercolani G, Grazi GL, Ravaioli M, Del Gaudio M, Gardini A, Cescon M, Varotti G, Cetta F, Cavallari A. Liver resection for hepatocellular carcinoma on cirrhosis: univariate and multivariate analysis of risk factors for intrahepatic recurrence. Ann Surg. 2003;237:536-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 262] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 19. | Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4333] [Cited by in RCA: 4508] [Article Influence: 225.4] [Reference Citation Analysis (0)] |
| 20. | Lok AS. Hepatitis B: liver fibrosis and hepatocellular carcinoma. Gastroenterol Clin Biol. 2009;33:911-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Cescon M, Cucchetti A, Grazi GL, Ferrero A, Viganò L, Ercolani G, Ravaioli M, Zanello M, Andreone P, Capussotti L. Role of hepatitis B virus infection in the prognosis after hepatectomy for hepatocellular carcinoma in patients with cirrhosis: a Western dual-center experience. Arch Surg. 2009;144:906-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Chen CH, Huang GT, Yang PM, Chen PJ, Lai MY, Chen DS, Wang JD, Sheu JC. Hepatitis B- and C-related hepatocellular carcinomas yield different clinical features and prognosis. Eur J Cancer. 2006;42:2524-2529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Hu Z, Zhou J, Wang H, Zhang M, Li S, Huang Y, Wu J, Li Z, Zhou L, Zheng S. Survival in liver transplant recipients with hepatitis B- or hepatitis C-associated hepatocellular carcinoma: the Chinese experience from 1999 to 2010. PLoS One. 2013;8:e61620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Xuan SY, Xin YN, Chen H, Shi GJ, Guan HS, Li Y. Significance of hepatitis B virus surface antigen, hepatitis C virus expression in hepatocellular carcinoma and pericarcinomatous tissues. World J Gastroenterol. 2007;13:1870-1874. [PubMed] |
| 25. | Messerini L, Novelli L, Comin CE. Microvessel density and clinicopathological characteristics in hepatitis C virus and hepatitis B virus related hepatocellular carcinoma. J Clin Pathol. 2004;57:867-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Rampone B, Schiavone B, Martino A, Viviano C, Confuorto G. Current management strategy of hepatocellular carcinoma. World J Gastroenterol. 2009;15:3210-3216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 94] [Cited by in RCA: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 27. | Chang WT, Kao WY, Chau GY, Su CW, Lei HJ, Wu JC, Hsia CY, Lui WY, King KL, Lee SD. Hepatic resection can provide long-term survival of patients with non-early-stage hepatocellular carcinoma: extending the indication for resection? Surgery. 2012;152:809-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 28. | Young AL, Malik HZ, Abu-Hilal M, Guthrie JA, Wyatt J, Prasad KR, Toogood GJ, Lodge JP. Large hepatocellular carcinoma: time to stop preoperative biopsy. J Am Coll Surg. 2007;205:453-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Vauthey JN, Dixon E, Abdalla EK, Helton WS, Pawlik TM, Taouli B, Brouquet A, Adams RB. Pretreatment assessment of hepatocellular carcinoma: expert consensus statement. HPB (Oxford). 2010;12:289-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 150] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 30. | Ayyappan AP, Jhaveri KS. CT and MRI of hepatocellular carcinoma: an update. Expert Rev Anticancer Ther. 2010;10:507-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Freeny PC, Grossholz M, Kaakaji K, Schmiedl UP. Significance of hyperattenuating and contrast-enhancing hepatic nodules detected in the cirrhotic liver during arterial phase helical CT in pre-liver transplant patients: radiologic-histopathologic correlation of explanted livers. Abdom Imaging. 2003;28:333-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Colli A, Fraquelli M, Casazza G, Massironi S, Colucci A, Conte D, Duca P. Accuracy of ultrasonography, spiral CT, magnetic resonance, and alpha-fetoprotein in diagnosing hepatocellular carcinoma: a systematic review. Am J Gastroenterol. 2006;101:513-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 388] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 33. | Willatt JM, Hussain HK, Adusumilli S, Marrero JA. MR Imaging of hepatocellular carcinoma in the cirrhotic liver: challenges and controversies. Radiology. 2008;247:311-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 270] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 34. | Yoo SH, Choi JY, Jang JW, Bae SH, Yoon SK, Kim DG, Yoo YK, Rha SE, Lee YJ, Jung ES. Gd-EOB-DTPA-enhanced MRI is better than MDCT in decision making of curative treatment for hepatocellular carcinoma. Ann Surg Oncol. 2013;20:2893-2900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 35. | Battaglia V, Bozzi E, Zingoni G, Bartolozzi C, Pisa IT. Correlation between histologic diagnosis ad MR signal intensity after Gd-EOB-DTPA administration of nodules detected within cirrhotic explanted livers: retrospective analysis. Eur Radiol. 2010;20 Suppl 1:S25. |
| 36. | Hammerstingl R, Huppertz A, Breuer J, Balzer T, Blakeborough A, Carter R, Fusté LC, Heinz-Peer G, Judmaier W, Laniado M. Diagnostic efficacy of gadoxetic acid (Primovist)-enhanced MRI and spiral CT for a therapeutic strategy: comparison with intraoperative and histopathologic findings in focal liver lesions. Eur Radiol. 2008;18:457-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 313] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 37. | Chau GY, Lui WY, Wu CW. Spectrum and significance of microscopic vascular invasion in hepatocellular carcinoma. Surg Oncol Clin N Am. 2003;12:25-34, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 38. | Lui WY, Chau GY, Loong CC, Tsay SH, Wu JC, King KL, Chiu JH, Lai CR, P’eng FK. Hepatic segmentectomy for curative resection of primary hepatocellular carcinoma. Arch Surg. 1995;130:1090-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 39. | Makuuchi M, Hasegawa H, Yamazaki S. Ultrasonically guided subsegmentectomy. Surg Gynecol Obstet. 1985;161:346-350. [PubMed] |
| 40. | Regimbeau JM, Kianmanesh R, Farges O, Dondero F, Sauvanet A, Belghiti J. Extent of liver resection influences the outcome in patients with cirrhosis and small hepatocellular carcinoma. Surgery. 2002;131:311-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 219] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 41. | Hasegawa K, Kokudo N, Imamura H, Matsuyama Y, Aoki T, Minagawa M, Sano K, Sugawara Y, Takayama T, Makuuchi M. Prognostic impact of anatomic resection for hepatocellular carcinoma. Ann Surg. 2005;242:252-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 503] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 42. | Shindoh J, Hasegawa K, Inoue Y, Ishizawa T, Nagata R, Aoki T, Sakamoto Y, Sugawara Y, Makuuchi M, Kokudo N. Risk factors of post-operative recurrence and adequate surgical approach to improve long-term outcomes of hepatocellular carcinoma. HPB (Oxford). 2013;15:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 101] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 43. | Cucchetti A, Cescon M, Ercolani G, Bigonzi E, Torzilli G, Pinna AD. A comprehensive meta-regression analysis on outcome of anatomic resection versus nonanatomic resection for hepatocellular carcinoma. Ann Surg Oncol. 2012;19:3697-3705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 44. | Lee CS, Sung JL, Hwang LY, Sheu JC, Chen DS, Lin TY, Beasley RP. Surgical treatment of 109 patients with symptomatic and asymptomatic hepatocellular carcinoma. Surgery. 1986;99:481-490. [PubMed] |
| 45. | Chau GY, Lui WY, Tsay SH, King KL, Loong CC, Chiu JH, Wu CW, P’eng FK. Prognostic significance of surgical margin in hepatocellular carcinoma resection: an analysis of 165 Childs’ A patients. J Surg Oncol. 1997;66:122-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 46. | Shi M, Guo RP, Lin XJ, Zhang YQ, Chen MS, Zhang CQ, Lau WY, Li JQ. Partial hepatectomy with wide versus narrow resection margin for solitary hepatocellular carcinoma: a prospective randomized trial. Ann Surg. 2007;245:36-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 404] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 47. | Chen MF, Hwang TL, Jeng LB, Wang CS, Jan YY, Chen SC. Postoperative recurrence of hepatocellular carcinoma. Two hundred five consecutive patients who underwent hepatic resection in 15 years. Arch Surg. 1994;129:738-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 132] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 48. | Masutani S, Sasaki Y, Imaoka S, Iwamoto S, Ohashi I, Kameyama M, Kabuto T, Ishikawa O, Furukawa H, Koyama H. The prognostic significance of surgical margin in liver resection of patients with hepatocellular carcinoma. Arch Surg. 1994;129:1025-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 49. | Nara S, Shimada K, Sakamoto Y, Esaki M, Kishi Y, Kosuge T, Ojima H. Prognostic impact of marginal resection for patients with solitary hepatocellular carcinoma: evidence from 570 hepatectomies. Surgery. 2012;151:526-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 50. | Matsui Y, Terakawa N, Satoi S, Kaibori M, Kitade H, Takai S, Kwon AH, Kamiyama Y. Postoperative outcomes in patients with hepatocellular carcinomas resected with exposure of the tumor surface: clinical role of the no-margin resection. Arch Surg. 2007;142:596-602; discussion 603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 51. | Makuuchi M, Yamamoto J, Takayama T, Kosuge T, Gunvén P, Yamazaki S, Hasegawa H. Extrahepatic division of the right hepatic vein in hepatectomy. Hepatogastroenterology. 1991;38:176-179. [PubMed] |
| 52. | Li CH, Chau GY, Lui WY, Tsay SH, King KL, Hsia CY, Wu CW. Risk factors associated with intra-operative major blood loss in patients with hepatocellular carcinoma who underwent hepatic resection. J Chin Med Assoc. 2003;66:669-675. [PubMed] |
| 53. | Lai EC, Fan ST, Lo CM, Chu KM, Liu CL. Anterior approach for difficult major right hepatectomy. World J Surg. 1996;20:314-317; discussion 318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 120] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 54. | Ozawa K. Hepatic function and liver resection. J Gastroenterol Hepatol. 1990;5:296-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 75] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 55. | Liu CL, Fan ST, Cheung ST, Lo CM, Ng IO, Wong J. Anterior approach versus conventional approach right hepatic resection for large hepatocellular carcinoma: a prospective randomized controlled study. Ann Surg. 2006;244:194-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 189] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 56. | Shah SA, Cleary SP, Wei AC, Yang I, Taylor BR, Hemming AW, Langer B, Grant DR, Greig PD, Gallinger S. Recurrence after liver resection for hepatocellular carcinoma: risk factors, treatment, and outcomes. Surgery. 2007;141:330-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 340] [Article Influence: 17.9] [Reference Citation Analysis (1)] |
| 57. | Wu CC, Cheng SB, Yeh DC, Wang J, P’eng FK. Second and third hepatectomies for recurrent hepatocellular carcinoma are justified. Br J Surg. 2009;96:1049-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 58. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5110] [Cited by in RCA: 5313] [Article Influence: 183.2] [Reference Citation Analysis (0)] |
| 59. | Yao FY, Bass NM, Nikolai B, Davern TJ, Kerlan R, Wu V, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: analysis of survival according to the intention-to-treat principle and dropout from the waiting list. Liver Transpl. 2002;8:873-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 312] [Article Influence: 13.6] [Reference Citation Analysis (1)] |
| 60. | Majno PE, Sarasin FP, Mentha G, Hadengue A. Primary liver resection and salvage transplantation or primary liver transplantation in patients with single, small hepatocellular carcinoma and preserved liver function: an outcome-oriented decision analysis. Hepatology. 2000;31:899-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 262] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 61. | Del Gaudio M, Ercolani G, Ravaioli M, Cescon M, Lauro A, Vivarelli M, Zanello M, Cucchetti A, Vetrone G, Tuci F. Liver transplantation for recurrent hepatocellular carcinoma on cirrhosis after liver resection: University of Bologna experience. Am J Transplant. 2008;8:1177-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 125] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 62. | Hu Z, Wang W, Li Z, Ye S, Zheng SS. Recipient outcomes of salvage liver transplantation versus primary liver transplantation: a systematic review and meta-analysis. Liver Transpl. 2012;18:1316-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 63. | Adam R, Azoulay D, Castaing D, Eshkenazy R, Pascal G, Hashizume K, Samuel D, Bismuth H. Liver resection as a bridge to transplantation for hepatocellular carcinoma on cirrhosis: a reasonable strategy? Ann Surg. 2003;238:508-518; discussion 518-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 239] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 64. | Margarit C, Escartín A, Castells L, Vargas V, Allende E, Bilbao I. Resection for hepatocellular carcinoma is a good option in Child-Turcotte-Pugh class A patients with cirrhosis who are eligible for liver transplantation. Liver Transpl. 2005;11:1242-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 161] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 65. | Sala M, Fuster J, Llovet JM, Navasa M, Solé M, Varela M, Pons F, Rimola A, García-Valdecasas JC, Brú C. High pathological risk of recurrence after surgical resection for hepatocellular carcinoma: an indication for salvage liver transplantation. Liver Transpl. 2004;10:1294-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 230] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 66. | Wu CC, Ho YZ, Ho WL, Wu TC, Liu TJ, P’eng FK. Preoperative transcatheter arterial chemoembolization for resectable large hepatocellular carcinoma: a reappraisal. Br J Surg. 1995;82:122-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 175] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 67. | Izumi R, Shimizu K, Iyobe T, Ii T, Yagi M, Matsui O, Nonomura A, Miyazaki I. Postoperative adjuvant hepatic arterial infusion of Lipiodol containing anticancer drugs in patients with hepatocellular carcinoma. Hepatology. 1994;20:295-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 91] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 68. | Hasegawa K, Takayama T, Ijichi M, Matsuyama Y, Imamura H, Sano K, Sugawara Y, Kokudo N, Makuuchi M. Uracil-tegafur as an adjuvant for hepatocellular carcinoma: a randomized trial. Hepatology. 2006;44:891-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 69. | Lai EC, Lo CM, Fan ST, Liu CL, Wong J. Postoperative adjuvant chemotherapy after curative resection of hepatocellular carcinoma: a randomized controlled trial. Arch Surg. 1998;133:183-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 131] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 70. | Lau WY, Lai EC, Leung TW, Yu SC. Adjuvant intra-arterial iodine-131-labeled lipiodol for resectable hepatocellular carcinoma: a prospective randomized trial-update on 5-year and 10-year survival. Ann Surg. 2008;247:43-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 71. | Takayama T, Sekine T, Makuuchi M, Yamasaki S, Kosuge T, Yamamoto J, Shimada K, Sakamoto M, Hirohashi S, Ohashi Y. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet. 2000;356:802-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 630] [Cited by in RCA: 653] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 72. | Chau GY. Adjuvant therapy for patients with hepatocellular carcinoma undergoing hepatic resection. Formas J Surg. 2009;42:133-138. |
| 73. | Lau WY, Lai EC, Lau SH. The current role of neoadjuvant/adjuvant/chemoprevention therapy in partial hepatectomy for hepatocellular carcinoma: a systematic review. Hepatobiliary Pancreat Dis Int. 2009;8:124-133. [PubMed] |
| 74. | Chau GY, Wu CW, Lui WY, Chang TJ, Kao HL, Wu LH, King KL, Loong CC, Hsia CY, Chi CW. Serum interleukin-10 but not interleukin-6 is related to clinical outcome in patients with resectable hepatocellular carcinoma. Ann Surg. 2000;231:552-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 121] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 75. | Vauthey JN, Klimstra D, Franceschi D, Tao Y, Fortner J, Blumgart L, Brennan M. Factors affecting long-term outcome after hepatic resection for hepatocellular carcinoma. Am J Surg. 1995;169:28-34; discussion 34-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 219] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 76. | Tsai TJ, Chau GY, Lui WY, Tsay SH, King KL, Loong CC, Hsia CY, Wu CW. Clinical significance of microscopic tumor venous invasion in patients with resectable hepatocellular carcinoma. Surgery. 2000;127:603-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 197] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 77. | Ikai I, Yamaoka Y, Yamamoto Y, Ozaki N, Sakai Y, Satoh S, Shinkura N, Yamamoto M. Surgical intervention for patients with stage IV-A hepatocellular carcinoma without lymph node metastasis: proposal as a standard therapy. Ann Surg. 1998;227:433-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 78] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 78. | Shi J, Lai EC, Li N, Guo WX, Xue J, Lau WY, Wu MC, Cheng SQ. Surgical treatment of hepatocellular carcinoma with portal vein tumor thrombus. Ann Surg Oncol. 2010;17:2073-2080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 222] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 79. | Marsh JW, Dvorchik I, Subotin M, Balan V, Rakela J, Popechitelev EP, Subbotin V, Casavilla A, Carr BI, Fung JJ. The prediction of risk of recurrence and time to recurrence of hepatocellular carcinoma after orthotopic liver transplantation: a pilot study. Hepatology. 1997;26:444-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 153] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 80. | Hung HH, Lei HJ, Chau GY, Su CW, Hsia CY, Kao WY, Lui WY, Wu WC, Lin HC, Wu JC. Milan criteria, multi-nodularity, and microvascular invasion predict the recurrence patterns of hepatocellular carcinoma after resection. J Gastrointest Surg. 2013;17:702-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 81. | Ho CM, Lee PH, Shau WY, Ho MC, Wu YM, Hu RH. Survival in patients with recurrent hepatocellular carcinoma after primary hepatectomy: comparative effectiveness of treatment modalities. Surgery. 2012;151:700-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 82. | Chau GY, Lui WY, Tsay SH, Chao Y, King KL, Wu CW. Postresectional adjuvant intraportal chemotherapy in patients with hepatocellular carcinoma: a case-control study. Ann Surg Oncol. 2006;13:1329-1337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 83. | Regimbeau JM, Abdalla EK, Vauthey JN, Lauwers GY, Durand F, Nagorney DM, Ikai I, Yamaoka Y, Belghiti J. Risk factors for early death due to recurrence after liver resection for hepatocellular carcinoma: results of a multicenter study. J Surg Oncol. 2004;85:36-41. [PubMed] |
| 84. | Chen XH, Zhang BH, Xin Y, Ren ZG, Fan J, Qiu SJ, Zhou J. Risk factors for residual tumor after resection of hepatocellular carcinoma. World J Gastroenterol. 2011;17:1889-1894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 85. | Villanueva A, Llovet JM. Targeted therapies for hepatocellular carcinoma. Gastroenterology. 2011;140:1410-1426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 369] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 86. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10272] [Article Influence: 604.2] [Reference Citation Analysis (2)] |
| 87. | Printz C. Clinical trials of note. Sorafenib as adjuvant treatment in the prevention of disease recurrence in patients with hepatocellular carcinoma (HCC) (STORM). Cancer. 2009;115:4646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 88. | Ferro V, Dredge K, Liu L, Hammond E, Bytheway I, Li C, Johnstone K, Karoli T, Davis K, Copeman E. PI-88 and novel heparan sulfate mimetics inhibit angiogenesis. Semin Thromb Hemost. 2007;33:557-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 118] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 89. | Liu CJ, Lee PH, Lin DY, Wu CC, Jeng LB, Lin PW, Mok KT, Lee WC, Yeh HZ, Ho MC. Heparanase inhibitor PI-88 as adjuvant therapy for hepatocellular carcinoma after curative resection: a randomized phase II trial for safety and optimal dosage. J Hepatol. 2009;50:958-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 121] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 90. | Lin SM, Sheen IS, Chien RN, Chu CM, Liaw YF. Long-term beneficial effect of interferon therapy in patients with chronic hepatitis B virus infection. Hepatology. 1999;29:971-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 324] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 91. | Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, Tanwandee T, Tao QM, Shue K, Keene ON, Dixon JS, Gray DF, Sabbat J. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1739] [Cited by in RCA: 1740] [Article Influence: 82.9] [Reference Citation Analysis (0)] |
| 92. | Hung IF, Poon RT, Lai CL, Fung J, Fan ST, Yuen MF. Recurrence of hepatitis B-related hepatocellular carcinoma is associated with high viral load at the time of resection. Am J Gastroenterol. 2008;103:1663-1673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 157] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 93. | Wei Q, Xu X, Ling Q, Zhou B, Zheng SS. Perioperative antiviral therapy for chronic hepatitis B-related hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2013;12:251-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 94. | Wu JC, Huang YH, Chau GY, Su CW, Lai CR, Lee PC, Huo TI, Sheen IJ, Lee SD, Lui WY. Risk factors for early and late recurrence in hepatitis B-related hepatocellular carcinoma. J Hepatol. 2009;51:890-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 355] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 95. | Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, Huang GT, Iloeje UH. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2309] [Cited by in RCA: 2365] [Article Influence: 124.5] [Reference Citation Analysis (0)] |
| 96. | Chan AC, Chok KS, Yuen WK, Chan SC, Poon RT, Lo CM, Fan ST. Impact of antiviral therapy on the survival of patients after major hepatectomy for hepatitis B virus-related hepatocellular carcinoma. Arch Surg. 2011;146:675-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 97. | Wu CY, Chen YJ, Ho HJ, Hsu YC, Kuo KN, Wu MS, Lin JT. Association between nucleoside analogues and risk of hepatitis B virus–related hepatocellular carcinoma recurrence following liver resection. JAMA. 2012;308:1906-1914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 657] [Cited by in RCA: 695] [Article Influence: 53.5] [Reference Citation Analysis (0)] |