Published online Sep 7, 2014. doi: 10.3748/wjg.v20.i33.11496
Revised: March 29, 2014
Accepted: May 23, 2014
Published online: September 7, 2014
Processing time: 198 Days and 17.8 Hours
Ulcerative colitis (UC) is a condition at increased risk for colorectal carcinoma (CRC) development. Nowadays, screening and follow-up programs are routinely performed worldwide to promote the early detection of CRCs in subjects with well known risk factors (extent, duration and severity of the disorder). The diffusion of these procedures is presumably the main reason for the marked reduction of cancer incidence and mortality in the course of UC. In addition, chemoprevention has been widely investigated and developed in many medical fields, and aspirin has shown a preventive effect against CRC, while mesalazine has been strongly invoked as a potential chemopreventive agent in UC. However, available studies show some limitations due to the obvious ethical implications of drug withdrawal in UC in order to design a control group. The estrogen receptors (ER) alpha/beta balance seems to have a relevant influence on colorectal carcinogenesis and ER beta appears to parallel apoptosis, and hence an anti-carcinogenic effect. Phytoestrogens are compounds acting as ER beta agonists and have shown a promising chemopreventive effect on sporadic as well as genetically inherited CRC. There is evidence suggesting a role for ERs in UC-related carcinogenesis. In this perspective, since these substances can be considered as dietary supplements and are completely free from side effects, phytoestrogens could be an interesting option for CRC prevention, even when the disease is a consequence of long-term chronic inflammation, as in the course of UC. Further studies of their effects are warranted in both the basic research and clinical fields.
Core tip: The present work outlines the main data regarding a possible involvement of estrogen receptors in colorectal carcinogenesis, paying particular attention to cancer arising in the course of ulcerative colitis. A protective role for beta receptors has been suggested by many studies. The challenge for the future could be to devise chemopreventive strategies against colorectal carcinoma employing estrogen receptor beta agonists, such as phytoestrogens.
- Citation: Principi M, Barone M, Pricci M, De Tullio N, Losurdo G, Ierardi E, Di Leo A. Ulcerative colitis: From inflammation to cancer. Do estrogen receptors have a role? World J Gastroenterol 2014; 20(33): 11496-11504
- URL: https://www.wjgnet.com/1007-9327/full/v20/i33/11496.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i33.11496
Ulcerative colitis (UC) is associated with an increased risk of colorectal cancer (CRC), which has been related to the long-standing chronic inflammation[1]. However, the magnitude of the risk is difficult to estimate, as many factors may bias study results[2] (i.e., patient selection, number of patients, completeness of case recruitment and ascertainment and duration of follow-up)[2,3].
Castańo-Milla et al[4] reported an overall incidence rate of CRC in UC of 1.67/1000 per year of disease (PYD) and incidence rates per decade were estimated at 1.01/1000, 3.75/1000 and 5.85/1000 PYD for the first, second and third decades, respectively. In a meta-analysis of prospective population-based studies, Jess et al[5] found that an average of 1.6% of patients with UC were diagnosed with CRC during the first 14 years of follow-up, and the estimated standardized incidence ratio (SIR) was 2.39 (2.1-2.7). Recent time-trend studies also demonstrate a decreasing risk of CRC in UC patients[6]. In a recent meta-analysis[4] the incidence rate was found to have decreased from 4.29/1000 PYD in studies published in the 1950s to 1.09/1000 PYD in the studies published between 2000 and 2011.
As known, reported risk factors for CRC include extensive disease[7,8], young age at diagnosis[9], a family history of CRC[10], co-existing primary sclerosing cholangitis (PSC)[11] and persistent inflammation of the colon[12,13].
The pathophysiology of colitis-associated cancer suggests the action of numerous positive and negative regulators[14]. Positive regulators are pro-carcinogenic cytokines such as tumor necrosis factor alpha (TNF alpha), that is over-expressed in a murine model of carcinoma arising on colitis[15], interleukin (IL)-6[16] and IL-21[17] and chemokines such as CCL2, whose expression is enhanced by TNF alpha, causing the recruitment of macrophages and monocytes[18]. Negative regulators include IL-10[19,20], transforming growth factor beta (TGF beta)[21] and MyD88, a Toll-like receptor adaptor, that has been found to significantly reduce tumor number and size in the Apcmin/+ mouse model of intestinal tumorigenesis[22,23].
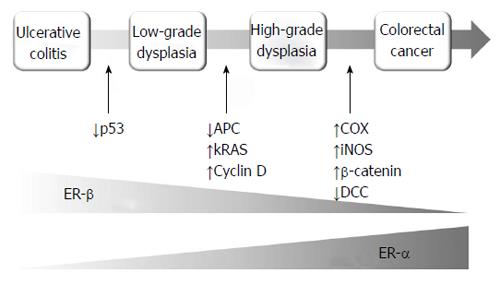
The progression from UC to CRC is a multistep process in which the accumulation of genetic mutations leads to the sequential evolution to low-grade dysplasia (LGD), high-grade dysplasia (HGD) and finally to cancer[24]. The p53 tumor suppressor gene appears to be a key factor in the initial steps of UC-associated colorectal carcinogenesis, being the most frequent single founding mutation in UC associated CRC[25]. p53 is overexpressed in 33%-67% of patients with dysplasia and in 83%-95% of patients with UC-associated CRC[26,27]. Other genes that undergo mutation in the following stages of carcinogenesis are kRAS, DCC, cyclin D, COX, iNOS, APC and beta-catenin (Figure 1), in a sequence that is substantially different from the classical adenoma-carcinoma pathway[28,29].
The essential morphological features of dysplasia, are (1) nuclear alterations such as increased nuclear to cytoplasmic ratios and hyperchromasia; (2) depletion of goblet cells; and (3) abnormal architectural patterns corresponding to dysregulated cellular proliferation, such as glandular crowding, a villous architecture and diminished surface maturation. HGD differs from LGD in that there are additional alterations, i.e., impaired cellular polarity including loss of nuclear parallelism, stratification of nuclei patterns such as a cribriform architecture. In most cases, the nuclei in HGD show severe cytological aberrations such as irregular nuclear membranes, abnormally prominent nucleoli or atypical mitotic figures[30]. The progression of such alterations is accompanied by both a progressive increase of epithelial proliferation and a reduction of apoptosis. This phenomenon starts as alterations of glandular architecture (i.e., shortening, loss of parallelism, ramification and branching) which anticipate the dysplasia onset[31].
The potential risk of malignant degeneration of UC to CRC has made it necessary to institute surveillance protocols to achieve early recognition and treatment of dysplastic lesions. The current evidence-based consensus for endoscopy in inflammatory bowel disease[32] suggests that surveillance should start when the risk starts to increase, i.e., after 8-10 years from the onset of disease[7]. This first colonoscopy also aims to reassess the extent of disease, since this parameter has an impact on the risk of CRC. After this first colonoscopy, patients with high risk features (stricture or dysplasia detected within the past 5 years, PSC, extensive colitis with severe active inflammation, or a family history of CRC in a first degree relative aged less than 50 years) should undergo surveillance colonoscopy annually. Conversely, patients with intermediate risk factors should have surveillance colonoscopy scheduled every 2 to 3 years and those without risk factors every 5 years. Biopsy sampling is fundamental: the American Gastroenterological Association recommends extensive sampling, of a minimum of 33 specimens[33], while, according to the British Society of Gastroenterology[34],two to four random biopsies every 10 centimetres should be taken.
Compliance to surveillance protocols, as well as a correct clinical overview of UC and the adequate pharmacological management of the disease, have led to a decreasing CRC incidence and mortality in UC[35,36]. In 1971, de Dombal[37] reported a 5% cumulative risk of CRC in a population from Leeds with extensive UC after 10 years and 41.8% after 25 years. Thirty years later, the cumulative risks reported by Lakatos et al[38] had dropped dramatically: 0.6% after 10 years, 5.4% after 20 years and 7.5% after 30 years of disease duration. These data testify to the exceptional impact of surveillance in the natural history of UC[39], but we must consider that it is not the only prevention strategy: other routes, such as chemoprevention, may have a remarkable effect.
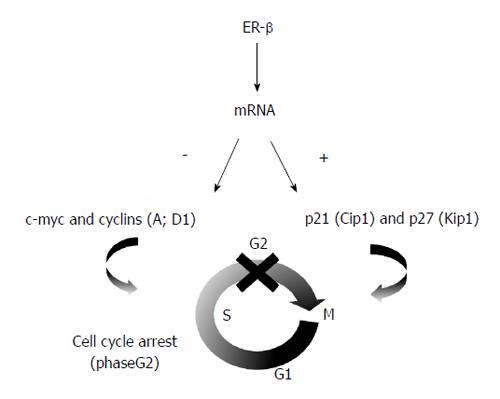
Modern medicine and oncology have been profoundly affected by the discovery of the estrogen receptors (ERs), a potential marker that plays a pivotal role in the pathogenesis, prognosis and therapy of various cancers, such as breast, prostate and colon. Estrogens can regulate the growth, differentiation, and function of various target tissues both within and outside the reproductive system[40,41]. The most relevant event after the initial discovery of these receptors[42] was the identification of two subtypes, ER alpha and ER beta, that are expressed at different levels in each organ of the human body[43]. Variations in the phenotype of knock-out mice lacking ER alpha or ER beta suggested that these receptors have different biological activities[44]. Moreover, in vitro and in vivo studies in ER beta knock-out mice demonstrated that ER beta is a modulator of ER activity, as it is able to reverse the effects of ER alpha and to inhibit estradiol-dependent proliferation[45,46]. These experiments demonstrated that ER alpha is a positive regulator of cellular growth, while ER beta has an antagonist inhibitory function, mediated by the down-regulation of proto-oncogenes (c-myc and cyclins) and up-regulation of oncosuppressants (p21 and p27), resulting in cell cycle arrest[47] (Figure 2). Experiments showing that in various cancers ER alpha is overexpressed and ER beta is down-regulated confirmed in vitro studies and demonstrated that cell proliferation is the result of a balance of ER alpha and ER beta[48,49].
The hypothesis of a possible link between CRC and ERs was advanced after the publication of epidemiological studies showing that females have a lower rate of colonic adenomas and cancers than males before menopause and that the differences progressively lessen after menopause[50]. Similarly, both observational and interventional data have shown that hormone replacement therapy decreases colonic adenoma and cancer risks[51,52]: in the last 40 years, a reduction of deaths from large bowel carcinoma has been observed in the United States. This reduction was significantly higher in women (30%) as compared to men (7%). In the same study, a link was observed between oral contraceptive use and a reduction of colorectal cancer, whereas there was a higher than expected frequency of colorectal tumors among non users[53].
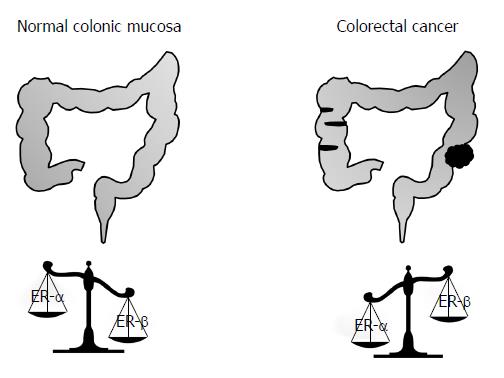
After the demonstration by our group that ERs are expressed in the colonic mucosa[54], Konstantinopoulos et al[55] demonstrated that ER beta is highly expressed in normal colonic mucosa in humans, while it is significantly reduced in CRC; this reduction is more pronounced in the case of poorly differentiated tumors. Since the majority of CRCs are derived from adenomatous polyps (a precancerous condition) our group recently evaluated the expression of ER alpha and ER beta in the colonic tissue of 25 patients with adenomatous polyps of the colon and in 25 normal subjects[56]. ERs expression was then correlated to proliferation and apoptosis. Our data confirmed that ER beta is the prevalent estrogen receptor in normal mucosa and shows a significantly reduced expression in adenomatous polyps (Figure 3). In a successive study, we confirmed that ER beta plays a primary role in the regulation of colonic mucosa proliferation in patients affected by Familial Adenomatous Polyposis (FAP)[57], an inherited disease characterized by an early inclination to develop hundreds of polyps and consequently CRC. Furthermore, ERs can even influence the prognosis of CRC, as it has been demonstrated that patients affected by CRCs with a minimal ERs expression had poor prognosis and short survival[58].
All these data confirm that sex steroid hormones are involved in CRC development and suggest that ER beta could play an important role in the early phase of the carcinogenic process and hence could be a target in the primary prevention of CRC[59].
ER beta has been suggested to exert anti-inflammatory and anti-tumorigenic effects in the colon, providing a translational potential to prevent and/or treat inflammatory bowel disease (IBD) and its progression to colitis-associated CRC[60,61]. Most studies in this field used a consolidated animal model which accurately mimics the carcinogenic model related to chronic bowel inflammation in mice (i.e., Azoxymethane/Dextran Sodium Sulfate - AOM/DSS)[62,63].
Saleiro et al[64] demonstrated that ER beta-deficient mice developed more severe clinical colitis compared to wild type mice, as evidenced by a significantly higher disease activity index after DSS treatment, as well as the inflammation score and grade of dysplasia. ER beta-deficient colons presented a greater number and size of polyps, and were characterized by a significant increase in IL-6, IL-17, TNF alpha and interferon-gamma mRNA levels as compared to wild type mice organs. Furthermore, higher protein expression levels of nuclear factor-kappa B, inducible nitric oxide synthase (iNOS), beta catenin, proliferating cell nuclear antigen, mucin-1, and significantly lower caveolin-1 and mucin-2 protein levels, were shown in ER beta knock-out mice compared to wild type. These data suggest a possible anti-inflammatory and anti-neoplastic mechanism of action of ER beta in UC-arisen CRC. These results suggest that ER beta may be protective in the AOM/DSS-induced CRC model in mice, supporting a preventive and/or therapeutic potential for the use of ER beta-selective agonists in IBD.
Fujii et al[65] performed a study to clarify whether methylation analysis of the ER gene in non-neoplastic epithelium can contribute to the prediction of an increased neoplasia risk in UC patients. The study was based on the assumption that the ER gene shows an age-related methylation in the colorectal epithelium and this phenomenon is frequently found in sporadic colorectal neoplasia, suggesting that it may predispose to colorectal neoplasia. The results suggested that the analysis of ER gene hypermethylation may be a potentially useful marker for identifying individuals at increased risk of neoplasia among those with long-standing and extensive UC. The same group confirmed that the quantitative analysis of ER gene methylation in non-neoplastic epithelium is a marker for identifying individuals at increased risk of neoplasia in long-standing and extensive UC[66].
A preliminary report by our group[67] assessed the pattern of ER-alpha/beta expression in relation to epithelial apoptosis and cell proliferation in long-lasting UC. We did not observe significant variations in ERs and their ratio in UC compared to UC-low degree dysplasia. However, there was a statistically significant progressive increase in apoptosis in UC and in UC-dysplasia that, despite Ki-67 expression, revealed a more marked significant increase at the same stages. This result, despite the small sample and the inclusion of only low-grade dysplasia, suggested that a possible ER-beta overseer of apoptosis/proliferation is operative until the investigated stage of carcinogenesis (Figure 1). In fact, in LGD we observed a high increase in cell proliferation with invariable levels of ER beta, accompanied by mild increased apoptosis, that was presumably unable to completely counter Ki-67 over-expression. Further, we investigated ER beta, ER alpha expression and their ratio in normal mucosa, in UC and in UC-low and high grade dysplasia and CRC. ERs did not show significant changes until LGD, while in HGD and UC-carcinoma there was a dramatic loss of ER beta expression and the ER beta/ER alpha ratio. Apoptosis and the TUNEL/Ki-67 ratio demonstrated a statistically significant progressive decrease from LGD to UC-carcinoma[68].
The main risk factors for colorectal cancer are not suitable targets for therapeutic intervention, but primary chemoprevention is an intriguing therapeutic option. The question whether mesalazine could exert a chemo-preventive effect has been raised and various studies have investigated this aspect.
The mechanisms by which aspirin or non-steroidal anti-inflammatory drugs (NSAIDs) act in the chemoprevention of CRC in non-IBD patients have not been entirely elucidated. However, data on the chemopreventive effect of aspirin and NSAIDs and CRC are supported by a series of independent lines of evidence. Indeed, several epidemiological studies have shown an inverse correlation between aspirin intake and the risk of CRC[69-71]. Furthermore, studies on secondary chemoprevention reported that aspirin intake was associated with a decreased risk of adenoma recurrence[72,73]. Aspirin and NSAIDs seem to act by inducing apoptosis in the colonic epithelium through the inhibition of cyclooxygenase (COX) activity and arachidonic acid accumulation[74]. Recent evidence suggests that COX inhibition can also change the activity of mitogen-activated protein kinases and NFκB[75,76].
The analogies between acetyl-salicylic acid and mesalazine (5-amino-salcylic acid), and the results obtained by using acetyl-salicylic acid as a chemopreventive agent in patients with sporadic colorectal cancer have prompted the study of potential chemopreventive effects of mesalazine in inflammatory bowel disease. The results of both epidemiological and experimental studies have shown that long-term 5-amino-salicylic acid treatments appear to have a chemopreventive effect. We can cite two studies, by Eaden and Lashner, in which the relative risk of CRC was estimated to be 0.18 and 0.88, respectively[77,78]. In a group of patients affected by UC and PSC, the risk was 0.88[79]. The evidence for this effect is provided by retrospective and case-control studies, however, whose results do not reach the highest grades for evidence-based recommendations. Indeed, not all clinical studies reported favorable results regarding CRC in IBD patients. Negative results were mainly reported in studies that elicited positive results with other drugs such as folate or ursodiol[80]. The peculiarities of the cohorts enrolled in these studies (disease refractory to conventional therapy, consideration for treatment with experimental therapy, consultation for surgery) may account for the negative outcome.
Positive results are supported by a series of experimental studies demonstrating the multiplicity of actions of 5-amino-salicylic acid, although data regarding the chemopreventive effect of 5-amino-salicylic acid may not be rigorous enough to meet the criteria for the highest evidence-based medicine recommendations. A final consideration is that suitable evidence may not be rationally gained in this case, because discontinuation of 5-amino-salicylic acid treatment would be unethical in patients with UC[81].
The data summarized in the previous sections suggest the hypothesis that the loss of ER beta expression could be a marker of colonic mucosa at increased risk for colonic neoplasia and that the induction of ER beta with ER beta-selective phytoestrogens could exert a chemopreventive effect against CRC.
Observational data also suggest that phytoestrogen intake may be associated with a decreased incidence of advanced lesions in both men and women[82-84]. The mechanism of the putative protective effect of estrogens and phytoestrogens on colonic neoplasia is not fully understood, but it seems to be markedly different from the one underlying the detrimental effect of estrogens in breast cancer. In the breast, it is well established that the detrimental effect is due to estrogen binding to the pro-proliferative ER alpha, since a similar effect is not found in women with ER-negative breast cancers[85].
Barone et al[86] have shown that the ER beta/ER alpha ratio was lower in the normal small intestinal mucosa of APCmin/+ mice than in syngenic APC wild type and this phenomenon was associated with a decreased apoptotic activity. The ER beta/ER alpha ratio and apoptosis were normalized by supplementation with a combination of silymarin and insoluble fibers. The combination also markedly decreased the number and size of intestinal tumors in APCmin/+ mice[86]. Silymarin displays a full ER beta agonist activity[87,88] and lignans also exert phytoestrogenic activity[89]. Another study by our group[90] was a randomized, double blind placebo-controlled trial in patients undergoing surveillance colonoscopy for previous sporadic colonic adenomas. Sixty eligible patients were randomized to receive a placebo or active dietary intervention with phytoestrogen supplements twice a day, for sixty days before surveillance colonoscopy. The phytoestrogen administration group showed a significant increase in ER beta protein and a general trend to an increase in ER beta, ER beta/ER alpha, TUNEL/Ki-67 ratio. Moreover, a significant increase of ER-beta protein, mRNA and labeling index (i.e., the percentage of ER-beta positive cells at immunohistochemistry) and a decrease of ER-alpha protein, as well as an increase in ER beta/ER beta protein were observed in phytoestrogen versus placebo group in patients without recurrent polyps. Therefore, the role of ER beta on the control of apoptosis, as well as its amenability to dietary intervention, were supported by this study.
Finally, 90-d supplementation with phytoestrogens was efficacious in reducing polyp number and size in recurrent duodenal adenomas of patients with FAP with an ileal pouch-anal anastomosis[91].
UC is a condition that increases affected patients’ risk for CRC development. Nowadays, specific screening and follow-up programs, based on epidemiological and clinical parameters, are routinely performed to promote the early detection of CRC onset. This practice has induced a marked reduction of the cancer incidence and mortality in subjects with UC.
Chemoprevention is an interesting topic which has been widely investigated and developed in many medical fields[92]. Aspirin has shown a preventive effect on CRC onset, and mesalazine has been strongly invoked as a potential chemopreventive agent against carcinoma arising in UC[93].
The ER alpha/beta balance seems to have a relevant influence on colorectal carcinogenesis and ER beta appears to parallel apoptosis, thus exerting an anti-carcinogenic effect[94]. In preliminary studies phytoestrogens, which are able to act as ER beta agonists, have shown promising chemopreventive effects on sporadic as well as genetically inherited CRC. In view of the strong evidence of a role for ERs in UC-related carcinogenesis, and taking into account the fact that phytoestrogens can be considered as dietary supplements and are completely free from side effects, they offer interesting prospects for CRC prevention even when the disease is the long term consequence of chronic inflammation.
In conclusion, ERs have a role in the development of all different types of CRC[95] (sporadic, genetic and post-inflammatory). Their targeted use is, therefore, a fascinating field for both basic and clinical investigations in order to elucidate the underlying pathophysiological, prognostic and therapeutic aspects.
P- Reviewer: Fujimori S, Shi C S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH
| 1. | Rubin DC, Shaker A, Levin MS. Chronic intestinal inflammation: inflammatory bowel disease and colitis-associated colon cancer. Front Immunol. 2012;3:107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 292] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 2. | Lakatos PL, Lakatos L. Challenges in calculating the risk for colorectal cancer in patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2012;10:1179; author reply 1179-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Katsanos KH, Stamou P, Tatsioni A, Tsianos VE, Zoumbas S, Kavvadia S, Giga A, Vagias I, Christodoulou DK, Tsianos EV. Prevalence of inflammatory bowel disease related dysplasia and cancer in 1500 colonoscopies from a referral center in northwestern Greece. J Crohns Colitis. 2011;5:19-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Castańo-Milla C, Chaparro M, Gisbert JP. Has the risk of developing colorectal cancer in patients with ulcerative colitis been overstated? A meta-analysis. Gastroenterology. 2012;142:S-251. |
| 5. | Jess T, Rungoe C, Peyrin-Biroulet L. Risk of colorectal cancer in patients with ulcerative colitis: a meta-analysis of population-based cohort studies. Clin Gastroenterol Hepatol. 2012;10:639-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 659] [Article Influence: 50.7] [Reference Citation Analysis (0)] |
| 6. | Jess T, Simonsen J, Jørgensen KT, Pedersen BV, Nielsen NM, Frisch M. Decreasing risk of colorectal cancer in patients with inflammatory bowel disease over 30 years. Gastroenterology. 2012;143:375-381.e1; quiz e13-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 384] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 7. | Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1985] [Cited by in RCA: 2075] [Article Influence: 86.5] [Reference Citation Analysis (1)] |
| 8. | Ekbom A, Helmick C, Zack M, Adami HO. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med. 1990;323:1228-1233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1294] [Cited by in RCA: 1198] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 9. | Baars JE, Kuipers EJ, van Haastert M, Nicolaï JJ, Poen AC, van der Woude CJ. Age at diagnosis of inflammatory bowel disease influences early development of colorectal cancer in inflammatory bowel disease patients: a nationwide, long-term survey. J Gastroenterol. 2012;47:1308-1322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 10. | Askling J, Dickman PW, Karlén P, Broström O, Lapidus A, Löfberg R, Ekbom A. Family history as a risk factor for colorectal cancer in inflammatory bowel disease. Gastroenterology. 2001;120:1356-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 283] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 11. | Soetikno RM, Lin OS, Heidenreich PA, Young HS, Blackstone MO. Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis: a meta-analysis. Gastrointest Endosc. 2002;56:48-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 383] [Article Influence: 16.7] [Reference Citation Analysis (1)] |
| 12. | Rutter MD, Saunders BP, Wilkinson KH, Rumbles S, Schofield G, Kamm MA, Williams CB, Price AB, Talbot IC, Forbes A. Cancer surveillance in longstanding ulcerative colitis: endoscopic appearances help predict cancer risk. Gut. 2004;53:1813-1816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 299] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 13. | Gupta RB, Harpaz N, Itzkowitz S, Hossain S, Matula S, Kornbluth A, Bodian C, Ullman T. Histologic inflammation is a risk factor for progression to colorectal neoplasia in ulcerative colitis: a cohort study. Gastroenterology. 2007;133:1099-1105; quiz 1340-1341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 617] [Cited by in RCA: 570] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 14. | Rizzo A, Pallone F, Monteleone G, Fantini MC. Intestinal inflammation and colorectal cancer: a double-edged sword? World J Gastroenterol. 2011;17:3092-3100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 56] [Reference Citation Analysis (0)] |
| 15. | Talero E, Sánchez-Fidalgo S, Villegas I, de la Lastra CA, Illanes M, Motilva V. Role of different inflammatory and tumor biomarkers in the development of ulcerative colitis-associated carcinogenesis. Inflamm Bowel Dis. 2011;17:696-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Atreya R, Mudter J, Finotto S, Müllberg J, Jostock T, Wirtz S, Schütz M, Bartsch B, Holtmann M, Becker C. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in crohn disease and experimental colitis in vivo. Nat Med. 2000;6:583-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 945] [Cited by in RCA: 1026] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 17. | Stolfi C, Rizzo A, Franzè E, Rotondi A, Fantini MC, Sarra M, Caruso R, Monteleone I, Sileri P, Franceschilli L. Involvement of interleukin-21 in the regulation of colitis-associated colon cancer. J Exp Med. 2011;208:2279-2290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 124] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 18. | Popivanova BK, Kostadinova FI, Furuichi K, Shamekh MM, Kondo T, Wada T, Egashira K, Mukaida N. Blockade of a chemokine, CCL2, reduces chronic colitis-associated carcinogenesis in mice. Cancer Res. 2009;69:7884-7892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 162] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 19. | Glocker EO, Kotlarz D, Boztug K, Gertz EM, Schäffer AA, Noyan F, Perro M, Diestelhorst J, Allroth A, Murugan D. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med. 2009;361:2033-2045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1149] [Cited by in RCA: 1091] [Article Influence: 68.2] [Reference Citation Analysis (0)] |
| 20. | Galatola M, Miele E, Strisciuglio C, Paparo L, Rega D, Delrio P, Duraturo F, Martinelli M, Rossi GB, Staiano A. Synergistic effect of interleukin-10-receptor variants in a case of early-onset ulcerative colitis. World J Gastroenterol. 2013;19:8659-8670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Becker C, Fantini MC, Neurath MF. TGF-beta as a T cell regulator in colitis and colon cancer. Cytokine Growth Factor Rev. 2006;17:97-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 22. | Rakoff-Nahoum S, Medzhitov R. Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science. 2007;317:124-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 456] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 23. | Fukata M, Abreu MT. Role of Toll-like receptors in gastrointestinal malignancies. Oncogene. 2008;27:234-243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 146] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 24. | Harpaz N, Ward SC, Mescoli C, Itzkowitz SH, Polydorides AD. Precancerous lesions in inflammatory bowel disease. Best Pract Res Clin Gastroenterol. 2013;27:257-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Leedham SJ, Graham TA, Oukrif D, McDonald SA, Rodriguez-Justo M, Harrison RF, Shepherd NA, Novelli MR, Jankowski JA, Wright NA. Clonality, founder mutations, and field cancerization in human ulcerative colitis-associated neoplasia. Gastroenterology. 2009;136:542-550.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 134] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 26. | Gerrits MM, Chen M, Theeuwes M, van Dekken H, Sikkema M, Steyerberg EW, Lingsma HF, Siersema PD, Xia B, Kusters JG. Biomarker-based prediction of inflammatory bowel disease-related colorectal cancer: a case-control study. Cell Oncol (Dordr). 2011;34:107-117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Pozza A, Scarpa M, Ruffolo C, Polese L, Erroi F, Bridda A, Norberto L, Frego M. Colonic carcinogenesis in IBD: molecular events. Ann Ital Chir. 2011;82:19-28. [PubMed] |
| 28. | Hardy RG, Meltzer SJ, Jankowski JA. ABC of colorectal cancer. Molecular basis for risk factors. BMJ. 2000;321:886-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Tanaka T. Development of an inflammation-associated colorectal cancer model and its application for research on carcinogenesis and chemoprevention. Int J Inflam. 2012;2012:658786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 30. | Magro F, Langner C, Driessen A, Ensari A, Geboes K, Mantzaris GJ, Villanacci V, Becheanu G, Borralho Nunes P, Cathomas G. European consensus on the histopathology of inflammatory bowel disease. J Crohns Colitis. 2013;7:827-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 466] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 31. | Ierardi E, Principi M, Francavilla R, Passaro S, Noviello F, Burattini O, Francavilla A. Epithelial proliferation and ras p21 oncoprotein expression in rectal mucosa of patients with ulcerative colitis. Dig Dis Sci. 2001;46:1083-1087. [PubMed] |
| 32. | Annese V, Daperno M, Rutter MD, Amiot A, Bossuyt P, East J, Ferrante M, Götz M, Katsanos KH, Kießlich R. European evidence based consensus for endoscopy in inflammatory bowel disease. J Crohns Colitis. 2013;7:982-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 584] [Article Influence: 48.7] [Reference Citation Analysis (1)] |
| 33. | Farraye FA, Odze RD, Eaden J, Itzkowitz SH. AGA technical review on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology. 2010;138:746-774, 774.e1-4; quiz e12-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 341] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 34. | Cairns SR, Scholefield JH, Steele RJ, Dunlop MG, Thomas HJ, Evans GD, Eaden JA, Rutter MD, Atkin WP, Saunders BP. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut. 2010;59:666-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 897] [Cited by in RCA: 808] [Article Influence: 53.9] [Reference Citation Analysis (2)] |
| 35. | van Rijn AF, Fockens P, Siersema PD, Oldenburg B. Adherence to surveillance guidelines for dysplasia and colorectal carcinoma in ulcerative and Crohn’s colitis patients in the Netherlands. World J Gastroenterol. 2009;15:226-230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 65] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 36. | Reenaers C, Belaiche J, Louis E. Impact of medical therapies on inflammatory bowel disease complication rate. World J Gastroenterol. 2012;18:3823-3827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 37. | de Dombal FT. Ulcerative colitis. Epidemiology and aetiology, course and prognosis. Br Med J. 1971;1:649-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 38. | Lakatos L, Mester G, Erdelyi Z, David G, Pandur T, Balogh M, Fischer S, Vargha P, Lakatos PL. Risk factors for ulcerative colitis-associated colorectal cancer in a Hungarian cohort of patients with ulcerative colitis: results of a population-based study. Inflamm Bowel Dis. 2006;12:205-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 151] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 39. | Andersen NN, Jess T. Has the risk of colorectal cancer in inflammatory bowel disease decreased? World J Gastroenterol. 2013;19:7561-7568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 55] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 40. | Pettersson K, Gustafsson JA. Role of estrogen receptor beta in estrogen action. Annu Rev Physiol. 2001;63:165-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 356] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 41. | Messa C, Russo F, Pricci M, Di Leo A. Epidermal growth factor and 17beta-estradiol effects on proliferation of a human gastric cancer cell line (AGS). Scand J Gastroenterol. 2000;35:753-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 42. | Jensen EV, DeSombre ER. Mechanism of action of the female sex hormones. Annu Rev Biochem. 1972;41:203-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 603] [Cited by in RCA: 505] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 43. | Mosselman S, Polman J, Dijkema R. ER beta: identification and characterization of a novel human estrogen receptor. FEBS Lett. 1996;392:49-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1630] [Cited by in RCA: 1527] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 44. | Couse JF, Curtis Hewitt S, Korach KS. Receptor null mice reveal contrasting roles for estrogen receptor alpha and beta in reproductive tissues. J Steroid Biochem Mol Biol. 2000;74:287-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 95] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 45. | Hall JM, McDonnell DP. The estrogen receptor beta-isoform (ERbeta) of the human estrogen receptor modulates ERalpha transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology. 1999;140:5566-5578. [PubMed] |
| 46. | Liu MM, Albanese C, Anderson CM, Hilty K, Webb P, Uht RM, Price RH, Pestell RG, Kushner PJ. Opposing action of estrogen receptors alpha and beta on cyclin D1 gene expression. J Biol Chem. 2002;277:24353-24360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 333] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 47. | Paruthiyil S, Parmar H, Kerekatte V, Cunha GR, Firestone GL, Leitman DC. Estrogen receptor beta inhibits human breast cancer cell proliferation and tumor formation by causing a G2 cell cycle arrest. Cancer Res. 2004;64:423-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 451] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 48. | Barone M, Lofano K, De Tullio N, Licinio R, Albano F, Di Leo A. Dietary, endocrine, and metabolic factors in the development of colorectal cancer. J Gastrointest Cancer. 2012;43:13-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 49. | Bardin A, Boulle N, Lazennec G, Vignon F, Pujol P. Loss of ERbeta expression as a common step in estrogen-dependent tumor progression. Endocr Relat Cancer. 2004;11:537-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 317] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 50. | Koo JH, Leong RW. Sex differences in epidemiological, clinical and pathological characteristics of colorectal cancer. J Gastroenterol Hepatol. 2010;25:33-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 51. | Woodson K, Lanza E, Tangrea JA, Albert PS, Slattery M, Pinsky J, Caan B, Paskett E, Iber F, Kikendall JW. Hormone replacement therapy and colorectal adenoma recurrence among women in the Polyp Prevention Trial. J Natl Cancer Inst. 2001;93:1799-1805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 52. | Solimando R, Bazzoli F, Ricciardiello L. Chemoprevention of colorectal cancer: a role for ursodeoxycholic acid, folate and hormone replacement treatment? Best Pract Res Clin Gastroenterol. 2011;25:555-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 53. | American Cancer Society. Cancer fact figures. American Cancer Society, Atlanta 1995. Available from: http://www.cancer.org/search/index?QueryText=Cancer fact figures. |
| 54. | Francavilla A, Di Leo A, Polimeno L, Conte D, Barone M, Fanizza G, Chiumarulo C, Rizzo G, Rubino M. Nuclear and cytosolic estrogen receptors in human colon carcinoma and in surrounding noncancerous colonic tissue. Gastroenterology. 1987;93:1301-1306. [PubMed] |
| 55. | Konstantinopoulos PA, Kominea A, Vandoros G, Sykiotis GP, Andricopoulos P, Varakis I, Sotiropoulou-Bonikou G, Papavassiliou AG. Oestrogen receptor beta (ERbeta) is abundantly expressed in normal colonic mucosa, but declines in colon adenocarcinoma paralleling the tumour’s dedifferentiation. Eur J Cancer. 2003;39:1251-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 250] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 56. | Di Leo A, Barone M, Maiorano E, Tanzi S, Piscitelli D, Marangi S, Lofano K, Ierardi E, Principi M, Francavilla A. ER-beta expression in large bowel adenomas: implications in colon carcinogenesis. Dig Liver Dis. 2008;40:260-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 57. | Barone M, Scavo MP, Papagni S, Piscitelli D, Guido R, Di Lena M, Comelli MC, Di Leo A. ERβ expression in normal, adenomatous and carcinomatous tissues of patients with familial adenomatous polyposis. Scand J Gastroenterol. 2010;45:1320-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 58. | Di Leo A, Messa C, Russo F, Misciagna G, Guerra V, Taveri R, Leo S. Prognostic value of cytosolic estrogen receptors in human colorectal carcinoma and surrounding mucosa. Preliminary results. Dig Dis Sci. 1994;39:2038-2042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 59. | Barone M, Tanzi S, Lofano K, Scavo MP, Guido R, Demarinis L, Principi MB, Bucci A, Di Leo A. Estrogens, phytoestrogens and colorectal neoproliferative lesions. Genes Nutr. 2008;3:7-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 60. | Harnish DC, Albert LM, Leathurby Y, Eckert AM, Ciarletta A, Kasaian M, Keith JC. Beneficial effects of estrogen treatment in the HLA-B27 transgenic rat model of inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol. 2004;286:G118-G125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 87] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 61. | Kennelly R, Kavanagh DO, Hogan AM, Winter DC. Oestrogen and the colon: potential mechanisms for cancer prevention. Lancet Oncol. 2008;9:385-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 119] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 62. | Tanaka T, Kohno H, Suzuki R, Yamada Y, Sugie S, Mori H. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer Sci. 2003;94:965-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 558] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 63. | De Robertis M, Massi E, Poeta ML, Carotti S, Morini S, Cecchetelli L, Signori E, Fazio VM. The AOM/DSS murine model for the study of colon carcinogenesis: From pathways to diagnosis and therapy studies. J Carcinog. 2011;10:9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 419] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 64. | Saleiro D, Murillo G, Benya RV, Bissonnette M, Hart J, Mehta RG. Estrogen receptor-β protects against colitis-associated neoplasia in mice. Int J Cancer. 2012;131:2553-2561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 65. | Fujii S, Tominaga K, Kitajima K, Takeda J, Kusaka T, Fujita M, Ichikawa K, Tomita S, Ohkura Y, Ono Y. Methylation of the oestrogen receptor gene in non-neoplastic epithelium as a marker of colorectal neoplasia risk in longstanding and extensive ulcerative colitis. Gut. 2005;54:1287-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 66. | Tominaga K, Fujii S, Mukawa K, Fujita M, Ichikawa K, Tomita S, Imai Y, Kanke K, Ono Y, Terano A. Prediction of colorectal neoplasia by quantitative methylation analysis of estrogen receptor gene in nonneoplastic epithelium from patients with ulcerative colitis. Clin Cancer Res. 2005;11:8880-8885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 67. | Principi M, De Tullio N, Scavo MP, Piscitelli D, Marzullo A, Russo S, Albano F, Lofano K, Papagni S, Barone M. Estrogen receptors expression in long-lasting ulcerative pancolitis with and without dysplasia: a preliminary report. Scand J Gastroenterol. 2012;47:1253-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 68. | Principi M, Scavo MP, Piscitelli D, Villanacci V, Contaldo A, Neve V, Lofano K, Piacentino G, De Tullio N, Ierardi E. The fall of estrogen receptors expression in long-lasting ulcerative-associated carcinoma. J Crohns Colitis. 2013;7:S15-S16. [DOI] [Full Text] |
| 69. | Smalley W, Ray WA, Daugherty J, Griffin MR. Use of nonsteroidal anti-inflammatory drugs and incidence of colorectal cancer: a population-based study. Arch Intern Med. 1999;159:161-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 195] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 70. | Courtney ED, Melville DM, Leicester RJ. Review article: chemoprevention of colorectal cancer. Aliment Pharmacol Ther. 2004;19:1-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 71. | Gwyn K, Sinicrope FA. Chemoprevention of colorectal cancer. Am J Gastroenterol. 2002;97:13-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 72. | Baron JA, Cole BF, Sandler RS, Haile RW, Ahnen D, Bresalier R, McKeown-Eyssen G, Summers RW, Rothstein R, Burke CA. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348:891-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1080] [Cited by in RCA: 1021] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 73. | Benamouzig R, Deyra J, Martin A, Girard B, Jullian E, Piednoir B, Couturier D, Coste T, Little J, Chaussade S. Daily soluble aspirin and prevention of colorectal adenoma recurrence: one-year results of the APACC trial. Gastroenterology. 2003;125:328-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 274] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 74. | Chan TA. Nonsteroidal anti-inflammatory drugs, apoptosis, and colon-cancer chemoprevention. Lancet Oncol. 2002;3:166-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 175] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 75. | Schwenger P, Alpert D, Skolnik EY, Vilcek J. Activation of p38 mitogen-activated protein kinase by sodium salicylate leads to inhibition of tumor necrosis factor-induced IkappaB alpha phosphorylation and degradation. Mol Cell Biol. 1998;18:78-84. [PubMed] |
| 76. | Kopp E, Ghosh S. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science. 1994;265:956-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1321] [Cited by in RCA: 1348] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 77. | Eaden J, Abrams K, Ekbom A, Jackson E, Mayberry J. Colorectal cancer prevention in ulcerative colitis: a case-control study. Aliment Pharmacol Ther. 2000;14:145-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 379] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 78. | Lashner BA, Provencher KS, Seidner DL, Knesebeck A, Brzezinski A. The effect of folic acid supplementation on the risk for cancer or dysplasia in ulcerative colitis. Gastroenterology. 1997;112:29-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 179] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 79. | Tung BY, Emond MJ, Haggitt RC, Bronner MP, Kimmey MB, Kowdley KV, Brentnall TA. Ursodiol use is associated with lower prevalence of colonic neoplasia in patients with ulcerative colitis and primary sclerosing cholangitis. Ann Intern Med. 2001;134:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 286] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 80. | Pardi DS, Loftus EV, Kremers WK, Keach J, Lindor KD. Ursodeoxycholic acid as a chemopreventive agent in patients with ulcerative colitis and primary sclerosing cholangitis. Gastroenterology. 2003;124:889-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 355] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 81. | Giannini EG, Kane SV, Testa R, Savarino V. 5-ASA and colorectal cancer chemoprevention in inflammatory bowel disease: can we afford to wait for ‘best evidence’? Dig Liver Dis. 2005;37:723-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 82. | Egeberg R, Olsen A, Loft S, Christensen J, Johnsen NF, Overvad K, Tjønneland A. Intake of wholegrain products and risk of colorectal cancers in the Diet, Cancer and Health cohort study. Br J Cancer. 2010;103:730-734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 83. | Kuijsten A, Hollman PC, Boshuizen HC, Buijsman MN, van ‘t Veer P, Kok FJ, Arts IC, Bueno-de-Mesquita HB. Plasma enterolignan concentrations and colorectal cancer risk in a nested case-control study. Am J Epidemiol. 2008;167:734-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 84. | Milder IE, Kuijsten A, Arts IC, Feskens EJ, Kampman E, Hollman PC, Van ‘t Veer P. Relation between plasma enterodiol and enterolactone and dietary intake of lignans in a Dutch endoscopy-based population. J Nutr. 2007;137:1266-1271. [PubMed] |
| 85. | Chang EC, Frasor J, Komm B, Katzenellenbogen BS. Impact of estrogen receptor beta on gene networks regulated by estrogen receptor alpha in breast cancer cells. Endocrinology. 2006;147:4831-4842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 255] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 86. | Barone M, Tanzi S, Lofano K, Scavo MP, Pricci M, Demarinis L, Papagni S, Guido R, Maiorano E, Ingravallo G. Dietary-induced ERbeta upregulation counteracts intestinal neoplasia development in intact male ApcMin/+ mice. Carcinogenesis. 2010;31:269-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 87. | Seidlová-Wuttke D, Becker T, Christoffel V, Jarry H, Wuttke W. Silymarin is a selective estrogen receptor beta (ERbeta) agonist and has estrogenic effects in the metaphysis of the femur but no or antiestrogenic effects in the uterus of ovariectomized (ovx) rats. J Steroid Biochem Mol Biol. 2003;86:179-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 74] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 88. | El-Shitany NA, Hegazy S, El-Desoky K. Evidences for antiosteoporotic and selective estrogen receptor modulator activity of silymarin compared with ethinylestradiol in ovariectomized rats. Phytomedicine. 2010;17:116-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 89. | Begum AN, Nicolle C, Mila I, Lapierre C, Nagano K, Fukushima K, Heinonen SM, Adlercreutz H, Rémésy C, Scalbert A. Dietary lignins are precursors of mammalian lignans in rats. J Nutr. 2004;134:120-127. [PubMed] |
| 90. | Principi M, Di Leo A, Pricci M, Scavo MP, Guido R, Tanzi S, Piscitelli D, Pisani A, Ierardi E, Comelli MC. Phytoestrogens/insoluble fibers and colonic estrogen receptor β: randomized, double-blind, placebo-controlled study. World J Gastroenterol. 2013;19:4325-4333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 91. | Calabrese C, Praticò C, Calafiore A, Coscia M, Gentilini L, Poggioli G, Gionchetti P, Campieri M, Rizzello F. Eviendep® reduces number and size of duodenal polyps in familial adenomatous polyposis patients with ileal pouch-anal anastomosis. World J Gastroenterol. 2013;19:5671-5677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 92. | Burn J, Mathers JC, Bishop DT. Chemoprevention in Lynch syndrome. Fam Cancer. 2013;12:707-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 93. | Mill J, Lawrance IC. Prevention of cancer in IBD - a balancing act. Minerva Gastroenterol Dietol. 2013;59:261-272. [PubMed] |
| 94. | Di Leo A, Linsalata M, Cavallini A, Messa C, Russo F. Sex steroid hormone receptors, epidermal growth factor receptor, and polyamines in human colorectal cancer. Dis Colon Rectum. 1992;35:305-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 95. | Di Leo A, Messa C, Cavallini A, Linsalata M. Estrogens and colorectal cancer. Curr Drug Targets Immune Endocr Metabol Disord. 2001;1:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 2.4] [Reference Citation Analysis (0)] |