INTRODUCTION
Pancreatic cancer is the fourth leading cause of cancer related death in developed countries[1,2]. From 1999 to 2008, the incidence rate of pancreatic cancer increased nearly 1% in United States, although the reason for this increase is unknown[3]. In general, pancreatic cancer refers to pancreatic ductal adenocarcinoma (PDAC) that accounts for around 90% of pancreatic cancer[4]. Since the clinical symptoms can be vague, patients are often diagnosed late, with regional invasion or distant metastasis already evident at first consultation[5-7]. The overall five-year survival rate of pancreatic cancer is approximately 6% in the United States[2], less than 6% across Europe[8] and 5% in Australia[9]. Despite the concerted endeavours of clinicians and scientists over several decades, pancreatic cancer remains a devastating disease with a poor outcome.
Risk factors for pancreatic cancer include age, smoking, race, diabetes and chronic pancreatitis, the last being the strongest known risk factor for this disease. Patients with a more than 5 year history of chronic pancreatitis have a greater than 14-fold risk of developing pancreatic cancer compared to the general population[10,11]. 40% of hereditary pancreatitis (a form of chronic pancreatitis) patients are likely to develop pancreatic cancer[12-14], while patients with tropical pancreatitis have been reported to have a 100-fold increased risk and an earlier onset of the disease compared to sporadic cases[15,16]. The mechanisms underlying the increased propensity for patients with chronic pancreatitis to develop pancreatic cancer are not fully elucidated. Recent studies suggest several signalling pathways known to be active in inflammatory disease, may be involved in the progression from pancreatitis to pancreatic cancer.
One such signalling molecule known to play a key role in inflammation is the transcription factor, nuclear factor κB (NF-κB). Activation of NF-κB leads to the release of several proinflammatory cytokines, such as interleukin (IL)-1β, IL-6, tumour necrosis factor-alpha (TNF-α), transforming growth factor-beta (TGF-β) and induces anti-apoptotic responses via Bcl-xL[17]. In addition to its observed activation in pancreatitis, NF-κB activity has also been observed in pancreatic cancer tissue. It has been shown to modulate angiogenesis via vascular endothelial growth factor (VEGF) and urokinase, and apoptosis possibly via antiapoptotic proteins such as Bcl-xL, cIAP1 (inhibitor of apoptosis protein), cIAP2, TRAF1 (TNF receptor-associated factor) and TRAF2[10,18]. NF-κB also negatively regulates the expression of p53, which is a tumour suppressor gene[19]. Further evidence for a role of NF-κB in cancer comes from an in vivo study using a NF-κB inhibitor (LC-1) in a xenograft pancreatic cancer mouse model. This inhibitor was found to reduce tumour growth and was associated with decreased expression of cyclin D1, a protein required in cell cycle G1/S transition[18,20].
K-Ras is another signalling pathway that is involved in both chronic pancreatitis and pancreatic cancer. K-Ras mutations exist in about a third of chronic pancreatitis patients[21]. Daniluk et al[22] reported that oncogenic K-Ras activation by inflammation in the mouse pancreas promoted development of chronic pancreatitis and pancreatic cancer precursor lesions. In another study, mutant K-Ras in acinar cells resulted in neoplastic lesions in mouse pancreas that progressed to pancreatic cancer in conjunction with p53 deletion[23]. Logsdon et al[24] have postulated that Ras activity is the direct link between chronic pancreatitis and pancreatic cancer. The induction of chronic pancreatitis in a genetically engineered mouse model with K-Ras overexpression led to the development of primary pancreatic tumours as well as metastasis[25-28]. Collins et al[29] have shown in mice bearing inducible K-Ras mutations, that oncogenic K-Ras initiates pancreatic carcinogenesis by hindering pancreatic repair after caerulein-induced pancreatitis. Importantly, inactivation of K-Ras mutation in these mice leads to tumour regression suggesting a role for oncogenic K-Ras in the maintenance of pancreatic cancer.
In addition to K-Ras mutations, a number of genetic mutations are frequently reported in pancreatic cancer. Biankin et al[30] performed exome sequencing and copy number analysis in a cohort of 142 sporadic PDAC cases and reported multiple significantly mutated genes, including the known mutations - KRAS, TP53, CDKN2A, SMAD4, MLL3, TGFBR2, ARID1A, SF3B1 and importantly, previously unidentified mutations such as EPC1, ARID2 (chromatin modifications), ATM (DNA damage repair), ZIM2 (transcription regulation), MAP2K4 (Toll-like receptor signalling pathway), NALCN (sodium channel activity), SLC16A4 (monocarboxylate transporter), MAGEA6 (protein binding). The accumulation of genetic mutations leads to the development of precursor lesions, the most common of which are pancreatic intraepithelial neoplasia (PanIN)[10,31,32]. PanINs are normally found in smaller diameter pancreatic ducts, with the microscopic features progressing from PanIN-1A to PanIN-3 and finally to overt PDAC.
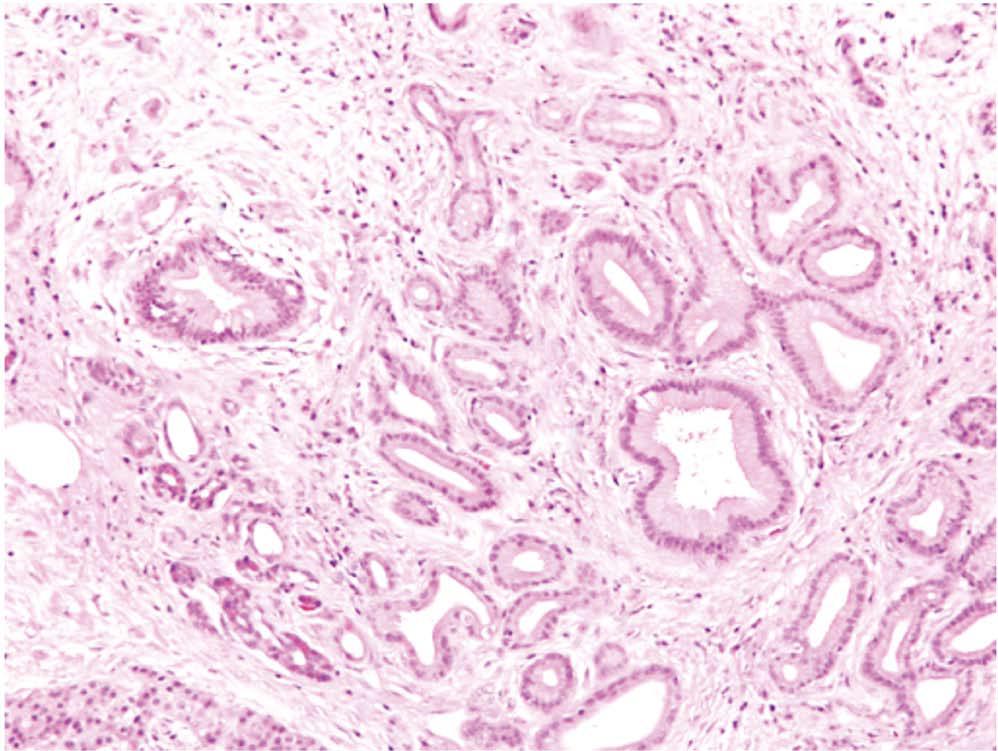
Histopathologically, PDAC is characterised by duct-like and tubular structures (malignant elements) infiltrating into and embedded in a highly fibrotic stromal reaction[5,33] (Figure 1). This stromal reaction is comprised of abundant extracellular matrix (ECM), stromal cells, blood vessels/endothelial cells, immune cells, nerves/neurons and other soluble proteins, e.g., cytokines, growth factors[10].
Figure 1 Pancreatic cancer and stromal reaction.
A representative HE stained human pancreatic cancer tissue section showing duct-like and tubular structures (malignant elements, examples highlighted by arrows) infiltrating into and embedded in a highly fibrotic stromal reaction (examples highlighted by asterisks).
The ECM itself is composed of proteins such as type I collagen, fibronectin and laminin as well as proteoglycans, such as hyaluronan, which is a non-sulphated glycosaminoglycan secreted by cancer cells. Hyaluronan is known to bind to CD44 (its receptor) and to influence angiogenesis, epithelial-mesenchymal transition (EMT) and chemo-resistance, possibly via the regulation of receptor tyrosine kinase and small GTPase[34]. Collagen I promotes pancreatic cancer cell adhesion, proliferation and migration via integrin α2β1[35]. Collagen, fibronectin and laminin are also found to be associated with increased chemo-resistance of pancreatic cancer cells in vitro[36].
There is now unequivocal evidence that fibrosis of the pancreas is produced by pancreatic stellate cells (PSCs)[33]. PSCs were first isolated from rat pancreas in 1998 by Apte et al[37] using a density centrifugation method. A similar method to isolate human PSCs from histologically normal human pancreas was later described by the same group[38]. Bachem et al[39,40] reported isolation of human PSCs from fibrotic pancreatic tissue of patients with chronic pancreatitis[39] and pancreatic cancer[40] using an explant technique. With the availability of these methods to isolate and culture of PSCs, researchers have been able to make significant advances in the understanding PSC biology.
PSCs are resident cells of the pancreas and comprise about 4%-7% of total parenchymal cells in the gland[37,41]. There are abundant vitamin A containing lipid droplets in the cytoplasm, which is a marker of quiescent PSCs. PSCs synthesise the ECM proteins collagen, fibronectin and laminin. They also express the matrix metalloproteinases (MMPs), MMP2, 9 and 13 that degrade ECM and the tissue inhibitors of metalloproteinases (TIMPs), TIMP 1 and 2 that inhibit the activity of MMPs. Therefore, PSCs are thought to play an important role in maintaining a balance between ECM synthesis and degradation to maintain normal pancreatic architecture in health[41]. During pancreatic injury, PSCs are activated by factors such as ethanol (a known cause of chronic pancreatitis) and its metabolites, bacterial endotoxin, oxidant stress, cytokines and growth factors. Activated PSCs lose vitamin A droplets, assume a myofibroblast-like phenotype, express the cytoskeletal protein α-smooth muscle actin (α-SMA) and synthesise excessive amount of ECM proteins leading to fibrosis[37,41-44].
In a bid to shed some light on the differences between stellate cells in health and diseases, we have conducted microarray studies to examine differences in gene expression in human PSCs obtained from normal pancreas (benign pancreatic diseases: serous cystadenoma, etc.) vs the disease-activated PSCs isolated from chronic pancreatitis and pancreatic cancer tissue[45]. Multiple genes were found to be differentially expressed. Validation studies confirmed that MMP3 was upregulated 32.25 fold, collagen type IVα1 (a basement membrane component) was downregulated 2.25 fold and syndecan-2 (a transmembrane heparan sulphate proteoglycan that plays a role in cell binding, cytoskeletal organization, migration and invasion[46]) was downregulated 2.04 fold. These three genes are postulated to be involved in ECM remodeling function and motility of PSCs. However, in depth characterisation of the role of these genes in the functional modulation of PSCs remains to be undertaken.
IDENTIFICATION OF PSCS AS SOURCE OF ECM DEPOSITION IN STROMA
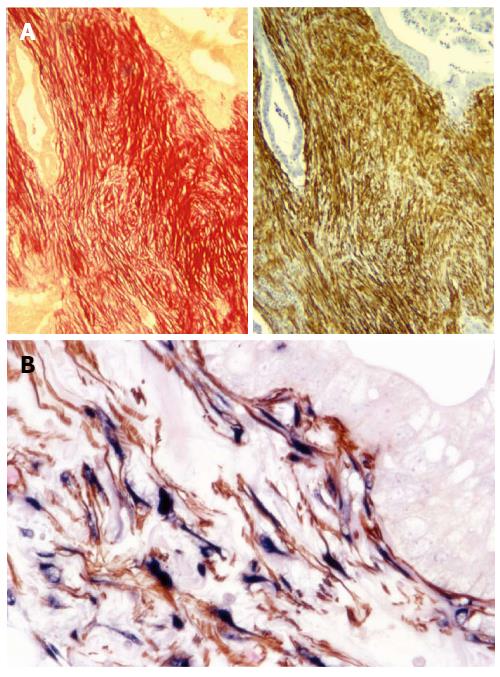
Up until just under a decade ago, the prominent stroma/fibrosis in pancreatic cancer had been largely ignored. In 2004, Apte et al[33] demonstrated that PSCs produced the collagenous stroma in pancreatic cancer. Using serial sections of human pancreatic cancer tissue, the authors showed that the PSC activation marker α-SMA, co-localised with Sirius red stain for collagen (Figure 2A), as well as with PSC selective markers, desmin and glial fibrillary acidic protein. Most importantly, co-localization of staining for α-SMA and procollagen mRNA (using in situ hybridization) indicated that activated PSCs were the predominant source of the collagen in stroma (Figure 2B). The authors also found that conditioned medium from human pancreatic cancer cell lines increased the proliferation and activation of PSCs in vitro[33]. This was one of the first studies to initiate investigations into tumour stroma interactions in pancreatic cancer.
Figure 2 Pancreatic stellate cells are the source of collagen in stroma.
A: A representative pair of serial sections of human pancreatic cancer tissue shows that Sirius Red staining for collagen (red) co-localises with immunohistochemical staining for α-smooth muscle actin (α-SMA) (brown), suggesting the presence of activated pancreatic stellate cells (PSCs) in the stroma of pancreatic cancer[33]. Reprinted with permission from Wolters Kluwer Health (Apte et al[33]); B: Immunohistochemistry for α-SMA (brown) and in situ hybridisation for procollagen α1 mRNA (blue), reveals colocalisation of α-SMA and procollagen mRNA on human pancreatic cancer tissue indicating that active PSCs are the major source of collagen in tumour stroma. Reprinted with permission from Elsevier (Apte et al[43]).
ROLE OF PSCS AS PROGENITOR CELLS
Recent evidence suggests that in addition to synthesising ECM proteins, PSCs may have other roles within the pancreas, for example, as progenitor cells. In this regard, Mato et al[47] isolated and expanded pancreatic cells from lactating rats using mitoxantrone (a drug that acts through multidrug transporter systems) selection. They have reported that the surviving, mitoxantrone-resistant cells showed a PSC-like morphology (fibroblast-like with vitamin A lipid droplets), expressed the stem cell marker ABCG2 transporter (ATP binding cassette G2 transporter) and were able to secrete insulin after cell differentiation. More intriguingly, a recent study by Kordes et al[48] has reported that clonally expanded rat PSCs, when injected into hepatectomised recipient rats, are able to migrate to the liver and to reconstitute large parts of the liver by differentiating into hepatocytes and cholangiocytes, whereas muscle fibroblast do not show any such transformations.
IN VITRO INTERACTION BETWEEN PANCREATIC CANCER CELLS AND PSCS
In vitro studies assessing interactions between pancreatic cancer cells and PSCs usually involve co-culture experiments with these two types of cells (from humans and rodents) and/or exposure of one type of cell to the conditioned medium from the other. Pancreatic cancer cells have been shown to increase the proliferation and migration of PSCs as well as to induce the synthesis of collagen type I and fibronectin by PSCs[33,40,49]. Studies using neutralising antibodies have indicated that cancer cell-induced PSC proliferation and migration is mediated by platelet derived growth factor (PDGF), while the increase in synthesis of collagen I and fibronectin is modulated by the pro-fibrogenic factors, basic fibroblast growth factor (FGF-2) and TGF-β1 from cancer cells[33,40,49].
Other recently identified factors that may play a role in the interaction between cancer cells and PSCs include: (1) Cyclooxygenase (COX)-2, this enzyme is known to be constitutively expressed by PSCs. It catalyses reactions that transform arachidonic acid to prostaglandin H2, the latter is then converted into prostaglandins, prostacyclin and thromboxanes to modulate inflammation, immune responses, mitogenesis, etc.[50]. Conditioned medium from cancer cells increases COX-2 expression and proliferation of PSCs. Mitogen-activated protein kinase kinase inhibitor, U0126, was shown to inhibit the cancer cell-induced increase in COX-2 expression in PSCs, while a COX-2 inhibitor, NS398, prevented cancer cell-stimulated PSCs proliferation, suggesting a role for COX-2 in cancer cell - PSC interactions[51]; and (2) Trefoil factors (TFF), a family of proteins secreted by the gastrointestinal mucosa, play a role in restitution after mucosal damage[52,53]. TFF1 expression is elevated in PDAC tissue and is detected in the majority of pancreatic cancer cell lines. It stimulates PSC proliferation and migration, as well as cancer cell invasion. The receptor and downstream signalling pathway for TFF1 are yet to be identified. TFF2 is expressed in chronic pancreatitis and PDAC, and has been shown to stimulate cancer cell migration in transwell membrane or wound healing experimental settings[53]. TFF2 is postulated to act via the chemokine receptor type 4 (CXCR4, a receptor for stromal derived factor-1) that is also expressed by pancreatic cancer cell lines and PSCs. Interestingly, CXCR4 expression is elevated in PanINs and in PDAC and promotes cancer cell metastasis, growth and survival[52,54].
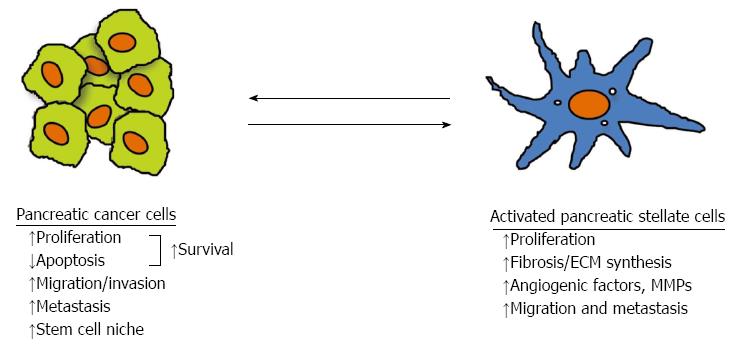
In parallel with the above described studies examining the influence of cancer cells on PSCs, researchers have been studying the effects of PSCs on cancer cells (Figure 3). Conditioned medium from PSCs has been shown to stimulate pancreatic cancer cell proliferation, and this effect is inhibited by pretreatment of the medium with a PDGF neutralising antibody. Since cancer cells express PDGF receptor, it is thought that PDGF in PSC conditioned medium mediates the observed effect[49]. Conditioned medium from PSCs also stimulates cancer cell migration, invasion and colony formation (again mediated by PDGF), but inhibits apoptosis, and increases resistance to chemotherapy and radiation. ERK1/2 and Akt kinases in cancer cells are known to increase after incubation with PSC conditioned medium[49,55], suggesting that these pathways mediate the responses of cancer cells to PSC conditioned medium.
Figure 3 Interaction between pancreatic cancer cells and pancreatic stellate cells.
Pancreatic cancer cells stimulate the proliferation, extracellular matrix (ECM) production, angiogenic factors and matrix metalloproteinase (MMP) expression, as well as migration of pancreatic stellate cells (PSCs); conversely PSCs increase proliferation and reduce apoptosis leading to increased survival, increase cancer cell migration and facilitate a cancer stem cell niche. The overall effect of interaction between pancreatic cancer cells and PSCs facilitates cancer progression[43].
PSCs secrete a cell adhesion protein named periostin, which has been found to stimulate cancer cell growth and to confer resistance on the latter to serum starvation and hypoxia. Periostin may also act in an autocrine manner on PSCs themselves leading to increased collagen I and fibronectin production. Collagen I might subsequently perpetuate the PSCs activation. Notably, cancer cells have been shown to induce periostin expression, and modulate collagen I and fibronectin expression in PSCs thus creating a supportive microenvironment for the tumour[56].
Evidence is now accumulating to indicate that PSCs may also influence EMT in cancer cells. A recent study has reported that a subpopulation of pancreatic cancer cells express endoglin (CD105, a TGF-β co-receptor); upon co-culture with PSCs, the proportion of CD105 positive cancer cells increases, and these cells exhibit a greater increase in migration activity compared to CD105 negative cells. Interestingly, in the CD105 positive population of cancer cells, mRNA expression of E-cadherin (an epithelial cell marker) is suppressed while vimentin (a mesenchymal cell marker) is over expressed, indicating that CD105 expression is associated with EMT in cancer cells[57]. These results suggest that PSCs may induce EMT in pancreatic cancer cells. This concept is supported by another study. Using organotypic in vitro cultures (collagen I coated culture wells), Froeling et al[58] reported that co-culture of cancer cells and immortalised human PSCs (obtained from donor pancreas and transfected with retroviruses containing cDNA encoding human telomerase reverse transcriptase) in this system resulted in decreased E-cadherin expression and increased beta-catenin expression of cancer cells, again signifying a transformation of the cells to a more mesenchymal phenotype.
While, as described above, there is strong evidence that PSCs significantly influence cancer cell function, some doubts have been raised in a recent study as to whether all PSCs uniformly exert such effects. Ikenaga et al[59] have demonstrated the existence of functional heterogeneity among the PSC population. They have reported that a sub-population of PSCs which express CD10, a cell membrane associated MMP, exerts a more inductive effect on cancer cell invasion and proliferation than CD10 negative PSCs. Thus, this study suggests that human PSCs (isolated from pancreatic tissue of pancreatic cancer patients) may differ in their ability to affect cancer cells, and explain, at least in part, the heterogeneity observed in patients with regard to rate of disease progression.
Recurrence is a well recognised feature of pancreatic cancer and recent studies suggest that this may be related to a population of cancer stem cells (identified by expression of markers such as CD24, CD44 and CD133) that are resistant to treatment[43,57]. Interestingly, PSCs have been reported to increase the stem cell characteristics of cancer cells by inducing the expression of cancer stem cell-related genes ABCG2, Nestin and LIN28[60]. This surviving cancer stem cell niche may be an important factor in pancreatic cancer recurrence.
IN VIVO INTERACTION BETWEEN PANCREATIC CANCER CELLS AND PSCS
While the in vitro studies noted above provided robust data on the direct interaction between cancer cells and PSCs, in vivo studies are essential in terms of biological/whole organism relevance. In this regard, two earlier clinical studies have reported findings to support a role for the stroma in cancer progression. Watanabe et al[61] have reported that the presence of fibrotic foci (which the authors postulated as representing intratumoural fibroblast proliferation) was associated with shorter survival in advanced pancreatic cancer, while Erkan et al[62] have reported that high α-SMA/collagen ratios in tumours correlated with poor prognosis. However, as detailed below, most of the in vivo evidence in support of tumour-stromal interactions in pancreatic cancer comes from experimental studies using tumour xenografts and genetically engineered mouse models.
Using a subcutaneous mouse model of pancreatic cancer, wherein tumours were produced by injecting a suspension of pancreatic cancer cells, alone or in combination with PSCs, into the flanks of mice, Bachem et al[40] showed that mice injected with both cell types exhibited larger tumours than those injected with cancer cells alone. Histological assessment of tumours indicated that cancer cell proliferation and stromal content were both increased in the presence of PSCs, an effect that would contribute to the observed increase in tumour volume. However, subcutaneous xenograft models have obvious limitations, since they do not replicate the appropriate microenvironment, nor can they provide information on metastasis.
In 2008, two research groups used similar approaches involving injection of a mixture of pancreatic cancer cells (cell lines MiaPaCa-2 or BxPC-3) and human PSCs (either primary culture[49] or immortalised cells[55]) into mouse pancreas. The results from these studies demonstrated that co-injection of cancer cells and PSCs yielded larger tumours with higher cancer cell density revealed by cytokeratin staining, increased fibrosis as determined by Masson’s trichrome staining and higher number of activated PSCs (increased α-SMA staining). The incidence of metastasis was also higher in the presence of PSCs in both studies. It is interesting to note that these facilitatory effects of PSCs on cancer progression are not restricted to the PSCs derived from resected pancreatic cancer tissue (i.e., PSCs that have been exposed to cancer cells prior to isolation). Xu et al[63] have demonstrated that normal human PSCs (isolated from normal pancreas) exert a similar facilitatory effect on tumour growth and metastasis in an orthotopic mouse model of pancreatic cancer. These findings suggest that PSCs are relatively quick to acquire tumour inductive properties after a relatively short exposure to cancer cells, supporting the concept that cancer cells are highly efficient and effective at recruiting surrounding PSCs, so as to set up a conducive microenvironment (such as ECM) for their own growth. Indeed, direct effect on cancer cells of ECM (produced by PSCs) have been demonstrated by Scaife et al[64]. The authors assessed cancer progression in an orthotopic mouse model of pancreatic cancer by implanting a mixture of cancer cells and synthetic ECM (a hyaluronan-based hydrogel) into the pancreas of nude mice; the encapsulation of cancer cells within ECM yielded larger tumours than cancer cells suspended in serum-free media.
In contrast to orthotopic models where tumours are produced in immunocompromised mice by xenografts of human pancreatic cancer cells and PSCs, genetically engineered mouse models exhibit the development of spontaneous pancreatic tumours with a prominent endogenously produced stromal reaction. These models include KPC mice (KrasLSL-G12D/+; Trp53LSL-R172H/+; Pdxcre/+), KPGC mice (KrasLSL-G12D/+; Trp53LSL-R172H/+; R26LSL-GFP/+; Pdxcre/+), and TGFβ type II receptor organ specific knockout in the mouse pancreas (KrasLSL-G12D/+; TGFβr2floxflox; Ptf1acre/+). The tumours develop spontaneously with lesions progressing from preinvasive ductal changes to overt carcinoma and metastases. The stromal reaction also increases over time and importantly, activated PSCs are observed at the earliest time point (PanIN stages)[65-68]. It is anticipated that such models will be increasingly utilised to assess mechanisms of stromal-tumour interaction and new therapeutic strategies in pancreatic cancer.
ANGIOGENESIS IN PANCREATIC CANCER
Pancreatic cancer is poorly perfused, with the blood vessels in the tumours being often disorganised with irregular diameters, abnormal multiple branching, disrupted endothelial cells junctions and missing or disordered basement membrane. These changes cause the microvasculature of tumours to become leaky[69]. Angiogenesis is a complex process, and the response of endothelial cells in different parts of the tumour likely depends on the balance of the pro-angiogenic and anti-angiogenic factors within the surrounding microenvironment. It is possible that as activated PSCs lay down increasing fibrous stroma in central areas of the tumour, the blood vessels in that area are compressed, leading to insufficient perfusion and hypoxia. However, at the invading front of the tumour, where the collagenous stroma is significantly less dense, endothelial cell proliferation in response to activated PSC secretions can occur in a relatively unrestricted manner.
Masamune et al[70] reported that hypoxia induces migration, type I collagen expression, and VEGF production in PSCs. Interestingly, conditioned medium of hypoxia-treated PSCs induces endothelial cell proliferation, migration, and angiogenesis in vitro, possibly via VEGF. Xu et al[63] demonstrated that even when collected under normoxic conditions, conditioned medium from PSCs stimulated tube formation (a method of measuring angiogenesis in vitro) by human microvascular endothelial cells, and that this effect was again mediated by VEGF. Other factors produced by PSCs under hypoxic conditions include FGF-2, angiopoietin-1, periostin and hypoxia inducible factor-1[70,71], which may also promote angiogenesis.
ROLE OF PANCREATIC STELLATE CELLS IN PANCREATIC CANCER METASTASIS
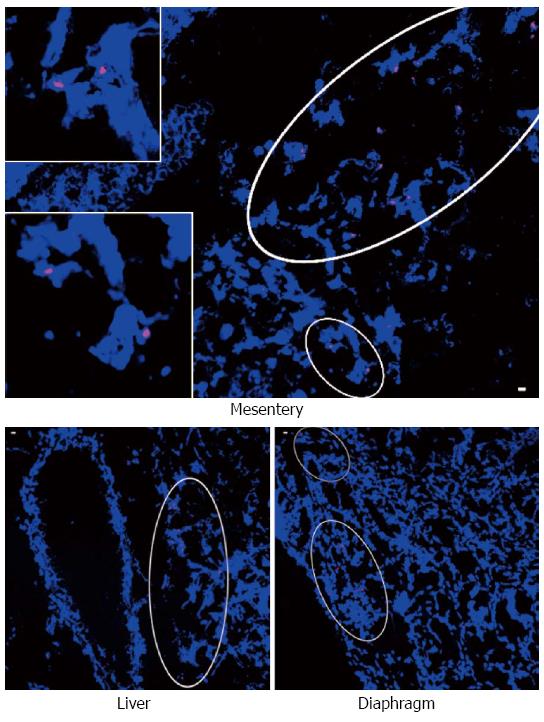
Metastasis occurs early in pancreatic cancer, and has long been regarded as a feature solely exhibited by cancer cells. However, this concept has been challenged in recent time with studies indicating that untransformed epithelial cells[72] and mesenchymal cells[73] may also have the capacity to metastasise. It is now accepted that cells can travel to metastatic sites through the circulation either as single cells, or more likely, as a cluster of cells. We believe that metastatic cell clusters in pancreatic cancer could comprise both cancer cells and PSCs from the primary tumour. In this regard, Xu et al[63] used a gender mismatch approach (female pancreatic cancer cells plus male human PSCs injected into the pancreas of female mice) to demonstrate the presence of Y-chromosome positive cells in metastatic nodules (Figure 4). These results indicated that male PSCs from primary tumours were able to (1) intravasate into blood vessels; (2) be transported in the circulation; and (3) extravasate from blood vessels at metastatic sites. This possibility was supported by in vitro studies showing that PSCs can migrate through an endothelial cell monolayer in vitro and this transendothelial migration is up-regulated by PDGF from cancer cells. In view of the above, we propose that PSCs that have travelled to the metastatic site perform a very important initial function at the metastatic sites, which is to facilitate seeding, survival and proliferation of the metastatic cancer cells at those sites. Also important is the likelihood that PSCs, via secretion of chemokines, subsequently recruit local stromal cells within the metastatic site, which further facilitates cancer cell growth.
Figure 4 Identification of human pancreatic stellate cells from primary tumour in metastatic nodules.
A representative photomicrograph showing Y chromosome positive cells in metastatic nodules in the mesentery (inserts are high power views of the circled regions), liver and diaphragm from mice (female) injected with female pancreatic cancer cells + male human pancreatic stellate cells, using fluorescent in situ hybridisation for the Y chromosome. Reprinted with permission from Elsevier (Xu et al[63]).
IMMUNE CELLS, IMMUNE EVASION AND PSCS IN PANCREATIC CANCER
It is well established that pancreatic cancer tissue is infiltrated with immune cells, such as T cells, B cells, NK cells, neutrophils and macrophages[43,74,75]. Higher levels of CD8+ T cell infiltration are correlated with a better survival[74,75], while macrophage and neutrophil infiltration show a negative correlation with survival[75]. Most recently, Ene-Obong et al[74] have reported that activated PSCs reduce the migration of CD8 positive T cell to cancer cells in human PDAC and the KPC mouse model of pancreatic cancer, indicating that PSCs may negatively modulate immune responses.
Immune evasion is also a characteristic of pancreatic cancer. Cancer cells have been shown to evade the host immune system by producing granulocte-macrophage colony-stimulating factor to suppress anti-tumour T cell immunity[76]. Recent studies suggest that PSCs may play a role in this process as well. PSCs in the stroma of PanIN lesions and around cancer cells produce galectin-1, a β-galactoside-binding protein[77,78], that binds to N-acetyllactosamine on membrane glycoproteins and induces apoptosis in T cells thus suppressing the immune response[79,80]. Fibroblast activation protein-α (FAP-α) known to be expressed by stromal cells, is another protein that has been reported to disrupt anti-tumour immunity. Depletion of the cells expressing FAP-α enabled immune response-associated tumour regression, supporting the notion that FAP-α might act as an immune suppressor in pancreatic cancer[81].
PANCREATIC STELLATE CELL AND ITS ROLE IN THE INITIATION OF NEOPLASIA
Evidence from recent studies is accumulating to indicate that PSCs might be activated at the earliest stages of carcinogenesis. Pandol et al[11] have found a distinct stromal reaction around PanIN lesions in a mouse model overexpressing KrasG12D that leads to pancreatic carcinogenesis. This stromal reaction is characterised by extensive collagen deposition and abundant α-SMA staining indicating the presence of activated PSCs around PanIN lesions. These findings corroborate those reported earlier by Fukushima et al[82] showing that periostin (solely expressed by PSCs) was observed in intraductal papillary mucinous neoplasms of the human pancreas. To assess the interaction between early neoplastic cells and PSCs, Pandol et al[11] isolated PanIN cells from the KrasG12D mice, and exposed PSCs to PanIN cell secretions. PSCs responded by increased proliferation, activation (α-SMA), fibronectin synthesis and MMP expression, indicating that preneoplastic cells have the capacity to activate PSCs in the early stages of carcinogenesis.
It is possible that in turn, these activated PSCs influence further progression of early lesions to established PDAC. In this regard, Funahashi et al[83] have reported that, nimesulide, a selective inhibitor of COX-2 (which as noted earlier is expressed by PSCs and implicated in PSC-cancer interactions[51]), retarded the progression of pancreatic cancer precursor lesions in a genetically engineered mouse model.
TARGETING THE STROMA OF PANCREATIC CANCER
The selection of treatment for pancreatic cancer patients depends on the stage of disease. The available options are surgery, chemotherapy, radiotherapy and recently developed targeted therapy, such as growth factor inhibition. Chemotherapy is the most frequently used treatment option for pancreatic cancer patients at different stages. Surgical resection with curative intent is only suitable for less than 20% of patients who have localised and early stage of pancreatic cancer[2,84]. Local recurrence is frequent and neoadjuvant and/or adjuvant therapies (chemotherapy and/or radiotherapy) are often required. For some advanced pancreatic cancers, surgery may be chosen to relieve obstruction and to improve the quality of life.
Gemcitabine was established as a first line chemotherapeutic drug for pancreatic cancer more than a decade ago, but it extends median overall survival only by several months[6]. Various combinations of chemotherapeutics have also been tried but regrettably the improvement has been negligible.
Based on an understanding of cancer cell biology and results from preclinical studies, several modalities targeting growth factor receptors and downstream signalling pathways have also been trialed. Unfortunately, these have not proved to be very successful. For example, the combination of erlotinib (an inhibitor of EGFR) and gemcitabine was shown to extend patient life by a mere two weeks vs gemcitabine alone. Other clinical trials involved inhibition of EGFR, VEGF and farnesyl-transferase by cetuximab, bevacizumab and tipifarnib respectively, but were not able to produce positive results[84]. The failure of translation of preclinical efficacy to the clinical situation may reflect the fact that many of the preclinical models used in these studies did not resemble human pancreatic cancer, in that, they lacked the stromal component.
In view of the above, it is clear that a comprehensive approach is needed to improve pancreatic cancer therapeutic efficacy. Given the increasingly recognised role of the stroma in cancer progression, there is a need to target not only cancer cells themselves but also the stromal elements in the tumour. The approaches discussed below have been built upon knowledge gained regarding PSC biology and ECM composition.
The Hedgehog signalling pathway is thought to play an important role in PSC activation[85]. This pathway is crucial to embryonic development, and stem cell regulation in adults, but has also been implicated in tumour development. There are three Hedgehog ligands in mammals: Sonic, Indian and Desert Hedgehog[86]. This signalling pathway is inactive in health, and therefore not detectable in healthy adult pancreas[87]. In the absence of Hedgehog ligand, its cell membrane bound receptor, named Patched, represses another transmembrane protein, called Smoothened. The binding of Hedgehog ligand to Patched causes the repression of Smoothened to be lifted, leading to activation of downstream Gli proteins, a family of transcription factors that regulate genes related to cell functions such as cell differentiation, proliferation, apoptosis, adhesion and migration[67,88-90]. Abnormal activation of Hedgehog pathway has been shown in basal cell carcinoma, as well as lung, prostate, pancreatic cancer[89]; this activation can be Hedgehog ligand dependent (as in pancreatic cancer) or due to mutation of Patched (as in basal cell carcinoma)[91]. In pancreatic cancer, Smoothened was shown to be highly expressed by PSCs, and Sonic Hedgehog ligand to be expressed by pancreatic cancer cells only[88]. Feldmann et al[92] administered cyclopamine, a Smoothened antagonist, in a Pdx1-Cre;LsL-KrasG12D; Ink4a/Arflox/lox transgenic pancreatic cancer mouse model (crossbred LsL-KrasG12D; Ink4a/Arflox/lox and Pdx1-Cre; Ink4a/Arflox/lox) resulting in an extension of the overall median survival from 61 to 67 d. Olive et al[67] administered IPI-926, a semisynthetic derivative of cyclopamine, alone or in combination with gemcitabine in a KPC pancreatic cancer mouse model. IPI-926 binds to and inhibits Smoothened to keep Gli in an inactive form[91]. IPI-926 decreased collagen 1 content in stroma associated with a decrease in the proliferation of α-SMA positive stromal cells and transiently increased blood vessel density in primary tumours in KPC mice. The authors reported an improvement in delivery of chemotherapeutic agent to the tumours and an extension of the median survival from 11 to 25 d. Hwang et al[88] applied another Smoothened inhibitor AZD8542 in an orthotopic xenograft model of pancreatic cancer produced by a mixture of PSCs and cancer cells in different proportions (0:1, 1:1 or 3:1) and showed that AZD8542 significantly reduced tumour volume, lowered metastasis, decreased Hedgehog downstream signalling activity via decreased GLI 1 expression and increased tumour vascularity in tumours with a 3:1 proportion of PSCs to cancer cells. These studies imply that Sonic Hedgehog acts in a paracrine manner on stroma to facilitate pancreatic cancer progression, and that Hedgehog inhibition represents a potentially useful additional treatment approach for pancreatic cancer.
There are now several clinical trials targeting Sonic Hedgehog pathway inhibition in pancreatic cancer[6]. Unfortunately, despite encouraging results in phase I trials, the most recent phase II trial of gemcitabine and IPI-926 has resulted in a disappointing outcome. The trial had to be halted due to progressive disease and decreased median overall survival in pancreatic cancer patients treated with IPI-926 and gemcitabine[6]. The reasons for the failure of this drug in the clinical setting are not entirely clear. The disappointing clinical outcome may reflect the fact that: (1) results from a single preclinical model are not sufficient to account for the heterogeneity of human pancreatic cancer; and (2) the effects described by Olive et al[67] in the preclinical model, particularly with regard to perfusion, were transient. Before taking treatments to the clinic, it would be prudent to ensure that robust, long lasting effects were demonstrable in the preclinical setting.
Most recently, researchers have utilised other compounds to target the stroma of pancreatic cancer. Kozono et al[93] administered pirfenidone (a pyridone compound that has been shown to be an effective antifibrotic agent in idiopathic pulmonary fibrosis) in subcutaneous and orthotopic models of pancreatic cancer. The results revealed that pirfenidone decreased the growth of tumours produced by the injection of a mixture of pancreatic cancer cells and PSCs, but not the growth of tumours produced by cancer cells alone. In vitro, the authors found that pirfenidone inhibited PSC proliferation, invasion and migration, and interrupted the interaction between pancreatic cancer cells and PSCs. These effects of pirfenidone were associated with decreased expression of PDGF-A, hepatocyte growth factor, periostin, collagen type I and fibronectin in PSCs, as well as reduced PSC activation as evidenced by decreased α-SMA expression in the cells. The findings suggest that pirfenidone regulates PSC function and inhibits cancer growth.
Angiotensin inhibitors, used routinely for treatment of hypertension, have been suggested as a potentially effective treatment in pancreatic cancer[94]. Angiotensin II is known to be able to stimulate PSC proliferation, migration, ECM production, and increase expression of FGF, TGF-β and VEGF[95,96]. Thus, angiotensin inhibition is postulated to prevent the activation of PSCs. Masamune et al[96] administered an angiotensin II type I receptor blocker, olmesartan, in a subcutaneous mouse model of pancreatic cancer. Similar to the effect of pirfenidone, olmesartan only inhibited the growth of tumours produced by injection of pancreatic cancer cells with PSCs, but not that of the tumours produced by cancer cells alone. The authors also reported that olmesartan reduced α-SMA expression and collagen deposition in tumours and decreased PSC proliferation and collagen I production in vitro. Similar to the results with olmesartan, Chauhan et al[97] have reported that another angiotensin II receptor inhibitor, losartan, decreased the density of α-SMA positive cells, collagen and hyaluronan production in the stroma of pancreatic cancer in an orthotopic mouse model. The effects of losartan might be mediated though reduction of TGF-β1, connective tissue growth factor and endothelin-1 (downstream target of TGF-β1) expression, all of which regulate ECM production by PSCs. The authors reported that the reduction in stroma decreased the physical pressure within the tumour, leading to improved perfusion and more effective drug delivery. The studies discussed above indicate that these compounds influence PSCs (stromal cells) function to inhibit tumour growth.
Taxanes [paclitaxel and docetaxel (semisynthetic analogue of paclitaxel)] have been widely used as chemotherapeutics for several cancers, such as breast, ovarian and non-small cell lung cancer. Paclitaxel inhibits the depolymerisation of microtubules in the cell and blocks cells in G2 and M phases resulting in cell death[98]. However, toxicity and insolubility in water of solvent-based paclitaxel significantly limit its clinical application. To overcome the toxicity of solvent, nab-paclitaxel was developed through homogenisation of paclitaxel and human serum albumin under high pressure to yield nano-particles about 130 nm in diameter. Paclitaxel is encapsulated by albumin in these nano-particles and becomes water soluble. As it is solvent free in comparison with parental compound, the toxicity of nab-paclitaxel is low and tolerated very well by pancreatic cancer patients. The albumin also enhances drug delivery through albumin facilitated receptor-mediated transcytosis[98].
Von Hoff et al[99] administered nab-paclitaxel alone or in combination with gemcitabine in a patient tumour-derived subcutaneous xenograft model. Nab-paclitaxel resulted in stromal depletion, increased the blood vessel diameter, increased expression of mNestin (an endothelial cell marker) in the tumour, and improved the delivery of gemcitabine. The mechanisms mediating these effects of nab-paclitaxel have not been fully elucidated. It has been observed that nab-paclitaxel accumulates in the proximity of tumour cells. Researchers have postulated that secreted protein acidic and rich in cysteine (SPARC), an albumin binding glycoprotein that is overexpressed in pancreatic cancer stroma[100], might contribute to the accumulation of nab-paclitaxel near tumour cells[98]. Analysis of SPARC expression in a clinical trial of gemcitabine and nab-paclitaxel combination has shown that high SPARC expression was correlated with significantly longer median overall survival compared to patients with low expression of SPARC[6]. Another mechanism that is also proposed to explain the synergistic effect of nab-paclitaxel and gemcitabine involves decreased metabolic inactivation of gemcitabine by cytidine deaminase which is destabilised by the increased production of reactive oxygen species in cancer cells following nab-paclitaxel administration[98]. The combination of nab-paclitaxel and gemcitabine is currently the subject of several ongoing clinical trials for locally advanced primary tumours and/or metastatic pancreatic cancer, as well as in neoadjuvant settings[6].
Two recent studies have directly targeted stromal ECM by using enzymes such as PEGylated human recombinant PH20 hyaluronidase (PEGPH20), to enzymatically degrade one of the predominant components of the ECM, hyaluronan. The authors reported that PEGPH20 treatment led to stromal depletion, resulting in decompression of tumour vessels and an increase in tumour vascular patency without increasing vessel density. PEGPH20 also increased fenestrations in endothelia and interendothelial junction gaps that increased the permeability of the endothelium to macromolecules. Thus, the delivery of gemcitabine was improved with the PEGPH20 and gemcitabine combination significantly inhibiting tumour growth and extending the median survival of KPC mice from 15 to 28.5 d compared to gemcitabine alone in the study done by Jacobetz et al[34] or 55.5-91.5 d in the study done by Provenzano et al[68].
Researchers have recently also turned their attention to immune cells in pancreatic cancer stroma. CD40 is a member of the TNF receptor family and plays an important role in the development of anti-tumour T cell immunity. Beatty et al[101] performed studies on the KPC mouse pancreatic cancer model showing that the administration of CD40 agonist antibody activated macrophages, induced caspase-3 expression (an indicator of apoptosis) and decreased collagen I content in tumours. The treatment of CD40 agonist antibody alone or in combination with gemcitabine induced a similar rate (30%) of tumour regression. This regression appeared not to be related to CD3+, CD4+ and CD8+ T cells in this in vivo study. The data from a clinical trial reported by the same group showed therapeutic efficacy of gemcitabine and CD40 agonist antibody on metastatic pancreatic cancer[101].
In summary, both in vitro and in vivo studies have clearly demonstrated a critical role of the stroma in the pathobiology of pancreatic cancer. PSCs interact closely with cancer cells to modulate cell proliferation, ECM production, migration and invasion of cancer cells. PSCs also play an important role in immune evasion, chemoresistance, angiogenesis and recurrence of pancreatic cancer (Figure 5). It is now increasingly clear that targeting tumour cells alone is insufficient to improve pancreatic cancer clinical outcome. Results from preclinical models and recent (albeit early) clinical trials provide vital evidence to support the concept that a comprehensive and combinatorial approach targeting both the cancer cells and stromal components in pancreatic cancer may represent the treatment strategy required to significantly improve the clinical outcome of this devastating disease.
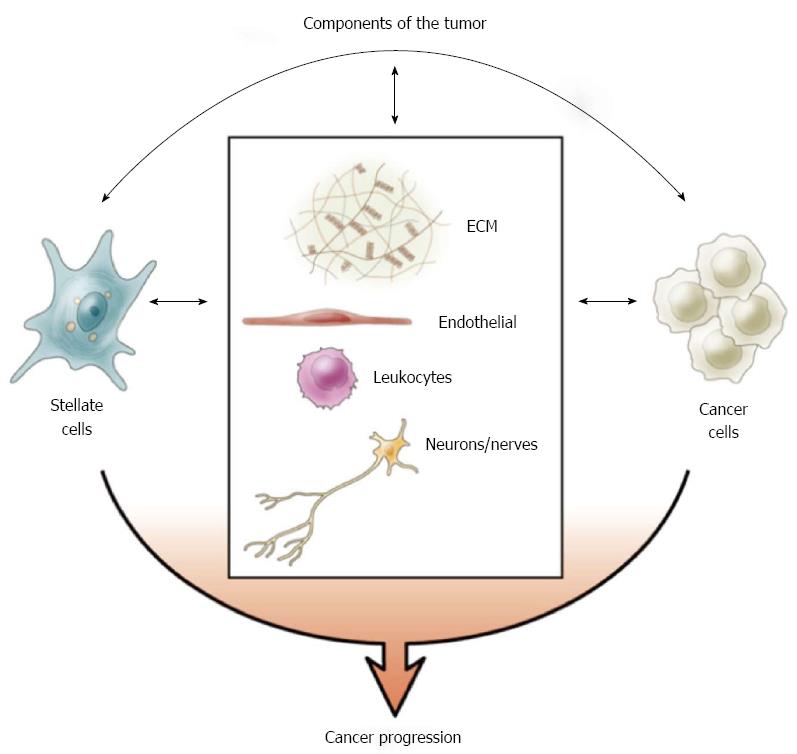
Figure 5 Tumour components.
The stromal reaction of pancreatic ductal adenocarcinoma is comprised of pancreatic stellate cells (stromal cells), abundant extracellular matrix (ECM), blood vessels/endothelial cells, immune cells and nerves/neurons[43]. The interaction between cancer cells and the components of stroma facilitates cancer progression. Reprinted with permission from Elsevier (Apte et al[43]).
ACKNOWLEDGEMENTS
Figures 2-5 are reprinted with permission from the publishers, Elsevier and Wolters Kluwer Health.