INTRODUCTION
Liver transplantation (LT) is the optimal treatment for patients with advanced-stage liver disease. However, the systemic and hepatic hemodynamic changes differ from patient to patient, such as the presence of collateral circulation, splenomegaly, or portal vein thrombus. Furthermore, the associated clinical parameters of portal vein flow (PVF), portal vein pressure (PVP), hepatic venous pressure gradient (HVPG), and hepatic artery flow (HAF) might interact, resulting in difficulties in decision making regarding whether to perform graft inflow modulation (GIM). Therefore, strategies aimed at obtaining an optimal blood supply to fulfill the oxygen and metabolic demands of the liver according to each individual represent a critical issue. In this review, we focus exclusively on the hemodynamic changes during LT and the possible correlations between clinically available parameters.
PHYSIOLOGY OF LIVER HEMODYNAMICS
The liver mass constitutes 2.5% of the total body weight or 33 g/kg of body weight[1,2]; however, the liver receives a total blood flow of 100-130 mL/min per 100 g liver and approximately 25% of the cardiac output[3,4]. The liver accounts for 10%-15% of the total blood volume and about 40% of that blood held in large vessels, such as the portal and hepatic veins, with the remaining 60% held in the sinusoids. Half of the hepatic blood can be rapidly expelled from the liver in response to both active and passive influences, thus providing the liver a major role as a blood volume reservoir[5,6].
The hepatic circulation is the most complex system among the organs owing to its dual blood supply from the portal vein and hepatic artery. The hepatic artery normally supplies about 25% of the portal blood flow to the liver, or 30 mL/min per 100 g liver weight (LW), and provides 30%-50% of the liver oxygen requirement with well-oxygenated blood. On the other hand, the portal vein carries about 75% of the total blood flow to the liver, or 90 mL/min per 100 g of liver tissue, and offers approximately 50%-70% of the normal liver oxygen requirement with partially deoxygenated blood of the venous outflow from the entire prehepatic splanchnic vascular bed. In the resting state, the liver accounts for approximately 20% of the total oxygen consumption of the body[7].
The valveless portal vein system is low pressure, low-resistance, and regulated mainly by mesenteric and splanchnic arteriole constriction as well as intrahepatic vascular resistance. The normal PVP is 5-10 mmHg as detected by direct cannulation[8] or the splenic puncture method[9,10], and the pressure in the sinusoid bed or clinical wedge hepatic venous pressure is estimated to be higher than that of the vena cava but slightly less than that of portal vein, with values of 3-10 mmHg[7,11-15]. In contrast, the hepatic artery is a high-pressure, high-resistance system regulated intrinsically by classical arterial autoregulation with a mean pressure similar to that of the aorta[15]. The normal hepatic artery and portal vein hemodynamic supply fluctuate and compensate for each other according to the physiological condition. The well-documented interaction between the hepatic artery and portal vein is termed the hepatic arterial buffer response (HABR)[16], which involves an increase of HAF to compensate for the reduced PVF to minimize the influence of PVF changes on hepatic clearance and to maintain the overall liver blood flow and adequate oxygen supply to tissues[17-20].
However, the PVF and PVP are less strongly correlated. In the normal liver, the PVP is relatively stable even when the PVF fluctuates. The sinusoidal structure and intrahepatic vasculature comprise a compliant vascular bed that can enlarge its volume to accommodate additional portal blood flow without changes in pressure[21-25].
Normally, hepatic hemodynamics change according to the encountered physiological condition and maintain the balance between systems and physical demands; however, this homeostasis is markedly altered in liver diseases and hepatic surgeries such as LT.
HEPATIC HEMODYNAMICS IN CIRRHOSIS-RELATED PORTAL HYPERTENSION
Portal hypertension is defined as a sustained increase in the intraluminal pressure of the portal vein and its collaterals with a mean pressure greater than 12 mmHg, the upper limit for variceal bleeding and other clinical consequences[26]. Cirrhosis-related portal hypertension may result from initial hepatocyte injury and inflammatory necrosis; then, activated stellate cells transform into contractile, fibrogenic myofibroblasts, which produce a large amount of extracellular matrix and inflammatory cytokines, and finally excessive fibrosis[21,27]. The increased sinusoidal resistance and structural changes of the sinusoidal endothelia result in diminished PVF and a reactive increase in PVP[28-31]. In contrast, the splanchnic vasculature undergoes progressive vasodilatation due to excess of vasodilators such as nitric oxide, which is related to increased vascular sheer stress and intestinal absorption of lipopolysaccharide[22,32,33]. Subsequently, vasodilators induce progressive vasodilatation of splanchnic circulation and a related PVF increase along with the development of systemic hyperdynamic circulation with reactive splenomegaly and portosystemic collateralization in multiple locations[34-37]. However, the progressive development of the collateral network and splenomegaly vary individually; it is thought that the development of collateral circulation was due to the opening of pre-existing vascular channels in response to increased PVP[37]. In cirrhotic patients, extrahepatic shunts may account for at least 50% of the portal flow, whereas 80% of the portal flow actually reaching the liver has been observed to bypass the sinusoidal vascular bed via intrahepatic shunts[7]. The azygos blood flow has been measured using a double thermodilution catheter directed under fluoroscopy in patients with alcoholic cirrhosis. The azygos blood flow was 596 ± 78 mL/min and 305 ± 29 mL/min in patients with repeated gastroesophageal variceal bleeding and others who underwent decompressive surgery of the portal system[38].
HEMODYNAMIC CHANGES DURING LIVER TRANSPLANTATION
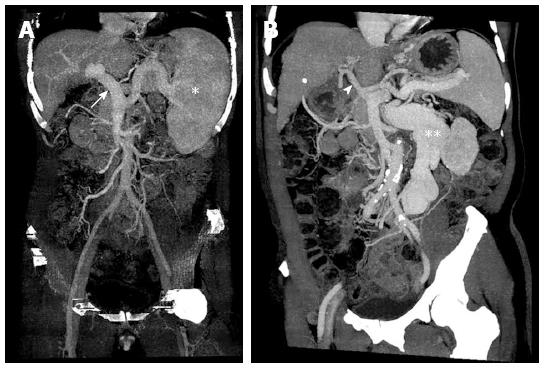
The hemodynamics vary widely among cirrhotic patients who undergo LT owing to different liver disease stages, underlying conditions, and disease nature; therefore, it is quite challenging for transplant surgeons to apply individualized transplant strategies. Cirrhotic patients exhibit different hepatic and splanchnic vasculature presentations at the time of transplantation. These are divided into two major types: (1) portal vein engorgement and splenomegaly without remarkable collateral formation; and (2) shrinkage of the portal vein with massive collateralization (Figure 1). The surgical strategies utilized during LT differ not only according to the vasculature but also depending on the graft type, such as a partial or full-sized graft. Thus, the hemodynamic changes during LT are important parameters for determining the optimal surgical strategy.
Figure 1 Hepatic and splanchnic vasculature at the time of transplantation in cirrhotic patients.
A: Portal vein engorgement (white arrow) and splenomegaly (white astrocyte) without remarkable collateral formation; B: Shrinkage of portal vein (white arrowhead) with massive collateralization (white double astrocytes).
LIVER TRANSPLANTATION WITH FULL-SIZED GRAFT
Adequate blood supply to the transplanted graft is essential to graft survival and function. In full-sized grafts, it was assumed that the PVF should be at least 1000 mL/min to maintain appropriate organ perfusion[39-41]. However, there are two major types of splanchnic vascular structures observed in patients before LT: (1) portal vein engorgement with splenomegaly but without massive collateral network formation; and (2) shrinkage of the portal vein with remarkable collateralization.
In the first situation, the possibility of developing small-for-size syndrome (SFSS) is less likely because of transplantation with a full-sized graft; however, the HABR becomes an important issue. Pratschke et al[42] reported that a decreased PVF of ≤ 1300 mL/min was associated with significantly lower organ survival in univariate analysis, but not in multivariate analysis; in contrast, diminished HAF was associated with an increased rate of primary nonfunction and impaired survival. In the same study, HAF was independent of other confounders with a hazard ratio of 2.5 for poor outcomes, and the clinical cutoff points of 100 and 240 mL/min were identified[42]. In another study, Spitzer et al[43] concluded that a target level of PVF > 1 L/min is required in LT to obtain better patient survival; in the hepatic artery, a baseline flow > 250 mL/min is a minimally acceptable level, but > 400 mL/min is ideal[43]. Therefore, when these patients undergo LT with full-sized grafts, two different types of GIM is recommended: splenic artery ligation (SAL) and splenectomy. We recommended that if the PVF is in the range of 1000-1300 mL/min and the HAF is < 100 mL/min, SAL may be the better choice. On the other hand, if the PVF is > 1300 mL/min and the HAF is far < 100 mL/min, splenectomy is likely the best choice if other possible structures or technical conditions are excluded. By performing SAL or splenectomy, excessive PVF can be prevented, which may impair HAF via the HABR.
In the second situation involving transplant patients with shrinkage of the portal vein and remarkable collateralization, including portal systemic shunting, the primary issue becomes the need for collateral or shunt ligation. Castillo-Suescun et al[44] presented a series of patients diagnosed with spontaneous splenorenal shunts (SSRSs) undergoing orthotopic LT, and shunt disconnection was performed when the post-reperfusion PVF was ≤ 1200 mL/min without any detrimental effects on renal function. However, strong clinical evidence is lacking regarding the performance of collateral or portosystemic shunt disconnection. Because the portal vein normally carries approximately 90 mL/min per 100 g LW[7], ligation of the major collateral vessels or shunts is reasonable and required if the portal perfusion is < 1000 mL/min, as the PVF would be shunted away from the new liver by old collaterals. Margarit et al[45] reported two patients underwent occlusion of distal splenorenal shunt during LT with an increase of PVF similar to that of splenorenal shunts. In addition, Esquivel et al[46] reported consistent anatomic changes in the portal vein diameter according to the presence of portosystemic shunt of 1.2-1.5 cm, which was smaller compared with other adult recipients without remarkable shunting or collaterals of an average of 2.5 cm[46,47]. Therefore, it is worth noting that when the portal vein diameter is smaller preoperatively by computed tomography or intraoperatively even without obvious collateral shunting, the possibility of portal hypoperfusion should always be taken into consideration.
LIVER TRANSPLANTATION WITH PARTIAL GRAFT
The hemodynamic changes are even more complicated when LT with partial graft is performed because of the higher risk of SFSS. The most appropriate hemodynamic parameter in deciding the application of GIM remains a topic of debate. PVP < 15 mmHg was reported as a key factor for successful adult living donor liver transplantation (LDLT) with better two-year survival[48]. In a subsequent study conducted by the same group, the cutoff point for intentional PVP modulation was 20 mmHg, which was mainly achieved by splenectomy or additional creation of a portosystemic shunt. Finally, the authors also concluded that intentional PVP modulation at < 15 mmHg is an effective surgical strategy for small-for-size grafts that establishes greater donor safety with good LDLT results[49]. Ito et al[50] reported that an elevated PVP of > 20 mmHg early in the first week post-transplantation is strongly associated with an increased incidence of bacteremia in the first three months and worse patient (graft) survival at six months. Other groups use PVP as an indicator for performing GIM, with the acceptable range of 15-20 mmHg[51,52]. The PVF is another parameter utilized by many groups; however, the proper range differs widely according to graft type. Sainz-Barriga et al[53] reported that the optimal threshold of four times the flow rate observed in healthy donors (360 mL/min per 100 g LW) is a risk factor for graft failure, and flow rates below the target of 180 mL/min per 100 g LW led to lower survival rates. This observation was confirmed by Hessheimer et al[54] in an experimental model. Shimamura et al[55] proposed that to avoid SFSS, a PVF of < 260 mL/min per 100 g LW is indicated. Troisi et al[56] also reported that a PVF value of 250 mL/min per 100 g LW predicted SFSS development.
In transplantation with partial grafts, HABR plays a crucial role despite the lack of consensus on the optimal range of HAF in LDLT. Sainz-Barriga et al[53] published a detailed report on the systemic and hepatic hemodynamics during LT. There was a significant difference in the median HAF between full-sized and partial grafts; however, no significant difference was found when the median HAF was indexed by graft weight. On the other hand, the ratio of PVF to HAF was elevated from the median of 6.6 to 15.4, which represented the effect of HABR[53]. Although no available data focus on the effect of the PVF to HAF ratio, it remains a potential predictor of surgical outcomes[57,58].
Similar to full-sized grafts, there are two major splanchnic vascular structures in patients before LT similarly as mentioned in the previous section. When the patients with engorged portal veins and splenomegaly undergo transplantation with partial grafts, the primary issue is the occurrence of SFSS. In the early era of LDLT, a graft versus recipient weight ratio (GRWR) of < 0.8 or a graft size of < 35% of the estimated standard graft weight were considered major risk factors for SFSS development[59,60]; however, with the evolution of surgical techniques and accumulation of clinical experience, the following investigations showed that the reduction of the lower limit of the GRWR to 0.6 in LDLT is safe[61-64]. The primary strategy employed to achieve better graft function and survival is strict inflow control including PVF and PVP instead of a smaller graft size. Asencio et al[65] hypothesized that the development of SFSS is not exclusively determined by the graft size, but instead by the hemodynamic parameters of the hepatic circulation, which indicates that hepatic hyperperfusion is a critical factor. Therefore, when cirrhotic patients with portal vein engorgement and splenomegaly undergo transplants with partial grafts, GIM is required in the majority owing to the potential risk of portal hyperperfusion. However, there is a lack of consensus about the clinical utility of using hemodynamic parameters to determine the need for and the type of GIM. It has been reported that splenic artery occlusion and coronary vein ligation can reduce the portal inflow by 52%[66], and SAL can reduce the portal inflow with a compensatory increase in HAF due to the HABR[67,68]. Troisi and de Hemptinne[56] reported that SAL resulted in a significant decrease in the PVF from 2600 ± 832 to 1700 ± 689 mL/min and a compensatory increase in the HAF from 87 ± 39 to 152 ± 64 mL; thus, SAL represents a simple and safe method that is sufficient to allow portal inflow modulation in most patients[69]. It is believed that intraoperative ligation of the splenic artery causes less than 50% of infarctions[70]. Furthermore, in cirrhotic patients with portal hypertension, splenic artery occlusion caused a significant reduction in PVP from the baseline of 21.5 ± 3.8 to 17.6 ± 3.2 mmHg 15 min after splenic artery occlusion (P < 0.0001) in the study conducted by Luca et al[71], Ito et al[50] reported similar results of immediate reduction of PVP with a median of 16-11 mmHg (P = 0.02) after SAL. Splenectomy or SAL is beneficial for improving outcomes of LDLT using a relatively smaller left lobe graft; however, splenectomy remains a life-threatening factor and is technically advanced compared with SAL[72,73]. However, Ikegami et al[74] began performing aggressive splenectomy using a vessel-sealing system to control PVP exceeding 20 mmHg, and a better graft survival rate was achieved compared to patients without strict portal pressure control (81.8% vs 90.6%, P < 0.01); in the same study, the complication rate of splenectomy was 10.1% (9/89), including pancreas leakage and overwhelming postsplenectomy sepsis[74]. Because of its technical simplicity and fewer postoperative complications, SAL can be considered the first-line GIM for portal hyperperfusion followed by subsequent splenectomy for more aggressive control[75].
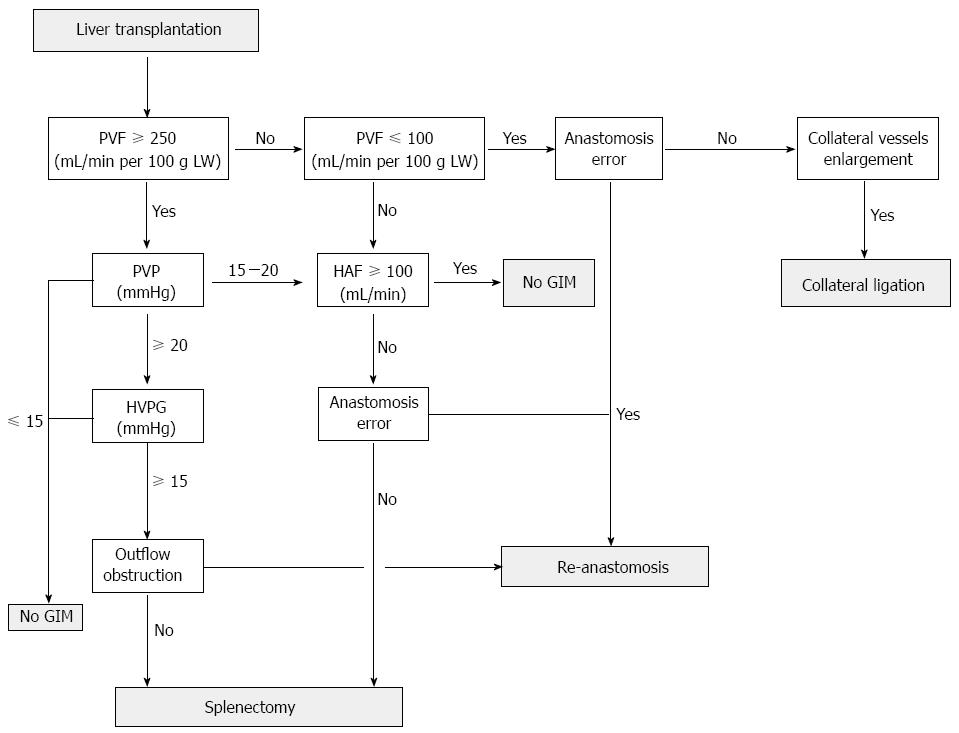
Flowcharts for GIM have been proposed by different groups primarily based on PVF and PVP; the Kyoto group uses PVP ≥ 20 mmHg as the cutoff point for performing splenectomy[49]. Asencio et al[65] proposed that either PVP > 20 mmHg or PVF > 250 mL/min per 100 g LWis indicated for implementing PVP control maneuvers. In a more detailed algorithm, the Italy group considers a PVF value greater than four times the normal baseline value (≥ 360 mL/min per 100 g LW) as the first-line determinant for performing portocaval shunt (PCS) or SAL, and then HVPG [HVPG = PVP - central venous pressure (CVP)] ≥ 15 mmHg is taken into consideration for possible PCS or SAL if the PVF does not exceed 4 times the normal baseline value. Finally, SAL is recommended if an HAF > 100 mL/min can be achieved after splenic artery isolation and clump testing under a normal gradient; otherwise, there is no need for further GIM, and the use of heparin and prostacyclin is considered[53]. After summarizing the flowcharts and recommended ranges for all clinically available parameters, the algorithm currently applied in our center for GIM is primarily based on the sequence of PVF, PVP, HVPG, hepatic outflow, and finally HAF (Figure 2). Hepatic outflow obstruction may aggravate the injury caused by excessive inflow with graft congestion, and it is essential to exclude hepatic outflow obstruction as well. Currently, clinical judgment of performing GIM is mainly based on the coloration, induration, and bile production of the graft at reperfusion along with objective parameters such as PVF, PVP, or HVPG, which varies in different centers. In the algorithm applied in our center, we strongly recommend measuring all available hemodynamic parameters required for delicate and individualized surgical strategies, particularly in transplant patients at high risk for developing SFSS.
Figure 2 Flowchart applied in our center for performing graft inflow modulation according to systemic and hepatic hemodynamic parameters in transplant patients with portal hyperperfusion.
Technical errors such as hepatic outflow obstruction, hepatic artery kinking, or anastomosis failure should be evaluated repeatedly. After graft inflow modulation (GIM) is performed, the portal vein flow (PVF), portal vein pressure (PVP), and central venous pressure should be re-measured to ensure optimal portal inflow. HAF: Hepatic artery flow; HVPG: Hepatic venous pressure gradient; LW: Liver weight.
In the second situation of transplant patients with shrinkage of portal vein and remarkable collateralization receiving partial grafts, the possibility of developing SFSS is lower; however, measurement of all hemodynamic parameters remains essential for determining the need for collateral ligation. Furthermore, portal hypoperfusion can occur when the collaterals are well developed, such as SSRS or coronary veins, and this is particularly common in recipients with portal vein thrombus, an important warning sign[70]. Sainz-Barriga et al[53] reported that flow rates below the target of 180 mL/min per 100 g LW led to lower survival rate; therefore, PVF is a major issue owing to the sinusoidal structure of the liver and its reservoir nature related to decreased PVP fluctuation if the veins are not fully distended[53,76-80]. Collateral shunting is a potential risk factor for portal hypoperfusion, and the need for disconnection remains controversial. In this circumstance, if the clinical decision is based solely the coloration and induration of the liver graft at reperfusion, the hypoperfusion state will be judged erroneously. It is worth noting that PVF measurement is required if the preoperative computed tomographic evaluations of the recipient shows a smaller portal vein caliber or the presence of remarkable collateral vessels. If the PVF is < 100 mL/min per 100 g LW, collateral ligation is definitely required, and if the PVF is between 100 to 180 mL/min per 100 g LW, collateral vessel isolation and clump testing should be performed. If the PVF exceeds 260 mL/min per 100 g LW, collateral ligation might not be needed in order to prevent portal hyperperfusion and decrease the HAF due to excessive HABR.
In order to describe the concept and rationale for GIM according to various hemodynamic parameters, we next describe two cases of LT performed in our hospital to illustrate how optimal graft inflow can be achieved.
CASE 1
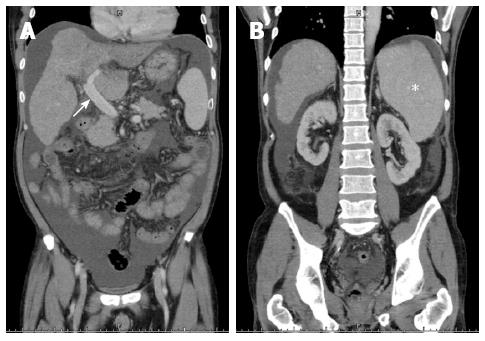
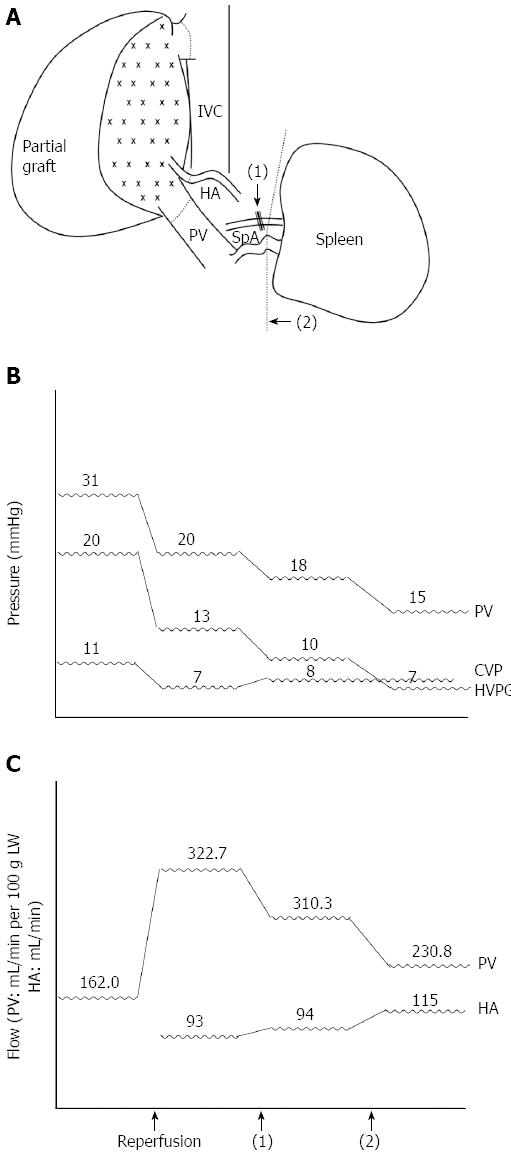
A 49-year-old man weighing 69 kg and diagnosed with hepatitis B-related liver cirrhosis, Child-Pugh B, and a hepatocellular carcinoma of approximately 6 cm in segment four, received a 600-g right lobe liver graft from his daughter. Preoperative computed tomography showed that the portal vein was 1.8 cm in diameter and splenomegaly was present with 15.4 cm at the longest axis without remarkable collateral vessels (Figure 3A and B). After portal vein isolation, the PVF before transplantation was 972 mL/min (162.0 mL/min per 100 g LW) measured by a transonic flowmeter with an ultrasonic probe encircling the main portal vein. The PVP was 31 mmHg detected by the direct puncture method and the CVP level was 11 mmHg. On reperfusion, the portal inflow was 1936 mL/min (322.7 mL/min per 100 g LW), the HAF was 93 mL/min, the PVP was 20 mmHg, and the CVP level was 7 mmHg. Considering the risk of SFSS development, SAL was performed first, and the PVF decreased to 1862 mL/min (310.3 mL/min per 100 g LW), the HAF was 94 mL/min, the PVP was 18 mmHg, and the CVP level was 8 mmHg. The PVF remained > 260 mL/min per 100 g LW; therefore, splenectomy was performed. After splenectomy, the PVF decreased to 1385 mL/min (230.8 mL/min per 100 g LW), the HAF increased to 115 mL/min, the PVP was 15 mmHg, and the CVP remained at 8 mmHg with an HVPG of 7 mmHg. The postoperative course was uneventful, and the patient was discharged from the hospital eight days after the operation (Figure 4).
Figure 3 Preoperative computed tomographic findings of case 1.
A: Engorged portal vein of 1.8 cm in diameter (white arrow); B: Splenomegaly with 15.4 cm at the longest axis without remarkable collaterals (white asterisk).
Figure 4 Sequential graft inflow modulation of case 1.
A: Splenic artery ligation [black arrow, (1)] followed with splenectomy [black arrow head, (2)]; B: Sequential changes of the portal vein (PV) pressure, central venous pressure (CVP), and hepatic venous pressure gradient (HVPG) during liver transplantation pre- and post-GIM; C: Sequential changes of the portal vein flow (mL/min per 100 g of LW) and hepatic artery (HA) flow (mL/min). IVC: Inferior vena cava; SpA: Splenic artery.
CASE 2
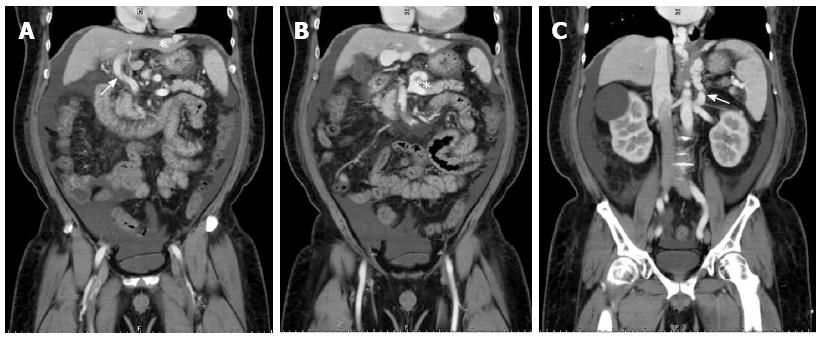
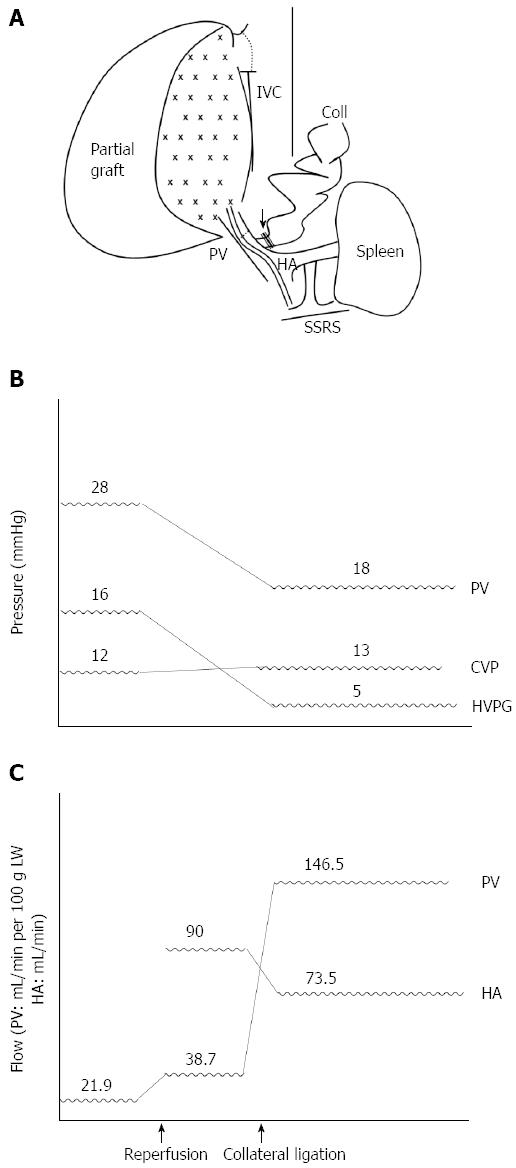
A 55-year-old man weighing 89 kg with hepatitis B-related liver cirrhosis complicated by bleeding esophageal varices and a 4-cm hepatocellular carcinoma in segments six and seven underwent LDLT with a 620-g right lobe graft from his son. Preoperative computed tomography showed that the right lobe portal vein could not be identified, likely due to hepatofugal flow-induced stasis and thrombosis; splenomegaly (long axis measuring about 12.5 cm), ascites, esophageal varices, a portal-systemic shunt from the left portal vein to the left pericardiac-phrenic vein, and SSRS were also noted (Figure 5). Before transplantation, the PVF was 136.0 mL/min (21.9 mL/min per 100 g LW), the PVP was 28 mmHg, and the CVP was 12 mmHg. At reperfusion, the PVF was 240 mL/min (38.7 mL/min per 100 g LW). Therefore, we performed hepatic artery anastomosis immediately with flow rate of 90 mL/min, and subsequent coronary vein ligation without measuring PVP because of portal hypoperfusion. Finally, the PVF was 908 mL/min (146.5 mL/min per 100 g LW), the HAF was 73.5 mL/min, the PVP was 18 mmHg, the CVP was 13 mmHg, and the HVPG was 5 mmHg. The patient recovered well after the operation and was discharged 10 d later (Figure 6).
Figure 5 Preoperative computed tomographic findings of case 2.
A: The right lobe portal vein could not be identified, likely owing to hepatofugal flow-induced stasis and thrombosis (white arrowhead) and portal-systemic shunting from the left portal vein to the left pericardiac-phrenic vein (white asterisk); B: Coronary vein engorgement (double white asterisks); C: Spontaneous splenorenal shunting was also noted (white arrow).
Figure 6 Sequential graft inflow modulation of case 2.
A: After transplantation with a right-lobe graft, hypoperfusion was noted and coronary vein ligation was performed immediately (black arrow); B: Sequential changes of the portal vein (PV) pressure, central venous pressure (CVP), and hepatic venous pressure gradient (HVPG) during liver transplantation pre- and post-coronary vein ligation; C: Sequential changes of the portal vein flow (mL/min per 100 g of LW) and hepatic artery flow (mL/min). Coll: Collateral; HA: Hepatic artery; IVC: Inferior vena cava; SSRS: Spontaneous splenorenal shunt.
DISCUSSION
These two cases focused primarily on the two most frequently encountered situations for transplant surgeons: portal hyperperfusion and hypoperfusion. In the first case, the preoperative evaluation showed an engorged portal vein with splenomegaly, which were both considered the presentation of severe portal hypertension without well-developed collaterals, thus GIM might be required even if the GRWR was more than 0.8. We had performed a series of measurements of PVF, PVP, HAF, and CVP; the GIM of SAL and splenectomy were considered sequentially with the consideration of all hemodynamic parameters instead of PVF or PVP alone. Finally, optimal PVF, PVP, and HVPG values were achieved without difficulty. There is always a debate about the best indicator for GIM, such as PVF, PVP, or even HVPG; however, our group takes all available parameters into account and proposes an individualized transplant procedure instead.
In the second case, preoperative computed tomography revealed the presence of possible portal vein thrombus and abundant portosystemic collaterals with portal vein shrinkage, which increased the risk of the development of portal hypoperfusion after transplantation even with a smaller partial graft (GRWR < 0.8). After reperfusion, PVF measurement was performed routinely despite subjective assessment of the graft being soft without congestion, and portal hypoperfusion was noted immediately. Therefore, hepatic artery anastomosis was performed without delay with subsequent ligation of the coronary vein, which was easier and less risky than splenorenal shunt ligation. Finally, satisfying results were also obtained without surgical complications.
We strongly recommend that all hemodynamic parameters should be monitored during surgery to evaluate the graft status instead of clinical subjective observations of the graft consistency and coloration. Additionally, transplant surgeons should review the preoperative studies carefully to evaluate the possibility of portal hypoperfusion or hyperperfusion instead of considering GRWR only.
CONCLUSION
The delicate control of all hemodynamic parameters during LT is critical for better graft survival and a reduced risk of perioperative complications. Although a lack of consensus remains regarding the best clinical parameter such as PVF or PVP, measurement of all available hemodynamic indices is essential for developing an individualized surgical plan not based on clinical judgment alone. Preoperative evaluation of the present portal and splanchnic vasculature is warranted; in addition to graft type, surgeons can predict the likelihood of developing portal hyperperfusion or hypoperfusion states, which may lead to poor surgical outcomes. For transplant surgeons, the most challenging aspect of LT is not only the complex surgical techniques used but also the modulation of portal inflow during the operation that require comprehensive consideration of all hepatic and systemic hemodynamics both anatomically and clinically. Presently, there is a lack of statistical correlation between PVF and PVP; therefore, there is no consensus about the clinical utility of these parameters to serve as a practical indicator for performing graft inflow modulations. Further investigation is required to identify a more generalized parameter derived from not only the hepatic but also the systemic hemodynamic status, which will represent a more reliable predictor for surgical outcomes and a potential intraoperative determinant of GIM.