Published online Aug 28, 2014. doi: 10.3748/wjg.v20.i32.11095
Revised: February 15, 2014
Accepted: May 29, 2014
Published online: August 28, 2014
Processing time: 245 Days and 6 Hours
Hepatitis C virus (HCV)-related cirrhosis represents the leading cause of liver transplantation in developed, Western and Eastern countries. Unfortunately, liver transplantation does not cure recipient HCV infection: reinfection universally occurs and disease progression is faster after liver transplant. In this review we focus on what happens throughout the peri-transplant phase and in the first 6-12 mo after transplantation: during this crucial period a completely new balance between HCV, liver graft, the recipient’s immune response and anti-rejection therapy is achieved that will deeply affect subsequent outcomes. Nearly all patients show an early graft reinfection, with HCV viremia reaching and exceeding pre-transplant levels; in this setting, histological assessment is essential to differentiate recurrent hepatitis C from acute or chronic rejection; however, differentiating the two patterns remains difficult. The host immune response (mainly cellular mediated) appears to be crucial both in the control of HCV infection and in the genesis of rejection, and it is also strongly influenced by immunosuppressive treatment. At present no clear immunosuppressive strategy could be strongly recommended in HCV-positive recipients to prevent HCV recurrence, even immunotherapy appears to be ineffective. Nonetheless it seems reasonable that episodes of rejection and over-immunosuppression are more likely to enhance the risk of HCV recurrence through immunological mechanisms. Both complete prevention of rejection and optimization of immunosuppression should represent the main goals towards reducing the rate of graft HCV reinfection. In conclusion, post-transplant HCV recurrence remains an unresolved, thorny problem because many factors remain obscure and need to be better determined.
Core tip: Hepatitis C virus (HCV) graft reinfection universally occurs post-liver transplantation and disease progression is accelerated. Differentiating recurrent hepatitis from rejection is essential in this setting; however, differentiation of the two pathological patterns remains difficult. The host immune response appears to be crucial both in the control of HCV infection and in the genesis of rejection: complete prevention of rejection and optimization of immunosuppression should represent the main goals. A proper graft allocation seems to be crucial to realize an ideal donor-to-recipient matching; however, many factors remain obscure.
- Citation: Grassi A, Ballardini G. Post-liver transplant hepatitis C virus recurrence: An unresolved thorny problem. World J Gastroenterol 2014; 20(32): 11095-11115
- URL: https://www.wjgnet.com/1007-9327/full/v20/i32/11095.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i32.11095
Since the liver transplant (LT) was approved as a life-saving intervention for end-stage liver disease in the 1980s, decompensated cirrhosis from hepatitis C virus (HCV) has become, and will remain, the leading cause of LT in developed, Western and Eastern countries[1-3]. Unfortunately, LT does not cure the recipient’s HCV infection. Reinfection occurs universally and disease progression is accelerated compared with the non-transplanted population. Histologically proven hepatitis C-related cirrhosis can be documented within a mean of five years after transplantation and, from that point on, the first episode of decompensation may occur in less than one year. Graft failure and loss are the unavoidable result for about 30%-35% of patients, resulting in poor outcomes for HCV-infected recipients compared with those who are HCV-negative[4-6].
In this review we discuss the current understanding of graft HCV reinfection in the LT recipient, with a focus on the peri-transplant phase and the first 6-12 mo after transplantation: during this crucial period, a completely new balance between HCV, liver graft, recipient immune response and anti rejection therapy is achieved, which will deeply affect subsequent outcomes. We have divided this article into five sections, focusing on the following pivotal topics: (1) early dynamics of graft HCV reinfection post LT; (2) differentiating acute cellular rejection (ACR) from early recurrent hepatitis C (RHC); (3) the role of genetic and host immune response; (4) immunosuppressive treatment and immunotherapy; and (5) graft-related factors.
Reinfection (measured by detectable serum HCV RNA) is the universal outcome after LT for HCV-related liver disease[7,8]. Serum viral load reflects a complex interaction between viral production by infected cells and clearance by the host immune system. After LT, the relative contribution of each of these factors likely differs at different sampling times.
Serum HCV RNA decreases rapidly during, and immediately after, the removal of the infected liver and the implantation of the new, uninfected graft. This is followed by a steady increase in viral concentrations within days. Garcia-Retortillo et al[9] showed that serum viral load rapidly decreases with reperfusion of the allograft, presumably as the liver removes virus from the circulation and the intrahepatic viral amount increases (intrahepatic viral load was not determined in this study); serum viral load reached a nadir 8-24 h after reperfusion, likely representing saturation of cell surface receptors for HCV in the allograft. The subsequent increase in the serum viral load should represent established infection and production of new virus by the infected allograft. During the first week following transplantation, viral kinetics appear highly variable between individuals and may be related to an attenuated immunological response of the recipient[9]. Once the new liver becomes infected, hepatic viral replication resumes, with serum HCV RNA reaching and exceeding pre-LT levels[4,10-17]. The rapid increase of HCV viral loads after LT proves the high capacity of HCV to adapt to a new environment. In particular, viral escape from a dominant immune response early after LT could play a central role in viral persistence by enhancing viral survival when it is most susceptible to immune selection, as in case of massive infection of the graft[18].
Not all allografts are equally efficient hosts for viral replication: Negro et al[11] showed that rates of viral replication in allografts (determined by anti-genomic strand-specific real time-polymerase chain reaction) appear to differ between patients and seem to be not related to immunosuppression. Limitations of this study were its semi-quantitative nature and the lack of clear defined protocol biopsy time-points[11].
The impact of post-LT serum viral load on clinical prognosis remains unclear: some authors argued that viral load does not seem to be correlated with worse outcomes in the post-transplant setting[19,20]; however, more recently, others have shown that high levels of replication at this time are correlated with the development of additional fibrosis in the allograft at one year post-transplant[21] and are associated with increased patient mortality and liver-related mortality[22].
These results are likely confounded by many factors: blood loss, transfusions and ongoing resuscitation during surgery; furthermore, secondary sites of viral infection may also contribute to variability in amount of virus available to infect the liver[23].
Powers et al[24] carefully evaluated six HCV-positive patients, collecting very frequent blood samples during and soon after the anhepatic phase in the course of LT. During the pre-anhepatic and anhepatic phases, HCV RNA levels dropped with an average half-life of 0.8 h and begin to rise (doubling-time 2.0 d) only 15 h after the anhepatic phase. Based on the decline in viral load over the first 24 h of the post-anhepatic phase, the authors estimated that a non-hepatic source might contribute up to 4% of total viral production, confirming data reported by Dahari et al[25] who evaluated that this extra-hepatic compartment is responsible for about 3.1% of virus in circulation.
Other studies of viral replication have also examined HCV quasispecies evolution during first few days post LT.
Feliu et al[26] showed a reduced viral complexity with respect to pre-transplant levels, suggesting a “bottleneck” effect, which arose soon after LT such that only one part of the pre-transplant variants reinfects the graft.
In contrast to the “bottleneck” scenario, Gray et al[27] revealed that multiple HCV lineages are transmitted at the time of LT, without a major decrease in viral genetic diversity. Although only some of the pre-transplant lineages were identified within the first 4 mo post-transplant, lineages are undoubtedly present because their ancestors were sampled at later time points. It should be underlined, (as correctly reported by the authors themselves) that all virus populations in that study were obtained from serum and, although such viruses are often assumed to represent the viral population in the liver, they may also contain variants from non-hepatic sites.
Other authors demonstrated that allografts remove from the circulation, and are infected by, certain HCV subpopulations over others in the immediate post-operative period. This selection for a fraction of HVR1 (the second envelope protein at hypervariable region 1) variants by allografts suggests that this area of the viral envelope contributes significantly to viral-allograft interaction. Additionally, after transplant, allografts express variable amounts of CD81, a multifunctional protein that has been demonstrated to act as a cell surface receptor for HCV and may interact directly with HVR1[19,28].
Other authors focused their attention on SR-BI, an 82-kDa glycoprotein highly expressed in the liver. SR-BI binds a variety of lipoproteins (HDL and LDL) and is involved in bidirectional cholesterol transport across the cell membrane[29]. It has been suggested that the interplay between lipoproteins, SR-BI, and HCV envelope glycoproteins is required for HCV entrance into liver cells[30,31]. In the setting of LT, Meuleman et al[32] demonstrated that a human monoclonal antibody targeting SR-BI efficiently precluded HCV infection and viral spread after LT, both in vitro and in vivo.
In a small, but very precise, analysis of six patients infected by HCV genotype 1b who underwent LT, HCV variants reinfecting the liver graft were characterized by efficient entry and poor neutralization by antibodies present in pre-transplant serum. Conversely, pre-transplant subvariants not detected soon after LT were characterized by less effective hepatocyte entry[33].
Nevertheless, the clinical significance of quasispecies evolution with established infection remains controversial. Sullivan et al[34] found that higher levels of diversity correlate with less severe recurrence (presumably because of a stronger immune response to the virus), whereas Pessoa et al[35] showed that immunosuppressed transplanted patients have greater quasispecies diversity than immunocompetent non-transplanted patients.
Hughes et al[28] demonstrated that only a portion of the complex population of quasispecies present in patient serum before reperfusion of allografts goes on to infect the liver, and that this quasispecies selection begins immediately upon reperfusion. It seems possible that persistence of a predominant variant from pre-transplant serum to post-perfusion liver would result in a greater magnitude of liver infection. This appears to be in agreement with previously reported data[36,37], where persistence of a predominant serum variant from pre- to post-transplant serum was associated with RHC, whereas failure of predominant variants to persist post-transplant was associated with no early recurrence. On the contrary, Pessoa et al[35] found that in the subset of patients with fibrosing cholestatic hepatitis (a severe form of HCV recurrence associated with early graft failure and death), divergence of quasispecies is enhanced, resulting in the emergence of many new variants. In a peculiar model of superinfection (HCV-infected liver into an HCV-positive recipient), Vargas et al[38] demonstrated that superinfection of the liver by the donor strain is associated with significantly milder disease than when the recipient strain becomes dominant. In addition, genotype 1 consistently predominates over non-1 genotypes in recipients of infected grafts, suggesting replicative differences among viral strains.
The influence of HCV genotypes on RHC is still controversial: some studies demonstrated that the severity of recurrence and the levels of HCV viral replication after LT are higher in patients with genotype 1b infection than in those with other genotypes[4,39-41]. By contrast, Gayowski et al[42] reported that the rate and the severity of RHC does not differ among genotypes, suggesting that HCV genotype might not be a significant factor influencing post-LT HCV hepatitis.
Some authors have proposed that cytomegalovirus (CMV) and human herpes virus-6 may have an immunomodulatory effect in transplanted recipients, and might play a role in promoting HCV replication[43,44]. Bosch et al[45] considered 347 LT recipients (donor or recipient CMV seropositive) transplanted for HCV related liver disease retrospectively to evaluate the associations of CMV infection and disease with RHC after LT. They demonstrated that CMV infection was associated with increased risk of fibrosis stage ≥ 2 and grade of inflammation ≥ 2. By contrast, Nebbia et al[46] reported that short term CMV viremia did not increase the replication of HCV after LT.
In light of these contrasting data, the clinical significance of the degree of, and variations in, early quasispecies complexity, and the influence of HCV genotype or other viruses on HCV replication post-LT, remain mostly unclear.
The measurement of the amount of HCV in the serum and its dynamic evolution may be less relevant than the amount of virus in the liver. Intrahepatic viral load rather than freely circulating virus likely causes liver injury; therefore, liver viral load may better reflect the magnitude of infection than serum viral load. While Terrault et al[47] found that serum and liver viral loads differed widely (ratio of liver/serum viral load ranged from 17 to 286), Sreekumar et al[21] demonstrated that serum and liver viral loads were significantly correlated (r = 0.77-0.93, P < 0.01), though intrahepatic levels were always higher (on average by 79-fold). It should be noted that the authors obtained different results despite using the same technique: these differences may reflect the narrow dynamic range of detection for their assays (early generation branched DNA), which allows discrimination of a 3-log range of concentrations only.
Fewer data are available concerning the dynamics of HCV reinfection within the graft and the liver expression of HCV antigens.
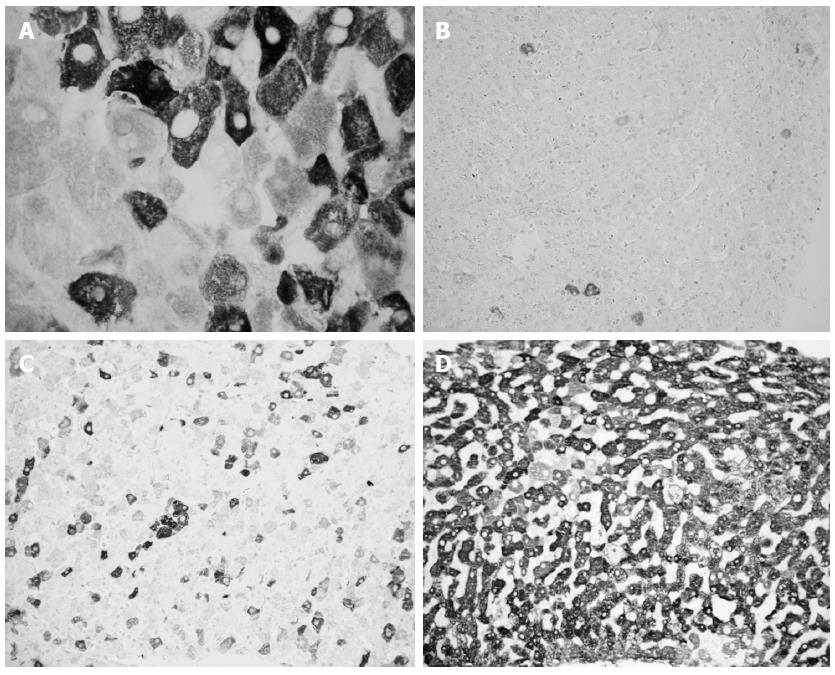
Liver HCV antigens expression is detected very early post-LT: 25% of liver specimens obtained within 10 d post LT show HCV antigens expression. This percentage rises to 66% and 90% when liver samples are collected between 11 and 20 or 21-60 d post-LT, respectively[48]. A subsequent paper demonstrated that the expression of liver HCV antigens is common until six months post-LT (92% of frozen liver specimens), while it declines after six months post-LT (74% of frozen liver specimens)[49], (Figure 1). Accordingly, Mensa et al[50] demonstrated on formalin fixed-paraffin embedded liver specimens that HCV core protein expression is present in 75% and 33% of acute phase and follow-up biopsies post-LT, respectively.
Differentiating between ACR and early RHC after LT is a challenging histological and clinical problem in the management of patients transplanted for HCV-related cirrhosis. In fact, both pathological conditions are associated with lymphocytic infiltration and variable degrees of bile duct injury in the portal tracts, as well as the presence of centrilobular necrosis. Clinically, increased aminotransferase and bilirubin levels characterize both diseases, whereas HCV blood viral load is of little help; moreover, both diseases may coexist.
The differentiation of RHC from ACR is crucial for appropriate treatment. Incorrect diagnosis may be detrimental, as failure to increase immunosuppression in patients with ACR may lead to acceleration of rejection. More importantly, increasing immunosuppression to treat presumed rejection may worsen RHC and lead to a faster progression to fibrosis and cirrhosis of the graft[13,51-54]. There is limited information on the reliability of histopathological assessment for the differentiation of RHC from ACR post-LT. One study in a small group of patients demonstrated relatively low interobserver and intraobserver agreement rates between two pathologists in early post-transplant liver biopsies[55]. More recently, Regev et al[56] evaluated interobserver agreement between five pathologists on the histopathological diagnosis in 102 liver biopsy specimens from post-LT HCV-positive patients. They revealed a slight agreement (K score = 0.12) on the histopathological diagnosis. All five pathologists agreed on the diagnosis of RHC in only five patients (5%) and on the diagnosis of ACR in only two patients (2%). Moreover, the intraobserver agreement also showed low reliability. Distinguishing RHC from ACR may be difficult, especially in the early stages of RHC, as both RHC and ACR may be associated with lymphocytic infiltration of the portal tracts and variable degree of bile duct injury with occasional lymphocytic aggregates. Thus, histology should be used very cautiously for differentiating RHC from ACR post-LT.
To improve the possibility of discriminating ACR from RHC we evaluated the percentage of HCV-infected hepatocytes using an immunohistochemical technique based on FITC-conjugated human polyclonal anti-HCV immunoglobulins in 55 frozen biopsy specimens from post-LT HCV recipients. The number of HCV-infected hepatocytes was never less than 40% in acute hepatitis specimens and never greater than 30% in the other cases; therefore, the detection of liver HCV antigens might be useful, combined with conventional histological evaluation, to make a diagnosis of RHC[48]. In a wider series (215 liver specimens) using the same technique, we found that in 15 out of 118 (13%) specimens obtained within six months post-LT, a final diagnosis of recurrent hepatitis occurred during the follow-up, despite previous inconclusive or discordant histological diagnosis. In all these patients, many infected hepatocytes were detected. Moreover, the presence of more than 30% HCV-infected hepatocytes confirmed the presence of RHC and “absolutely” excluded the presence of significant rejection[49]. These data were confirmed by Sadamori et al[57] using a similar immunohistochemical technique with a monoclonal antibody against HCV-envelope 2 in a series of 84 liver biopsies.
Other authors considered 65 liver specimens comparing tissue HCV quantification and HCV immunohistochemistry (IHC) to histology. They demonstrated that HCV RNA, HCV IHC, and Councilman body/portal tract ratio are the only variables able to discriminate ACR. They therefore proposed to routinely perform at least HCV RNA tissue quantification, in addition to histology, in all initial biopsies performed after LT in HCV-positive patients[58]. The same authors described stratification of the risk of RHC post-LT using tissue and serum HCV RNA quantification. In a series of 83 post-LT liver specimens they reported that when tissue HCV RNA is ≤ 1.5 IU/ng with any serum HCV RNA, the recurrent hepatitis rate was 61%. By contrast, when tissue HCV RNA was > 1.5 IU/ng the recurrent hepatitis rate was 91%, if serum HCV RNA < 40 × 106 copies/mL, and 100%, if serum HCV RNA > 40 × 106 copies/mL[59].
Ciccorossi et al[60] focused their attention on IgM anti-HCV in a series of 98 consecutive HCV-positive LT patients. They found that the serum IgM anti-HCV titer increased in 82% of cases with RHC, while remaining unchanged in all rejection cases. Moreover, the IgM anti-HCV titer increased in 10 of 11 histologically doubtful cases that were diagnosed as hepatitis at the subsequent liver biopsy. Thus, they proposed the quantitative monitoring of IgM anti-HCV as an additional diagnostic tool for distinguishing RHC from graft rejection.
Other authors reported that C4d (a marker of the activated complement cascade) is detectable in hepatic specimens in acute rejection after LT. They analyzed retrospectively 97 paraffin embedded specimens by immunohistochemistry, and demonstrated that 67.7% of patients with ACR showed C4d-positive staining in liver biopsy compared with 11.8% of patients with hepatitis C reinfection. The hypothesis is that humoral components, represented by C4d deposition, might play a role in ACR after LT and might be helpful to distinguish between acute rejection and HCV reinfection[61]. Nevertheless, the same authors were not able to confirm these results using ELISA measurement of C4d concentration in a prospective series of cryo-preserved liver biopsies from post-LT patients[62].
Transcriptional analysis has also been applied to explore potential pathways defining the presence of ACR in the setting of recurrent HCV infection after LT. Microarray analysis has identified differentially expressed genes associated with a variety of pathways, including apoptosis, as potentially targeting the presence of ACR in this setting[63].
Joshi et al[64] analyzed liver micro RNA (miRNA) expression in a carefully matched series of patients who had previously undergone LT for HCV-related liver disease, comparing those with slow vs rapid fibrosis progression, individuals with ACR, and control subjects without viral hepatitis. A clear segregation of miRNA expression patterns was seen for all four groups. A pathway analysis that compared subjects with slow fibrosis and subjects with rapid fibrosis revealed differences in miRNA expressions influencing antifibrotic, antiangiogenic, anti-inflammatory and antiapoptotic pathways. These results identified a number of potential pathways for further exploration with respect to the pathogenesis of RHC after LT, as well as potential biomarkers useful to detect rapid fibrosis progression and ACR in this setting. The main bias of this otherwise intriguing paper is the different timing of sampling biopsies. Patients with slow or rapid fibrosis progression had protocol liver biopsy one year after transplant, when fibrosis was already well established. By contrast, liver biopsies from patients with histologically diagnosed ACR were obtained at the time of suspected rejection. Thus it is not clear whether the observed changes in mRNA expression predict the development of a specific injury phenotype, or simply are the result of established differing patterns of injury within the allograft[64].
Recently Cabrera et al[65] proposed a blood test to discriminate ACR from RHC post LT, focusing on what happens in the blood rather than in the liver. Using the ImmuKnow assay, which measures the amount of ATP produced by CD4 lymphocytes after stimulation, they studied 42 transplanted patients. Patients with ACR presented a significantly stronger immune response than those with active RHC, while patients with mixed features of ACR and mild RHC showed an intermediate immune response[65]. The main advantage of this assay is the rapid assessment of nonspecific CD4 effector T-cells. Responses within 24 h offer real-time results on the status of the cell-mediated function, whereas the traditional functional immunological assays need long incubation periods. Obviously, as correctly reported by the authors, these data need to be confirmed in a larger population of transplanted patients.
The major histocompatibility complex (MHC) in LT plays a less important role than in other solid organs because the liver is more tolerogenic and most allograft losses are caused by recurrence of the primary disease rather than by rejection. Even if human leukocyte antigen (HLA) mismatching contributes to liver allograft rejection, lower graft survival rates have been reported when HLA compatibility between donor and recipient is present[66-68]. To explain these apparently contrasting data, Mañez et al[69] evaluated 58 patients transplanted for HCV-related end stage liver disease and proposed a dualistic role of HLA in LT: HLA matching reduces ACR but increases the risk of RHC post LT, favoring a more efficient MHC-restricted antigen presentation, thereby increasing cell-mediated immune responses toward HCV-infected liver allografts. Langrehr et al[70] confirmed this observation in a larger retrospective analysis of 165 HCV-positive transplanted patients. The number of rejection episodes increased significantly in patients with more HLA mismatches (P < 0.05), whereas fibrosis progression (presumably related to RHC) was significantly faster in patients with 0-5 HLA mismatches compared with patients with a complete HLA mismatch. Globally, there is no correlation between number of HLA mismatches and graft survival. These data are in agreement with ours and other reports showing that MHC-I restricted T cells mighty be involved in the control of post-operative HCV spread[48,71]. In contrast, Belli et al[72], evaluated two separate cohorts of 120 and 190 patients with liver graft for HCV-related disease and found that HLA-DRB1 mismatch affected the risk of RHC and its severity, both in univariate analysis and, after correction for known clinical factors, in multivariate analysis. Similarly, Balan et al[73] demonstrated that HLA mismatching in the A locus significantly increases the rates of HCV recurrence.
More recently, in a retrospective study of 163 patients with documented post-LT RHC, Audet et al[74] could not find any relationship between the total score of HLA mismatches and HCV recurrence. On the contrary, a significant relationship between the individual scores of HLA mismatches and the recurrence of HCV were observed for some recipient HLA genotypes (HLA-A3, HLA-B35, HLA-DR3, HLA-DR7, HLA-DQ2 and HLA-DQ2-0)[74]. It should be noted that in the two studies above, different end points were used according to the length of follow-up, making them poorly comparable with each other. Furthermore, the differences regarding ethnic background, immunosuppressive protocols, HLA typing methods and the definition of hepatitis recurrence, particularly when protocol liver biopsies were not performed[75], make the comparison difficult.
The association of allelic variation in the interleukin 28B (IL-28B) gene with HCV eradication after antiviral therapy provided new insight into the complex relationship between HCV and the human immune system. The initial report showed that patients homozygous for the C single-nucleotide polymorphism at position rs12979860 of chromosome 19q (corresponding to 3 kb upstream of the IL-28B gene) are twice as likely to achieve a sustained virological response to antiviral therapy than patients with either the CT or TT variant[76]. Subsequently, it was suggested that the CC variant is also associated with spontaneous viral clearance after acute HCV infection[77].
The evaluation of IL-28B gene in the setting of post-LT HCV infection is particularly complicated because recipients have two contributing sources of IL-28B genotypes: the recipient and the donor allograft. The impact of IL-28B on the antiviral treatment of HCV infection in the setting of LT goes beyond the purposes of this review, but has been fully analyzed[78]. Here, we focus on the impact of IL-28B in the spontaneous clearance of HCV and, if present, in the risk of ACR.
The spontaneous clearance of HCV infection is rare in the post-transplant setting, being limited to case reports. Only two studies of spontaneous clearance of HCV post-LT have included IL-28B genotyping, with three cases overall. Two out of the three cases involved recipients with the CT genotype, one had the CC genotype and all three patients received organs from CC donors[79,80]. Despite the limitations of the available data, it seems that the IL-28B gene of the donor has more influence than the recipient’s in this setting. By contrast, Biggins et al[81] recently suggested favorable effects of the CC genotype in the non-transplant setting or when present in the recipient, but unfavorable when present in a donor liver graft.
Bitetto et al[82] assessed the risk of ACR in 251 consecutive patients undergoing LT (40% with HCV-related cirrhosis). They found a significantly lower risk of ACR in recipients with the CC genotype (20.6% with CC, 34.1% with CT and 47.8% with TT, P = 0.003), but the association was weaker in patients with HCV infection vs those with other etiologies of liver disease. Other authors have studied the association between ACR and IL-28B SNPs in HCV-only cohorts, finding no significant variation in risk among the available genotypes for the IL-28B gene (donor and recipient)[83,84].
Overall, in this setting, the role of IL-28B gene remains uncertain.
There is evidence of a direct cytopathic effect of HCV in post-LT, which is supported by data demonstrating a higher viral load in patients with fibrosing cholestatic hepatitis with severe histology compared with patients with milder forms of HCV recurrence[14,85]. However, the evidence supporting a role for indirect immune-mediated mechanism in liver damage may even be more convincing.
In contrast to what happens in the vast majority of infections, specific antibodies to HCV, although diagnostic of infection, do not protect the host from subsequent damage from the same virus. Jain et al[86] quantified HCV antibody levels in 141 blood samples from 39 HCV-positive LT patients and confirmed that the antibody concentration did not correlate with viral load or hepatic injury in the post-LT setting.
In the non-transplant setting, immune responses appear to be crucial in the control of HCV infection. Patients with a self-limited course of acute HCV infection show activation of viral-specific CD4 and CD8 T-cells producing type 1 cytokines, such as interferon gamma (IFN-γ) and tumor necrosis factor-α (TNF-α)[87]. Presumably, the adaptive immune responses (CD4 helper T-cells and CD8 cytotoxic T-cells) and innate immune responses [natural killer (NK) and natural killer T (NKT)-cells] play a pivotal role in liver injury associated with RHC post-LT.
The majority of intrahepatic lymphocytes in patients with RHC after LT are represented by the CD8 T-cell subset: their presence is not proof of their role in liver injury, although it is unlikely that the predominant immune cells within the liver are simply bystanders. Asanza et al[88] demonstrated that patients with a more severe and progressive form of RHC after LT had higher numbers of activated lymphocytes, which implied that these activated CD8 T-cells play a critical role in injury and progression of liver disease. Corresponding to the main importance of CD8 T-cells in this setting, Rosen et al[71] described the presence of HLA-A2-restricted, HCV-specific CD8 T-cells in LT recipients, in whom the allograft was HLA-A2 positive, but the recipient was HLA-A2 negative. These cells are memory-effector recipient-derived T-cells that recognize HCV peptides uniquely in the context of HLA-A2. They are absent before the transplant, suggesting that the allograft is capable of selectively expanding naive CD8 T-cells that may function to control HCV spread in the allograft.
Evidence also suggest that not only CD8, but also CD4 T-cells play an important role in post-LT HCV recurrence.
Rosen et al[89] demonstrated that despite immunosuppression, HCV-specific, MHC class II-restricted CD4 T-cell responses are detectable in patients with minimal histological recurrence after LT. By contrast, peripheral blood mononuclear cells from patients with severe HCV recurrence, despite being able to proliferate in response to non-HCV antigens, fail to respond to the HCV antigens. These findings suggest that the inability to generate virus-specific T-cell responses plays a contributory role in the pathogenesis of HCV-related graft injury after LT. Other authors reported that the HCV-specific CD4 T-cell response after LT occurs early, is multispecific, compartmentalizes to the liver and does not correlate with recurrent disease[90], while another study reported that robust CD4 T-cell immunity is associated with milder recurrence of HCV[91]. Mendler et al[92] evaluated peripheral blood CD4 T-cell ATP activity in an LT cohort and concluded that after LT, global cellular immune function appeared depressed at baseline in HCV-positive vs HCV-negative patients and remained significantly lower in case of RHC with respect to non-recurrence. This has been subsequently confirmed also by Te et al[93]. In addition, Alkhouri et al[94], using the same technique, revealed that a greater suppression CD4 T-cells was associated with more rapid progression of fibrosis in patients with RHC post-LT.
Other authors focused their attention on regulatory T-cells (Tregs) and their contribution to HCV disease. The Treg population, which accounts for 5%-10% of peripheral CD4 T-cells, constitutively expresses CD25[95] and can suppress host immune responses in the setting of autoimmune diseases, transplantation and antitumor immunity[96,97].
Carpentier et al[98] showed that CD4/CD25 Tregs are overexpressed, both peripherally and in the liver, in HCV-positive patients after LT, compared with HCV-negative patients. Moreover, Tregs were significantly overexpressed in patients with severe RHC compared with those with mild recurrence. These data agree with the findings of Perrella et al[99] who showed that transplanted patients with HCV recurrence show an increased frequency and function of CD4/CD25 Tregs, similar to patients with acute hepatitis C who develop persistent infection.
Although direct HCV infection of dendritic cells (DCs) is rare, HCV is associated with decreased numbers of peripheral DCs in patients with chronic HCV-related liver disease[100,101]; however, very limited data exist for the post-LT setting. Ocaña et al[102] studied two LT patients demonstrating an inadequate maturation of DCs with relapsing HCV infection. According to these preliminary data, Schvoever et al[103] studied a small series of 16 transplanted patients (eight of them HCV-positive) and showed a significant decrease in the relative and absolute values of blood DCs at day seven after LT compared with the values obtained before transplant. The number increased again one month later in both HCV-infected patients and controls. The authors suggested that this could partially explain the early and systematic recurrence of HCV infection in the liver graft.
More recently, because of their fundamental role in the spectrum of host immune responses in chronic HCV infection, greater attention has been given to NK and NKT-cells and the innate immune response. Studies suggest that NK and NKT-cells are involved in HCV clearance and in liver injury in the post-LT setting[104,105]. Rosen et al[106] demonstrated that patients who develop severe RHC after LT have a lower frequency of NK and NKT-cells in peripheral blood before LT, suggesting a protective role of these immune cells in the post-transplant period after exposure of the graft to HCV. Furthermore, they demonstrated that the presence of HCV infection is associated with impaired cytolytic activity of NK and NKT-cells, providing evidence for quantitative and qualitative defects in innate immunity associated with severe RHC after LT. Varchetta et al[107] analyzed the dynamics of NK-cells after LT and demonstrated a significant reduction of this subset of cells seven days post-LT, probably as a result of graft repopulation, returning to baseline values thereafter. Moreover, in contrast with Rosen, they revealed a significant correlation between expression of the natural cytotoxicity receptors on NK-cells and ALT (P < 0.05), supporting the hypothesis that NK-cells participate in the necro-inflammatory process. Recently, Howell et al[108] studied 70 patients with RHC post-LT and demonstrated an impaired function of NK-cells (comprising reduction of IFN-γ secretion) without impairment of NK-cell cytotoxicity in patients with rapid fibrosis.
Other authors evaluated KIRs (KIRs are a family of activatory and inhibitory receptors present on NK-cell surface interacting with self-MHC class I ligands) and demonstrated that the mismatching of KIR-HLA-C ligands between donor-recipient pairs is associated with recurrent hepatitis, and that the presence of KIR2DL3 in the recipient is correlated with fibrosis progression[109]. In fact, KIR-HLA disease association studies are intriguing, but complex and difficult to evaluate. The interpretation of these data is largely speculative and often based on simplified models of MHC-KIR functional interactions.
When considering the Th1/Th2 paradigm, RHC post-LT appears related to an imbalance towards Th2 prevalence and vice versa. Tambur et al[110] studied 68 LT recipients and found that among patients without RHC, the percentage of genetically low IL-10 (Th2-cytokine) producers was higher than among patients with RHC. Furthermore, a genetic tendency to produce higher levels of IFN-γ (Th1-cytokine) was noted among LT recipients with no RHC than among those with RHC. These findings have been confirmed by Ocaña et al[102], who described a loss of IFN-γ and TNF-α (Th1-citokine) production in the LT recipient with relapsing HCV infection. In addition, Carpentier et al[98] suggested that high levels of IL-10 could be predictive of severe RHC post-LT.
Many data confirm the pivotal role of T-cells in the post-LT RHC setting, but they are essentially restricted to research field and are not usable in everyday clinical practice. A recent study by Nagai et al[111] appears particularly interesting because of its potential impact on daily clinical settings. They investigated the impact of peri-transplant absolute lymphocyte count (ALC) on HCV recurrence following LT in 289 patients and found that peri-transplant lymphopenia is significantly associated with higher rates of HCV recurrence. Furthermore, severe pre-LT lymphopenia appears to be an independent negative prognostic factor for overall survival. Therefore, the authors have proposed peri-transplant ALC as a novel and useful surrogate marker for prediction of HCV recurrence and patient survival, suitable for transplant physicians, surgeons and general practitioners.
A comprehensive summary of the role of each immune cell line is reported in Table 1.
| HCV antibodies | No protective role against HCV reinfection | [86] |
| CD8 T-cells | Correlation with recurrent hepatitis C (RHC) | [71,88] |
| CD4 T-cells | Protective role against RHC | [89-94] |
| CD4/CD25 T-cells | Correlation with RHC | [98,99] |
| Dendritic cells | Defective in case of RHC | [102,103] |
| NK/NKT-cells | Controversial: defective in case of RHC; damaging role in RHC | [106-109] |
| Th1/Th2 paradigm | RHC is related to an imbalance towards Th2 prevalence | [98,102,110] |
Corticosteroids are administered as an induction protocol during LT, and low doses combined with other immunosuppressants are used as maintenance immunosuppression after surgery. In cases of acute rejection, recipients receive pulse methylprednisolone to reverse the rejection.
In transplanted patients for HCV related liver disease, serum viral load increases very early post LT (typically by postoperative day two), during the induction steroid treatment[17,112], and methylprednisolone treatment for acute rejection leads to a 4-100-fold increase in serum HCV RNA[14,21]. Subsequently, the use of steroid boluses leads to an increased frequency of acute hepatitis, an earlier time to recurrence, a higher risk of progression to cirrhosis, and a higher risk of early post-transplant mortality[1,113,114]. Corticosteroids specifically increase HCV entry by upregulating factors like occludin and scavenger receptor class B type I; therefore, the use of corticosteroids on HCV infection in vivo may cause increased HCV dissemination[115]. In addition, Boor et al[116] showed that prednisolone suppresses the functions of plasmacytoid DCs (capable of producing IFN-α against HCV) by promoting their apoptosis.
However, despite the risks associated with steroid boluses, they remain the cornerstone of treatment for ACR, and corticosteroid maintenance therapy in association with newer immunosuppressive drugs has been evaluated significantly.
Klintmalm et al[117] considered 312 patients, randomized to one of three arms: tacrolimus (Tac) and corticosteroids vs Tac, corticosteroids and mycophenolate mofetil (MMF) vs Tac, daclizumab and MMF. They found no significant differences in graft or patient survival or HCV recurrence between the three groups; however, they found less risk of rejection in the corticosteroid-sparing arm. A subsequent study by the same group in 2011 showed there were still no differences in ACR, RHC, patient or graft survival at two years post-LT[118].
Kato et al[119] randomized 70 patients to Tac and; daclizumab vs Tac; and steroids vs Tac, MMF and daclizumab. They reported no significant difference in mean fibrosis stage between the three arms. Lladó et al[120] considered 198 patients randomized to basiliximab and cyclosporine with or without a 90-d prednisone taper, and reported similar fibrosis in the two groups. Both authors reported a reduction in bacterial infections and less post-transplant diabetes mellitus in the steroid-free groups.
Manousou et al[121] studied 103 patients and found that patients treated with Tac, azathioprine and maintenance steroids vs those not receiving maintenance steroids showed a lower incidence of severe fibrosis, suggesting a beneficial effect of maintenance steroids. Weiler et al[122] studied 30 HCV-positive patients who had received (after two weeks of Tac and corticosteroids) steroids vs placebo, in addition to Tac. They found that progression to cirrhosis was not influenced by continuing steroid therapy, but was more frequent in those receiving steroid boluses. Recently, Neumann et al[123] reported no significant differences in viral load, fibrosis score, or graft survival at 12 mo in 135 HCV-positive recipients randomized to Tac and daclizumab vs Tac and corticosteroids; however, these results appear inconclusive, mainly because of the higher dropout rates in the Tac and daclizumab group (55%) compared with the Tac and corticosteroids group (18%)[123].
Whether tapering-off of steroids might be more influential on outcomes than the avoidance or continued use of steroids is another matter of debate. Brillanti et al[124] studied 80 patients with RHC retrospectively and found that the slow tapering-off of steroids was the only factor associated with reduced recurrence and minor severity of post-transplant hepatitis C. Later Vivarelli et al[125] confirmed these data in a prospective randomized controlled trial, which showed that a rapid tapering (< 3 mo) is associated with more severe RHC. Finally, in 2008, Segev et al[126] performed a meta-analysis of 19 randomized trials that compared steroid-free with steroid-based immunosuppression. Although no individual trial reached statistical significance, the meta-analysis demonstrated that HCV recurrence is lower with steroid avoidance (RR 0.90, 95%CI: 0.82-0.99, P = 0.03). However, the authors themselves emphasized the heterogeneity of trials performed to date and, as such, did not recommend basing clinical guidelines on their conclusions.
Calcineurin inhibitors (CNIs) have been a cornerstone for immunosuppression since the National Institute of Health Consensus Conference approved LT for the treatment of end-stage liver disease in 1983[127]. Both cyclosporine A (CyA) and Tac bind with high affinity to a family of cytoplasmic proteins (called immunophilins), present in many immune cells. Immunophilin-dependent signal transduction via calcineurin leads to the activation of T-cell proliferation by regulating expression of the gene that encodes IL-2. The binding of CNIs blocks the activity of calcineurin and subsequently inhibits T-cell proliferation by the blockage of IL-2 production.
CyA has an antiviral effect against HCV: Watashi et al[128] showed an inhibitory effect of cyclosporin in vitro on HCV protein expression and replicon HCV ribonucleic acid levels, an effect that was not detected with Tac. Nakagawa et al[129] later confirmed these results; however, it remains unclear whether this finding reflects the in vivo situation.
Numerous retrospective studies have compared CyA with Tac in terms of the endpoints of patient/graft survival and HCV recurrence in HCV-positive recipients. Berenguer et al[130] reported a very comprehensive summary of 33 retrospective studies. In 28 studies, no consistent differences between CyA-based or Tac-based immunosuppressive regimens and recurrent disease were noted, while five studies suggested worse outcomes related with the use of Tac. In the same paper, the authors performed a meta-analysis on five prospective studies in the HCV-positive LT setting (366 patients), demonstrating that mortality, graft survival, acute rejection and fibrosing cholestatic hepatitis are comparable, independently of the CNI selected as the basic immunosuppressant. More recently, Irish et al[131] analyzed retrospectively data received from the United Network for Organ Sharing on 8809 HCV-positive LT recipients receiving either cyclosporine microemulsion (CSA ME) or Tac as maintenance immunosuppression. The results suggest that LT recipients receiving CSA-ME have an increased risk of death and graft loss because of HCV recurrent disease compared to those receiving Tac. These findings appear to contradict the above-mentioned previous results; indeed the explanation for the worse outcomes is not known. It may be related, however, to the higher rate of ACR and steroid-resistant ACR in the CSA-ME group: higher rejection rates could require multiple treatments of corticosteroid boluses, which are associated with more severe post-LT HCV recurrence.
MMF belongs to the class of anti-metabolite immunosuppressive agents. In addition to its potent immunosuppressive capacity, mycophenolic acid (MPA), the active metabolite of MMF, has an in vitro antiviral effect against HCV[132]. Moreover, in HCV cell culture models, MPA could induce the expression of important antiviral interferon-stimulated genes, probably involved in anti HCV activity[133]. Many studies have established that MMF monotherapy is ineffective because of unacceptably high incidences of ACR and chronic rejection[134,135]; therefore, in clinical practice, MMF is usually administered with lower doses of CyA or TAC, as a CNIs sparing agent, especially in cases of CNIs-related nephrotoxicity. In 2009, Germani et al[136] published a review based on 17 studies focusing the role of MMF in acute rejection and RHC. They showed that only two studies found a decreased severity of HCV recurrence with MMF, nine studies documented similar severities of HCV recurrence, and six studies showed increased severity of HCV recurrence. Subsequently, Manzia et al[137] showed, in a small retrospective study, a favorable effect of MMF monotherapy on the progression of liver fibrosis in HCV-positive LT patients.
Sirolimus (otherwise named rapamycin, originally known as a macrolid antibiotic) inhibits the mammalian target of the rapamycin (mTOR) pathway by directly binding to the mTOR complex 1, resulting in blockage of cell cycle progression from the G1 to S phase, thereby causing inhibition of T-cell proliferation. It reduces transforming growth factor beta and procollagen, which are both important factors in the development of hepatic fibrosis; therefore, it has been proposed that immunosuppression with sirolimus could reduce fibrosis progression. In addition, sirolimus reduces the in vivo phosphorylation of NS5A phosphopeptides (which enhance HCV virus replication) and therefore might inhibit HCV replication[138]. Additionally, mTOR proteins were found to protect HCV against apoptosis; therefore, sirolimus might improve apoptosis of HCV infected hepatocytes[139]. There are few studies describing the role of mTOR inhibitors in HCV recipients and that confirm the data in the clinical setting. Wagner et al[140] studied 67 post-LT HCV-positive patients, 39 received a regimen including sirolimus and 28 patients received CNIs. The sirolimus patients showed a significant decrease in HCV RNA levels and a significantly higher survival rate than the CNIs cohort. Other studies demonstrated that sirolimus is associated with slower progression towards advanced fibrosis in transplanted patients with HCV recurrence, but did not find any effect on the timing or severity of post-transplant RHC[141,142]. Notably, the United States Food and Drug Administration has issued a black-box warning against the use of sirolimus in LT patients because of significantly higher rates of hepatic artery thrombosis, graft loss and death[143]. Moreover, recently, Watt et al[144] analyzed 26414 patients (12589 HCV-positive) in the American Scientific Registry of Transplant Recipients database, and found that the use of sirolimus is strongly associated with increased mortality in the HCV group, but not in patients without HCV. Thus, sirolimus should be used with great caution in HCV-positive LT recipients.
OKT3 is a monoclonal antibody targeted at the CD3 receptor, a membrane protein on the surface of T-cells. It is approved for the therapy of acute, glucocorticoid-resistant rejection of allogeneic LT but, unfortunately, the use of OKT3 is associated with early and severe RHC after LT[54].
Alemtuzumab (campath-1H) is a humanized, recombinant anti-CD52 monoclonal antibody that depletes circulating lymphocytes but spares stem cells. It has been used as an induction agent in LT; however, there is little data about its use in HCV-positive recipients. Many abstracts have suggested extreme caution when using alemtuzumab in HCV-positive liver recipients. This appears to be confirmed by Marcos et al[145], who studied a cohort of 38 HCV-positive recipients treated with alemtuzumab as an induction agent: they reported a low rate of patient and graft survival (71% and 70%, respectively) after a follow up of 14-22 mo.
Antithymocyte globulin (ATG) is a rabbit-derived polyclonal antibody directed against human thymocytes. It has been administered mainly as an immunosuppressive induction agent, with the intent of sparing steroids. Many studies have compared the impact of ATG vs steroids in post-LT HCV-positive patients, revealing no significant differences in terms of HCV recurrence and patient/graft survival[146-150]. De Ruvo et al[151] compared ATG and Tac vs Tac and steroids in HCV-positive liver recipients. They confirmed no difference in the rate of RHC; however, significantly lower HCV RNA levels were seen in the ATG arm. Finally, Uemura et al[152] evaluated the UNOS database, including 16898 adult primary LT patients who received ATG alone, ATG and steroids, daclizumab alone or steroids alone as induction immunosuppression. In the subgroup with HCV, the use of ATG with steroids was associated with significantly inferior graft survival compared with daclizumab alone or steroids alone.
Daclizumab and basiliximab are antibodies against the IL-2 receptor, originally developed in an attempt to reduce CNIs use in patients with renal dysfunction. To date, there have been few studies specifically in HCV-positive recipients. As above mentioned neither daclizumab[117-119] nor basiliximab[120] showed any effect on HCV recurrence post-LT. In a non-randomized study, Nelson et al[153] demonstrated that early RHC and more rapid histological progression was associated with the use of daclizumab.
In the long term, because of the important role played by immunosuppression in HCV recurrence patients, the goal is to utilize the least number of drugs at the lowest dose, while still providing effective immunosuppression. Yoshizawa et al[154] anecdotally reported two cases of living donor LT for patients with HCV-related cirrhosis who received right-lobe grafts from an identical twin, in which, thanks to genetic identity, no immunosuppressive drugs needed to be administered. HCV RNA kinetics showed a rapid increase following LT and liver biopsies performed one month after transplant showed acute lobular hepatitis in both cases. In the more common setting of LT without genetic identity, a permanent immunosuppression free state (IFS) can be achieved in almost 25% of cases[155]. Manzia et al[156] performed a meticulous review on this topic in 2012, evaluating globally 91 HCV-positive recipients included in immunosuppression withdrawal studies worldwide. Twenty-three HCV-positive patients (25%) achieved a sustainable IFS with more than one year of follow-up; and 19 of 23 (83%) did not show HCV recurrence/progression in the long term. The same authors recently reevaluated their own data on six HCV-positive recipients who completed 10 years of IFS follow up and demonstrated that maintaining IFS appears beneficial towards a reduction in fibrosis progression in the long term[157]. In conclusion, even though few studies have reported long-term outcomes of IFS in HCV-positive recipients, withdrawal of immunosuppression seems to have a favorable effect on HCV disease progression after LT, avoids side effects such as dyslipidemia and diabetes, and permits sparing of other drugs that might negatively impact the natural history of post-LT disease.
Adoptive immunotherapy has only been studied in a phase-1 trial. Lymphocytes extracted from liver allograft perfusate were able to generate an anti-HCV response, so that activated graft-derived NK-cells were isolated from the perfusate and injected intravenously into the transplanted recipients. Early data from the pilot study reported lower HCV RNA titers at one-month post-LT; however, the effect was not confirmed in the long term[158].
Prophylactic therapy with neutralizing antibodies is effective in patients transplanted for HBV-related liver disease; however, currently, there is no evidence that this strategy is effective in preventing HCV recurrence. HCV antibody therapy usually starts in the anhepatic phase and is then continued for 12 to 14 wk after LT. Gurusamy et al[159] performed a Cochrane meta-analysis on three trials comparing high dose HCV antibody vs low dose HCV antibody. No differences in patient and graft survival, virological response and fibrosis progression were observed. Discontinuation of therapy occurred in 35% of patients with the high dose antibody and in 17% of patients with the low dose antibody.
Recently, Chung et al[160] tested a human monoclonal antibody targeting the HCV E2 glycoprotein (MBL-HCV1) in a small pilot study (six patients). They demonstrated that this treatment delays median time to viral rebound compared with placebo treatment, even if it is not able to prevent it. Considering the lack of clinical benefit and occurrence of side effects, there is currently no evidence supporting the use of prophylactic HCV antibody treatment.
In Western countries, living donor LT (LDLT) is usually performed to decrease the mortality among patients awaiting transplant because of the shortage of donor organs. In Eastern regions, with low deceased donor organ availability, LDLT represents the standard of care for HCV end stage liver disease, with indications similar to those of deceased donor LT (DDLT) in Western world.
Early studies reported a worse graft outcome and earlier and more aggressive RHC after LDLT compared with DDLT[161-163]. To explain these findings it has been hypothesized that the more severe HCV recurrence in LDLT is related to the genetic similarity between donor and recipient[164] and that the intense hepatocyte proliferation that occurs in partial liver grafts may induce enhanced HCV replication[165,166].
Nevertheless, more recent studies did not confirm these findings, on the contrary, they often revealed improved results in LDLT recipients compared with DDLT, possibly because of the young age of the donor and shorter ischemic time of LDLT grafts[167-173]. Compared with LDLT, DDLT recipients usually also have a higher model for end stage liver disease score (MELD-score), which is considered an independent prognostic factor for severe RHC and worse patient/graft outcome; therefore, the above data should be evaluated with caution[174]. In agreement with these considerations, Jain et al[173], in a subanalysis of their study, adjusted for MELD score (< 25) and donor age (< 50 years), and revealed similar outcomes between LDLT and DDLT.
In light of that, LDLT appears to be recommended for HCV-positive patients, whenever it is available.
The impact of donor age on outcome has become more and more important because of the increased use of liver grafts from older donors, reflecting the absolute shortage of available organs. Grafts from older donors are at greater risk of more severe HCV disease progression and impaired graft/patient survival compared with those from younger donors[175-179].
Lake et al[180] analyzed data from the American Scientific Registry of Transplant Recipients, looking at the effect of donor age on the outcome of 3463 HCV-positive transplanted patients. Donor age was the strongest predictor for graft loss in HCV-positive recipients, with hazard ratios of 1.67 and 2.21 for donors > 40 years and > 60 years, respectively. In a multicenter study of more than 500 HCV-positive recipients, the risk of severe RHC following LT from a donor older than 60 years old was doubled in female compared with male recipients. This gender impact on HCV recurrence is not observed with younger donors and remains unexplained[181]. Recently, Avolio et al[182] analyzed 5946 liver transplants on a national Italian database and proposed that the MELD score adjusted by donor age (D-MELD: calculated as donor age × MELD) could accurately predict the outcome of HCV-infected recipients. In conclusion, it remains very difficult to define an age cut-off level beyond which older donors should not be used for HCV-positive recipients.
The increasing organ shortage prompted transplant centers to use grafts from HCV-positive donors. Several studies demonstrated that in HCV-positive recipients, grafts from HCV-positive donors are as safe as those from HCV-negative donors[177,183-186]. Wilson et al[187] evaluated data from the United Network for Organ Sharing (UNOS) and demonstrated that receiving a graft from an HCV-positive donor might be more favorable. They performed a case-control study (published only in abstract form and not in extenso) evaluating 38 HCV-infected recipients of HCV-infected grafts compared with 76 LT recipients of livers strictly meeting UNOS criteria. One-year patient survival rates of 97% favored recipients of HCV-infected grafts compared with rates of 87.5% for recipients of organs meeting the UNOS criteria. The same results have been noted for progression of fibrosis one-year post-LT: a 26% increase in fibrosis in HCV-infected organs compared with a 69% increase in the UNOS-approved group.
Nevertheless, considering the risk of super-infection and the impaired response of genotype 1 to antiviral treatment, it remains advisable that HCV-positive grafts should be used only in HCV genotype 1-positive recipients.
The impact of allograft steatosis on fibrosis progression and on the outcome of HCV-positive recipients remains controversial. Two studies indicated that moderate/severe donor graft steatosis (> 30%-35%) might induce more frequent, earlier and more severe HCV recurrence[188], and might contribute to fibrosis progression and poor outcome[189] post-LT. Nevertheless, Botha et al[190] found that recipients receiving grafts with mild steatosis (< 15% in their classification) had a good outcome, although only three out of 113 donors presented steatosis greater than 30%. Burra et al[191] reached the same conclusion, although they classified mild steatosis as < 30% and only five patients in their cohort presented steatosis > 30%. In light of that, the grade of steatosis seems to represent a crucial factor: grafts with mild steatosis are expected to be as safe as non-steatotic grafts.
Prolonged liver ischemia followed by reperfusion, which occurs during LT, results in severe injury that contributes to increased morbidity and mortality after LT. This phenomenon is defined as ischemia-reperfusion injury (IRI). IRI of the graft depends on many peri-operative factors: cold and warm ischemia time; preservation solution and technical factors during graft removal; donor status (cardiac or brain death); and type of reperfusion used. Its complexity depends on many variables; therefore, the majority of studies have found it difficult to focus on IRI. Within these limitations, ischemic injury to the graft seems to have a serious impact on patient/graft survival and disease progression in HCV recipients[192-194]. However, Killackey et al[195] reported a significant correlation between peak alanine transaminase and the severity of IRI on reperfusion biopsy among 477 HCV-positive recipients, but did not identify a correlation between the severity of IRI and the incidence or timing of HCV recurrence or incidence of ACR. When IRI is associated with moderate/severe steatosis (> 30%), the impact on graft survival becomes more and more important[188].
Liver ischemic preconditioning (IPC) is an endogenous mechanism consisting of brief and repetitive episodes of vascular occlusion, followed by reperfusion that makes the liver more tolerant to subsequent prolonged episodes of ischemia[196]. Several studies have demonstrated that IPC might have protective effects on IRI, but minimal or no clinical benefit[196,197] and a Cochrane systematic review confirmed this result[198]. No specific data exist for HCV-positive recipients.
In HCV-positive recipients, a balance between HCV, liver graft, recipient immune response and anti-rejection therapy is achieved in a few months after LT. During this period, almost all patients show an early graft reinfection, with HCV viremia reaching and exceeding pre-LT levels. Histological assessment for differentiating RHC from acute or chronic rejection is essential in this setting; however, differentiating the two pathological patterns remains difficult. The host immune response (mainly cellular mediated) appears to be crucial both in the control of HCV infection and in the genesis of ACR; however, it is also strongly influenced by anti-rejection immunosuppressive treatment. Currently, there is no clear immunosuppressive strategy to prevent HCV recurrence that could be strongly recommended for HCV-positive LT. Similarly, immunotherapy appears to be ineffective. It seems reasonable that ACR episodes and over-immunosuppression are more likely to enhance the risk of HCV recurrence through immunological mechanisms; therefore, both complete prevention of ACR and optimization of immunosuppression (possibly up to IFS) should represent the main goals for reducing the rate of graft HCV reinfection. Other factors that might be modified by clinicians, include proper graft allocation and preservation injury to realize an ideal donor-to-recipient matching; however, many aspects related to these factors remain to be better determined in well-designed prospective studies. At present, post-LT HCV recurrence remains an unresolved thorny problem.
Evaluation of current treatment options for HCV in the transplant setting was not an aim of this review. Nevertheless, it should be stated that clinical concerns regarding HCV recurrence and needs of differentiation from rejection are strongly related to the available treatment options for the two conditions. Interferon-based treatments are unsatisfactory[199] and triple treatment with boceprevir and telaprevir is hampered by side effects and interaction with CyA and Tac[200]. In the near future, new drugs like sofosbuvir, that are better tolerated and with no interactions with CNIs, might represent the basis for reliable interferon-free treatment options for RHC[201]. Pre-emptive treatments to prevent HCV recurrence have been unsuccessful until now; however, newer drugs have the potential to change the natural history of HCV infection in transplanted patients.
The authors wish to thank Ilaria Panzini, Francesca Cellauro and Andrew John Lawless for their valuable help in performing English proofreading.
P- Reviewer: Boin IFSF, Sugawara Y S- Editor: Gou SX L- Editor: Stewart G E- Editor: Zhang DN
| 1. | Wiesner RH, Sorrell M, Villamil F. Report of the first International Liver Transplantation Society expert panel consensus conference on liver transplantation and hepatitis C. Liver Transpl. 2003;9:S1-S9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 329] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 2. | Adam R, McMaster P, O’Grady JG, Castaing D, Klempnauer JL, Jamieson N, Neuhaus P, Lerut J, Salizzoni M, Pollard S. Evolution of liver transplantation in Europe: report of the European Liver Transplant Registry. Liver Transpl. 2003;9:1231-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 414] [Article Influence: 18.8] [Reference Citation Analysis (1)] |
| 3. | Sugawara Y, Makuuchi M. Living donor liver transplantation to patients with hepatitis C virus cirrhosis. World J Gastroenterol. 2006;12:4461-4465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Gane EJ, Portmann BC, Naoumov NV, Smith HM, Underhill JA, Donaldson PT, Maertens G, Williams R. Long-term outcome of hepatitis C infection after liver transplantation. N Engl J Med. 1996;334:815-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 787] [Cited by in RCA: 736] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 5. | Berenguer M. Natural history of recurrent hepatitis C. Liver Transpl. 2002;8:S14-S18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 189] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 6. | Burra P. Hepatitis C. Semin Liver Dis. 2009;29:53-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Wright TL, Donegan E, Hsu HH, Ferrell L, Lake JR, Kim M, Combs C, Fennessy S, Roberts JP, Ascher NL. Recurrent and acquired hepatitis C viral infection in liver transplant recipients. Gastroenterology. 1992;103:317-322. [PubMed] |
| 8. | Rosen HR. Hepatitis C virus in the human liver transplantation model. Clin Liver Dis. 2003;7:107-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Garcia-Retortillo M, Forns X, Feliu A, Moitinho E, Costa J, Navasa M, Rimola A, Rodes J. Hepatitis C virus kinetics during and immediately after liver transplantation. Hepatology. 2002;35:680-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 381] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 10. | Böker KH, Dalley G, Bahr MJ, Maschek H, Tillmann HL, Trautwein C, Oldhaver K, Bode U, Pichlmayr R, Manns MP. Long-term outcome of hepatitis C virus infection after liver transplantation. Hepatology. 1997;25:203-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Negro F, Giostra E, Krawczynski K, Quadri R, Rubbia-Brandt L, Mentha G, Colucci G, Perrin L, Hadengue A. Detection of intrahepatic hepatitis C virus replication by strand-specific semi-quantitative RT-PCR: preliminary application to the liver transplantation model. J Hepatol. 1998;29:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Di Martino V, Saurini F, Samuel D, Gigou M, Dussaix E, Reynès M, Bismuth H, Féray C. Long-term longitudinal study of intrahepatic hepatitis C virus replication after liver transplantation. Hepatology. 1997;26:1343-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 59] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Petrovic LM, Villamil FG, Vierling JM, Makowka L, Geller SA. Comparison of histopathology in acute allograft rejection and recurrent hepatitis C infection after liver transplantation. Liver Transpl Surg. 1997;3:398-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 40] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Gane EJ, Naoumov NV, Qian KP, Mondelli MU, Maertens G, Portmann BC, Lau JY, Williams R. A longitudinal analysis of hepatitis C virus replication following liver transplantation. Gastroenterology. 1996;110:167-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 352] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 15. | Gretch DR, Bacchi CE, Corey L, dela Rosa C, Lesniewski RR, Kowdley K, Gown A, Frank I, Perkins JD, Carithers RL. Persistent hepatitis C virus infection after liver transplantation: clinical and virological features. Hepatology. 1995;22:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Vargas V, Krawczynski K, Castells L, Martinez N, Esteban J, Allende H, Esteban R, Guardia J. Recurrent hepatitis C virus infection after liver transplantation: immunohistochemical assessment of the viral antigen. Liver Transpl Surg. 1998;4:320-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Fukumoto T, Berg T, Ku Y, Bechstein WO, Knoop M, Lemmens HP, Lobeck H, Hopf U, Neuhaus P. Viral dynamics of hepatitis C early after orthotopic liver transplantation: evidence for rapid turnover of serum virions. Hepatology. 1996;24:1351-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 126] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 18. | Rosen HR, Schwartz JM. Hepatitis C quasispecies and severity of recurrence: cause, consequence, or coincidence? Liver Transpl. 2002;8:646-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Chazouilleres O, Kim M, Combs C, Ferrell L, Bacchetti P, Roberts J, Ascher NL, Neuwald P, Wilber J, Urdea M. Quantitation of hepatitis C virus RNA in liver transplant recipients. Gastroenterology. 1994;106:994-999. [PubMed] |
| 20. | Freeman RB, Tran S, Lee YM, Rohrer RJ, Kaplan MM. Serum hepatitis C RNA titers after liver transplantation are not correlated with immunosuppression or hepatitis. Transplantation. 1996;61:542-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Sreekumar R, Gonzalez-Koch A, Maor-Kendler Y, Batts K, Moreno-Luna L, Poterucha J, Burgart L, Wiesner R, Kremers W, Rosen C. Early identification of recipients with progressive histologic recurrence of hepatitis C after liver transplantation. Hepatology. 2000;32:1125-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 152] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 22. | Shackel NA, Jamias J, Rahman W, Prakoso E, Strasser SI, Koorey DJ, Crawford MD, Verran DJ, Gallagher J, McCaughan GW. Early high peak hepatitis C viral load levels independently predict hepatitis C-related liver failure post-liver transplantation. Liver Transpl. 2009;15:709-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Pal S, Sullivan DG, Kim S, Lai KK, Kae J, Cotler SJ, Carithers RL, Wood BL, Perkins JD, Gretch DR. Productive replication of hepatitis C virus in perihepatic lymph nodes in vivo: implications of HCV lymphotropism. Gastroenterology. 2006;130:1107-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 107] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 24. | Powers KA, Ribeiro RM, Patel K, Pianko S, Nyberg L, Pockros P, Conrad AJ, McHutchison J, Perelson AS. Kinetics of hepatitis C virus reinfection after liver transplantation. Liver Transpl. 2006;12:207-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Dahari H, Feliu A, Garcia-Retortillo M, Forns X, Neumann AU. Second hepatitis C replication compartment indicated by viral dynamics during liver transplantation. J Hepatol. 2005;42:491-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 60] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Feliu A, Gay E, García-Retortillo M, Saiz JC, Forns X. Evolution of hepatitis C virus quasispecies immediately following liver transplantation. Liver Transpl. 2004;10:1131-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Gray RR, Strickland SL, Veras NM, Goodenow MM, Pybus OG, Lemon SM, Fried MW, Nelson DR, Salemi M. Unexpected maintenance of hepatitis C viral diversity following liver transplantation. J Virol. 2012;86:8432-8439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Hughes MG, Chong TW, Smith RL, Evans HL, Iezzoni JC, Sawyer RG, Rudy CK, Pruett TL. HCV infection of the transplanted liver: changing CD81 and HVR1 variants immediately after liver transplantation. Am J Transplant. 2005;5:2504-2513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Krieger M. Scavenger receptor class B type I is a multiligand HDL receptor that influences diverse physiologic systems. J Clin Invest. 2001;108:793-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 280] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 30. | Maillard P, Huby T, Andréo U, Moreau M, Chapman J, Budkowska A. The interaction of natural hepatitis C virus with human scavenger receptor SR-BI/Cla1 is mediated by ApoB-containing lipoproteins. FASEB J. 2006;20:735-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 110] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 31. | Hishiki T, Shimizu Y, Tobita R, Sugiyama K, Ogawa K, Funami K, Ohsaki Y, Fujimoto T, Takaku H, Wakita T. Infectivity of hepatitis C virus is influenced by association with apolipoprotein E isoforms. J Virol. 2010;84:12048-12057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 109] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 32. | Meuleman P, Catanese MT, Verhoye L, Desombere I, Farhoudi A, Jones CT, Sheahan T, Grzyb K, Cortese R, Rice CM. A human monoclonal antibody targeting scavenger receptor class B type I precludes hepatitis C virus infection and viral spread in vitro and in vivo. Hepatology. 2012;55:364-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 33. | Fafi-Kremer S, Fofana I, Soulier E, Carolla P, Meuleman P, Leroux-Roels G, Patel AH, Cosset FL, Pessaux P, Doffoël M. Viral entry and escape from antibody-mediated neutralization influence hepatitis C virus reinfection in liver transplantation. J Exp Med. 2010;207:2019-2031. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 34. | Sullivan DG, Wilson JJ, Carithers RL, Perkins JD, Gretch DR. Multigene tracking of hepatitis C virus quasispecies after liver transplantation: correlation of genetic diversification in the envelope region with asymptomatic or mild disease patterns. J Virol. 1998;72:10036-10043. [PubMed] |
| 35. | Pessoa MG, Bzowej N, Berenguer M, Phung Y, Kim M, Ferrell L, Hassoba H, Wright TL. Evolution of hepatitis C virus quasispecies in patients with severe cholestatic hepatitis after liver transplantation. Hepatology. 1999;30:1513-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 57] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 36. | Gretch DR, Polyak SJ, Wilson JJ, Carithers RL, Perkins JD, Corey L. Tracking hepatitis C virus quasispecies major and minor variants in symptomatic and asymptomatic liver transplant recipients. J Virol. 1996;70:7622-7631. [PubMed] |
| 37. | Doughty AL, Painter DM, McCaughan GW. Post-transplant quasispecies pattern remains stable over time in patients with recurrent cholestatic hepatitis due to hepatitis C virus. J Hepatol. 2000;32:126-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 38. | Vargas HE, Laskus T, Wang LF, Lee R, Radkowski M, Dodson F, Fung JJ, Rakela J. Outcome of liver transplantation in hepatitis C virus-infected patients who received hepatitis C virus-infected grafts. Gastroenterology. 1999;117:149-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 113] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 39. | Féray C, Gigou M, Samuel D, Paradis V, Mishiro S, Maertens G, Reynés M, Okamoto H, Bismuth H, Bréchot C. Influence of the genotypes of hepatitis C virus on the severity of recurrent liver disease after liver transplantation. Gastroenterology. 1995;108:1088-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 206] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 40. | Pageaux GP, Ducos J, Mondain AM, Costes V, Picot MC, Perrigault PF, Domergue J, Larrey D, Michel H. Hepatitis C virus genotypes and quantitation of serum hepatitis C virus RNA in liver transplant recipients: relationship with severity of histological recurrence and implications in the pathogenesis of HCV infection. Liver Transpl Surg. 1997;3:501-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 41. | Féray C, Caccamo L, Alexander GJ, Ducot B, Gugenheim J, Casanovas T, Loinaz C, Gigou M, Burra P, Barkholt L. European collaborative study on factors influencing outcome after liver transplantation for hepatitis C. European Concerted Action on Viral Hepatitis (EUROHEP) Group. Gastroenterology. 1999;117:619-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 268] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 42. | Gayowski T, Singh N, Marino IR, Vargas H, Wagener M, Wannstedt C, Morelli F, Laskus T, Fung JJ, Rakela J. Hepatitis C virus genotypes in liver transplant recipients: impact on posttransplant recurrence, infections, response to interferon-alpha therapy and outcome. Transplantation. 1997;64:422-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 64] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 43. | Humar A, Kumar D, Raboud J, Caliendo AM, Moussa G, Levy G, Mazzulli T. Interactions between cytomegalovirus, human herpesvirus-6, and the recurrence of hepatitis C after liver transplantation. Am J Transplant. 2002;2:461-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 74] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 44. | Singh N, Husain S, Carrigan DR, Knox KK, Weck KE, Wagener MM, Gayowski T. Impact of human herpesvirus-6 on the frequency and severity of recurrent hepatitis C virus hepatitis in liver transplant recipients. Clin Transplant. 2002;16:92-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 45. | Bosch W, Heckman MG, Pungpapong S, Diehl NN, Shalev JA, Hellinger WC. Association of cytomegalovirus infection and disease with recurrent hepatitis C after liver transplantation. Transplantation. 2012;93:723-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 46. | Nebbia G, Mattes FM, Cholongitas E, Garcia-Diaz A, Samonakis DN, Burroughs AK, Emery VC. Exploring the bidirectional interactions between human cytomegalovirus and hepatitis C virus replication after liver transplantation. Liver Transpl. 2007;13:130-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 47. | Terrault NA, Dailey PJ, Ferrell L, Collins ML, Wilber JC, Urdea MS, Bhandari BN, Wright TL. Hepatitis C virus: quantitation and distribution in liver. J Med Virol. 1997;51:217-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 48. | Ballardini G, De Raffele E, Groff P, Bioulac-Sage P, Grassi A, Ghetti S, Susca M, Strazzabosco M, Bellusci R, Iemmolo RM. Timing of reinfection and mechanisms of hepatocellular damage in transplanted hepatitis C virus-reinfected liver. Liver Transpl. 2002;8:10-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 73] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 49. | Grassi A, Quarneti C, Ravaioli M, Bianchini F, Susca M, D’Errico A, Piscaglia F, Tamè MR, Andreone P, Grazi G. Detection of HCV antigens in liver graft: relevance to the management of recurrent post-liver transplant hepatitis C. Liver Transpl. 2006;12:1673-1681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 50. | Mensa L, Pérez-del-Pulgar S, Crespo G, Koutsoudakis G, Fernández-Carrillo C, Coto-Llerena M, Miquel R, Allende H, Castells L, Navasa M. Imaging of hepatitis C virus infection in liver grafts after liver transplantation. J Hepatol. 2013;59:271-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 51. | Ferrell LD, Wright TL, Roberts J, Ascher N, Lake J. Hepatitis C viral infection in liver transplant recipients. Hepatology. 1992;16:865-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 150] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 52. | Sheiner PA, Schwartz ME, Mor E, Schluger LK, Theise N, Kishikawa K, Kolesnikov V, Bodenheimer H, Emre S, Miller CM. Severe or multiple rejection episodes are associated with early recurrence of hepatitis C after orthotopic liver transplantation. Hepatology. 1995;21:30-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 254] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 53. | Greenson JK, Svoboda-Newman SM, Merion RM, Frank TS. Histologic progression of recurrent hepatitis C in liver transplant allografts. Am J Surg Pathol. 1996;20:731-738. [PubMed] |
| 54. | Rosen HR, Shackleton CR, Higa L, Gralnek IM, Farmer DA, McDiarmid SV, Holt C, Lewin KJ, Busuttil RW, Martin P. Use of OKT3 is associated with early and severe recurrence of hepatitis C after liver transplantation. Am J Gastroenterol. 1997;92:1453-1457. [PubMed] |
| 55. | Younossi ZM, Boparai N, Gramlich T, Goldblum J, George P, Mayes J. Agreement in pathologic interpretation of liver biopsy specimens in posttransplant hepatitis C infection. Arch Pathol Lab Med. 1999;123:143-145. [PubMed] |
| 56. | Regev A, Molina E, Moura R, Bejarano PA, Khaled A, Ruiz P, Arheart K, Berho M, Drachenberg CB, Mendez P. Reliability of histopathologic assessment for the differentiation of recurrent hepatitis C from acute rejection after liver transplantation. Liver Transpl. 2004;10:1233-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 91] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 57. | Sadamori H, Yagi T, Iwagaki H, Matsuda H, Shinoura S, Umeda Y, Ohara N, Yanai H, Ogino T, Tanaka N. Immunohistochemical staining of liver grafts with a monoclonal antibody against HCV-Envelope 2 for recurrent hepatitis C after living donor liver transplantation. J Gastroenterol Hepatol. 2009;24:574-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 58. | D’Errico-Grigioni A, Fiorentino M, Vasuri F, Gruppioni E, Fabbrizio B, Zucchini N, Ballardini G, Morelli C, Pinna AD, Grigioni WF. Tissue hepatitis C virus RNA quantification and protein expression help identify early hepatitis C virus recurrence after liver transplantation. Liver Transpl. 2008;14:313-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 59. | Vasuri F, Morelli MC, Gruppioni E, Fiorentino M, Ercolani G, Cescon M, Pinna AD, Grigioni WF, D’Errico-Grigioni A. The meaning of tissue and serum HCV RNA quantitation in hepatitis C recurrence after liver transplantation: a retrospective study. Dig Liver Dis. 2013;45:505-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 60. | Ciccorossi P, Filipponi F, Oliveri F, Campani D, Colombatto P, Bonino F, Campa M, Maltinti G, Mosca F, Brunetto MR. Increasing serum levels of IgM anti-HCV are diagnostic of recurrent hepatitis C in liver transplant patients with ALT flares. J Viral Hepat. 2003;10:168-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 61. | Schmeding M, Dankof A, Krenn V, Krukemeyer MG, Koch M, Spinelli A, Langrehr JM, Neumann UP, Neuhaus P. C4d in acute rejection after liver transplantation--a valuable tool in differential diagnosis to hepatitis C recurrence. Am J Transplant. 2006;6:523-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 62. | Schmeding M, Kienlein S, Röcken C, Neuhaus R, Neuhaus P, Heidenhain C, Neumann UP. ELISA-based detection of C4d after liver transplantation--a helpful tool for differential diagnosis between acute rejection and HCV-recurrence? Transpl Immunol. 2010;23:156-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 63. | Asaoka T, Kato T, Marubashi S, Dono K, Hama N, Takahashi H, Kobayashi S, Takeda Y, Takemasa I, Nagano H. Differential transcriptome patterns for acute cellular rejection in recipients with recurrent hepatitis C after liver transplantation. Liver Transpl. 2009;15:1738-1749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 64. | Joshi D, Salehi S, Brereton H, Arno M, Quaglia A, Heaton N, O’Grady J, Agarwal K, Aluvihare V. Distinct microRNA profiles are associated with the severity of hepatitis C virus recurrence and acute cellular rejection after liver transplantation. Liver Transpl. 2013;19:383-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 65. | Cabrera R, Ararat M, Soldevila-Pico C, Dixon L, Pan JJ, Firpi R, Machicao V, Levy C, Nelson D, Morelli G. Using an immune functional assay to differentiate acute cellular rejection from recurrent hepatitis C in liver transplant patients. Liver Transpl. 2009;15:216-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 66. | Donaldson PT, Alexander GJ, O’Grady J, Neuberger J, Portmann B, Thick M, Davis H, Calne RY, Williams R. Evidence for an immune response to HLA class I antigens in the vanishing-bileduct syndrome after liver transplantation. Lancet. 1987;1:945-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 99] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 67. | Yagihashi A, Kobayashi M, Noguchi K, Konno A, Yoshida Y, Terasawa K, Starzl TE, Iwaki Y. HLA matching effect in liver transplantation. Transplant Proc. 1992;24:2432-2433. [PubMed] |
| 68. | Thorogood J. Relationship between HLA compatibility and first liver allograft survival. l’ESPRIT Study Group. Transplant Proc. 1993;25:2655-2656. [PubMed] |
| 69. | Mañez R, Mateo R, Tabasco J, Kusne S, Starzl TE, Duquesnoy RJ. The influence of HLA donor-recipient compatibility on the recurrence of HBV and HCV hepatitis after liver transplantation. Transplantation. 1995;59:640-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 70. | Langrehr JM, Puhl G, Bahra M, Schmeding M, Spinelli A, Berg T, Schönemann C, Krenn V, Neuhaus P, Neumann UP. Influence of donor/recipient HLA-matching on outcome and recurrence of hepatitis C after liver transplantation. Liver Transpl. 2006;12:644-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 71. | Rosen HR, Hinrichs DJ, Leistikow RL, Callender G, Wertheimer AM, Nishimura MI, Lewinsohn DM. Cutting edge: identification of hepatitis C virus-specific CD8+ T cells restricted by donor HLA alleles following liver transplantation. J Immunol. 2004;173:5355-5359. [PubMed] |
| 72. | Belli LS, Burra P, Poli F, Battista Alberti A, Silini E, Zavaglia C, Fagiuoli S, Prando D, Espadas de Arias A, Boninsegna S. HLA-DRB1 donor-recipient mismatch affects the outcome of hepatitis C disease recurrence after liver transplantation. Gastroenterology. 2006;130:695-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 73. | Balan V, Ruppert K, Demetris AJ, Ledneva T, Duquesnoy RJ, Detre KM, Wei YL, Rakela J, Schafer DF, Roberts JP. Long-term outcome of human leukocyte antigen mismatching in liver transplantation: results of the National Institute of Diabetes and Digestive and Kidney Diseases Liver Transplantation Database. Hepatology. 2008;48:878-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 81] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 74. | Audet M, Piardi T, Cag M, Navarro F, Ornis S, Cinqualbre J, Wolf P, Panaro F. Hepatitis C recurrence after liver transplantation: has the human leukocyte antigen mismatching at individual loci a role? J Gastroenterol Hepatol. 2011;26:1772-1778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 75. | Berenguer M, Rayón JM, Prieto M, Aguilera V, Nicolás D, Ortiz V, Carrasco D, López-Andujar R, Mir J, Berenguer J. Are posttransplantation protocol liver biopsies useful in the long term? Liver Transpl. 2001;7:790-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 112] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 76. | Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, Heinzen EL, Qiu P, Bertelsen AH, Muir AJ. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2776] [Cited by in RCA: 2723] [Article Influence: 170.2] [Reference Citation Analysis (0)] |
| 77. | Thomas DL, Thio CL, Martin MP, Qi Y, Ge D, O’Huigin C, Kidd J, Kidd K, Khakoo SI, Alexander G. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009;461:798-801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1706] [Cited by in RCA: 1687] [Article Influence: 105.4] [Reference Citation Analysis (0)] |
| 78. | Duarte-Rojo A, Deneke MG, Charlton MR. Interleukin-28B polymorphism in hepatitis C and liver transplantation. Liver Transpl. 2013;19:49-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 79. | Duarte-Rojo A, Veldt BJ, Goldstein DD, Tillman HL, Watt KD, Heimbach JK, McHutchison JG, Poterucha JJ, Vargas-Vorackova F, Charlton MR. The course of posttransplant hepatitis C infection: comparative impact of donor and recipient source of the favorable IL28B genotype and other variables. Transplantation. 2012;94:197-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 80. | Chin JL, Nicholas RM, Russell J, Carr M, Connell J, Stewart S, McCormick PA. Spontaneous clearance of hepatitis C infection after liver transplantation from IL28B rs12979860 CC donors. Eur J Gastroenterol Hepatol. 2012;24:1110-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 81. | Biggins SW, Trotter J, Gralla J, Burton JR, Bambha KM, Dodge J, Brocato M, Cheng L, McQueen M, Forman L. Differential effects of donor and recipient IL28B and DDX58 SNPs on severity of HCV after liver transplantation. J Hepatol. 2013;58:969-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 82. | Bitetto D, Fabris C, Falleti E, Fornasiere E, Avellini C, Cmet S, Cussigh A, Fontanini E, Pirisi M, Corradini SG. Recipient interleukin-28B Rs12979860 C/T polymorphism and acute cellular rejection after liver transplantation: role of the calcineurin inhibitor used. Transplantation. 2012;93:1038-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 83. | Lange CM, Moradpour D, Doehring A, Lehr HA, Müllhaupt B, Bibert S, Bochud PY, Antonino AT, Pascual M, Farnik H. Impact of donor and recipient IL28B rs12979860 genotypes on hepatitis C virus liver graft reinfection. J Hepatol. 2011;55:322-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 84. | Eurich D, Boas-Knoop S, Ruehl M, Schulz M, Carrillo ED, Berg T, Neuhaus R, Neuhaus P, Neumann UP, Bahra M. Relationship between the interleukin-28b gene polymorphism and the histological severity of hepatitis C virus-induced graft inflammation and the response to antiviral therapy after liver transplantation. Liver Transpl. 2011;17:289-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 85. | Doughty AL, Spencer JD, Cossart YE, McCaughan GW. Cholestatic hepatitis after liver transplantation is associated with persistently high serum hepatitis C virus RNA levels. Liver Transpl Surg. 1998;4:15-21. [PubMed] |
| 86. | Jain A, Menegus M, Mohanka R, Orloff M, Abt P, Mantry P, Bozorgzadeh A. HCV antibody quantitative levels in liver transplant patients: do they have any relevance in clinical practice? Exp Clin Transplant. 2006;4:475-480. [PubMed] |
| 87. | Rehermann B, Chisari FV. Cell mediated immune response to the hepatitis C virus. Curr Top Microbiol Immunol. 2000;242:299-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 88. | Asanza CG, García-Monzón C, Clemente G, Salcedo M, García-Buey L, García-Iglesias C, Bañares R, Alvarez E, Moreno-Otero R. Immunohistochemical evidence of immunopathogenetic mechanisms in chronic hepatitis C recurrence after liver transplantation. Hepatology. 1997;26:755-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 89. | Rosen HR, Hinrichs DJ, Gretch DR, Koziel MJ, Chou S, Houghton M, Rabkin J, Corless CL, Bouwer HG. Association of multispecific CD4(+) response to hepatitis C and severity of recurrence after liver transplantation. Gastroenterology. 1999;117:926-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 123] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 90. | Schirren CA, Jung MC, Worzfeld T, Mamin M, Baretton G, Gerlach JT, Gruener NH, Zachoval R, Houghton M, Rau HG. Hepatitis C virus-specific CD4+ T cell response after liver transplantation occurs early, is multispecific, compartmentalizes to the liver, and does not correlate with recurrent disease. J Infect Dis. 2001;183:1187-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 91. | Weston SJ, Leistikow RL, Reddy KR, Torres M, Wertheimer AM, Lewinsohn DM, Chou S, Davey MP, Corless C, O’Farrelly C. Reconstitution of hepatitis C virus-specific T-cellmediated immunity after liver transplantation. Hepatology. 2005;41:72-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 92. | Mendler M, Kwok H, Franco E, Baron P, Weissman J, Ojogho O. Monitoring peripheral blood CD4+ adenosine triphosphate activity in a liver transplant cohort: insight into the interplay between hepatitis C virus infection and cellular immunity. Liver Transpl. 2008;14:1313-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 93. | Te HS, Dasgupta KA, Cao D, Satoskar R, Mohanty SR, Reau N, Millis JM, Jensen DM. Use of immune function test in monitoring immunosuppression in liver transplant recipients. Clin Transplant. 2012;26:826-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 94. | Alkhouri N, Hanouneh IA, Lopez R, Zein NN. Monitoring peripheral blood CD4+ adenosine triphosphate activity in recurrent hepatitis C and its correlation to fibrosis progression. Liver Transpl. 2010;16:155-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 95. | Sakaguchi S, Sakaguchi N, Shimizu J, Yamazaki S, Sakihama T, Itoh M, Kuniyasu Y, Nomura T, Toda M, Takahashi T. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev. 2001;182:18-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 96. | Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151-1164. [PubMed] |
| 97. | Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002;3:135-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1294] [Cited by in RCA: 1293] [Article Influence: 56.2] [Reference Citation Analysis (0)] |
| 98. | Carpentier A, Conti F, Stenard F, Aoudjehane L, Miroux C, Podevin P, Morales O, Chouzenoux S, Scatton O, Groux H. Increased expression of regulatory Tr1 cells in recurrent hepatitis C after liver transplantation. Am J Transplant. 2009;9:2102-2112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 99. | Perrella A, Arenga G, Pisaniello D, Rampone B, Di Costanzo GG, Atripaldi L, Esposito C, Di Florio E, Perrella O, Cuomo O. Elevated CD4+/CD25+ T-cell frequency and function during hepatitis C virus recurrence after liver transplantation. Transplant Proc. 2009;41:1761-1766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 100. | Kanto T, Inoue M, Miyatake H, Sato A, Sakakibara M, Yakushijin T, Oki C, Itose I, Hiramatsu N, Takehara T. Reduced numbers and impaired ability of myeloid and plasmacytoid dendritic cells to polarize T helper cells in chronic hepatitis C virus infection. J Infect Dis. 2004;190:1919-1926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 174] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 101. | Longman RS, Talal AH, Jacobson IM, Rice CM, Albert ML. Normal functional capacity in circulating myeloid and plasmacytoid dendritic cells in patients with chronic hepatitis C. J Infect Dis. 2005;192:497-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 102. | Ocaña L, Cos J, Quer J, Bilbao I, Palou E, Parra R, Sauleda S, Esteban JI, Guàrdia J, Massuet LI. Analysis of INF-gamma, TNF-alpha and dendritic cells to predict hepatitis C virus recurrence in liver transplant patients. Transplant Proc. 2005;37:3951-3956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 103. | Schvoerer E, Thumann C, Spohrer S, Soulier E, Royer C, Brignon N, Doridot S, Meyer N, Ellero B, Woehl-Jaegle ML. Early decrease in circulating dendritic cells number after liver transplantation could favor hepatitis C virus recurrence. J Med Virol. 2006;78:1070-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 104. | Corado J, Toro F, Rivera H, Bianco NE, Deibis L, De Sanctis JB. Impairment of natural killer (NK) cytotoxic activity in hepatitis C virus (HCV) infection. Clin Exp Immunol. 1997;109:451-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 105. | Deignan T, Curry MP, Doherty DG, Golden-Mason L, Volkov Y, Norris S, Nolan N, Traynor O, McEntee G, Hegarty JE. Decrease in hepatic CD56(+) T cells and V alpha 24(+) natural killer T cells in chronic hepatitis C viral infection. J Hepatol. 2002;37:101-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 77] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 106. | Rosen HR, Doherty DG, Madrigal-Estebas L, O’Farrelly C, Golden-Mason L. Pretransplantation CD56(+) innate lymphocyte populations associated with severity of hepatitis C virus recurrence. Liver Transpl. 2008;14:31-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 107. | Varchetta S, Oliviero B, Francesca Donato M, Agnelli F, Rigamonti C, Paudice E, Arosio E, Berra M, Rossi G, Tinelli C. Prospective study of natural killer cell phenotype in recurrent hepatitis C virus infection following liver transplantation. J Hepatol. 2009;50:314-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 108. | Howell J, Sawhney R, Skinner N, Gow P, Angus P, Ratnam D, Visvanathan K. Toll-like receptor 3 and 7/8 function is impaired in hepatitis C rapid fibrosis progression post-liver transplantation. Am J Transplant. 2013;13:943-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 109. | de Arias AE, Haworth SE, Belli LS, Burra P, Pinzello G, Vangeli M, Minola E, Guido M, Boccagni P, De Feo TM. Killer cell immunoglobulin-like receptor genotype and killer cell immunoglobulin-like receptor-human leukocyte antigen C ligand compatibility affect the severity of hepatitis C virus recurrence after liver transplantation. Liver Transpl. 2009;15:390-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 110. | Tambur AR, Ortegel JW, Ben-Ari Z, Shabtai E, Klein T, Michowiz R, Tur-Kaspa R, Mor E. Role of cytokine gene polymorphism in hepatitis C recurrence and allograft rejection among liver transplant recipients. Transplantation. 2001;71:1475-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 111. | Nagai S, Yoshida A, Kohno K, Altshuler D, Nakamura M, Brown KA, Abouljoud MS, Moonka D. Peritransplant absolute lymphocyte count as a predictive factor for advanced recurrence of hepatitis C after liver transplantation. Hepatology. 2014;59:35-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 112. | Berg T, Müller AR, Platz KP, Höhne M, Bechstein WO, Hopf U, Wiedenmann B, Neuhaus P, Schreier E. Dynamics of GB virus C viremia early after orthotopic liver transplantation indicates extrahepatic tissues as the predominant site of GB virus C replication. Hepatology. 1999;29:245-249. [PubMed] |
| 113. | Lake JR. The role of immunosuppression in recurrence of hepatitis C. Liver Transpl. 2003;9:S63-S66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 97] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 114. | Samuel D, Forns X, Berenguer M, Trautwein C, Burroughs A, Rizzetto M, Trepo C. Report of the monothematic EASL conference on liver transplantation for viral hepatitis (Paris, France, January 12-14, 2006). J Hepatol. 2006;45:127-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 128] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 115. | Ciesek S, Steinmann E, Iken M, Ott M, Helfritz FA, Wappler I, Manns MP, Wedemeyer H, Pietschmann T. Glucocorticosteroids increase cell entry by hepatitis C virus. Gastroenterology. 2010;138:1875-1884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 116. | Boor PP, Metselaar HJ, Mancham S, Tilanus HW, Kusters JG, Kwekkeboom J. Prednisolone suppresses the function and promotes apoptosis of plasmacytoid dendritic cells. Am J Transplant. 2006;6:2332-2341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 117. | Klintmalm GB, Washburn WK, Rudich SM, Heffron TG, Teperman LW, Fasola C, Eckhoff DE, Netto GJ, Katz E. Corticosteroid-free immunosuppression with daclizumab in HCV(+) liver transplant recipients: 1-year interim results of the HCV-3 study. Liver Transpl. 2007;13:1521-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 118. | Klintmalm GB, Davis GL, Teperman L, Netto GJ, Washburn K, Rudich SM, Pomfret EA, Vargas HE, Brown R, Eckhoff D. A randomized, multicenter study comparing steroid-free immunosuppression and standard immunosuppression for liver transplant recipients with chronic hepatitis C. Liver Transpl. 2011;17:1394-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 119. | Kato T, Gaynor JJ, Yoshida H, Montalvano M, Takahashi H, Pyrsopoulos N, Nishida S, Moon J, Selvaggi G, Levi D. Randomized trial of steroid-free induction versus corticosteroid maintenance among orthotopic liver transplant recipients with hepatitis C virus: impact on hepatic fibrosis progression at one year. Transplantation. 2007;84:829-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 120. | Lladó L, Fabregat J, Castellote J, Ramos E, Xiol X, Torras J, Serrano T, Baliellas C, Figueras J, Garcia-Gil A. Impact of immunosuppression without steroids on rejection and hepatitis C virus evolution after liver transplantation: results of a prospective randomized study. Liver Transpl. 2008;14:1752-1760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 121. | Manousou P, Samonakis D, Cholongitas E, Patch D, O’Beirne J, Dhillon AP, Rolles K, McCormick A, Hayes P, Burroughs AK. Outcome of recurrent hepatitis C virus after liver transplantation in a randomized trial of tacrolimus monotherapy versus triple therapy. Liver Transpl. 2009;15:1783-1791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 122. | Weiler N, Thrun I, Hoppe-Lotichius M, Zimmermann T, Kraemer I, Otto G. Early steroid-free immunosuppression with FK506 after liver transplantation: long-term results of a prospectively randomized double-blinded trial. Transplantation. 2010;90:1562-1566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 123. | Neumann U, Samuel D, Trunečka P, Gugenheim J, Gerunda GE, Friman S. A Randomized Multicenter Study Comparing a Tacrolimus-Based Protocol with and without Steroids in HCV-Positive Liver Allograft Recipients. J Transplant. 2012;2012:894215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 124. | Brillanti S, Vivarelli M, De Ruvo N, Aden AA, Camaggi V, D’Errico A, Furlini G, Bellusci R, Roda E, Cavallari A. Slowly tapering off steroids protects the graft against hepatitis C recurrence after liver transplantation. Liver Transpl. 2002;8:884-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 112] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 125. | Vivarelli M, Burra P, La Barba G, Canova D, Senzolo M, Cucchetti A, D’Errico A, Guido M, Merenda R, Neri D. Influence of steroids on HCV recurrence after liver transplantation: A prospective study. J Hepatol. 2007;47:793-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 126. | Segev DL, Sozio SM, Shin EJ, Nazarian SM, Nathan H, Thuluvath PJ, Montgomery RA, Cameron AM, Maley WR. Steroid avoidance in liver transplantation: meta-analysis and meta-regression of randomized trials. Liver Transpl. 2008;14:512-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 125] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 127. | National Institutes of Health. Liver Transplantation. Consensus Development Conference Summary. Washington, DC: NIH Consens Statement Online 1983; 1-15. |
| 128. | Watashi K, Hijikata M, Hosaka M, Yamaji M, Shimotohno K. Cyclosporin A suppresses replication of hepatitis C virus genome in cultured hepatocytes. Hepatology. 2003;38:1282-1288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 409] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 129. | Nakagawa M, Sakamoto N, Tanabe Y, Koyama T, Itsui Y, Takeda Y, Chen CH, Kakinuma S, Oooka S, Maekawa S. Suppression of hepatitis C virus replication by cyclosporin a is mediated by blockade of cyclophilins. Gastroenterology. 2005;129:1031-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 184] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 130. | Berenguer M, Royuela A, Zamora J. Immunosuppression with calcineurin inhibitors with respect to the outcome of HCV recurrence after liver transplantation: results of a meta-analysis. Liver Transpl. 2007;13:21-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 116] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 131. | Irish WD, Arcona S, Bowers D, Trotter JF. Cyclosporine versus tacrolimus treated liver transplant recipients with chronic hepatitis C: outcomes analysis of the UNOS/OPTN database. Am J Transplant. 2011;11:1676-1685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 132. | Henry SD, Metselaar HJ, Lonsdale RC, Kok A, Haagmans BL, Tilanus HW, van der Laan LJ. Mycophenolic acid inhibits hepatitis C virus replication and acts in synergy with cyclosporin A and interferon-alpha. Gastroenterology. 2006;131:1452-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 133. | Pan Q, de Ruiter PE, Metselaar HJ, Kwekkeboom J, de Jonge J, Tilanus HW, Janssen HL, van der Laan LJ. Mycophenolic acid augments interferon-stimulated gene expression and inhibits hepatitis C Virus infection in vitro and in vivo. Hepatology. 2012;55:1673-1683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 134. | Schlitt HJ, Barkmann A, Böker KH, Schmidt HH, Emmanouilidis N, Rosenau J, Bahr MJ, Tusch G, Manns MP, Nashan B. Replacement of calcineurin inhibitors with mycophenolate mofetil in liver-transplant patients with renal dysfunction: a randomised controlled study. Lancet. 2001;357:587-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 181] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 135. | Stewart SF, Hudson M, Talbot D, Manas D, Day CP. Mycophenolate mofetil monotherapy in liver transplantation. Lancet. 2001;357:609-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 87] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 136. | Germani G, Pleguezuelo M, Villamil F, Vaghjiani S, Tsochatzis E, Andreana L, Burroughs AK. Azathioprine in liver transplantation: a reevaluation of its use and a comparison with mycophenolate mofetil. Am J Transplant. 2009;9:1725-1731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 137. | Manzia TM, Angelico R, Toti L, Bellini MI, Sforza D, Palmieri G, Orlando G, Tariciotti L, Angelico M, Tisone G. Long-term, maintenance MMF monotherapy improves the fibrosis progression in liver transplant recipients with recurrent hepatitis C. Transpl Int. 2011;24:461-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 138. | Coito C, Diamond DL, Neddermann P, Korth MJ, Katze MG. High-throughput screening of the yeast kinome: identification of human serine/threonine protein kinases that phosphorylate the hepatitis C virus NS5A protein. J Virol. 2004;78:3502-3513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 139. | Mannová P, Beretta L. Activation of the N-Ras-PI3K-Akt-mTOR pathway by hepatitis C virus: control of cell survival and viral replication. J Virol. 2005;79:8742-8749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 148] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 140. | Wagner D, Kniepeiss D, Schaffellner S, Jakoby E, Mueller H, Fahrleitner-Pammer A, Stiegler P, Tscheliessnigg KH, Iberer F. Sirolimus has a potential to influent viral recurrence in HCV positive liver transplant candidates. Int Immunopharmacol. 2010;10:990-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 141. | Asthana S, Toso C, Meeberg G, Bigam DL, Mason A, Shapiro J, Kneteman NM. The impact of sirolimus on hepatitis C recurrence after liver transplantation. Can J Gastroenterol. 2011;25:28-34. [PubMed] |
| 142. | McKenna GJ, Trotter JF, Klintmalm E, Onaca N, Ruiz R, Jennings LW, Neri M, O’Leary JG, Davis GL, Levy MF. Limiting hepatitis C virus progression in liver transplant recipients using sirolimus-based immunosuppression. Am J Transplant. 2011;11:2379-2387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 143. | Trotter JF. Hot-topic debate on hepatitis C virus: the type of immunosuppression matters. Liver Transpl. 2011;17 Suppl 3:S20-S23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 144. | Watt KD, Dierkhising R, Heimbach JK, Charlton MR. Impact of sirolimus and tacrolimus on mortality and graft loss in liver transplant recipients with or without hepatitis C virus: an analysis of the Scientific Registry of Transplant Recipients Database. Liver Transpl. 2012;18:1029-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 145. | Marcos A, Eghtesad B, Fung JJ, Fontes P, Patel K, Devera M, Marsh W, Gayowski T, Demetris AJ, Gray EA. Use of alemtuzumab and tacrolimus monotherapy for cadaveric liver transplantation: with particular reference to hepatitis C virus. Transplantation. 2004;78:966-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 110] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 146. | Eason JD, Loss GE, Blazek J, Nair S, Mason AL. Steroid-free liver transplantation using rabbit antithymocyte globulin induction: results of a prospective randomized trial. Liver Transpl. 2001;7:693-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 93] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 147. | Eason JD, Nair S, Cohen AJ, Blazek JL, Loss GE. Steroid-free liver transplantation using rabbit antithymocyte globulin and early tacrolimus monotherapy. Transplantation. 2003;75:1396-1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 134] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 148. | Tector AJ, Fridell JA, Mangus RS, Shah A, Milgrom M, Kwo P, Chalasani N, Yoo H, Rouch D, Liangpunsakul S. Promising early results with immunosuppression using rabbit anti-thymocyte globulin and steroids with delayed introduction of tacrolimus in adult liver transplant recipients. Liver Transpl. 2004;10:404-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 149. | Horton PJ, Tchervenkov J, Barkun JS, Rochon C, Chaudhury PK, Znajda TL, Martinie JB, Metrakos P. Antithymocyte globulin induction therapy in hepatitis C-positive liver transplant recipients. J Gastrointest Surg. 2005;9:896-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 150. | Nair S, Loss GE, Cohen AJ, Eason JD. Induction with rabbit antithymocyte globulin versus induction with corticosteroids in liver transplantation: impact on recurrent hepatitis C virus infection. Transplantation. 2006;81:620-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 151. | De Ruvo N, Cucchetti A, Lauro A, Masetti M, Cautero N, Di Benedetto F, Dazzi A, Del Gaudio M, Ravaioli M, Di Francesco F. Preliminary results of a “prope” tolerogenic regimen with thymoglobulin pretreatment and hepatitis C virus recurrence in liver transplantation. Transplantation. 2005;80:8-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 152. | Uemura T, Schaefer E, Hollenbeak CS, Khan A, Kadry Z. Outcome of induction immunosuppression for liver transplantation comparing anti-thymocyte globulin, daclizumab, and corticosteroid. Transpl Int. 2011;24:640-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 153. | Nelson DR, Soldevila-Pico C, Reed A, Abdelmalek MF, Hemming AW, Van der Werf WJ, Howard R, Davis GL. Anti-interleukin-2 receptor therapy in combination with mycophenolate mofetil is associated with more severe hepatitis C recurrence after liver transplantation. Liver Transpl. 2001;7:1064-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 109] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 154. | Yoshizawa A, Takada Y, Fujimoto Y, Koshiba T, Haga H, Nabeshima S, Uemoto S. Liver transplantation from an identical twin without immunosuppression, with early recurrence of hepatitis C. Am J Transplant. 2006;6:2812-2816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 155. | Orlando G, Soker S, Wood K. Operational tolerance after liver transplantation. J Hepatol. 2009;50:1247-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 127] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 156. | Manzia TM, Angelico R, Toti L, Lai Q, Ciano P, Angelico M, Tisone G. Hepatitis C virus recurrence and immunosuppression-free state after liver transplantation. Expert Rev Clin Immunol. 2012;8:635-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 157. | Manzia TM, Angelico R, Baiocchi L, Toti L, Ciano P, Palmieri G, Angelico M, Orlando G, Tisone G. The Tor Vergata weaning of immunosuppression protocols in stable hepatitis C virus liver transplant patients: the 10-year follow-up. Transpl Int. 2013;26:259-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 158. | Ohira M, Ishiyama K, Tanaka Y, Doskali M, Igarashi Y, Tashiro H, Hiraga N, Imamura M, Sakamoto N, Asahara T. Adoptive immunotherapy with liver allograft-derived lymphocytes induces anti-HCV activity after liver transplantation in humans and humanized mice. J Clin Invest. 2009;119:3226-3235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 159. | Gurusamy KS, Tsochatzis E, Davidson BR, Burroughs AK. Antiviral prophylactic intervention for chronic hepatitis C virus in patients undergoing liver transplantation. Cochrane Database Syst Rev. 2010;CD006573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 160. | Chung RT, Gordon FD, Curry MP, Schiano TD, Emre S, Corey K, Markmann JF, Hertl M, Pomposelli JJ, Pomfret EA. Human monoclonal antibody MBL-HCV1 delays HCV viral rebound following liver transplantation: a randomized controlled study. Am J Transplant. 2013;13:1047-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 161. | Gaglio PJ, Malireddy S, Levitt BS, Lapointe-Rudow D, Lefkowitch J, Kinkhabwala M, Russo MW, Emond JC, Brown RS. Increased risk of cholestatic hepatitis C in recipients of grafts from living versus cadaveric liver donors. Liver Transpl. 2003;9:1028-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 88] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 162. | Thuluvath PJ, Yoo HY. Graft and patient survival after adult live donor liver transplantation compared to a matched cohort who received a deceased donor transplantation. Liver Transpl. 2004;10:1263-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 108] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 163. | Garcia-Retortillo M, Forns X, Llovet JM, Navasa M, Feliu A, Massaguer A, Bruguera M, Fuster J, Garcia-Valdecasas JC, Rimola A. Hepatitis C recurrence is more severe after living donor compared to cadaveric liver transplantation. Hepatology. 2004;40:699-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 137] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 164. | Everson GT, Trotter J. Role of adult living donor liver transplantation in patients with hepatitis C. Liver Transpl. 2003;9:S64-S68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 165. | Olthoff KM. Hepatic regeneration in living donor liver transplantation. Liver Transpl. 2003;9:S35-S41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 166. | Zimmerman MA, Trotter JF. Living donor liver transplantation in patients with hepatitis C. Liver Transpl. 2003;9:S52-S57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 167. | Shiffman ML, Stravitz RT, Contos MJ, Mills AS, Sterling RK, Luketic VA, Sanyal AJ, Cotterell A, Maluf D, Posner MP. Histologic recurrence of chronic hepatitis C virus in patients after living donor and deceased donor liver transplantation. Liver Transpl. 2004;10:1248-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 96] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 168. | Humar A, Horn K, Kalis A, Glessing B, Payne WD, Lake J. Living donor and split-liver transplants in hepatitis C recipients: does liver regeneration increase the risk for recurrence? Am J Transplant. 2005;5:399-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 169. | Guo L, Orrego M, Rodriguez-Luna H, Balan V, Byrne T, Chopra K, Douglas DD, Harrison E, Moss A, Reddy KS. Living donor liver transplantation for hepatitis C-related cirrhosis: no difference in histological recurrence when compared to deceased donor liver transplantation recipients. Liver Transpl. 2006;12:560-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 170. | Terrault NA, Shiffman ML, Lok AS, Saab S, Tong L, Brown RS, Everson GT, Reddy KR, Fair JH, Kulik LM. Outcomes in hepatitis C virus-infected recipients of living donor vs. deceased donor liver transplantation. Liver Transpl. 2007;13:122-129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 171. | Schmeding M, Neumann UP, Puhl G, Bahra M, Neuhaus R, Neuhaus P. Hepatitis C recurrence and fibrosis progression are not increased after living donor liver transplantation: a single-center study of 289 patients. Liver Transpl. 2007;13:687-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 172. | Selzner N, Girgrah N, Lilly L, Guindi M, Selzner M, Therapondos G, Adeyi O, McGilvray I, Cattral M, Greig PD. The difference in the fibrosis progression of recurrent hepatitis C after live donor liver transplantation versus deceased donor liver transplantation is attributable to the difference in donor age. Liver Transpl. 2008;14:1778-1786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 173. | Jain A, Singhal A, Kashyap R, Safadjou S, Ryan CK, Orloff MS. Comparative analysis of hepatitis C recurrence and fibrosis progression between deceased-donor and living-donor liver transplantation: 8-year longitudinal follow-up. Transplantation. 2011;92:453-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 174. | Akamatsu N, Sugawara Y. Liver transplantation and hepatitis C. Int J Hepatol. 2012;2012:686135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 175. | Berenguer M, Prieto M, San Juan F, Rayón JM, Martinez F, Carrasco D, Moya A, Orbis F, Mir J, Berenguer J. Contribution of donor age to the recent decrease in patient survival among HCV-infected liver transplant recipients. Hepatology. 2002;36:202-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 494] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 176. | Wali M, Harrison RF, Gow PJ, Mutimer D. Advancing donor liver age and rapid fibrosis progression following transplantation for hepatitis C. Gut. 2002;51:248-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 228] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 177. | Khapra AP, Agarwal K, Fiel MI, Kontorinis N, Hossain S, Emre S, Schiano TD. Impact of donor age on survival and fibrosis progression in patients with hepatitis C undergoing liver transplantation using HCV+ allografts. Liver Transpl. 2006;12:1496-1503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 178. | Rayhill SC, Wu YM, Katz DA, Voigt MD, Labrecque DR, Kirby PA, Mitros FA, Kalil RS, Miller RA, Stolpen AH. Older donor livers show early severe histological activity, fibrosis, and graft failure after liver transplantation for hepatitis C. Transplantation. 2007;84:331-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 179. | Maluf DG, Edwards EB, Stravitz RT, Kauffman HM. Impact of the donor risk index on the outcome of hepatitis C virus-positive liver transplant recipients. Liver Transpl. 2009;15:592-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 180. | Lake JR, Shorr JS, Steffen BJ, Chu AH, Gordon RD, Wiesner RH. Differential effects of donor age in liver transplant recipients infected with hepatitis B, hepatitis C and without viral hepatitis. Am J Transplant. 2005;5:549-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 181. | Belli LS, Burroughs AK, Burra P, Alberti AB, Samonakis D, Cammà C, De Carlis L, Minola E, Quaglia A, Zavaglia C. Liver transplantation for HCV cirrhosis: improved survival in recent years and increased severity of recurrent disease in female recipients: results of a long term retrospective study. Liver Transpl. 2007;13:733-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 182. | Avolio AW, Cillo U, Salizzoni M, De Carlis L, Colledan M, Gerunda GE, Mazzaferro V, Tisone G, Romagnoli R, Caccamo L. Balancing donor and recipient risk factors in liver transplantation: the value of D-MELD with particular reference to HCV recipients. Am J Transplant. 2011;11:2724-2736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 183. | Arenas JI, Vargas HE, Rakela J. The use of hepatitis C-infected grafts in liver transplantation. Liver Transpl. 2003;9:S48-S51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 184. | Berenguer M. Risk of extended criteria donors in hepatitis C virus-positive recipients. Liver Transpl. 2008;14 Suppl 2:S45-S50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 185. | Northup PG, Argo CK, Nguyen DT, McBride MA, Kumer SC, Schmitt TM, Pruett TL. Liver allografts from hepatitis C positive donors can offer good outcomes in hepatitis C positive recipients: a US National Transplant Registry analysis. Transpl Int. 2010;23:1038-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 186. | Ballarin R, Cucchetti A, Spaggiari M, Montalti R, Di Benedetto F, Nadalin S, Troisi RI, Valmasoni M, Longo C, De Ruvo N. Long-term follow-up and outcome of liver transplantation from anti-hepatitis C virus-positive donors: a European multicentric case-control study. Transplantation. 2011;91:1265-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 187. | Wilson S, Mangus RS, Tector A, Fridell JA, Vianna R, Liangpunsakul S, Misra V, Bhardwaj S, Kwo P. Use of infected liver donors in liver transplantation: a case-control study. Gastroenterology. 2007;132:A278. |
| 188. | Briceño J, Ciria R, Pleguezuelo M, de la Mata M, Muntané J, Naranjo A, Sánchez-Hidalgo J, Marchal T, Rufián S, López-Cillero P. Impact of donor graft steatosis on overall outcome and viral recurrence after liver transplantation for hepatitis C virus cirrhosis. Liver Transpl. 2009;15:37-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 189. | Subramanian V, Seetharam AB, Vachharajani N, Tiriveedhi V, Angaswamy N, Ramachandran S, Crippin JS, Shenoy S, Chapman WC, Mohanakumar T. Donor graft steatosis influences immunity to hepatitis C virus and allograft outcome after liver transplantation. Transplantation. 2011;92:1259-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 190. | Botha JF, Thompson E, Gilroy R, Grant WJ, Mukherjee S, Lyden ER, Fox IJ, Sudan DL, Shaw BW, Langnas AN. Mild donor liver steatosis has no impact on hepatitis C virus fibrosis progression following liver transplantation. Liver Int. 2007;27:758-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 191. | Burra P, Loreno M, Russo FP, Germani G, Galligioni A, Senzolo M, Cillo U, Zanus G, Fagiuoli S, Rugge M. Donor livers with steatosis are safe to use in hepatitis C virus-positive recipients. Liver Transpl. 2009;15:619-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 192. | Baron PW, Sindram D, Higdon D, Howell DN, Gottfried MR, Tuttle-Newhall JE, Clavien PA. Prolonged rewarming time during allograft implantation predisposes to recurrent hepatitis C infection after liver transplantation. Liver Transpl. 2000;6:407-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 61] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 193. | Watt KD, Lyden ER, Gulizia JM, McCashland TM. Recurrent hepatitis C posttransplant: early preservation injury may predict poor outcome. Liver Transpl. 2006;12:134-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 194. | Briceño J, Ciria R, Pleguezuelo M, Naranjo A, Sánchez-Hidalgo J, Ruiz-Rabelo J, López-Cillero P, Luque A, de la Mata M, Rufián S. Contribution of marginal donors to liver transplantation for hepatitis C virus infection. Transplant Proc. 2007;39:2297-2299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 195. | Killackey MT, Gondolesi GE, Liu LU, Paramesh AS, Thung SN, Suriawinata A, Nguyen E, Roayaie S, Schwartz ME, Emre S. Effect of ischemia-reperfusion on the incidence of acute cellular rejection and timing of histologic hepatitis C virus recurrence after liver transplantation. Transplant Proc. 2008;40:1504-1510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 196. | Gomez D, Homer-Vanniasinkam S, Graham AM, Prasad KR. Role of ischaemic preconditioning in liver regeneration following major liver resection and transplantation. World J Gastroenterol. 2007;13:657-670. [PubMed] |
| 197. | Desai KK, Dikdan GS, Shareef A, Koneru B. Ischemic preconditioning of the liver: a few perspectives from the bench to bedside translation. Liver Transpl. 2008;14:1569-1577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 198. | Gurusamy KS, Kumar Y, Sharma D, Davidson BR. Ischaemic preconditioning for liver transplantation. Cochrane Database Syst Rev. 2008;CD006315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 199. | Gurusamy KS, Tsochatzis E, Toon CD, Xirouchakis E, Burroughs AK, Davidson BR. Antiviral interventions for liver transplant patients with recurrent graft infection due to hepatitis C virus. Cochrane Database Syst Rev. 2013;12:CD006803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 200. | Pungpapong S, Aqel BA, Koning L, Murphy JL, Henry TM, Ryland KL, Yataco ML, Satyanarayana R, Rosser BG, Vargas HE. Multicenter experience using telaprevir or boceprevir with peginterferon and ribavirin to treat hepatitis C genotype 1 after liver transplantation. Liver Transpl. 2013;19:690-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 201. | Fontana RJ, Hughes EA, Bifano M, Appelman H, Dimitrova D, Hindes R, Symonds WT. Sofosbuvir and daclatasvir combination therapy in a liver transplant recipient with severe recurrent cholestatic hepatitis C. Am J Transplant. 2013;13:1601-1605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |