INTRODUCTION
Children in the United States requiring liver transplantation are prioritized organs according to the pediatric end-stage liver disease (PELD) scoring system. PELD estimates mortality risk in order to assign deceased donor organs to the children who need them most. PELD weighs three variables - total bilirubin, albumin, and INR - and gives special consideration to patients who are less than two years old and/or weigh two standard deviations below normal[1]. Initially, the PELD system functioned successfully, with only 3% of children in the early PELD era (2000-2004) dying while waiting for transplant[2]. Unfortunately, in recent years, the PELD system has not been as efficacious for the youngest children, i.e., those less than 2 years old. Over the past 10 years, approximately 15% of these children with chronic liver disease either died while waiting on the transplant list or were removed from the list because they became too ill[3]. Similarly, other countries using the PELD system, such as Brazil, experience similar waiting list death rates of approximately 15%[4].
A large number of these young children requiring liver transplant suffer from the same disease: biliary atresia. Biliary atresia (BA) is a progressive cholestatic and fibrotic disease of unknown etiology which occurs only in infants. Children often appear normal at birth but become jaundiced in the first weeks of life. Those diagnosed early receive a Kasai portoenterostomy, which removes the obstructed extra-hepatic bile ducts in an attempt to restore normal bile flow. Some Kasai operations are successful; however, many will fail. Children with a “failed Kasai” will require liver transplant in infancy to survive. In addition, infants who are diagnosed late often have too much liver damage to benefit from the Kasai operation and will also require an early transplant, usually in the first year of life. Unfortunately these two groups encompass anywhere from one-third to greater than one half of the BA population[5]. For example in the United States, a multicenter study observed that 46 out of 104 infants with BA either required liver transplant or died by 24 mo of age[6].
Infants with BA requiring liver transplant are especially vulnerable[7-9] as they do not fare well in the current PELD system. First, PELD does not account for acute life-threatening complications that commonly develop in BA, such as refractory ascites, acute variceal bleeds, and infections such as cholangitis. Second, PELD calculations award points to patients with low albumin and weight Z-scores, both of which are often artificially normal in infants with BA. Low serum albumin concentrations can be masked by infusions, and weight can be elevated by fluid overload and enhanced by parenteral nutrition. Third, PELD automatically diverts the already rare small organs away from infants with BA - no matter how high their PELD score - by allocating them to infants with metabolic or oncological disorders first.
In this review, we discuss ways to improve outcomes in infants with BA requiring liver transplant. Our focus is on interventions beyond the PELD system, and falls into two categories: (1) optimizing the success of the Kasai operation; and (2) maximizing liver transplants when needed, before BA liver disease progresses too far to make transplant unsafe.
OPTIMIZING KASAI OUTCOMES
The Kasai portoenterostomy is the only treatment for infants with BA other than liver transplant. The Kasai operation has variable outcomes, with a 53.7% and 46.7% 1- and 2-year transplant-free success rate in a North American study[10]. Hence, improving the success rate could dramatically decrease the need for liver transplants in infancy. The Kasai operation’s success rate may be influenced by at least 4 factors: (1) time of Kasai; (2) surgeon experience; (3) post-operative nutrition; and (4) post-operative medications.
Early diagnosis of BA and referral for Kasai
Many studies have correlated earlier Kasai portoenterostomies with the best outcomes and reduced need for transplant[5]. For example, in one large study of 743 infants, 66% of infants receiving a Kasai portoenterostomy before 30 days of life (DoL) were transplant-free at two years, compared to 58% with Kasai performed between 45 and 60 DoL and 42% when performed at greater than 90 DoL[11]. Similarly, a North American study of 104 infants detected a trend, though not statistically significant, toward better outcomes with greatest transplant-free survival when the Kasai portoenterostomy was performed at less than 30 DoL[6]. It is important to note that all these studies demonstrate correlation rather than causality. However, given the strong correlation a more definitive randomized-controlled trial would be unethical to perform.
The main barrier to an early Kasai portoenterostomy is that BA is difficult to diagnose. In the US, we have no standard way to identify it early. The disease starts insidiously, with newborns typically appearing healthy and only developing jaundice later. Furthermore, practitioners often mistake the disease’s jaundice as “physiological” or “breast milk” jaundice. In other countries such as Taiwan, infants are screened for BA with a stool color card (SCC) that parents use to detect the acholic stools characteristic of extrahepatic biliary obstruction. The SCC is an effective screen, with a sensitivity of 82.9% for detection before 45 DoL and 97.1% at 60 DoL[12].
Early detection of BA may also be achieved by measurement of newborn serum direct/conjugated bilirubin[13]. This test has a number of advantages. Newborn direct/conjugated bilirubin measurements are very sensitive for BA, as infants with the disease have high direct/conjugated bilirubin levels at birth. The test is also very specific if all abnormal levels are confirmed with a single repeat test at the two-week well child check (Harpavat S, in preparation). The test has the additional advantage of already being used in clinical practice and thus does not require any additional infrastructure or training. Newborn bilirubin screening is now being tested prospectively to determine whether it could be a standard-of-care test in the newborn nursery to help detect BA earlier.
Enhancing surgical outcomes via experience
Because BA has an incidence of only one in 8000 to 18000 infants[5,10], few surgeons have the opportunity to perform multiple Kasai operations. Several studies have suggested that successful outcomes for the Kasai are dependent on the experience of the operative surgeon and the presence of an organized team of medical and surgical personnel to care for children with BA[5,14,15]. Studies in the United Kingdom demonstrated a clear distinction in jaundice-free two-year survival among high activity centers, with those performing > 5 Kasai portoenterostomies/year enjoying 43% success, those performing 2-5 Kasais/year 29% success, and those performing 1 Kasai/year only 11% success[14]. As a result of the study, all Kasai portoenterostomies in the United Kingdom are now performed in large volume centers. Similar trends were found in high vs low volume centers in France, which spurred a collaborative initiative among centers in an attempt to equalize outcomes[15].
Surgeons performing the Kasai operation face a number of technical challenges which may explain why those most experienced have the greatest success. There are many anatomical variants in BA, leading to different appearances of the central, distal, and proximal biliary trees[10]; experience with these variations certainly can improve outcomes. Surgeons also vary in how deep into the hilum they dissect when removing the proximal portions of the obstructed duct. Whether a deeper dissection improves outcomes remains a topic for further study[16]. Furthermore, after removing the obstruction, surgeons can connect the liver to intestine in various ways. For example, some were previously attaching the intestine to a patent gallbladder before this technique was proven inefficacious[10].
Aggressive post-Kasai nutrition
Nutrition is vital in BA patients post-Kasai; as with many chronic diseases, there can be both increased caloric demands and decreased oral intake in patients with BA[17,18] Adequate nutrition correlates with both improved post-Kasai and post-transplant outcomes. In a study of 100 infants, those surviving two years jaundice-free with their native liver had higher weight and length Z-scores when compared to those requiring liver transplant[19]. The differences in weight and height were statistically significant 6 and 18 mo after the Kasai operation, respectively; however, it is important to note that both groups had lower weight and length Z-scores when compared to the general population[19]. Additionally, better nutrition at time of transplant is associated with decreased mortality post-transplant[18].
Aggressive nutritional therapy is thus warranted as it may improve both post-Kasai and post-transplant outcomes. While there are no validated nutrition protocols for infants with BA, we follow a protocol based on a number of parameters. First, infants receive nutrition in the first 24 h after the Kasai procedure. This often involves temporary parenteral nutrition while awaiting resumption of gut function. Second, infants are started on enteral feeds of breast milk supplemented with medium chain triglycerides (MCT) or MCT-based formulas, to achieve 120-150 kcal/kg per day. However, infants are often unable to take this increased volume orally, as cholestasis can contribute to poor appetite[20], and require naso-gastric feeds to meet these goals. Third, if infants are still unable to gain weight with enteral feeds, parenteral nutrition is started. Parenteral nutrition both increases the nutritional status and equalizes outcomes post-transplant of malnourished BA patients when compared to their better-nourished counterparts[21]. Fourth, infants receive high doses of fat-soluble vitamins in an attempt to increase vitamin D, A and E levels, as well as to help maintain normal clotting parameters[22].
Post-operative medications
Several medications are administered post-Kasai to improve outcomes, with variable success and data to support their use. Antibiotics are typically given following surgery to prevent gut bacteria from entering the intrahepatic biliary tree and causing cholangitis. A prospective randomized study found that patients receiving either sulfamethoxazole/trimethoprim (average 14.6 mo treatment) or neomycin (average 14.7 mo treatment) had decreased rates of cholangitis and increased survival when compared to historical controls[23]. Another study detected a trend towards improved two year liver transplant-free survival if antibiotics were given for more than three months after Kasai; however, interestingly, the rates of cholangitis were statistically no different between groups of children receiving antibiotics (55%) and those not (49%)[6].
Ursodiol is also commonly prescribed and continued indefinitely, usually at a dose of 15-30 mg/kg per day[6]. Ursodiol is a naturally-occurring bile acid thought to help flush bile through ducts and out of the liver. Ursodiol has a suggested benefit in several observational and case-control studies, including one in which sixteen 18-mo old post-Kasai BA children stopped Ursodiol for 3 mo. Prior to discontinuation, their mean dose of Ursodiol was 25 mg/kg per day (range: 20-36 mg/kg per day). Twelve of these children had worsened liver panels, and one child developed jaundice when Ursodiol was discontinued[24]. Not all trials, however, support Ursodiol use. Other studies report no benefit and perhaps even harm with Ursodiol at a dose of 20 mg/kg per day[25]. A prospective randomized controlled trial for Ursodiol would better assess the drug’s benefit.
Steroids are the most tested, and the most controversial, of the post-Kasai medications. Steroids are used because many consider the progressive fibrosis of BA to be due to an inflammatory immune-mediated process[26]. Corticosteroids were recently evaluated in the large double-blind randomized placebo-controlled START trial of 140 infants. Corticosteroids were given to 70 infants after the Kasai operation, at a daily dose of 4 mg/kg iv methylprednisolone for two weeks, followed by 2 mg/kg oral prednisolone for two weeks, followed by a taper over the next nine weeks. Compared to the 70 control infants receiving placebo, the experimental subjects had equivalent rates of restored bile flow at six months and two years, as well as similar rates of liver transplant-free survival at two years. However, those receiving steroids had a shorter time to first serious adverse event compared to controls[27].
In part because of this trial we do not prescribe steroids, though many practitioners do because of potential benefits previously reported. An earlier two-center, double-blind, randomized, placebo-controlled trial showed that steroids reduced serum bilirubin levels without improving transplant-free survival[28]. An additional follow-up study demonstrated decreased bilirubin levels with high dose (5 mg/kg per day) vs low dose (2 mg/kg per day) corticosteroids, but again demonstrated no improved survival effect[29]. Recently a study from Japan also reported decreased bilirubin levels with high (4 mg/kg per day) vs low (2 mg/kg per day) dose corticosteroids[30]. However, they did not report improvements in transplant-free survival.
MAXIMIZING TRANSPLANT OPTIONS FOR CHILDREN WITH BA-ASSOCIATED LIVER FAILURE
As mentioned above, infants with BA who need a liver transplant will often have low natural PELD scores. To circumvent this, infants must earn “exception points,” granted to them at the request of their physicians by an anonymous board reviewing their case. Still, even with these exception points, many infants receive a transplant only after long waits, worsening disease, and deteriorating health. There are at least three ways for infants to avoid these delays and expedite liver transplant: (1) performing living-related donor (LRD) liver transplants; (2) increasing “split” transplants; and (3) permitting ABO-incompatible transplants (ILTs).
Living-related donor transplants
Living-related donor (LRD) transplants have excellent outcomes. In Japan, where deceased-donor liver transplants are not always accepted culturally, LRD transplants are standard with 88.3% one-year, 85.4% 5-year, 82.8% 10-year and 79.6% 20-year survival rates[7]. Survival for BA, which accounted for 66% of all Japanese pediatric transplants, was just as successful for LRD with a 20-year survival rate of 84%[7]. LRD transplants are especially advantageous because they can occur when they are most likely to result in the best outcome: when transplant is needed but before the infant becomes too ill to safely tolerate the operation. In contrast, with deceased donor transplants, infants with BA can spend many months waiting for a suitable organ to become available and must compete with other infants in the PELD system[31].
Despite these good outcomes, LRD transplants do pose a number of challenges. First, LRD transplants raise ethical issues regarding the choice of donor. It is unclear who should be considered, i.e., only parents vs extended family vs others. Second, LRD does pose a risk to those caregivers most vital to the care of an infant after transplant. If a patient’s parent decides to donate and then suffers from operative complications, the infant may no longer have stable support for further care. Third, in the US, LRD transplants create a “two-tier” system of transplant because public insurers are less likely to cover the donor’s surgery[31]. As a result, LRD raises the prospect of privately-insured infants with BA surviving longer than publically-insured infants because the former are able to receive an earlier transplant. In our experience, this insurance issue precludes many of our BA patients from qualifying for a LRD transplant.
Increasing “split” liver transplants
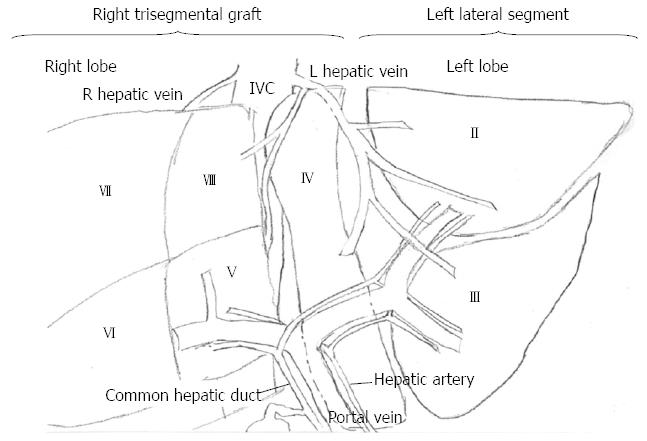
“Split” liver transplants maximize the deceased-donor organs available by allowing for one donor to benefit two recipients. The procedure involves splitting the liver into a left lateral segment and a right trisegmental graft, with the smaller left lateral segment allocated to a child (Figure 1). “Split” transplants are technically more challenging, and in previous eras, split transplants had more complications when compared to whole graft transplants[32]. However, recently these differences have been resolved, with no significant survival differences between whole and split liver transplants. Both the children and adults receiving left lateral segments and right trisegmental grafts, respectively, have the same low rates of graft failure and mortality when compared to those patients receiving whole organs[32,33].
Figure 1 Diagram of split liver transplant.
The left lateral segment is donated to a child and the right trisegmental graft is designated for an adult.
Unfortunately, at this time, less than 10% of donor liver that could be split are split[32]. This may be due to a number of factors, including surgeon comfort with the technique. Another barrier is that recipients currently decide whether they prefer a split or whole liver, with most recipients opting for a whole liver. To address this, the OPTN/UNOS Ethics committee now suggests that patients not be given this choice in centers where split liver transplants have equal outcomes to whole liver transplants. Their White Paper statement endorses “splitting these optimum livers should be considered the benchmark rather than the exception. Fostering maximum utilization of these organs is ethically proper and should be required”[34]. This has the potential to significantly improve organ availability for young infants with BA. However, these are only recommendations, and a major avenue for future advocacy should be in converting these recommendations into formal policy.
ABO-incompatible liver transplants
ABO-incompatible donor liver transplants (ILT) represent one of the next frontiers of pediatric liver transplant. Traditionally, matching a donor liver to recipient is based solely on ABO compatibility, unlike heart and kidney transplantations which also rely on HLA-typing[35,36]. However, good results have been demonstrated with ILT, which questions the need for ABO-compatibility in children. A 2011 study in the United Kingdom demonstrated equal outcomes in 5 infant ILT recipients when compared to 25 infants who received ABO-compatible liver transplants (CLT); there was no difference in rejection, biliary complications or patient survival after an average of 3 years[37]. Additionally, a 2011 meta-analysis encompassing greater than 40 years of over 3500 pediatric and adult ILT patients around the world showed no difference in 1-, 3-, 5- and 10-year outcomes in pediatric ILT patients when compared to pediatric CLT[38]. Similarly, in Japan, ILT survival in 185 recipients transplanted at less than 2 years of age was 81% at 15 years, further validating the safety of ILT[7]. Given the outcomes described and the need for organs for the sickest pediatric liver patients, especially patients with BA, ABO-incompatible liver transplantation may be another way to limit waiting list mortality.
CONCLUSION
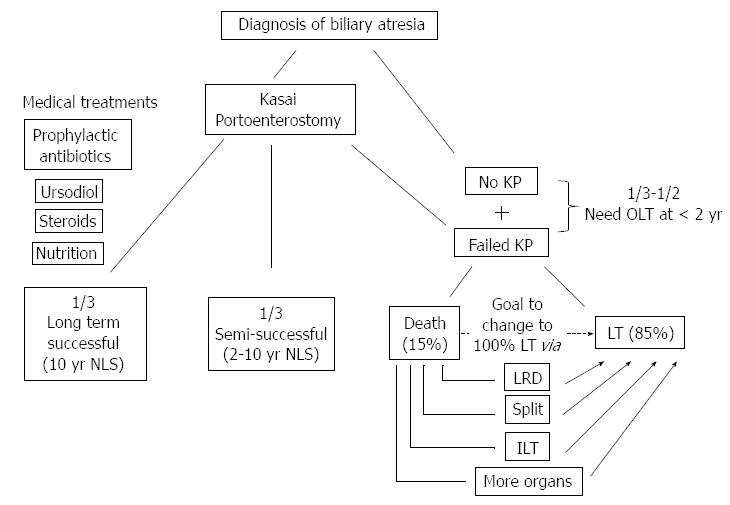
Infants with chronic liver failure from BA are a vulnerable group; the natural history of their disease course is grim. The Kasai portoenterostomy can result in excellent outcomes in a small subset of children with BA, with resulting normal liver function and quality of life for decades. However, the majority of children with BA will ultimately require transplantation, and infants with BA who need a liver transplant are particularly at risk. These infants wait on the transplant list based on their PELD score, while their clinical condition invariably worsens. Fortunate infants are matched with a deceased-donor organ in a timely fashion and often have excellent outcomes. However, too many infants with BA receive a liver transplant later than ideal or never have the chance to receive one at all. To help these infants, practitioners spend considerable energy applying for PELD “exception points” and extending life by managing serious complications as they arise. We suggest that practitioners could serve infants with BA better (Figure 2) with two additional interventions: (1) preventing/delaying need for liver transplant, by optimizing the success of Kasai operation; and (2) maximizing the availability of liver transplants when needed, through LRD transplants, “split” transplants, and ILTs.
Figure 2 Outcomes in the current era of biliary atresia and ways to improve patient outcomes.
KP: Kasai portoenterostomy; NLS: Native liver survival; LT: Liver transplant; LRD: Living related donor; ILT: Incompatible donor liver transplant.