Published online Aug 7, 2014. doi: 10.3748/wjg.v20.i29.9976
Revised: February 28, 2014
Accepted: April 21, 2014
Published online: August 7, 2014
Processing time: 266 Days and 20 Hours
Endoscopic ultrasound (EUS) is an important part of modern gastrointestinal endoscopy and now has an integral role in the diagnostic evaluation of pancreatic diseases. Furthermore, as EUS technology has advanced, it has increasingly become a therapeutic procedure, and the prospect of multiple applications of interventional EUS for the pancreas is truly on the near horizon. However, this review focuses on the established diagnostic and therapeutic roles of EUS that are used in current clinical practice. In particular, the diagnostic evaluation of acute pancreatitis, chronic pancreatitis, cystic pancreatic lesions and solid masses of the pancreas are discussed. The newer enhanced imaging modalities of elastography and contrast enhancement are evaluated in this context. The main therapeutic aspects of pancreatic EUS are then considered, namely celiac plexus block and celiac plexus neurolysis for pain control in chronic pancreatitis and pancreas cancer, and EUS-guided drainage of pancreatic fluid collections.
Core tip: This is an invited review paper that provides a comprehensive review of the current diagnostic and therapeutic roles of endoscopic ultrasound in pancreatic disease.
- Citation: Teshima CW, Sandha GS. Endoscopic ultrasound in the diagnosis and treatment of pancreatic disease. World J Gastroenterol 2014; 20(29): 9976-9989
- URL: https://www.wjgnet.com/1007-9327/full/v20/i29/9976.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i29.9976
Endoscopic ultrasound (EUS) is an important technology with many established applications that has become a necessary component of any referral center endoscopy unit. In particular, EUS has emerged as an integral component in the evaluation of the pancreas, both from a diagnostic and an increasingly therapeutic perspective. In both respects, innovations continue to expand the capabilities of EUS and push the boundaries of its indications (Table 1). In this review, the current diagnostic and therapeutic aspects of EUS with respect to pancreatic diseases will be explored. Emerging imaging technologies and interventional applications of EUS that remain experimental and not yet ready for clinical practice are beyond the scope of this paper and will not be discussed.
| Diagnostic | Therapeutic | Applications under investigation |
| Acute biliary pancreatitis | CPB/CPN | Biliary access and drainage |
| Chronic pancreatitis | Pseudocyst drainage | Pancreas duct access |
| Solid masses | Necrosectomy for WON | Cyst ablation |
| Cystic lesions | Fiducial marking in tumors | |
| Local tumor injection therapy or ablation | ||
| 3-dimensional EUS | ||
| nCLE | ||
| Pancreatic cystoscopy |
The diagnosis of acute pancreatitis should be evident from clinical and biochemical parameters and EUS is not necessary for this purpose. Furthermore, EUS does not have proven utility in the assessment of the severity of acute pancreatitis, for which clinical scoring systems[1] and computed tomography (CT)[2] have established roles. However, EUS may be useful in confirming the biliary origin of acute pancreatitis when in doubt, and more commonly, for determining the need for endoscopic retrograde cholangiopancreatography (ERCP) when the likelihood of persistent choledocholithiasis is deemed to be low or moderate probablility. EUS has high 94% sensitivity and 95% specificity for the detection of common bile duct stones[3], and is superior to transabdominal ultrasound, CT and magnetic resonance cholangiopancreatography (MRCP), with MRCP being inferior for small stones in particular[4-6]. In fact, EUS has a greater than 95% negative predictive value for choledocholithiasis[7]. For this reason, patients with acute biliary pancreatitis who have a normal EUS can avoid the need for ERCP, and thus the risk of post-ERCP pancreatitis or other complications. In this context, performing EUS prior to ERCP has been shown to decrease the need for ERCP by nearly 70%, while significantly reducing the overall risk of complications (relative risk 0.35) and of post-ERCP pancreatitis (relative risk 0.21)[8,9]. EUS may also be beneficial in the evaluation of patients with recurrent idiopathic acute pancreatitis, particularly for the exclusion of structural anomalies such as cystic or solid pancreatic neoplasms obstructing the main pancreatic duct (PD), PD strictures, ampullary tumors, annular pancreas and pancreas divisum[10]. While similar information can be obtained by MRCP, secretin stimulation is probably necessary for MRCP to achieve a high level of accuracy for pancreas divisum[11,12], although doubts remain about the reliability of EUS for making this diagnosis as well[13]. Finally, when autoimmune pancreatitis is suspected, EUS can demonstrate characteristic imaging patterns and enables histologic tissue biopsies to confirm the diagnosis[14,15].
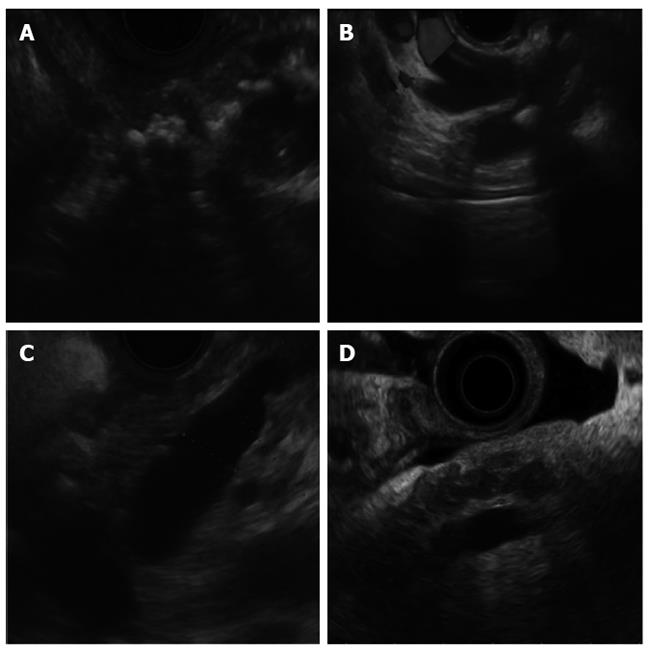
EUS is a well-established and accepted test for the diagnosis of chronic pancreatitis[16]. The EUS criteria are based on nine parenchymal and ductal features (Table 2), with the detection of ≥ 5 features typically considered diagnostic[17-19], while some studies suggest that 4 features are sufficient to make the diagnosis[20]. The clinical relevance of the presence of fewer features remains unclear and no clear correlation with early chronic pancreatitis has been confirmed. In fact, as imaging technology has advanced, there is concern that EUS may now be overly sensitive to the identification of subtle abnormalities in the pancreas in patients without any clinical pancreatic disease. Recently, the Rosemont classification scheme was proposed by a meeting of experts that divided the EUS features (Figure 1) into major and minor criteria and increased the stringency of each criterion[21]. In addition, it provided graded levels of the likelihood of chronic pancreatitis, compared to the all-or-nothing stratification of older schemes. However, the Rosemont classification was never validated and has not been shown to improve interobserver agreement among endosonographers compared to the traditional scoring system[22-24]. Thus, the ideal classification scheme remains undefined and uncertainty remains about how exactly EUS should be used to make this diagnosis. In addition, while EUS can clearly discriminate between patients with advanced chronic pancreatitis and those with a normal pancreas, it is unclear how well current criteria can differentiate patients with early chronic pancreatitis from those who have no clinical disease.
| Parenchymal features | Ductal features |
| Hyperechoic foci | Calcification |
| Hyperechoic strands | Main PD dilation |
| Lobularity | Dilated side branches |
| Cysts | Hyperechoic PD margins |
| Irregular PD contour |
Recently, the image enhancement technique of elastography has been used to help improve the diagnostic capabilities of EUS for chronic pancreatitis. Elastography is a technology available with Pentax echoendoscopes (Pentax Inc.; Tokyo, Japan) and Hitachi processors (Hitachi Inc.; Tokyo, Japan) that measures the relative stiffness of tissues based on deformation or “strain” caused by compression, which is presented as a color scheme that is superimposed on the standard B-mode image[25]. Initially, elastography was a purely subjective technique that relied on the interpretation of relative color patterns, and was limited by inconsistent results and concerns over its operator dependence[26,27]. However, newer methods of quantitative elastography have demonstrated promising early results that may aid the diagnosis of chronic pancreatitis by EUS. One such quantitative method is the strain ratio, which measures the strain in a reference area relative to that in the area of interest (both of which are manually selected), reflected as a numerical score[28]. A recent Spanish study prospectively examined 191 patients with known chronic pancreatitis or unexplained epigastric abdominal pain using quantitative EUS elastography, measuring the strain ratio in the head, body and tail of the pancreas, as well as standard EUS criteria for chronic pancreatitis and the Rosemont classification[29]. Patients who had ≥ 5 EUS criteria were considered to have a positive diagnosis of chronic pancreatitis whereas those with ≤ 2 EUS criteria were considered negative. Patients with inconclusive EUS findings, defined as the presence of 3 or 4 criteria, then underwent secretin-stimulated MRCP and contrast-enhanced pancreas MRI to further confirm or refute the suspicion of chronic pancreatitis. In all regions of the pancreas, the strain ratio was significantly higher in patients with chronic pancreatitis compared to those without, with a strain ratio cut-off of ≥ 2.25 diagnosing chronic pancreatitis with 91% accuracy. Furthermore, a direct linear correlation was found between the strain ratio and the number of EUS criteria seen, while the strain ratio differed significantly between the different Rosemont classification groups. This study is interesting because it suggests the possibility of using EUS elastography to quantify the severity of pancreatic fibrosis, which potentially could then be used to follow the progression of the disease over time. In addition, of the 92 patients in this study[29] who had chronic pancreatitis, 22 had inconclusive results based on the presence of only 3 or 4 EUS criteria and needed additional MRI studies to confirm the diagnosis. Of these 22 patients, 17 had a mean strain ratio greater than 2.25 and would have been correctly identified by means of EUS elastography alone. Thus, quantitative elastography has the potential to improve the diagnostic capabilities of EUS for chronic pancreatitis, particularly for early or less severe stages of the disease for which current EUS criteria have been inadequate. However, this study was performed at a leading EUS centre by an expert in elastrography and it remains to be seen whether similar results can be consistently replicated.
With significant improvements in cross-sectional abdominal imaging, pancreatic cysts are being increasingly identified not only in patients being investigated for non-specific abdominal complaints but also in those undergoing investigations for other unrelated reasons. In recent studies, the prevalence of incidental pancreatic cysts in patients undergoing cross-sectional abdominal imaging was 2.4%-13.6%[30-32]. Pancreatic cysts are categorized into non-neoplastic cysts and cystic neoplasms. Non-neoplastic cysts include the rare true simple cysts, retention cysts and lymphoepithelial cysts, or the more common inflammatory cysts (pseudocysts) and serous cystadenomas. There is no known risk of malignant transformation of cysts in this category. On the other hand, cystic neoplasms include mucinous cyst neoplasms (MCN), intraductal papillary mucinous neoplasms (IPMN) and solid pseudo papillary neoplasms (SPN). Based on morphologic criteria, IPMN are categorized into main duct (MD-IPMN) and branch duct (BD-IPMN) subtypes. Also, based on the presence and degree of dysplasia, MCN and IPMN are further divided into low-grade dysplasia, high-grade dysplasia and adenocarcinoma subcategories[33]. The malignant potential of cystic neoplasms varies based on the histological type. A recent surgical series of 163 patients with MCN reported an 18% risk of malignant transformation[34]. MD-IPMN has the highest malignant potential ranging from 57%-92% in various series whereas the risk for BD-IPMN is < 20%[35-37]. SPN are rare, low-grade malignant tumors with excellent prognosis after surgical resection[38]. However, given that these are all surgical studies, there is likely substantial selection bias that results in an overestimation of the true risk of malignant transformation for patients with cystic neoplasms being followed as part of surveillance protocols[39].
Pancreatic cysts have characteristic imaging features, which means EUS is ideally suited for their evaluation. However, a study from expert EUS centers only achieved 51% accuracy for the correct prediction of cyst diagnosis based on echosonographic features alone[40]. For this reason, EUS-guided fine needle aspiration (EUS-FNA) has formed the cornerstone of the diagnosis of pancreatic cysts, with analysis of cyst fluid markers such as carcinoembryonic antigen (CEA) and amylase helping to discriminate mucinous from non-mucinous lesions, and to confirm communication with the pancreatic duct[41]. Traditionally, a cut-off of a CEA greater than 192 ng/mL has been used to predict the likelihood of mucinous cyst lesion[40], but this threshold is neither sensitive nor specific and so only CEA values that are very low (non-mucinous) or very high (mucinous) are truly helpful. The assessment of cystic pancreatic lesions by EUS is made more challenging by the fact that FNA rarely yields diagnostic cytology. In a prospective study of 143 consecutive patients undergoing EUS at two leading tertiary referral centers in The Netherlands, adequate cellular material to enable cytology was present in only 31% of cases[42]. Even more sobering was that sufficient cyst fluid for biochemical analysis was present in only half of cases. Because of the limitations of traditional cyst fluid analysis, considerable research efforts have focused on genetic markers such as DNA analysis, allelic loss analysis and K-ras mutation with some promise[43], but are not yet ready for clinical application.
One challenge for endosonographers who follow patients with cystic pancreatic neoplasms is to know which patients should be sent to surgery and which patients can be safely followed, and to know how frequently and by what method that follow-up should occur. Fortunately, international consensus guidelines for the management of IPMN and MCN have recently been updated[44]. Accordingly, all surgically fit patients with MCN or MD-IPMN should undergo surgery, as well as surgically fit patients with BD-IPMN and “high risk stigmata” such as obstructive jaundice, an enhancing solid component within the cyst, or a main PD ≥ 10 mm. Patients with BD-IPMN but without high risk stigmata are instead followed closely for the development of “worrisome features,” which include cyst size ≥ 3 cm, main PD 5-9 mm, thickened/enhancing cyst walls, a mural nodule, and an abrupt change in the caliber of the PD with distal pancreatic atrophy. Thus, the main role for EUS is to assist in the initial diagnosis of a cyst neoplasm and then to watch for the development of “worrisome features” in subsequent surveillance that is often done in combination with MRI. To this end, an interesting meta-analysis was recently performed that sought to determine the risk of malignancy that is associated with each of these different features[45]. They determined that the OR for malignancy were: cyst size ≥ 3 cm (OR = 62), mural nodule (OR = 9.3), dilated main PD ≥ 6 mm (OR = 7.3), and MD-IPMN vs BD-IPMN (OR = 4.7). This is particularly interesting since cyst size is no longer considered an absolute criterion for surgery in the new guidelines[44] but carried the highest risk of malignancy in this study[45]. However, another study that followed elderly patients who met criteria for resection but declined surgery demonstrated that most patients with BD-IPMN with lesions ≥ 3 cm had a good outcome over long-term follow-up, suggesting that a conservative approach may be reasonable in certain circumstances. Nevertheless, cystic neoplasms larger than 2 cm need to be monitored closely, often using a combination of both EUS and MRI. Recently, considerable interest has been placed on methods for EUS-guided cyst ablation[46], but this remains experimental and under ongoing development.
EUS has a high sensitivity and specificity for the detection of focal masses in the pancreas, and has greater sensitivity and accuracy compared to CT, especially for smaller lesions < 3 cm[47]. Thus, it is particularly useful for the diagnosis and staging of pancreas cancer[48]. In addition, EUS has a high negative predictive value and can reliably exclude pancreatic tumors[49]. Yet, it is the ability of EUS to enable tissue biopsies of pancreatic lesions via FNA that makes it such an appealing test. EUS-FNA is a well-established, safe, and effective technique to confirm the diagnosis of pancreas cancer, neuroendocrine tumors and other neoplasms in the pancreas[50-56]. However, EUS-FNA does have its limitations, including significantly reduced sensitivity of only 54%-73% in the setting of chronic pancreatitis compared to 85%-91% without chronic pancreatitis[57,58]. To optimize the technical performance of EUS-FNA, much research has focused on improving the needle design as well as improving the FNA technique. Numerous studies have sought to determine the preferred needle size for pancreatic biopsies, since different 19-gauge (G), 22 G and 25 G needles exist. A recent meta-analysis that examined 8 studies that directly compared 25 G and 22 G needles for FNA of pancreatic mass lesions in 1292 patients found that 25 G needles had a higher sensitivity (93% vs 85%, P = 0.0003) but comparable specificity (97% vs 100%, P = 0.97) to 22 G needles[59]. In a separate study that developed an algorithm for needle selection, technical performance of FNA was optimized when 25 G needles were used for transduodenal biopsies and when 22 G needles were used for other biopsy locations[60]. Other studies have considered whether negative pressure applied by suction helps or hinders biopsy results. Suction increases cellular yield but also increases the likelihood of bloody contamination. Therefore, whether or not to use suction remains the source of considerable debate. Some studies have found that increased suction compromises specimen quality and is only required if initially biopsy passes do not obtain a sample[61]. However, other studies have found that use of suction improves the diagnostic yield[62]. A recently proposed alternative involves creating slight negative pressure by slowly pulling back the stylet timed with the to-and-fro movements of the needle during the biopsy, termed the “capillary suction” technique, which has quickly become a popular method when performing FNA of solid pancreatic lesions[63]. In addition, “fanning” of the needle by moving it in multiple trajectories through different parts of a lesion rather than back-and-forth along the same needle track further enhances the biopsy technique[64]. While it is clear that the presence of an on-site cytopathologist greatly enhances the success and diagnostic yield from EUS-FNA[65-67], most centers cannot afford this luxury and therefore, 6 to 7 passes have been considered necessary to optimize diagnostic yield for pancreatic masses[68,69]. The desire to reliably obtain a sufficient sample with fewer passes in the absence of a cytopathologist was one of the motivating factors behind the creation of a new core biopsy needle (ProCore; Cook Medical, Bloomington, Indiana) that was designed to obtain material for both cytology and histology. In so doing, this core needle would also enable histological analysis that is considered crucial for the diagnosis of certain conditions such as autoimmune pancreatitis and gastrointestinal stromal tumors. The initial study reported excellent outcomes using the 19-gauge ProCore needle, with histology obtained in 89% of cases and a sensitivity, specificity and diagnostic accuracy of 90%, 100% and 93%[70]. Use of this larger needle for transduodenal FNA of lesions in the head of the pancreas or uncinate process remained technically challenging due to difficulty using the needle in the long position and with acute angulation of the echoendoscope, and so 22 G and 25 G versions of the ProCore needle have since been developed. However, it remains to be seen whether the smaller versions of this core biopsy needle will prove to be superior to conventional FNA needles of the same size, or even if they are more likely to obtain sufficient tissue for histology. In fact, a recent study that used both the new 22 G ProCore needle and a conventional 22 G FNA needle to biopsy the same lesion in 144 patients, with randomization to needle sequence, found that there was no difference between the core and conventional needles in terms of high quality tissue core (69% vs 66%), sample adequacy for histologic analysis (86% vs 88%), or correct diagnosis verified on follow-up (79% vs 81%), although the core needle required fewer passes on average to obtain adequate tissue[71]. Furthermore, a study of the 25 G ProCore needle in which biopsy specimens were sent for both cytology and histology showed that most of the diagnostic information from this core needle was actually provided by the cytological analysis[72]. The cumulative sensitivity of the results from histology compared to cytology was 63% vs 83% after the first pass and 87% vs 96% after the fourth pass, with a combined sensitivity of both histology and cytology (96%) no better than the sensitivity of cytology alone (96%) after 4 passes. In fact, while the expert endoscopist who conducted this study believed that a core sample was visible in 92% of cases, a true histologic core was only present 32% of the time. Thus, it has not yet been proven that the new 22- and 25 G ProCore needles confer a clinical advantage that justifies their increased cost compared to conventional FNA needles. Finally, while excellent results have been obtained using the larger 19 G ProCore needle, previous work has demonstrated that sufficient samples for histology can be obtained in 97% of cases using a conventional 19 G FNA needle[73]. This means that in situations such as autoimmune pancreatitis where histological analysis is mandatory and for which the new core biopsy needles have been advocated, it is possible that a conventional 19 G FNA needle may suffice. Clearly, further work is necessary to clarify the optimal needle type and method for biopsy of pancreas masses. Ultimately, there is unlikely to be a unifying answer, but rather a tailored approach that modifies the type and size of needle, as well as needle biopsy technique based upon the location, vascularity, extent of fibrosis, and suspected etiology of a given lesion.
To further aid in the evaluation of pancreatic masses, enhanced imaging techniques such as elastography and contrast-enhanced EUS have been recently studied, although neither has yet to become an established part of widespread clinical practice. As discussed earlier, elastography measures the relative stiffness of tissues based on their resistance to compression, producing a color pattern that is superimposed onto the standard B-mode ultrasound image. Early studies using qualitative elastography demonstrated great promise using color patterns to predict benign from malignant status of lesions that were subsequently verified on biopsy or surgery, achieving sensitivities, specificities and diagnostic accuracies of 92%, 81%, and 89% and 100%, 86%, and 94% respectively, as well as substantial interobserver agreement with kappa scores of 0.79 and 0.77[74,75]. However, other studies have produced disappointing results, yielding sensitivity, specificity and accuracy as low as 41%, 53% and 45%[27]. This inconsistency in the results has led to criticisms that qualitative elastography is too subjective and overly operator-dependent, which provided the impetus for the development of quantitative elastography. The previously described strain ratio is the most widely studied quantitative method and it has been shown to accurately discriminate between normal pancreas (lowest strain ratio), inflammatory masses, pancreas adenocarcinoma and neuroendocrine tumors (highest strain ratio)[25]. However, while the patterns are consistent within a given study, the actual strain ratio values overlap considerably between studies. For instance, in one study from Spain[28], the mean strain ratio was 3.3 for inflammatory masses and 18.1 for pancreas cancer, but in a study from Japan[76] the mean strain ratio was 23.7 for inflammatory masses and 39.1 for pancreas cancer. Thus, the broader meaning of any particular strain ratio does not seem to apply outside of a given study population, which once again must call into question the operator-dependent nature of this technology and its lack of generalizability. That said, more rigorous quantitative methods based on postprocessing computer analysis to generate a numerical scale of firmness referred to as hue-histograms[77], or using artificial neural networks[78], continue to be developed and hold the promise of more objectively rated quantitative scoring with elastography. A recent meta-analysis has sought to provide some clarity on the usefulness of EUS elastography for the characterization of pancreatic masses by synthesizing the data from available studies, including both qualitative and quantitative techniques[79]. The pooled sensitivity, specificity, odds ratio for distinguishing benign from malignant masses, and area under the receiver operator curve (AUROC) for 13 studies involving 1044 patients was 95%, 67%, 42.3 and 0.90. Thus, while elastography has a high sensitivity that may be useful in the detection of tumors, its limited specificity means that FNA remains necessary to confirm any suspected diagnosis, and so at least for the foreseeable future, this technology must only complement rather than replace tissue biopsy.
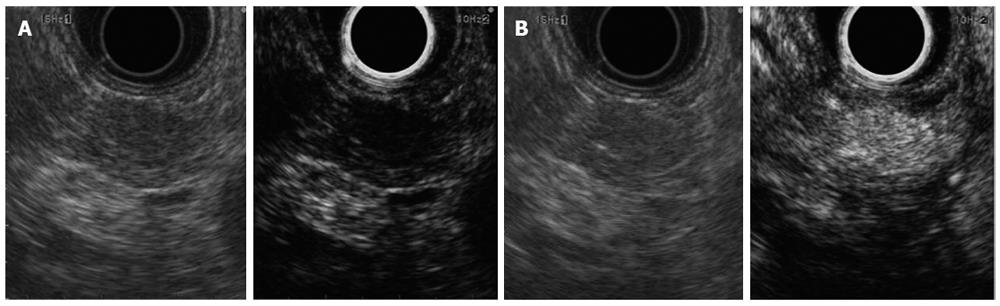
Contrast enhanced EUS offers similar promise to aid in the diagnosis of pancreatic mass lesions, while facing many of the same limitations as elastography. The technique has been usefully integrated into the practice of transabdominal ultrasound[80], particularly for lesions in the liver, and the same principles apply for its use with EUS. The contrast agents consist of microbubbles composed of an inert gas surrounded by a lipid membrane that is administered intravenously to characterize flow within blood vessels[81]. The first generation of contrast enhanced EUS relied on Doppler signaling, which created significant artifacts and had limited usefulness[82]. However, with the advent of second generation contrast agents and advances in echoendoscope capabilities, the newer technique of contrast-enhanced harmonic EUS (CEH-EUS) can depict vessels with high resolution while detecting microbubbles within the microvasculature, thereby demonstrating tissue perfusion[83]. It is this capability of illustrating areas of relative tissue perfusion within the pancreas that is beneficial for the diagnosis of pancreas masses. In most cases, pancreas adenocarcinoma has a hypovascular appearance, often with an irregular network of vessels. In contrast, benign nodules arising within chronic pancreatitis are typically isovascular to the rest of the pancreas, whereas neuroendocrine tumors are characteristically hypervascular (Figure 2)[84-86]. In addition, CEH-EUS may help localize a mass lesion within the pancreas that is suspected on cross-sectional imaging but not initially visualized on EUS, can improve the staging of pancreatic cancers with respect to vascular involvement, and may help guide the target of biopsies within a particular lesion when performing FNA[87].
The early data regarding the use of CEH-EUS for pancreas masses has been promising. In a study from France, the diagnostic acumen of CEH-EUS was compared to the results of FNA in 35 patients presenting with a solid pancreatic mass, with the final diagnosis determined from surgical pathology or long-term clinical follow-up[85]. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and accuracy of CEH-EUS was 89%, 88%, 89%, 88% and 89% compared to 72%, 100%, 100%, 77% and 86% for FNA with respect to pancreas adenocarcinoma. Thus, CEH-EUS significantly improved both the sensitivity and NPV compared to EUS-FNA, which was particularly true when all pancreatic masses (and not just adenocarcinoma) were considered, in which case FNA had a NPV of only 54%. In fact, of 5 pancreas cancers that had negative FNA, 4 were correctly characterized by their hypo-enhancing patterns on CEH-EUS. However, there is insufficient specificity to consider using contrast enhancement patterns as a diagnostic method to replace tissue biopsy. Since then, several additional studies have been published demonstrating sensitivities and specificities for CEH-EUS in similar ranges between 90%-96% and 64%-89%[84,86]. A recent meta-analysis has summarized the results from 1139 patients, and has found a pooled sensitivity, specificity and AUROC of 94%, 89% and 0.97 for the diagnosis of pancreas adenocarcinoma[88]. However, while these data appear extremely promising, CEH-EUS has been criticized for being unduly subjective with regards to the interpretation of contrast enhancement patterns, in a manner similar to qualitative elastography. Novel efforts to quantify the extent of contrast enhancement using computer software to generate time intensity curves offers a more objective measurement system and perhaps will prove to be a more reliable method, although this is yet to be determined[89,90]. Currently, the main clinical role of CEH-EUS is to strengthen the diagnostic interpretation of FNA, particularly when a biopsy is negative. For instance, when a pancreatic mass has a hypoenhancing contrast pattern that is characteristic of adenocarcinoma but a negative FNA, it would provide further argument for the need to repeat the biopsy. On the other hand, if there was an isoenhancing contrast pattern more typical of a benign lesion, a negative FNA could be accepted with greater confidence. Nonetheless, the true role of CEH-EUS in clinical practice, if any, requires further study and remains unclear. In any event, a major limiting factor for the widespread adoption of this technology is the lack of availability of the contrast agents themselves in the United States and many other countries outside of Europe and Asia due to lack of regulatory approval.
Chronic abdominal pain arising from locally invasive pancreatic cancer or from chronic pancreatitis has a considerable negative impact on quality of life. A significant proportion of this patient population requires narcotic analgesia, mostly in escalating doses, which may result in systemic consequences and dependency[91,92]. In an effort to decrease the requirement for narcotic analgesics, attempts have been made to interrupt the transmission of pain signals through the celiac plexus. Traditional methods of performing celiac plexus block (CPB) for chronic pancreatitis and/or celiac plexus neurolysis (CPN) for pancreas cancer involved a variety of different percutaneous approaches from the para-spinal region, most of which involved a blind needle puncture guided by CT scan measurements[93,94]. In contrast, CPB/CPN performed under the guidance of EUS can be effectively performed as a safe day procedure. The first report of EUS-guided CPB/CPN was by Wiersema et al[95]. Since then, there have been numerous studies demonstrating the benefit of the EUS-guided approach that have been summarized by two recent meta-analyses[96,97]. The included studies have significant heterogeneity in terms of the definitions of what is considered a positive response to therapy, but in most cases the aim has been a reduction in the daily usage of narcotic analgesia. Most benefit has clearly been observed with CPN for patients with chronic pain from pancreatic malignancy where 73%-85% demonstrated a significant response. CPB has not proven to be as effective for patients with abdominal pain from chronic pancreatitis, where the response rate is only 51%-60%. The reasons for this variation in benefit are unclear but may include different mechanisms of pain causation and characteristics of the underlying patient population itself.
EUS-guided CPB/CPN is performed using a linear echoendoscope via a trans-gastric approach from the proximal stomach. The celiac plexus is a conglomerate of nerve plexi wrapped around the origin of the celiac artery. A local anesthetic (typically bupivacaine) is injected alone for CPB whereas the combination of anesthetic and neurolytic agent (usually 100% ethanol) is injected for CPN using a FNA needle positioned in the angle between the celiac artery and the abdominal aorta. Single midline injection in the angle has been compared with injection to either side of this angle. A superior response was observed using the bilateral injection technique with a mean pain reduction of 70% compared with 46%, respectively (P = 0.002)[98]. Since the outcomes with CPB/CPN have remained less than optimal, there has been a recent shift toward directly targeting the celiac ganglia instead of the celiac plexus. Levy et al[99] first reported the endosonographic visualization of the celiac ganglia in 2006. Since then, other investigators have been successful in visualizing the celiac ganglia in a significant number of their patients. A recent randomized, controlled trial demonstrated a significant improvement in both the partial and complete response rates in patients with pain from abdominal cancer undergoing celiac ganglia neurolysis (CGN) compared with CPN (74% vs 46%, P = 0.026)[100]. It has also been recently suggested that earlier performance of CPN for patients with inoperable pancreas cancer may be preferable by reducing their need for, and mitigate some of the consequences of, increasing narcotic analgesic usage[101]. However, additional studies are required to verify all these recent findings. In summary, EUS-guided CPN and CGN are safe procedures with minimal side effects that provide significant benefit for chronic abdominal pain due to pancreatic cancer, but have more limited benefit in patients with chronic pancreatitis.
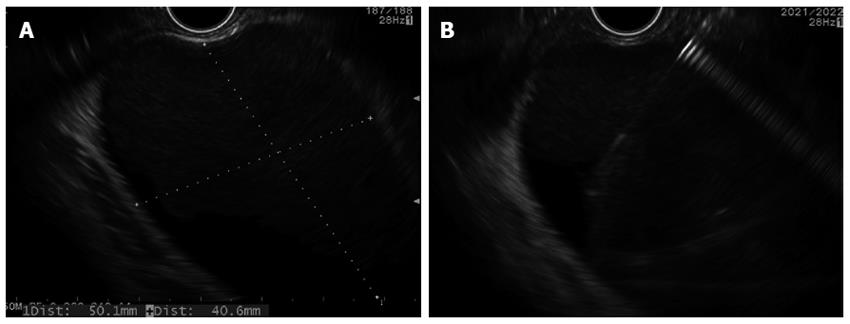
The management of pancreatic fluid collections (PFC), including both pseudocysts and walled-off necrosis (WON), is a complicated topic that has been discussed in detail by recent reviews and guideline statements[102,103], which increasingly falls within the purview of EUS and interventional endoscopy. While the traditional management of PFC was open surgery, consensus has emerged in recent years focusing on a more conservative approach, delaying or avoiding invasive procedures where possible and favoring minimally invasive or endoscopic methods when such interventions are necessary[103]. Furthermore, distinguishing the different types of PFC is now recognized as important since this has an important impact on the therapeutic approach and expected outcomes[104]. In the revised Atlanta classification of acute pancreatitis, PFC are now divided into acute fluid collections and acute necrotic collections when occurring less than 4 wk from an attack of acute interstitial pancreatitis and acute necrotizing pancreatitis respectively, and are considered pseudocysts when more than 4 wk after an episode of interstitial pancreatitis, have an encapsulated wall and no internal debris, and are considered WON when more than 4 wk from an episode of necrotizing pancreatitis, have an encapsulated wall and contain internal debris[105]. Most PFC will resolve on their own, which means that intervention is only required when pseudocysts or WON are symptomatic, typically because of persistent abdominal pain, luminal or biliary compression, or most importantly, infection[106]. Endoscopic strategies for the drainage of PFC have been used for over 2 decades[107] and have evolved from a direct endoscopic approach dependent upon visualization of a large bulge into the lumen created by the PFC, to an EUS-guided approach that appears to be safer and more effective[108-110], and is increasingly considered standard-of-care[103]. There are many variations of the technique, but the principle involves combining endoscopic, EUS, and fluoroscopic imaging to facilitate the creation of a transgastric or transduodenal fistulous tract into the encapsulated PFC, through which stents (plastic pigtail or covered metal) are placed to maintain the patency of the cyst-gastrostomy or cyst-duodenostomy tract to enable ongoing drainage. The basic steps are as follows: (1) Delay intervention for at least 4 wk until PFC has become encapsulated and adherent to the gastric or duodenal wall; (2) Perform recent cross-sectional imaging (CT or MRI) to provide “road map” for drainage procedure and to help differentiate a pseudocyst from WON (however internal debris within the PFC is sometimes only seen on EUS when not previously detected by other imaging modalities). Also helpful for excluding a possible pseudoaneurysm; (3) The procedure should be performed with peri-procedural antibiotics, use of CO2 insufflation to avoid rare risk of air embolism, and endotracheal intubation to avoid aspiration risk; (4) The PFC is carefully examined under EUS to determine the optimal location for cyst puncture and to verify that the PFC is adherent to the luminal wall (i.e., they move synchronously) and is located within reasonably proximity (< 10-20 mm) to the luminal wall (Figure 3A); (5) Doppler imaging is used to verify the absence of intervening vessels (such as varices from splenic vein thrombosis) or a pseudoaneurysm communicating with the PFC; (6) EUS-FNA is performed using a 19 G needle. Fluid is aspirated to give a gross sense of the nature of the PFC (i.e., does it appear as clear liquid consistent with pseudocyst, thick, murky fluid consistent with WON, or purulent fluid suggestive of infection?) and is sent off for cyst fluid analysis to confirm that the PFC is truly secondary to pancreatitis and not a primary cyst neoplasm (Figure 3B); (7) Contrast may be injected via the FNA needle to delineate the dimensions of the PFC. A soft tip guide wire is then inserted under fluoroscopic guidance and should be seen to coil within the PFC, demonstrating adequate guide wire advancement; (8) The fistula tract is then enlarged by advancement of a balloon dilator over the wire and into the PFC. Occasionally it is difficult to advance the balloon catheter through the gastric or duodenal wall, in which case the needle tract needs to first be enlarged by passing a Soehendra stent retriever or by using electrocautery with a needle-knife device or cystotome over the wire. Once this is done, the balloon dilator will pass easily over the wire into the cyst; (9) Balloon dilation and placement of stents: For pseudocysts: balloon dilation to 10 mm, followed by placement of 2 plastic pigtail stents (usually 7 Fr or 8.5 Fr) over a wire across the dilated fistula tract. For WON: progressive balloon dilation to 18-20 mm, followed by placement of 2 plastic pigtail stents (7 or 8.5 Fr and 10 Fr) or a fully-covered, self-expanding metal stent, and then placement of a naso-cystic tube for intermittent flushing of the WON collection. For WON, direct endoscopic necrosectomy is performed by inserting a gastroscope into the collection via the dilated cyst-gastrostomy or cyst-duodenostomy tract, followed by careful debridement of the necrotic contents (Figure 4); and (10) The ideal duration of transluminal stent placement remains unclear and is the source of ongoing debate. At a minimum, the stents should be left in place until repeat imaging demonstrates complete resolution of the PFC, although in some cases the stents should be left for much longer[111].
Recent innovations continue to modify and improve this basic technique and should result in a more effective and perhaps technically simpler procedure, particularly as dedicated devices for EUS-guided PFC drainage emerge. While these developments are likely unnecessary for pseudocyst drainage, they will probably improve the management of WON. One such innovation is the multiple transluminal gateway technique proposed by Varadarajulu et al[112] whereby 2 or more separate fistulous tracts are made across the gastric wall into one WON collection under EUS guidance, with placement of multiple plastic pigtail stents in each. A nasocystic tube is then used to flush normal saline through one of the fistula tracts, with the expectation that the additional transluminal tracts will enable greater drainage of necrotic debris. In the initial retrospective study, patients with symptomatic WON who underwent the multiple transluminal gateway technique were significantly more likely to have treatment success compared to those who received the conventional single-port drainage technique (92% vs 52%, P = 0.01) and much less likely to ultimately require surgery (0% vs 35%)[112]. However, prospective comparative studies are needed to verify these findings. Another recent innovation is the development of the first fully covered, metal stents specifically designed for EUS-guided drainage of PFC. These include the Nagi stent (Taewoong Medical Co.; Gyeonggi-do, South Korea) and the AXIOS stent (Xlumina Inc., Mountain View, CA). Both stents have a similar design that exhibit flared ends that securely anchor the walls of the cyst and lumen while preventing migration. While the Nagi stent requires balloon dilation of the fistula tract prior to its deployment[113], the AXIOS stent may be deployed as a single-step device over a guide wire, which can be done entirely under EUS and endoscopic guidance without fluoroscopy[114]. Balloon dilation can then be performed inside of the stent to increase the diameter to enable endoscopic necrosectomy for WON if necessary. However, these systems have not yet been studied in prospective clinical trials to determine their merits and to define when they should be used in the treatment of pseudocysts and WON.
Where data do exist is the increasing demonstration of the superior efficacy and safety of endoscopic methods for the treatment of PFC, as well as the benefits of an overall more conservative, step-up approach. A recent randomized, controlled trial of EUS-guided vs surgical cystgastrostomy for pseudocyst drainage demonstrated no differences in treatment success, complications, need for re-intervention or pseudocyst recurrence, but there was significantly shorter hospital length of stay (median 2 d vs 6 d, P < 0.001) and reduced costs in the endoscopic group[115]. Thus, endoscopic approaches clearly seem to be the preferable strategy for uncomplicated pseudocysts since there is no apparent advantage to surgery. Management of WON, particularly when direct endoscopic necrosectomy is required, is more difficult and poses greater risk of complications. In a prospective series from major tertiary centers in the United States, endoscopic necrosectomy was successful in 91% of patients but there was a 14% complication rate[116]. The Japanese and German experiences also demonstrate good treatment success rates (75% and 80% resolution, respectively) but a concerning 33% and 25% rate of complications, including mortality rates of 10% and 7.5%[117,118]. Such a high rate of potential complications should give caution to those who would consider taking on this procedure outside of specialized centers or without adequate training. That said, the experience of the Dutch Pancreatitis Study Group demonstrates that less invasive approaches, including endoscopy, may indeed be safer than surgery for the treatment of WON. In a prospective, randomized trial of primary open necrosectomy vs a step-up approach consisting of percutaneous drainage followed by minimally invasive retroperitoneal necrosectomy when necessary, the primary composite endpoint of major complications, multi-organ failure or death occurred in 69% of the open surgical group compared to 40% of the step-group (P = 0.006)[119]. Furthermore, more than one-third of patients in the step-up group were managed by percutaneous drainage only, suggesting that a more conservative approach is adequate for a significant proportion of patients. The same group then published a small follow-up paper in which patients with infected WON who failed to respond to percutaneous drainage were randomized to either endoscopic necrosectomy or to minimally invasive surgery using video-assisted retroperitoneal debridement[120]. In this study, the composite endpoint of multi-organ failure, intra-abdominal bleeding, fistula formation or death occurred in 20% of the endoscopic group and in 80% of the surgical group (P = 0.03), illustrating a clear advantage for the endoscopic approach. However, the number of patients included in this study was small. Additional insight will become available once this Dutch group completes their ongoing randomized trial that is comparing endoscopic necrosectomy to a conservative, step-up approach. Furthermore, with the development of dedicated metal stents for PFC drainage and innovations such as the multiple transluminal gateway technique, it is possible that the endoscopic approach may become safer by reducing the need for direct endoscopic debridement within the necrotic collection while still achieving successful outcomes. This too remains speculative and will require significant ongoing study.
In summary, EUS has a clearly established role in the diagnosis of pancreatic diseases, particularly in the evaluation of acute and chronic pancreatitis, and in the diagnosis and management of cystic and solid pancreatic lesions. Furthermore, established therapeutic applications of EUS include CPB/CPN and management of PFC. In the future, the diagnostic capabilities of EUS will continue to expand while its therapeutic potential should blossom as it increasingly becomes an interventional procedure for local tumor treatments, cyst ablation, as well as access and drainage of the PD and biliary system. The future of pancreatic EUS is exciting indeed.
We would like to thank Dr. Tara Lynn Stewart for her assistance with the figures in this manuscript.
P- Reviewer: Ciccone MM, Gow KW S- Editor: Gou SX L- Editor: A E- Editor: Ma S
| 1. | Wu BU, Banks PA. Clinical management of patients with acute pancreatitis. Gastroenterology. 2013;144:1272-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 125] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 2. | Bollen TL. Imaging of acute pancreatitis: update of the revised Atlanta classification. Radiol Clin North Am. 2012;50:429-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 3. | Tse F, Liu L, Barkun AN, Armstrong D, Moayyedi P. EUS: a meta-analysis of test performance in suspected choledocholithiasis. Gastrointest Endosc. 2008;67:235-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 129] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 4. | Maluf-Filho F, Dotti CM, Halwan B, Queiros AF, Kupski C, Chaves DM, Nakao FS, Kumar A. An evidence-based consensus statement on the role and application of endosonography in clinical practice. Endoscopy. 2009;41:979-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Ortega AR, Gómez-Rodríguez R, Romero M, Fernández-Zapardiel S, Céspedes Mdel M, Carrobles JM. Prospective comparison of endoscopic ultrasonography and magnetic resonance cholangiopancreatography in the etiological diagnosis of “idiopathic” acute pancreatitis. Pancreas. 2011;40:289-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Zhan X, Guo X, Chen Y, Dong Y, Yu Q, Wang K, Li Z. EUS in exploring the etiology of mild acute biliary pancreatitis with a negative finding of biliary origin by conventional radiological methods. J Gastroenterol Hepatol. 2011;26:1500-1503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Napoléon B, Dumortier J, Keriven-Souquet O, Pujol B, Ponchon T, Souquet JC. Do normal findings at biliary endoscopic ultrasonography obviate the need for endoscopic retrograde cholangiography in patients with suspicion of common bile duct stone? A prospective follow-up study of 238 patients. Endoscopy. 2003;35:411-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Petrov MS, Savides TJ. Systematic review of endoscopic ultrasonography versus endoscopic retrograde cholangiopancreatography for suspected choledocholithiasis. Br J Surg. 2009;96:967-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 9. | De Lisi S, Leandro G, Buscarini E. Endoscopic ultrasonography versus endoscopic retrograde cholangiopancreatography in acute biliary pancreatitis: a systematic review. Eur J Gastroenterol Hepatol. 2011;23:367-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Mariani A, Arcidiacono PG, Curioni S, Giussani A, Testoni PA. Diagnostic yield of ERCP and secretin-enhanced MRCP and EUS in patients with acute recurrent pancreatitis of unknown aetiology. Dig Liver Dis. 2009;41:753-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Mosler P, Akisik F, Sandrasegaran K, Fogel E, Watkins J, Alazmi W, Sherman S, Lehman G, Imperiale T, McHenry L. Accuracy of magnetic resonance cholangiopancreatography in the diagnosis of pancreas divisum. Dig Dis Sci. 2012;57:170-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Kushnir VM, Wani SB, Fowler K, Menias C, Varma R, Narra V, Hovis C, Murad FM, Mullady DK, Jonnalagadda SS. Sensitivity of endoscopic ultrasound, multidetector computed tomography, and magnetic resonance cholangiopancreatography in the diagnosis of pancreas divisum: a tertiary center experience. Pancreas. 2013;42:436-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Lai R, Freeman ML, Cass OW, Mallery S. Accurate diagnosis of pancreas divisum by linear-array endoscopic ultrasonography. Endoscopy. 2004;36:705-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Iwashita T, Yasuda I, Doi S, Ando N, Nakashima M, Adachi S, Hirose Y, Mukai T, Iwata K, Tomita E. Use of samples from endoscopic ultrasound-guided 19-gauge fine-needle aspiration in diagnosis of autoimmune pancreatitis. Clin Gastroenterol Hepatol. 2012;10:316-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 15. | Kanno A, Ishida K, Hamada S, Fujishima F, Unno J, Kume K, Kikuta K, Hirota M, Masamune A, Satoh K. Diagnosis of autoimmune pancreatitis by EUS-FNA by using a 22-gauge needle based on the International Consensus Diagnostic Criteria. Gastrointest Endosc. 2012;76:594-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 16. | Stevens T, Parsi MA. Endoscopic ultrasound for the diagnosis of chronic pancreatitis. World J Gastroenterol. 2010;16:2841-2850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (2)] |
| 17. | Conwell DL, Zuccaro G, Purich E, Fein S, Vargo JJ, Dumot JA, VanLente F, Lopez R, Trolli P. Comparison of endoscopic ultrasound chronic pancreatitis criteria to the endoscopic secretin-stimulated pancreatic function test. Dig Dis Sci. 2007;52:1206-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Stevens T, Dumot JA, Zuccaro G, Vargo JJ, Parsi MA, Lopez R, Kirchner HL, Purich E, Conwell DL. Evaluation of duct-cell and acinar-cell function and endosonographic abnormalities in patients with suspected chronic pancreatitis. Clin Gastroenterol Hepatol. 2009;7:114-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Gardner TB, Levy MJ. EUS diagnosis of chronic pancreatitis. Gastrointest Endosc. 2010;71:1280-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Varadarajulu S, Eltoum I, Tamhane A, Eloubeidi MA. Histopathologic correlates of noncalcific chronic pancreatitis by EUS: a prospective tissue characterization study. Gastrointest Endosc. 2007;66:501-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 21. | Catalano MF, Sahai A, Levy M, Romagnuolo J, Wiersema M, Brugge W, Freeman M, Yamao K, Canto M, Hernandez LV. EUS-based criteria for the diagnosis of chronic pancreatitis: the Rosemont classification. Gastrointest Endosc. 2009;69:1251-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 346] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 22. | Stevens T, Lopez R, Adler DG, Al-Haddad MA, Conway J, Dewitt JM, Forsmark CE, Kahaleh M, Lee LS, Levy MJ. Multicenter comparison of the interobserver agreement of standard EUS scoring and Rosemont classification scoring for diagnosis of chronic pancreatitis. Gastrointest Endosc. 2010;71:519-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 23. | Kalmin B, Hoffman B, Hawes R, Romagnuolo J. Conventional versus Rosemont endoscopic ultrasound criteria for chronic pancreatitis: comparing interobserver reliability and intertest agreement. Can J Gastroenterol. 2011;25:261-264. [PubMed] |
| 24. | Del Pozo D, Poves E, Tabernero S, Beceiro I, Moral I, Villafruela M, Sanz C, Borrego G. Conventional versus Rosemont endoscopic ultrasound criteria for chronic pancreatitis: interobserver agreement in same day back-to-back procedures. Pancreatology. 2012;12:284-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Iglesias-Garcia J, Domínguez-Muñoz JE. Endoscopic ultrasound image enhancement elastography. Gastrointest Endosc Clin N Am. 2012;22:333-348, x-xi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Janssen J, Schlörer E, Greiner L. EUS elastography of the pancreas: feasibility and pattern description of the normal pancreas, chronic pancreatitis, and focal pancreatic lesions. Gastrointest Endosc. 2007;65:971-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 178] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 27. | Hirche TO, Ignee A, Barreiros AP, Schreiber-Dietrich D, Jungblut S, Ott M, Hirche H, Dietrich CF. Indications and limitations of endoscopic ultrasound elastography for evaluation of focal pancreatic lesions. Endoscopy. 2008;40:910-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 100] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 28. | Iglesias-Garcia J, Larino-Noia J, Abdulkader I, Forteza J, Dominguez-Munoz JE. Quantitative endoscopic ultrasound elastography: an accurate method for the differentiation of solid pancreatic masses. Gastroenterology. 2010;139:1172-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 195] [Article Influence: 13.0] [Reference Citation Analysis (1)] |
| 29. | Iglesias-Garcia J, Domínguez-Muñoz JE, Castiñeira-Alvariño M, Luaces-Regueira M, Lariño-Noia J. Quantitative elastography associated with endoscopic ultrasound for the diagnosis of chronic pancreatitis. Endoscopy. 2013;45:781-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 30. | de Jong K, Nio CY, Hermans JJ, Dijkgraaf MG, Gouma DJ, van Eijck CH, van Heel E, Klass G, Fockens P, Bruno MJ. High prevalence of pancreatic cysts detected by screening magnetic resonance imaging examinations. Clin Gastroenterol Hepatol. 2010;8:806-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 376] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 31. | Lee KS, Sekhar A, Rofsky NM, Pedrosa I. Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am J Gastroenterol. 2010;105:2079-2084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 436] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 32. | Laffan TA, Horton KM, Klein AP, Berlanstein B, Siegelman SS, Kawamoto S, Johnson PT, Fishman EK, Hruban RH. Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am J Roentgenol. 2008;191:802-807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 724] [Cited by in RCA: 658] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 33. | Yoon WJ, Brugge WR. Pancreatic cystic neoplasms: diagnosis and management. Gastroenterol Clin North Am. 2012;41:103-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 34. | Crippa S, Salvia R, Warshaw AL, Domínguez I, Bassi C, Falconi M, Thayer SP, Zamboni G, Lauwers GY, Mino-Kenudson M. Mucinous cystic neoplasm of the pancreas is not an aggressive entity: lessons from 163 resected patients. Ann Surg. 2008;247:571-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 274] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 35. | Sugiyama M, Izumisato Y, Abe N, Masaki T, Mori T, Atomi Y. Predictive factors for malignancy in intraductal papillary-mucinous tumours of the pancreas. Br J Surg. 2003;90:1244-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 308] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 36. | Salvia R, Fernández-del Castillo C, Bassi C, Thayer SP, Falconi M, Mantovani W, Pederzoli P, Warshaw AL. Main-duct intraductal papillary mucinous neoplasms of the pancreas: clinical predictors of malignancy and long-term survival following resection. Ann Surg. 2004;239:678-685; discussion 685-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 545] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 37. | Schmidt CM, White PB, Waters JA, Yiannoutsos CT, Cummings OW, Baker M, Howard TJ, Zyromski NJ, Nakeeb A, DeWitt JM. Intraductal papillary mucinous neoplasms: predictors of malignant and invasive pathology. Ann Surg. 2007;246:644-651; discussion 651-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 295] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 38. | Butte JM, Brennan MF, Gönen M, Tang LH, D’Angelica MI, Fong Y, Dematteo RP, Jarnagin WR, Allen PJ. Solid pseudopapillary tumors of the pancreas. Clinical features, surgical outcomes, and long-term survival in 45 consecutive patients from a single center. J Gastrointest Surg. 2011;15:350-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 39. | Tanno S, Nakano Y, Nishikawa T, Nakamura K, Sasajima J, Minoguchi M, Mizukami Y, Yanagawa N, Fujii T, Obara T. Natural history of branch duct intraductal papillary-mucinous neoplasms of the pancreas without mural nodules: long-term follow-up results. Gut. 2008;57:339-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 175] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 40. | Brugge WR, Lewandrowski K, Lee-Lewandrowski E, Centeno BA, Szydlo T, Regan S, del Castillo CF, Warshaw AL. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330-1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1016] [Cited by in RCA: 901] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 41. | Al-Haddad M, Schmidt MC, Sandrasegaran K, Dewitt J. Diagnosis and treatment of cystic pancreatic tumors. Clin Gastroenterol Hepatol. 2011;9:635-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 42. | de Jong K, Poley JW, van Hooft JE, Visser M, Bruno MJ, Fockens P. Endoscopic ultrasound-guided fine-needle aspiration of pancreatic cystic lesions provides inadequate material for cytology and laboratory analysis: initial results from a prospective study. Endoscopy. 2011;43:585-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 43. | Khalid A, Zahid M, Finkelstein SD, LeBlanc JK, Kaushik N, Ahmad N, Brugge WR, Edmundowicz SA, Hawes RH, McGrath KM. Pancreatic cyst fluid DNA analysis in evaluating pancreatic cysts: a report of the PANDA study. Gastrointest Endosc. 2009;69:1095-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 314] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 44. | Tanaka M, Fernández-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, Kimura W, Levy P, Pitman MB, Schmidt CM. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1714] [Cited by in RCA: 1614] [Article Influence: 124.2] [Reference Citation Analysis (0)] |
| 45. | Anand N, Sampath K, Wu BU. Cyst features and risk of malignancy in intraductal papillary mucinous neoplasms of the pancreas: a meta-analysis. Clin Gastroenterol Hepatol. 2013;11:913-921; quiz e59-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 154] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 46. | Oh HC, Brugge WR. EUS-guided pancreatic cyst ablation: a critical review (with video). Gastrointest Endosc. 2013;77:526-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 47. | Othman MO, Wallace MB. The role of endoscopic ultrasonography in the diagnosis and management of pancreatic cancer. Gastroenterol Clin North Am. 2012;41:179-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 48. | Săftoiu A, Vilmann P. Role of endoscopic ultrasound in the diagnosis and staging of pancreatic cancer. J Clin Ultrasound. 2009;37:1-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 49. | Klapman JB, Chang KJ, Lee JG, Nguyen P. Negative predictive value of endoscopic ultrasound in a large series of patients with a clinical suspicion of pancreatic cancer. Am J Gastroenterol. 2005;100:2658-2661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 91] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 50. | Giovannini M, Seitz JF, Monges G, Perrier H, Rabbia I. Fine-needle aspiration cytology guided by endoscopic ultrasonography: results in 141 patients. Endoscopy. 1995;27:171-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 264] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 51. | Wiersema MJ, Vilmann P, Giovannini M, Chang KJ, Wiersema LM. Endosonography-guided fine-needle aspiration biopsy: diagnostic accuracy and complication assessment. Gastroenterology. 1997;112:1087-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 874] [Cited by in RCA: 736] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 52. | Bhutani MS, Hawes RH, Baron PL, Sanders-Cliette A, van Velse A, Osborne JF, Hoffman BJ. Endoscopic ultrasound guided fine needle aspiration of malignant pancreatic lesions. Endoscopy. 1997;29:854-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 170] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 53. | Chang KJ, Nguyen P, Erickson RA, Durbin TE, Katz KD. The clinical utility of endoscopic ultrasound-guided fine-needle aspiration in the diagnosis and staging of pancreatic carcinoma. Gastrointest Endosc. 1997;45:387-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 402] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 54. | Voss M, Hammel P, Molas G, Palazzo L, Dancour A, O’Toole D, Terris B, Degott C, Bernades P, Ruszniewski P. Value of endoscopic ultrasound guided fine needle aspiration biopsy in the diagnosis of solid pancreatic masses. Gut. 2000;46:244-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 248] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 55. | Eloubeidi MA, Jhala D, Chhieng DC, Chen VK, Eltoum I, Vickers S, Mel Wilcox C, Jhala N. Yield of endoscopic ultrasound-guided fine-needle aspiration biopsy in patients with suspected pancreatic carcinoma. Cancer. 2003;99:285-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 249] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 56. | Early DS, Acosta RD, Chandrasekhara V, Chathadi KV, Decker GA, Evans JA, Fanelli RD, Fisher DA, Fonkalsrud L, Hwang JH. Adverse events associated with EUS and EUS with FNA. Gastrointest Endosc. 2013;77:839-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 149] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 57. | Fritscher-Ravens A, Brand L, Knöfel WT, Bobrowski C, Topalidis T, Thonke F, de Werth A, Soehendra N. Comparison of endoscopic ultrasound-guided fine needle aspiration for focal pancreatic lesions in patients with normal parenchyma and chronic pancreatitis. Am J Gastroenterol. 2002;97:2768-2775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 168] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 58. | Varadarajulu S, Tamhane A, Eloubeidi MA. Yield of EUS-guided FNA of pancreatic masses in the presence or the absence of chronic pancreatitis. Gastrointest Endosc. 2005;62:728-736; quiz 751, 753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 260] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 59. | Madhoun MF, Wani SB, Rastogi A, Early D, Gaddam S, Tierney WM, Maple JT. The diagnostic accuracy of 22-gauge and 25-gauge needles in endoscopic ultrasound-guided fine needle aspiration of solid pancreatic lesions: a meta-analysis. Endoscopy. 2013;45:86-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 157] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 60. | Bang JY, Ramesh J, Trevino J, Eloubeidi MA, Varadarajulu S. Objective assessment of an algorithmic approach to EUS-guided FNA and interventions. Gastrointest Endosc. 2013;77:739-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 61. | Varadarajulu S, Fockens P, Hawes RH. Best practices in endoscopic ultrasound-guided fine-needle aspiration. Clin Gastroenterol Hepatol. 2012;10:697-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 62. | Lee JK, Choi JH, Lee KH, Kim KM, Shin JU, Lee JK, Lee KT, Jang KT. A prospective, comparative trial to optimize sampling techniques in EUS-guided FNA of solid pancreatic masses. Gastrointest Endosc. 2013;77:745-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 63. | Iwashita T, Nakai Y, Samarasena JB, Park DH, Lee JG, Chang KJ. Endoscopic ultrasound-guided fine needle aspiration and biopsy (EUS-FNAB) using a novel 25-gauge core biopsy needle: optimizing the yield of both cytology and histology. Gastrointest Endosc. 2012;75:AB183. [DOI] [Full Text] |
| 64. | Bang JY, Magee SH, Ramesh J, Trevino JM, Varadarajulu S. Randomized trial comparing fanning with standard technique for endoscopic ultrasound-guided fine-needle aspiration of solid pancreatic mass lesions. Endoscopy. 2013;45:445-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 192] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 65. | Klapman JB, Logrono R, Dye CE, Waxman I. Clinical impact of on-site cytopathology interpretation on endoscopic ultrasound-guided fine needle aspiration. Am J Gastroenterol. 2003;98:1289-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 362] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 66. | Alsohaibani F, Girgis S, Sandha GS. Does onsite cytotechnology evaluation improve the accuracy of endoscopic ultrasound-guided fine-needle aspiration biopsy? Can J Gastroenterol. 2009;23:26-30. [PubMed] |
| 67. | Iglesias-Garcia J, Dominguez-Munoz JE, Abdulkader I, Larino-Noia J, Eugenyeva E, Lozano-Leon A, Forteza-Vila J. Influence of on-site cytopathology evaluation on the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) of solid pancreatic masses. Am J Gastroenterol. 2011;106:1705-1710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 270] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 68. | LeBlanc JK, Ciaccia D, Al-Assi MT, McGrath K, Imperiale T, Tao LC, Vallery S, DeWitt J, Sherman S, Collins E. Optimal number of EUS-guided fine needle passes needed to obtain a correct diagnosis. Gastrointest Endosc. 2004;59:475-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 182] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 69. | Nguyen YP, Maple JT, Zhang Q, Ylagan LR, Zhai J, Kohlmeier C, Jonnalagadda S, Early DS, Edmundowicz SA, Azar RR. Reliability of gross visual assessment of specimen adequacy during EUS-guided FNA of pancreatic masses. Gastrointest Endosc. 2009;69:1264-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 70. | Iglesias-Garcia J, Poley JW, Larghi A, Giovannini M, Petrone MC, Abdulkader I, Monges G, Costamagna G, Arcidiacono P, Biermann K. Feasibility and yield of a new EUS histology needle: results from a multicenter, pooled, cohort study. Gastrointest Endosc. 2011;73:1189-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 232] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 71. | Hucl T, Wee E, Anuradha S, Gupta R, Ramchandani M, Rakesh K, Shrestha R, Reddy DN, Lakhtakia S. Feasibility and efficiency of a new 22G core needle: a prospective comparison study. Endoscopy. 2013;45:792-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 72. | Iwashita T, Nakai Y, Samarasena JB, Park do H, Zhang Z, Gu M, Lee JG, Chang KJ. High single-pass diagnostic yield of a new 25-gauge core biopsy needle for EUS-guided FNA biopsy in solid pancreatic lesions. Gastrointest Endosc. 2013;77:909-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 125] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 73. | Larghi A, Verna EC, Ricci R, Seerden TC, Galasso D, Carnuccio A, Uchida N, Rindi G, Costamagna G. EUS-guided fine-needle tissue acquisition by using a 19-gauge needle in a selected patient population: a prospective study. Gastrointest Endosc. 2011;74:504-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 74. | Giovannini M, Thomas B, Erwan B, Christian P, Fabrice C, Benjamin E, Geneviève M, Paolo A, Pierre D, Robert Y. Endoscopic ultrasound elastography for evaluation of lymph nodes and pancreatic masses: a multicenter study. World J Gastroenterol. 2009;15:1587-1593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 204] [Cited by in RCA: 201] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 75. | Iglesias-Garcia J, Larino-Noia J, Abdulkader I, Forteza J, Dominguez-Munoz JE. EUS elastography for the characterization of solid pancreatic masses. Gastrointest Endosc. 2009;70:1101-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 124] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 76. | Itokawa F, Itoi T, Sofuni A, Kurihara T, Tsuchiya T, Ishii K, Tsuji S, Ikeuchi N, Umeda J, Tanaka R. EUS elastography combined with the strain ratio of tissue elasticity for diagnosis of solid pancreatic masses. J Gastroenterol. 2011;46:843-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 77. | Săftoiu A, Vilmann P, Gorunescu F, Janssen J, Hocke M, Larsen M, Iglesias-Garcia J, Arcidiacono P, Will U, Giovannini M. Accuracy of endoscopic ultrasound elastography used for differential diagnosis of focal pancreatic masses: a multicenter study. Endoscopy. 2011;43:596-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 78. | Săftoiu A, Vilmann P, Gorunescu F, Janssen J, Hocke M, Larsen M, Iglesias-Garcia J, Arcidiacono P, Will U, Giovannini M. Efficacy of an artificial neural network-based approach to endoscopic ultrasound elastography in diagnosis of focal pancreatic masses. Clin Gastroenterol Hepatol. 2012;10:84-90.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 129] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 79. | Mei M, Ni J, Liu D, Jin P, Sun L. EUS elastography for diagnosis of solid pancreatic masses: a meta-analysis. Gastrointest Endosc. 2013;77:578-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 80. | Morin SH, Lim AK, Cobbold JF, Taylor-Robinson SD. Use of second generation contrast-enhanced ultrasound in the assessment of focal liver lesions. World J Gastroenterol. 2007;13:5963-5970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in CrossRef: 20] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 81. | Sanchez MV, Varadarajulu S, Napoleon B. EUS contrast agents: what is available, how do they work, and are they effective? Gastrointest Endosc. 2009;69:S71-S77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 82. | Dietrich CF, Ignee A, Braden B, Barreiros AP, Ott M, Hocke M. Improved differentiation of pancreatic tumors using contrast-enhanced endoscopic ultrasound. Clin Gastroenterol Hepatol. 2008;6:590-597.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 122] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 83. | Kitano M, Sakamoto H, Matsui U, Ito Y, Maekawa K, von Schrenck T, Kudo M. A novel perfusion imaging technique of the pancreas: contrast-enhanced harmonic EUS (with video). Gastrointest Endosc. 2008;67:141-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 141] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 84. | Fusaroli P, Spada A, Mancino MG, Caletti G. Contrast harmonic echo-endoscopic ultrasound improves accuracy in diagnosis of solid pancreatic masses. Clin Gastroenterol Hepatol. 2010;8:629-634.e1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 153] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 85. | Napoleon B, Alvarez-Sanchez MV, Gincoul R, Pujol B, Lefort C, Lepilliez V, Labadie M, Souquet JC, Queneau PE, Scoazec JY. Contrast-enhanced harmonic endoscopic ultrasound in solid lesions of the pancreas: results of a pilot study. Endoscopy. 2010;42:564-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 128] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 86. | Kitano M, Kudo M, Yamao K, Takagi T, Sakamoto H, Komaki T, Kamata K, Imai H, Chiba Y, Okada M. Characterization of small solid tumors in the pancreas: the value of contrast-enhanced harmonic endoscopic ultrasonography. Am J Gastroenterol. 2012;107:303-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 242] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 87. | Kitano M, Sakamoto H, Kudo M. Endoscopic ultrasound: contrast enhancement. Gastrointest Endosc Clin N Am. 2012;22:349-358, xi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 88. | Gong TT, Hu DM, Zhu Q. Contrast-enhanced EUS for differential diagnosis of pancreatic mass lesions: a meta-analysis. Gastrointest Endosc. 2012;76:301-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 89. | Matsubara H, Itoh A, Kawashima H, Kasugai T, Ohno E, Ishikawa T, Itoh Y, Nakamura Y, Hiramatsu T, Nakamura M. Dynamic quantitative evaluation of contrast-enhanced endoscopic ultrasonography in the diagnosis of pancreatic diseases. Pancreas. 2011;40:1073-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 90. | Imazu H, Kanazawa K, Mori N, Ikeda K, Kakutani H, Sumiyama K, Hino S, Ang TL, Omar S, Tajiri H. Novel quantitative perfusion analysis with contrast-enhanced harmonic EUS for differentiation of autoimmune pancreatitis from pancreatic carcinoma. Scand J Gastroenterol. 2012;47:853-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 91. | Ventafridda V, Tamburini M, Caraceni A, De Conno F, Naldi F. A validation study of the WHO method for cancer pain relief. Cancer. 1987;59:850-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 92. | Ventafridda GV, Caraceni AT, Sbanotto AM, Barletta L, De Conno F. Pain treatment in cancer of the pancreas. Eur J Surg Oncol. 1990;16:1-6. [PubMed] |
| 93. | Wong GY, Schroeder DR, Carns PE, Wilson JL, Martin DP, Kinney MO, Mantilla CB, Warner DO. Effect of neurolytic celiac plexus block on pain relief, quality of life, and survival in patients with unresectable pancreatic cancer: a randomized controlled trial. JAMA. 2004;291:1092-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 289] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 94. | Yan BM, Myers RP. Neurolytic celiac plexus block for pain control in unresectable pancreatic cancer. Am J Gastroenterol. 2007;102:430-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 133] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 95. | Wiersema MJ, Wiersema LM. Endosonography-guided celiac plexus neurolysis. Gastrointest Endosc. 1996;44:656-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 270] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 96. | Puli SR, Reddy JB, Bechtold ML, Antillon MR, Brugge WR. EUS-guided celiac plexus neurolysis for pain due to chronic pancreatitis or pancreatic cancer pain: a meta-analysis and systematic review. Dig Dis Sci. 2009;54:2330-2337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 198] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 97. | Kaufman M, Singh G, Das S, Concha-Parra R, Erber J, Micames C, Gress F. Efficacy of endoscopic ultrasound-guided celiac plexus block and celiac plexus neurolysis for managing abdominal pain associated with chronic pancreatitis and pancreatic cancer. J Clin Gastroenterol. 2010;44:127-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 181] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 98. | Sahai AV, Lemelin V, Lam E, Paquin SC. Central vs. bilateral endoscopic ultrasound-guided celiac plexus block or neurolysis: a comparative study of short-term effectiveness. Am J Gastroenterol. 2009;104:326-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 99. | Levy M, Rajan E, Keeney G, Fletcher JG, Topazian M. Neural ganglia visualized by endoscopic ultrasound. Am J Gastroenterol. 2006;101:1787-1791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 100. | Doi S, Yasuda I, Kawakami H, Hayashi T, Hisai H, Irisawa A, Mukai T, Katanuma A, Kubota K, Ohnishi T. Endoscopic ultrasound-guided celiac ganglia neurolysis vs. celiac plexus neurolysis: a randomized multicenter trial. Endoscopy. 2013;45:362-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 101. | Wyse JM, Carone M, Paquin SC, Usatii M, Sahai AV. Randomized, double-blind, controlled trial of early endoscopic ultrasound-guided celiac plexus neurolysis to prevent pain progression in patients with newly diagnosed, painful, inoperable pancreatic cancer. J Clin Oncol. 2011;29:3541-3546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 165] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 102. | Gardner TB. Endoscopic management of necrotizing pancreatitis. Gastrointest Endosc. 2012;76:1214-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 103. | Freeman ML, Werner J, van Santvoort HC, Baron TH, Besselink MG, Windsor JA, Horvath KD, vanSonnenberg E, Bollen TL, Vege SS. Interventions for necrotizing pancreatitis: summary of a multidisciplinary consensus conference. Pancreas. 2012;41:1176-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 264] [Article Influence: 20.3] [Reference Citation Analysis (1)] |
| 104. | Varadarajulu S, Bang JY, Phadnis MA, Christein JD, Wilcox CM. Endoscopic transmural drainage of peripancreatic fluid collections: outcomes and predictors of treatment success in 211 consecutive patients. J Gastrointest Surg. 2011;15:2080-2088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 184] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 105. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4932] [Cited by in RCA: 4342] [Article Influence: 361.8] [Reference Citation Analysis (45)] |
| 106. | van Brunschot S, Bakker OJ, Besselink MG, Bollen TL, Fockens P, Gooszen HG, van Santvoort HC. Treatment of necrotizing pancreatitis. Clin Gastroenterol Hepatol. 2012;10:1190-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 107. | Binmoeller KF, Seifert H, Soehendra N. Endoscopic pseudocyst drainage: a new instrument for simplified cystoenterostomy. Gastrointest Endosc. 1994;40:112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 108. | Varadarajulu S, Christein JD, Tamhane A, Drelichman ER, Wilcox CM. Prospective randomized trial comparing EUS and EGD for transmural drainage of pancreatic pseudocysts (with videos). Gastrointest Endosc. 2008;68:1102-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 278] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 109. | Park DH, Lee SS, Moon SH, Choi SY, Jung SW, Seo DW, Lee SK, Kim MH. Endoscopic ultrasound-guided versus conventional transmural drainage for pancreatic pseudocysts: a prospective randomized trial. Endoscopy. 2009;41:842-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 217] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 110. | Fisher JM, Gardner TB. Endoscopic therapy of necrotizing pancreatitis and pseudocysts. Gastrointest Endosc Clin N Am. 2013;23:787-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 111. | Varadarajulu S, Wilcox CM. Endoscopic placement of permanent indwelling transmural stents in disconnected pancreatic duct syndrome: does benefit outweigh the risks? Gastrointest Endosc. 2011;74:1408-1412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 112. | Varadarajulu S, Phadnis MA, Christein JD, Wilcox CM. Multiple transluminal gateway technique for EUS-guided drainage of symptomatic walled-off pancreatic necrosis. Gastrointest Endosc. 2011;74:74-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 190] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 113. | Yamamoto N, Isayama H, Kawakami H, Sasahira N, Hamada T, Ito Y, Takahara N, Uchino R, Miyabayashi K, Mizuno S. Preliminary report on a new, fully covered, metal stent designed for the treatment of pancreatic fluid collections. Gastrointest Endosc. 2013;77:809-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 114. | Itoi T, Binmoeller KF, Shah J, Sofuni A, Itokawa F, Kurihara T, Tsuchiya T, Ishii K, Tsuji S, Ikeuchi N. Clinical evaluation of a novel lumen-apposing metal stent for endosonography-guided pancreatic pseudocyst and gallbladder drainage (with videos). Gastrointest Endosc. 2012;75:870-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 311] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 115. | Varadarajulu S, Bang JY, Sutton BS, Trevino JM, Christein JD, Wilcox CM. Equal efficacy of endoscopic and surgical cystogastrostomy for pancreatic pseudocyst drainage in a randomized trial. Gastroenterology. 2013;145:583-590.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 332] [Article Influence: 27.7] [Reference Citation Analysis (1)] |
| 116. | Gardner TB, Coelho-Prabhu N, Gordon SR, Gelrud A, Maple JT, Papachristou GI, Freeman ML, Topazian MD, Attam R, Mackenzie TA. Direct endoscopic necrosectomy for the treatment of walled-off pancreatic necrosis: results from a multicenter U.S. series. Gastrointest Endosc. 2011;73:718-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 207] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 117. | Yasuda I, Nakashima M, Iwai T, Isayama H, Itoi T, Hisai H, Inoue H, Kato H, Kanno A, Kubota K. Japanese multicenter experience of endoscopic necrosectomy for infected walled-off pancreatic necrosis: The JENIPaN study. Endoscopy. 2013;45:627-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 118. | Seifert H, Biermer M, Schmitt W, Jürgensen C, Will U, Gerlach R, Kreitmair C, Meining A, Wehrmann T, Rösch T. Transluminal endoscopic necrosectomy after acute pancreatitis: a multicentre study with long-term follow-up (the GEPARD Study). Gut. 2009;58:1260-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 305] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 119. | van Santvoort HC, Besselink MG, Bakker OJ, Hofker HS, Boermeester MA, Dejong CH, van Goor H, Schaapherder AF, van Eijck CH, Bollen TL. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med. 2010;362:1491-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1038] [Cited by in RCA: 1037] [Article Influence: 69.1] [Reference Citation Analysis (0)] |
| 120. | Bakker OJ, van Santvoort HC, van Brunschot S, Geskus RB, Besselink MG, Bollen TL, van Eijck CH, Fockens P, Hazebroek EJ, Nijmeijer RM. Endoscopic transgastric vs surgical necrosectomy for infected necrotizing pancreatitis: a randomized trial. JAMA. 2012;307:1053-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 498] [Article Influence: 38.3] [Reference Citation Analysis (0)] |