INTRODUCTION
Neuroendocrine tumors (NETs) are a heterogeneous group of neoplasms, most often derived from the GastroEnteroPancreatic system or the bronchopulmonary system[1]. NETs are still considered rare, but in the last few decades, increased incidence has been observed, due in part to advances in detection through improved imaging, endoscopy and histopathological standards. NETs are most often sporadic, but there are also some familial syndromes with a high penetrance of NETs[2], such as multiple endocrine neoplasia type 1[3,4]. Despite the introduction of novel therapeutic strategies for inoperable metastasized NETs[5-8], the only curative therapeutic option remains complete surgical resection of the primary tumor. Despite complete (R0) surgery, however, many patients experience recurrence of the disease. For example, following a complete resection of pancreatic NETs, the recurrence rate was 23% within a median follow-up time of 31.5 mo[9]. Surgery to remove the liver metastases of pancreatic NETs has been reported to result in 5-year disease-free survivals of only 6%-30%[10,11]. Despite these high tumor recurrences in NETs, no adjuvant treatment is currently available for patients with R0 tumor status after surgery[12].
Aspirin (acetylsalicylic acid) is a more than 100-year-old drug widely used due to its antipyretic, analgesic, anti-inflammatory and anti-platelet properties[13]. Recently, several epidemiological studies have demonstrated the risk reduction of various malignancies by aspirin. The chemopreventive mechanisms and the antitumoral effects of aspirin in cancer are not fully understood[14]. In addition to chemopreventive mechanisms mediated by cyclooxygenase (COX) inhibition[15], aspirin has also recently been reported to inhibit the mammalian target of rapamycin (mTOR) signaling in colorectal cancer (CRC) cells[16]. A large case-control study demonstrated that a low dose of aspirin (75 mg) has a protective effect against the development of CRC[17]. A recently published systematic review[18] demonstrated a good agreement between case-control studies and randomized controlled trials (RCTs) and concluded that the regular use of aspirin reduces both the risk of CRC and the 20-year risk of death due to CRC. A pooled analysis of 51 RCTs examining the short term effect of daily aspirin on cancer demonstrated that treatment with aspirin reduced cancer deaths, particularly after 5 years[19]. A meta-analysis conducted on 15 case-control studies and 15 cohort studies concluded that there was evidence of a 27% CRC risk reduction under aspirin[20].
Current treatment options for metastasized NETs also involve targeting of the phosphatidylinositol-3-kinase (PI3K)-AKT-mTOR signaling cascade. Pancreatic NETs may harbor mutations in the mTOR signaling cascade[21], and NETs with activated pAKT have been suggested to demonstrate increased antitumoral sensitivity to mTOR inhibition[22]. Preclinical data have shown the antitumoral efficacy of mTOR inhibition in various neuroendocrine tumor cell models[23,24]. The mTOR inhibitor everolimus has been shown to improve progression free survival in two large clinical phase 3 studies in pancreatic NETs[25] and extrapancreatic NETs[26], respectively.
Here, we report the anti-proliferative effects of aspirin on pancreatic, midgut and bronchopulmonary neuroendocrine tumor cells and characterize the intracellular signaling pathways that are involved in aspirin-mediated neuroendocrine cell regulation.
MATERIALS AND METHODS
Materials
Dulbecco’s Modified Eagle Medium (DMEM)/F12 media, penicillin and streptomycin were purchased from Gibco by Life Technologies (Paisley, United Kingdom). Roswell Park Memorial Institute (RPMI) media, phosphate-buffered saline (PBS) and trypsin-ethylenediaminetetraacetic acid (EDTA) were obtained from PAA (Pasching, Austria). Fetal bovine serum (FBS) and amphotericin B were acquired from Biochrom (Berlin, Germany). Aspirin (acetylsalicylic acid) was provided by Sigma-Aldrich (St. Louis, United States).
Cell culture
Human pancreatic neuroendocrine BON1 tumor cells[27] were originally provided by Göke R (Marburg, Germany) and cultured in DMEM/F12 (1:1) medium as described[23]. Human bronchopulmonary neuroendocrine NCI-H727 tumor cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA, United States) and human midgut carcinoid GOT1 cells[28] were kindly provided by Ola Nilsson (Göteborg, Sweden). NCI-H727 and GOT1 cells were cultured in RPMI medium as described[23]. All media were supplemented with 10% FBS, 1% penicillin/streptomycin, and 0.4% amphotericin B. GOT1 culture medium was additionally supplemented with 0.135 IU/mL insulin and 5 μg/mL apo-transferrin.
Assessment of cell viability
BON1 and NCI-H727 cells were seeded into 96-well plates at a density of 750 cells/well and grown for 24 h. Due to their slower growth rate, GOT1 cells were seeded at a density of 50000 cells/well. The next day, the cells were incubated with various concentrations of aspirin in medium containing 10% FBS. The metabolic activity of the cells was measured using the Cell Titer 96 Aqueous One Solution Cell Proliferation Assay (Promega) after 72, 144 and 216 h of incubation according to the manufacturer’s instructions. After 3 h of incubation with the Cell Titer 96 solution, the absorbance at 492 nm was determined using an enzyme-linked immunosorbent assay (elisa) plate reader.
SYBR-DNA-labeling assay
A synergy brands (SYBR)-DNA-labeling experiment was performed exactly as described for the Cell Titer 96 aqueous One Solution Cell Proliferation Assay. The assays were stopped after the indicated time intervals. The cells were washed with PBS and incubated for 40 min with distilled water. The cells were then stained with 100 μL/well of a 1:500 dilution of SYBR® Green I (Lonza, Rockland, United States) in distilled water for 5 min and then quantified by cytofluorimetry at 530 nm with 485 nm excitation, measured using a CytoFluor® Multi-Well Plate Reader Series 4000 (PerSeptive Biosystems, Framingham, MA, United States).
Cell cycle analysis
Propidium iodide (PI) staining and flow cytometry were used to measure cell cycle distribution and apoptosis in the cells. The cells were cultured in 6-well plates (3 × 105 BON1 cells per well and 4 × 105 NCI-H727 cells per well), washed with PBS and treated with 400 mL trypsin at 37 °C for 4 min. The cells were then collected and centrifuged at 2000 g for 5 min. The pellets were washed with PBS and then centrifuged again. The cells were resuspended in 350 μL PI before being analyzed by flow cytometry (BD Accuri C6 Flow Cytometer). Nuclei that fell to the left of the G0/1-peak containing hypodiploid DNA were considered to be apoptotic.
Protein extraction and Western blotting
For Western blot experiments, 3 × 105 cells (BON1) or 4 × 105 cells (NCI-H727) or 1 × 106 cells (GOT1) were seeded in 6-well plates and grown for 24 h in 1 mL of complete medium. Afterwards, the growth medium was replaced by fresh serum-free medium, and the cells were incubated with aspirin at different concentrations (1 and 5 mM) for the indicated times. The cells were lysed in 200 μL of lysis buffer (M-PER® Mammalian Protein Extraction Reagent added with Halt TM Protease Phosphatase Inhibitor Cocktail, EDTA free, Thermo Scientific, Rockford, United States). The lysates were centrifuged at 13000 rpm for 10 min, and the supernatants were adjusted to the same protein concentration (20-50 μg/50 μL) (Rotiquant Universal, Carl Roth, Karlsruhe, Germany). Sodium dodecyl sulfate (SDS) sample buffer (0.25 mol/L Tris HCl, 40% glycerol, 2% SDS, 1% dithiothreitol, and bromophenol blue, pH 8.8) was added, and the samples were boiled for 5 min and separated on a SDS polyacrylamide gel. The proteins were electrotransferred for 60 min onto polyvinylidene difluoride (PVDF) membranes (Immobilone; Millipore, Eschborn, Germany) using a semi-dry Western-blot transfer technique. After blocking in 2% non-fat dried milk, the membranes were incubated overnight in appropriate dilutions of antibodies against phosphorylated protein kinase B pAKT (Ser 473) (#4060) and AKT (#2920), phosphorylated extracellular signal-regulated kinases (pERK) (Thr202/Tyr204) 1/2 (#4370), phosphorylated serine/threonine kinase P70S6K (pP70S6K) (Thr389) (#9234), P70S6K (#9202), phosphorylated 4E binding protein (p4EBP1) (Ser65) (#9451), 4EBP1 (#9644), phosphorylated S6 ribosomal protein (pS6) (Ser235/6 (#4858) and Ser240/4 (#5364)), S6 (#2317), phosphorylated glycogen synthase kinase 3 (pGSK3) (Ser21/9) (#9331), GSK3 (#9315), phosphorylated-CAMP response element-binding protein (pCREB) (Ser133) (#9198), CREB (#9197), pmTOR (Ser2448) (#2971), mTOR (#2972), Cyclin Dependent Kinase 4 CDK4 (#2906), Cyclin D3 (#2936), phosphorylated epidermal growth factor receptor (pEGFR) (Tyr1068) (#3777, EGFR (#4267), phosphorylated human proto-oncogene c-MET (pMET)(Tyr1234/1235) (#3077), MET (#3127), phosphorylated tuberous sclerosis 2 (pTSC2) (Thr1462) (#3617), TSC2 (#4308) (all of the preceding were obtained from Cell Signaling, Danvers, MA), ERK 1/2 (06-182; Millipore), or p21cip (610233, BD Transduction Laboratories, Franklin Lakes, NJ, United States). The membranes were washed with PBS and incubated with a peroxidase-conjugated secondary antibody (1:25000) for 2 h. The blots were washed and immersed in the SuperSignal West Dura chemiluminescent substrate (Thermo Scientific, Rockford, United States) and exposed to Super RX X-ray film (FUJIFILM Corporation, Tokyo, Japan).
Statistical analysis
For the proliferation assays and cell cycle analyses, the comparisons were evaluated using 2-tailed Student’s t-tests. The results are expressed as the mean ± SD of 3 or 4 independently performed experiments. Statistical significance was set at P < 0.05.
RESULTS
Aspirin inhibits neuroendocrine tumor cell proliferation
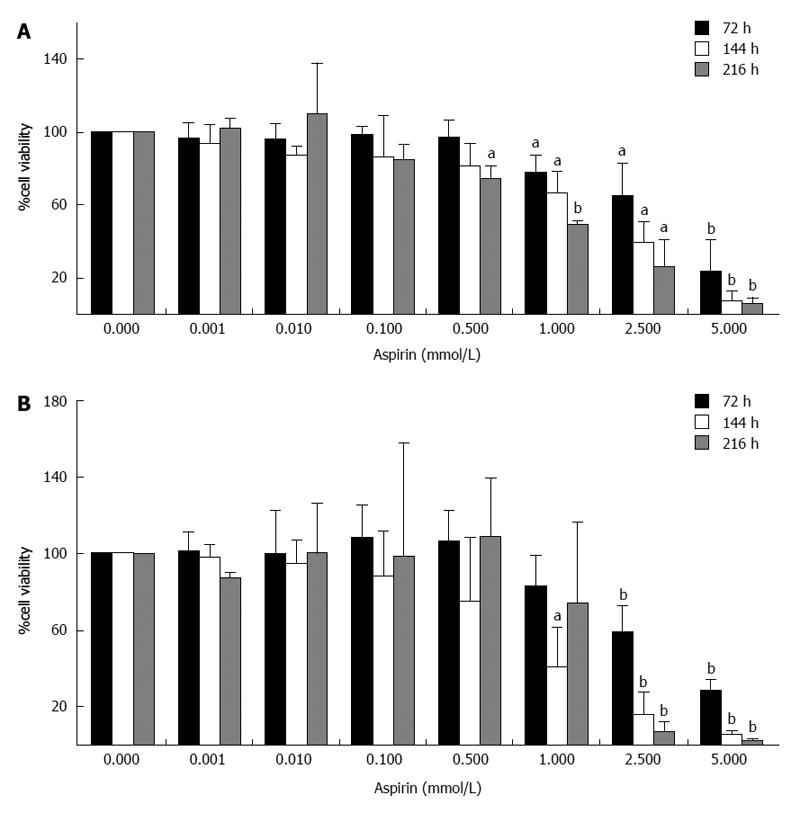
The treatment of human pancreatic neuroendocrine BON1 cells with aspirin suppressed cell viability in a time- and dose-dependent manner (Figure 1). Significant effects were observed at the starting aspirin doses of 0.5 and 1 mmol/L and peaking at the highest concentration tested (5 mmol/L). Treatment with 1 mmol/L aspirin for 72, 144 and 216 h decreased cell viability (as assessed by cell titer) to 78% ± 10% (P < 0.05), 66% ± 13% (P < 0.05) and 50% ± 2% (P < 0.001), respectively (Figure 1A). Similar results were obtained in the SYBR green experiments (Figure 1B).
Figure 1 Inhibition of neuroendocrine BON1 cell viability by aspirin.
Human pancreatic neuroendocrine BON1 cells were treated with the indicated concentrations of aspirin for 72, 144 and 216 h. The viability of the cells was measured based on both metabolic activity using the Cell Titer 96 kit (Promega) (A) and DNA labeling experiments using SYBR green (Lonza) (B). The data shown are the mean ± SD of 3 independently performed experiments. aP < 0.05, bP < 0.01 vs untreated control.
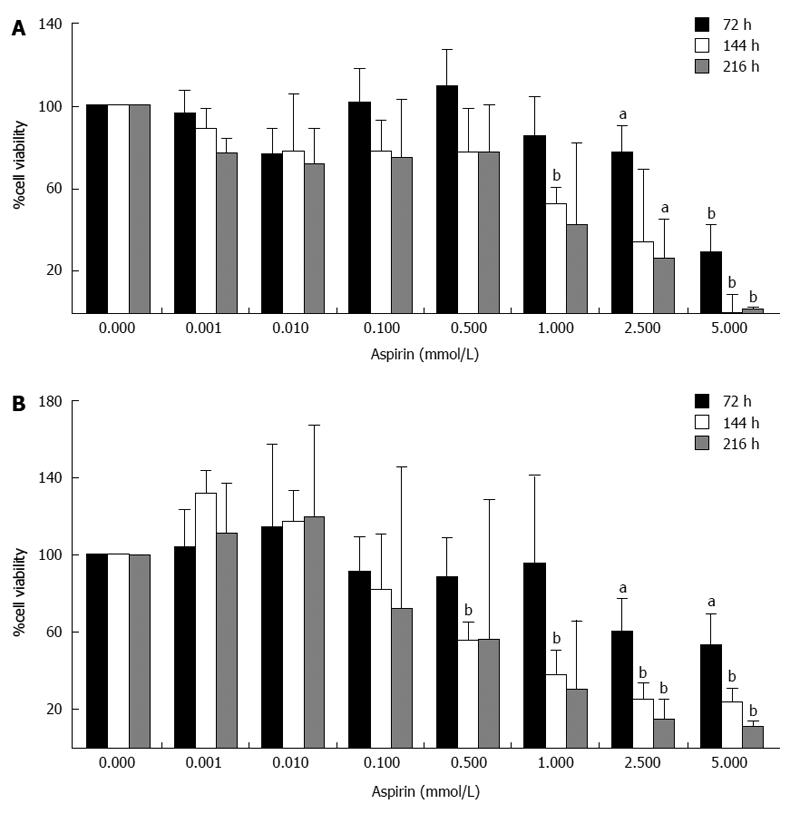
Treatment of NCI-H727 cells with the same concentrations of aspirin also caused a time- and dose-dependent decrease in cell viability (Figure 2). Significant effects were observed in this cell line at the starting aspirin doses of 0.5 and 1 mmol/L and peaking at the highest dose tested (5 mmol/L). Treatment with 1 mmol/L aspirin for 72, 144 and 216 h suppressed cell viability to 85% ± 19% (P = 0.227), 53% ± 8% (P < 0.01) and 42% ± 39% (P = 0.129), respectively (Figure 2A). Similar effects were observed in the SYBR green experiments (Figure 2B).
Figure 2 Inhibition of neuroendocrine NCI-H727 cell viability by aspirin.
Human bronchopulmonary neuroendocrine NCI-H727 cells were treated with the indicated concentrations of aspirin for 72, 144 and 216 h. The viability of the cells was measured based both metabolic activity using the Cell Titer 96 kit (Promega) (A) and DNA labeling experiments using SYBR green (Lonza) (B). The data shown are the mean ± SD of 3 independently performed experiments. aP < 0.05, bP < 0.01 vs untreated control.
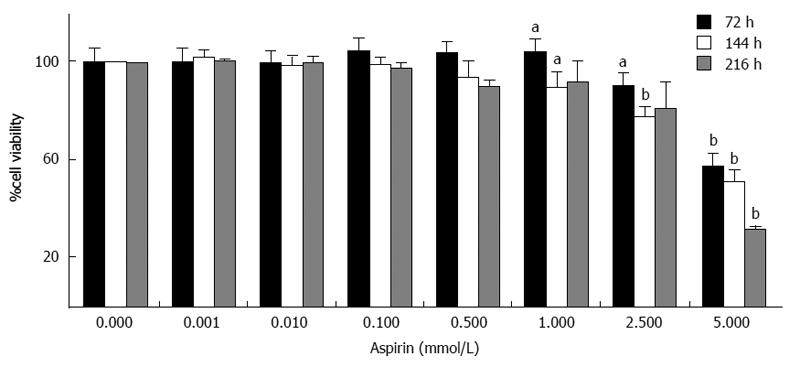
Treatment of human midgut neuroendocrine GOT1 cells with aspirin also suppressed cell viability in a dose-dependent manner (Figure 3). Significant effects were observed at the starting aspirin dose of 1 mmol/L and peaked at the highest concentration tested (5 mmol/L). Treatment with 2.5 mmol/L aspirin for 72, 144 and 216 h decreased cell viability (as assessed by cell titer) to 90% ± 4% (P < 0.05), 78% ± 4% (P < 0.01) and 81% ± 11% (NS), respectively (Figure 3).
Figure 3 Inhibition of neuroendocrine GOT1 cell viability by aspirin.
Human midgut neuroendocrine GOT1 cells were treated with the indicated concentrations of aspirin for 72, 144 and 216 h. The viability of the cells was measured based on metabolic activity using the Cell Titer 96 kit (Promega). The data shown are the mean ± SD of 4 independently performed experiments. aP < 0.05, bP < 0.01 vs untreated control.
Time- and dose-dependent effects of aspirin on PI3K/AKT/mTOR signaling in neuroendocrine tumor cells
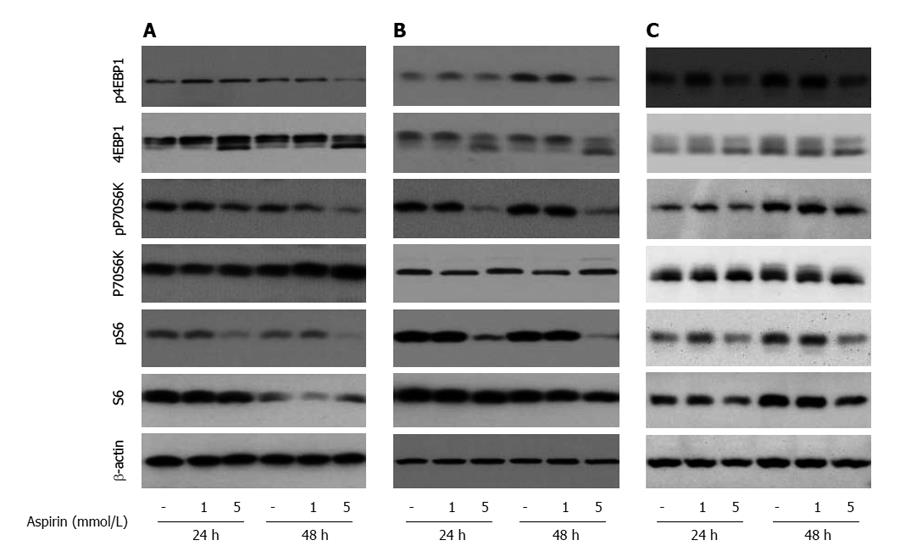
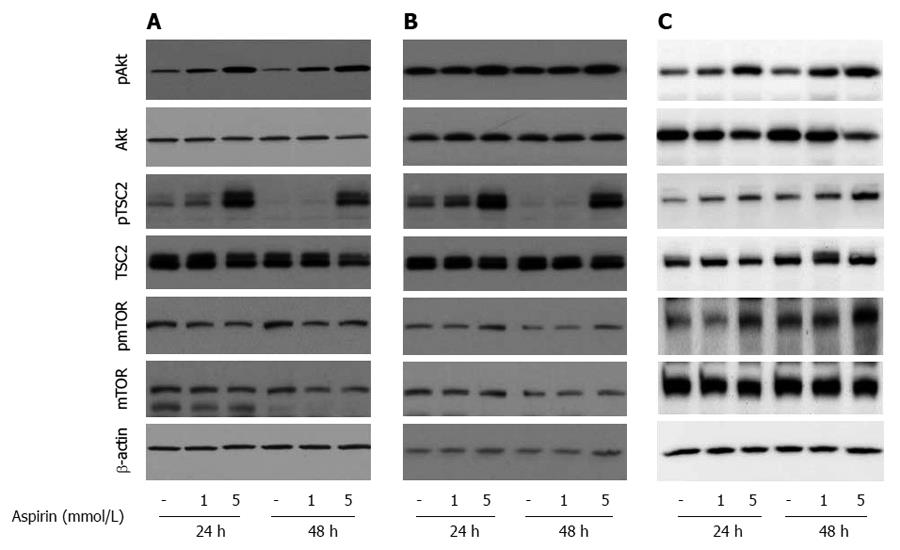
To explore the mechanisms of aspirin-mediated inhibition of cell proliferation in neuroendocrine tumor cells, we examined the time- and dose-dependent effects of aspirin on mTOR signaling. Treatment of human pancreatic BON-1 cells (Figure 4A) with increasing concentrations of aspirin for 24 and 48 h resulted in modest inhibition of 4EBP1 and P70S6K phosphorylation (particularly at 48 h) and potent suppression of S6 phosphorylation at both time points tested. Similar effects were observed with bronchopulmonary neuroendocrine NCI-H727 (Figure 4B) and midgut GOT1 tumor cells (Figure 4C). In all 3 cell lines (Figure 5), treatment with aspirin induced AKT and TSC2 phosphorylation in a dose-dependent manner, and modest effects were observed on mTOR phosphorylation (at serine 2448).
Figure 4 The effect of aspirin on mTORC1 downstream signaling in neuroendocrine BON1, NCI-H727 and GOT1 tumor cells.
Human pancreatic neuroendocrine BON1 (A), bronchopulmonary NCI-H727 (B) and midgut GOT1 (C) cells were treated with the indicated concentrations of aspirin for 24 and 48 h. The expression of p4EBP1, 4EBP1, pP70S6K, P70S6K, pS6, S6 and a β-actin loading control was subsequently evaluated using Western blot analysis. A representative blot from 3 independently performed experiments is shown.
Figure 5 The effect of aspirin on AKT/mTOR signaling in neuroendocrine BON1, NCI-H727 and GOT1 tumor cells.
Human pancreatic neuroendocrine BON1 (A), human bronchopulmonary NCI-H727 (B) and midgut GOT1 (C) cells were treated with the indicated concentrations of aspirin for 24 and 48 h. The expression of pAkt, Akt, pTSC2, TSC2, pmTOR, mTOR and a β-actin loading control was subsequently evaluated using Western blot analysis. A representative blot from 3 independently performed experiments is shown.
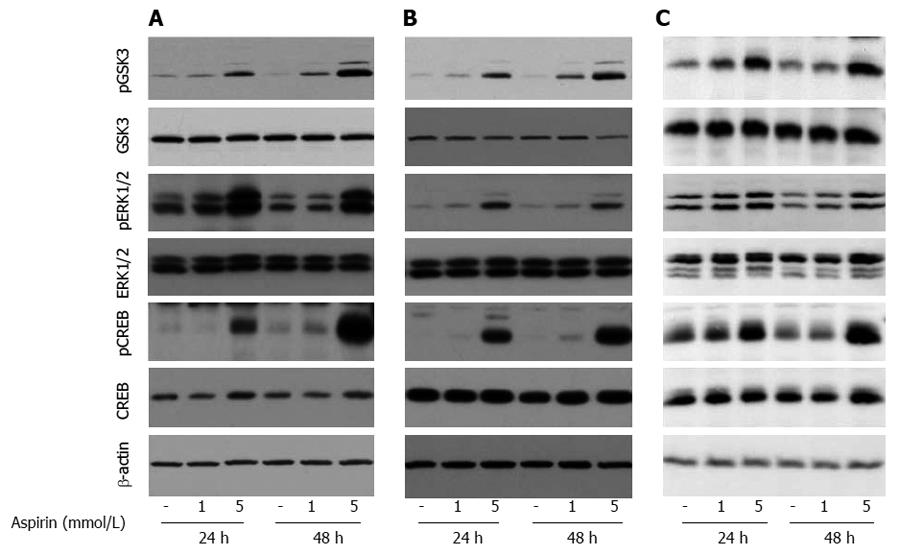
Time- and dose-dependent effects of aspirin on ERK, GSK3 and growth factor signaling in neuroendocrine tumor cells
Treatment of pancreatic BON1 cells with aspirin induced the phosphorylation of ERK1/2 and CREB, members of the mitogen- activated protein kinase (MAPK) survival pathway, in a time-and dose-dependent manner (Figure 6A). In addition, aspirin concentrations as low as 1 mmol/L induced the phosphorylation of GSK3; maximal effects on GSK3 phosphorylation were observed at 48 h. Similar effects were observed for bronchopulmonary NCI-H727 (Figure 6B) and midgut GOT1 tumor cells (Figure 6C).
Figure 6 The effect of aspirin on GSK3 and ERK1/2 signaling in neuroendocrine BON1, NCI-H727 and GOT1 tumor cells.
Human pancreatic neuroendocrine BON1 (A), human bronchopulmonary NCI-H727 (B) and midgut GOT1 (C) cells were treated with the indicated concentrations of aspirin for 24 and 48 h. The expression of pGSK3, GSK3, pERK1/2, ERK1/2, pCREB, CREB and a β-actin loading control was subsequently evaluated by Western blot analysis. A representative blot from 3 independently performed experiments is shown.
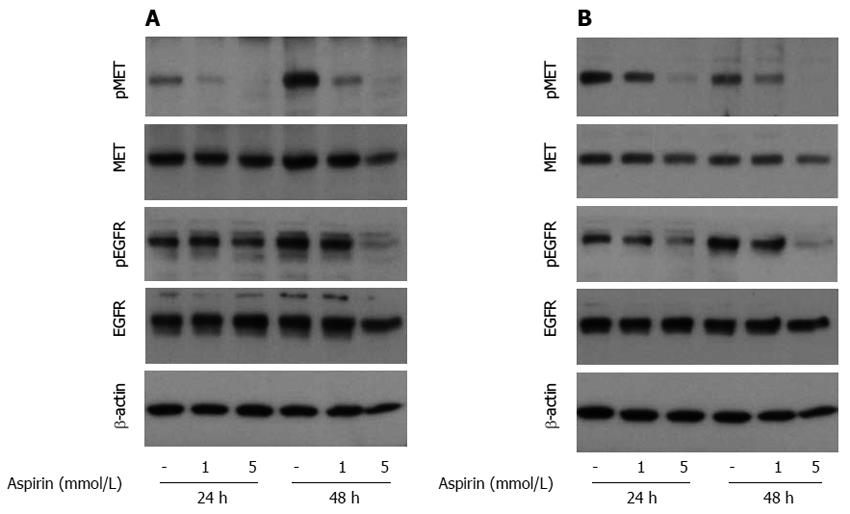
The induction of ERK and GSK signaling in BON1, NCI-H727 and GOT1 cells was not the result of increased upstream growth factor signaling because aspirin suppressed the phosphorylation of the tumorigenic growth factors EGFR and cMET (Figure 7A and B).
Figure 7 The effect of aspirin on EGFR and c-MET signaling in neuroendocrine BON1 and NCI-H727 tumor cells.
Human pancreatic neuroendocrine BON1 (A) and bronchopulmonary NCI-H727 (B) cells were treated with the indicated concentrations of aspirin for 24 and 48 h. The expression of pMET, MET, pEGFR, EGFR and a β-actin loading control was evaluated using Western blot analysis. A representative blot from 3 independently performed experiments is shown.
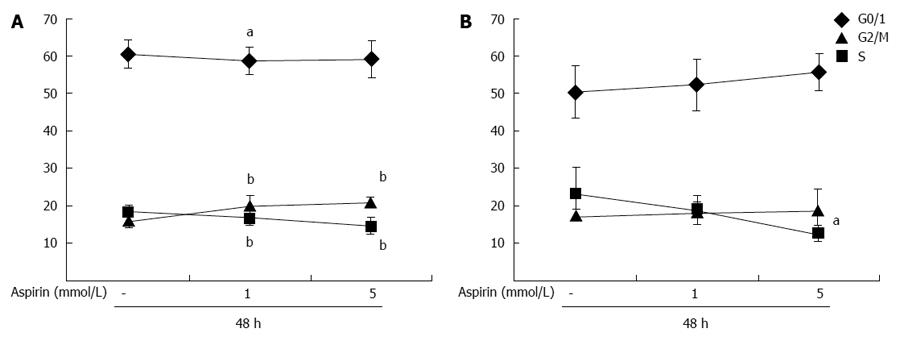
Effects of aspirin on cell cycle progression in neuroendocrine tumor cells
Treatment of partially synchronized (culture in 0.2% BSA for 24 h) pancreatic neuroendocrine BON1 cells with aspirin for 48 h decreased the percentage of cells in S phase in a dose-dependent manner from 18% ± 2% to 14% ± 2% (P < 0.01; 5 mmol/L) and increased the percentage of cells in G2/M phase from 16% ± 2% to 21% ± 1% (P < 0.01; 5 mmol/L) (Figure 8A). Similar effects were observed in NCI-H727 cells (Figure 8B); aspirin dose-dependently decreased entry into S phase from 23% ± 8% to 12% ± 2% (P < 0.05; 5 mmol/L) and had minor effects on G2/M phase (Figure 8B).
Figure 8 The effect of aspirin on cell cycle distribution of neuroendocrine BON1 and NCI-H727 tumor cells.
Human pancreatic neuroendocrine BON1 (A) and bronchopulmonary NCI-H727 (B) cells were cultured in serum-free medium (0.2% BSA) for 24 h and then treated with the indicated concentrations of aspirin for 48 h. The mean ± SDs of 8 independently performed experiments in duplicates are shown; aP < 0.05, bP < 0.01 vs untreated control.
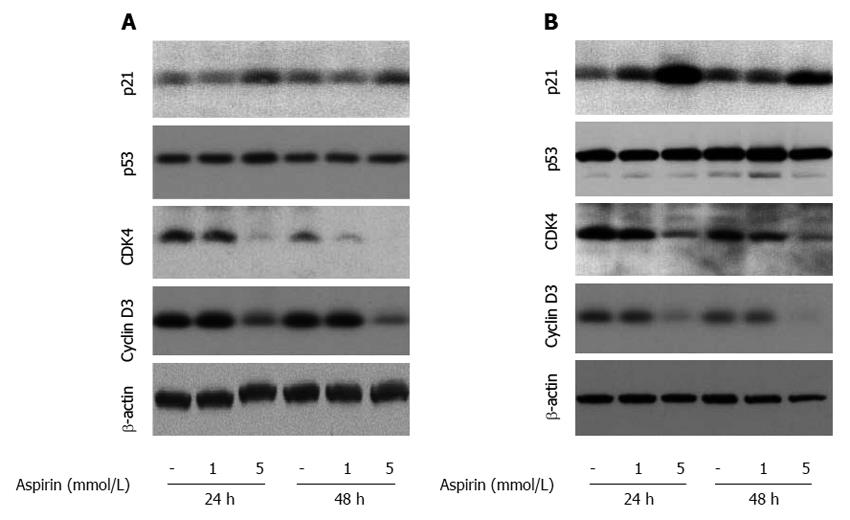
Western blot analysis revealed aspirin-mediated induction of p21 expression at 24 and 48 h in pancreatic BON1 (Figure 9A) and bronchopulmonary NCI-H727 (Figure 9B) cells (particularly at 5 mmol/L). Aspirin also suppressed the expression of cyclin dependent kinase 4 (CDK4) and cyclin D3 in both cell lines tested (Figure 9), while no major effect was observed on tumor suppression protein p53 (Figure 9), cyclin-dependent kinase inhibitor p27, cyclin dependent kinase 6 (CDK6) or cyclin D1 (not shown).
Figure 9 The effect of aspirin on proteins involved in cell cycle progression in neuroendocrine BON1 and NCI-H727 tumor cells.
Human pancreatic neuroendocrine BON1 (A) and human bronchopulmonary NCI-H727 (B) cells were treated with the indicated concentrations of aspirin for 24 and 48 h. The expression of p21, CDK4, p53, cyclin D3 and a β-actin loading control was subsequently evaluated by Western blot analysis. A representative blot from 3 independently performed experiments is shown.
DISCUSSION
The mechanism through which aspirin might affect carcinogenesis is still not clear[14,15]. COX-2 is a known target of aspirin, and the inhibition of COX-2 results in the reduced production of potentially neoplastic prostaglandins[29]. Other mechanisms by which aspirin may regulate inflammation, apoptosis and tumorigenesis include inhibition of the transcription factor NFκB and the upregulation of tumor suppressor genes such as tumor protein 53 (TP53), cyclin-dependent kinase inhibitor 1A (CDKN1A) and B Cell Lymphoma (BCL)-associated X (BAX)[29].
Din et al[16] demonstrated that aspirin reduces mTOR signaling in CRC through the inhibition of the mTOR effectors S6K1 and 4EBP1. The regular use of aspirin after diagnosis of CRC in patients with a mutated phosphatidylinositol-4,5-biphosphonate 3-kinase catalytic subunit alpha polypeptide gene (PIK3CA), who show up-regulation of the PI3K/AKT/mTOR pathway, is associated with superior CRC specific survival[30]. Thus, aspirin may suppress cancer cell growth by inhibiting the PI3K/mTOR signaling pathway[30].
PI3K/AKT/mTOR signaling is critical for neuroendocrine tumor cell growth[23-26]. This pathway demonstrates aberrant activity in NET and is therefore considered an attractive therapeutic target in NETs[2,7,23,29,31,32]. The mTOR inhibitor everolimus has been approved for the treatment of advanced progressive pancreatic NETs due to its significant improvement of progression free survival[25]. Furthermore, in extrapancreatic NETs, everolimus may be considered as an off-label therapeutic option due to the promising results of a large clinical phase 3 study[28]. The effects of aspirin on neuroendocrine tumor cell growth and signaling are not known. The aim of this study was to examine the effect of aspirin on neuroendocrine tumor cell regulation.
We examined the effect of aspirin in human pancreatic neuroendocrine BON1, human bronchopulmonary NCI-H727 and midgut GOT1 cells and evaluated aspirin concentrations ranging from 0.001 to 5 mmol/L. According to the literature, the therapeutic peak serum levels of aspirin range from approximately 0.003 to 5.2 mmol/L; the aspirin oral doses used in vivo vary from approximately 75 mg total dose up to 25 mg/kg body weight every 8 h[33-37]. Aspirin suppressed cell proliferation in all of the NET cell lines in a time- and dose-dependent manner, starting at concentrations of 0.5 to 1 mmol/L, which are within the peak therapeutic serum levels of aspirin after oral (anti-inflammatory) use. The inhibition of cell proliferation was confirmed by SYBR green DNA-labeling in BON1 and NCI-H727 cells and cell titer proliferation assays in all 3 cell lines.
To explore the mechanisms of aspirin-mediated inhibition of cell proliferation, we examined the effect of aspirin on major NET cell signaling pathways, and observed the suppression of 4EBP1 and (to a lesser extent) P70S6K phosphorylation; both of these factors are downstream substrates of mTOR signaling. The phosphorylation of the P70S6K substrate S6 was also potently suppressed, indicating a central role of mTOR downstream signaling in the aspirin-mediated inhibition of NET cell proliferation; this effect is similar to those observed in CRC[16]. S6 and 4EBP1 normally promote proliferation, coupling cell growth with cell cycle progression. The dephosphorylation of 4EBP1, p70S6K and S6 may cause inhibition of cell proliferation and survival, mainly through a reduction in protein synthesis[38]. We observed increased upstream AKT and (possibly AKT-mediated[39]) tuberin phosphorylation at Thr1462 (pTSC2); therefore, the aspirin-mediated inhibition of S6 and 4EBP1 activity may reflect TSC1/TSC2-mediated inhibition of mTORC1 downstream signaling[40,41]. Despite the increased phosphorylation of TSC2, we observed only minor effects on mTOR phosphorylation at serine 2448; however, decreased phosphorylation of mTOR at other sites (and the associated suppression of mTORC1 activity) or compensatory mTORC2-mediated positive feedback signalling[42] cannot be excluded.
Treatment with aspirin and the associated inhibition of downstream mTOR signalling was also associated with increased activation of the rapid accelerated fibrosarcoma (Raf1)/MAP kinase/ERK kinase (MEK)/ERK1/2 pathway, as shown by increased phosphorylation of ERK1/2 and CREB. Similar to the observed effects on AKT, ERK/CREB activation may reflect a compensatory mechanism of the tumor cell machinery. It remains unclear whether such compensatory mechanisms in response to mTOR inhibition are a sign of clinical resistance[43,44] or treatment effectiveness[22]. The activation of ERK has also been associated with the inhibition of gastrointestinal carcinoid cell growth[45] and the regulation of the G2/M checkpoint[45,46]. Simultaneous inhibition of mTOR and compensatory AKT (and/or ERK) activity may potentiate the inhibition of NET cell growth[44].
These compensatory effects on AKT and ERK were likely not mediated by the induction of upstream growth factor signaling because aspirin suppressed the phosphorylation of EGFR and cMET in a dose-dependent manner; both of these receptors represent important growth factors that are implicated in tumorigenesis and considered for targeted therapy[47-49]. Indeed, aspirin has been shown to inhibit specificity protein (Sp)-regulated gene products, such as c-met, in colon cancer cells[50], which may partially explain the potential anti-cancer effects of this drug.
In all 3 neuroendocrine cell lines, aspirin induced the phosphorylation of GSK3 in a time- and dose-dependent manner; GSK3 is a serine/threonine protein kinase that can be phosphorylated by either the AKT or MAP kinase pathways[51]. The phosphorylation of GSK3 causes its inactivation and is the primary mechanism responsible for growth factor inhibition of this protein kinase[52,53]. The suppression of GSK3 plays an important role in survival signaling, consistent with the proapoptotic effects caused by transgenic overexpression of GSK3[52]. However, the exact role of GSK3 in carcinogenesis is still elusive, and GSK3 inhibitors have been proposed as a novel class of therapeutic agents for colon cancer[53,54].
Aspirin-mediated inhibition of cell proliferation was not associated with induction of apoptosis in BON1 and NCI-H727 cells because we did not observe increases in the sub-G0/1 fraction or PARP cleavage (not shown), but instead observed alterations in the distribution of cells through the cell cycle and decreased S phase entry. Treatment with aspirin induced the expression of p21 and suppressed the expression of CDK4 and cyclin D3. Because p21 may inhibit cell cycle progression by binding to cyclin/CDK complexes and preventing the activation of apoptosis[55], the aspirin-mediated inhibition of neuroendocrine cell proliferation may involve increased p21 signalling, similar to the effects observed in hepatocellular carcinoma HepG2 cells[56].
In this study, we show for the first time that aspirin mediates the inhibition of neuroendocrine tumor cell growth and signaling. Given the lack of established pharmacologic adjuvant therapies following complete (R0) resection of NETs, further preclinical and clinical studies are required to determine the potential use of aspirin in neuroendocrine tumor disease.
COMMENTS
Background
Neuroendocrine tumors (NETs) are rare neoplasms, but in recent years, the incidence and prevalence of these tumors have increased significantly. Although available treatments have improved in the last few decades, they cannot offer a real increase in disease free survival. New therapeutic options are therefore required. Aspirin has been shown to have antitumor activity in several cancer cell lines.
Research frontiers
In the current study, authors investigated the potential effects of aspirin, a common co-medication in many patients, on pancreatic, bronchopulmonary and midgut NETs cell lines. Specifically, they studied the effects of aspirin on cell viability/cell proliferation, cell signaling and the cell cycle.
Innovations and breakthroughs
Din et al recently demonstrated that aspirin caused the inhibition of mammalian target of rapamycin (mTOR) downstream signaling in colorectal cancer cells (CRC). NETs have different treatments strategies; one of these strategies is targeted therapy with everolimus, an inhibitor of mTOR. With this background, their purpose was to investigate the effects of aspirin on NETs. No data on these effects have been previously published.
Applications
If further preclinical and clinical studies confirm the results by showing antiproliferative effects, aspirin could be used as an adjuvant therapy, particularly for patients with neuroendocrine tumors and R0 status after surgery.
Terminology
Aspirin or acetylsalicylic acid was first isolated in 1897 by Felix Hoffmann. Since then, it has been widely used for its anti-inflammatory, antipyretic, analgesic and antiplatelet properties. More recently, anticancer effects have been described, particularly for CRC. NETs are neoplasms that arise from the diffuse endocrine system. The incidence of NETs is rising, along with the need for more effective drugs. mTOR is one of the molecular targets of anticancer strategies. The inhibition of mTOR signaling after treatment with aspirin has already been described in CRC cells.
Peer review
Summarizing, the authors analyzed the time-and dose-dependent effects of aspirin on mTOR signaling in in vitro NETs cells. They found that aspirin suppressed phosphorylation of tumorigenic growth factors EGFR and cMET also. They show the inhibition of NETs cell growth and signaling by aspirin. The methods are well described and the results are very interesting. It is a valuable paper, with pretty new research in NETs. It can be a powerful step towards new knowledge in NETs cell biology.