Published online Jul 28, 2014. doi: 10.3748/wjg.v20.i28.9578
Revised: April 20, 2014
Accepted: May 19, 2014
Published online: July 28, 2014
Processing time: 285 Days and 16.5 Hours
AIM: To investigate enough valid measurements (VMs) to assess liver fibrosis in chronic hepatitis B patients (CHB).
METHODS: One hundred and twelve CHB patients (25 women, 87 men) with a mean age of 38.43 years received liver stiffness evaluations using real-time shear wave elastography for 10 VMs. All patients underwent liver biopsy. Based on the biopsy pathology, the liver stiffness data obtained from different VMs (1, 2, 3, 5 and 10 times) were compared for the evaluation of liver fibrosis. The correlation between the elastic modulus means of the liver obtained from different VMs of detection at each pathological stage was analysed. The receiver operating characteristic (ROC) curve was employed to determine the diagnostic performance of different VMs of detection, and the areas under the ROC curve of different groups were compared.
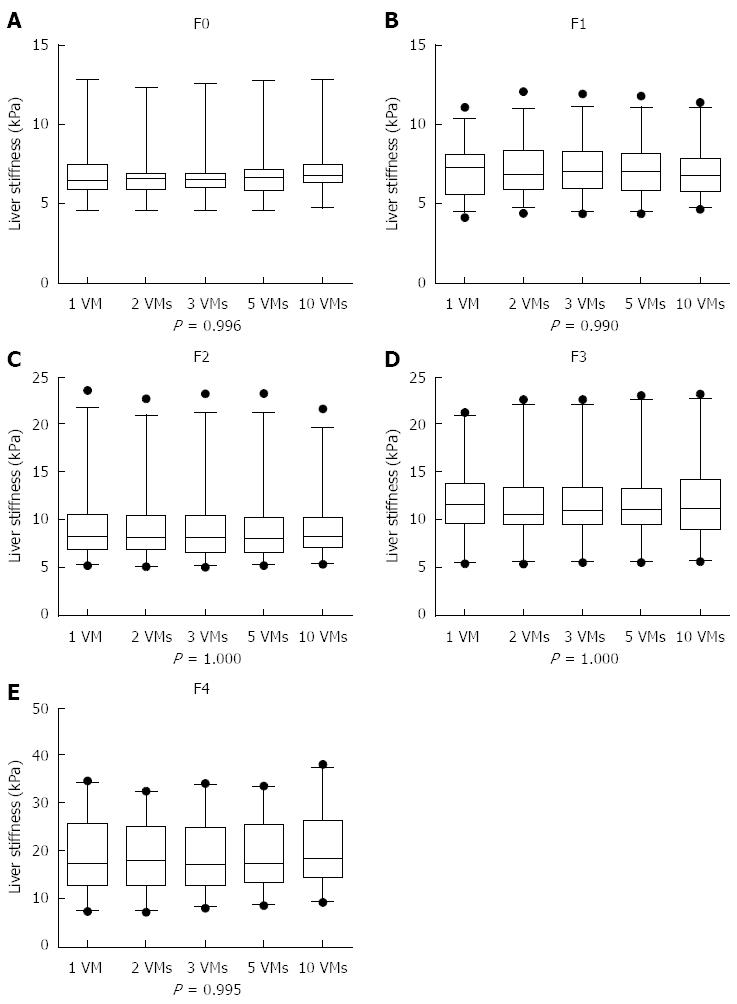
RESULTS: The liver stiffness values obtained from 1 VM, 2 VMs, 3 VMs, 5 VMs and all 10 VMs for stage F0 were 6.95 ± 2.01 kPa, 6.87 ± 1.83 kPa, 6.90 ± 1.88 kPa, 6.95 ± 1.93 kPa and 7.15 ± 1.89 kPa, respectively (F = 0.043, P = 0.996). For stage F1, these values were 7.12 ± 1.72 kPa, 7.24 ± 1.72 kPa, 7.21 ± 1.74 kPa, 7.10 ± 1.78 kPa and 7.04 ± 1.70 kPa, respectively (F = 0.075, P = 0.990). For stage F2, they were 9.37 ± 3.87 kPa, 9.18 ± 3.68 kPa, 9.19 ± 3.81 kPa, 9.18 ± 3.81 kPa and 9.19 ± 3.53 kPa, respectively (F = 0.012, P = 1.000). For stage F3, these were 11.91 ± 3.88 kPa, 11.78 ± 4.04 kPa, 11.83 ± 4.07 kPa, 11.94 ± 4.17 kPa and 12.00 ± 4.02 kPa, respectively (F = 0.010, P = 1.000). For stage F4, the readings were 19.30 ± 7.63 kPa, 19.40 ± 7.36 kPa, 19.54 ± 7.43 kPa, 19.73 ± 7.21 kPa and 20.25 ± 7.22 kPa, respectively (F = 0.054, P = 0.995). There were no significant differences between these groups. Intraclass correlation coefficients among different pathological stages (F0-F4) with different detection VMs were 0.995, 0.993, 0.996, 0.994 and 0.996, respectively. The mean elasticity values from 1 VM, 2 VMs, 3 VMs, 5 VMs and 10 VMs can accurately distinguish fibrosis stages (F0 vs F1234, F01 vs F234, F012 vs F34 and F0123 vs F4) with no significant differences in the five groups (P > 0.05 for all).
CONCLUSION: One VM may be sufficient to assess liver fibrosis by using SWE without any significant loss of accuracy in patients with CHB. However, future studies of larger patient samples are necessary for the validation of this method.
Core tip: We evaluated the impact of different valid measurements (VMs) on the liver stiffness measurement by shear wave elastography in 112 chronic hepatitis B patients. Liver stiffness values obtained from 1 VM, 2 VMs, 3 VMs, 5 VMs and all 10 VMs were similar, and there were no significant differences between these groups. The repeatability was excellent among different pathological stages in different groups. The mean elasticity values can accurately distinguish fibrosis stages with no significant differences seen in the five groups. One VM may be enough to assess liver fibrosis using shear wave elastography without any significant loss of accuracy.
- Citation: Huang ZP, Zhang XL, Zeng J, Zheng J, Wang P, Zheng RQ. Study of detection times for liver stiffness evaluation by shear wave elastography. World J Gastroenterol 2014; 20(28): 9578-9584
- URL: https://www.wjgnet.com/1007-9327/full/v20/i28/9578.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i28.9578
Liver fibrosis is a progressive change in a variety of chronic liver diseases, including cirrhosis. The early and accurate determination of the degree of liver fibrosis in patients with chronic liver disease and effective therapy can significantly improve the prognosis of patients, which is of important clinical significance[1]. The current “gold standard” for the clinical diagnosis of liver fibrosis and cirrhosis is liver biopsy, but it still has certain risks and limitations, such as the fact that it is invasive, painful and costly, and there is intra- and inter-observer variability[2,3]. Therefore, non-invasive technology for the accurate diagnosis of liver fibrosis has become an urgent clinical need.
Liver stiffness measurements using elastography for the non-invasive evaluation of liver fibrosis have been developed in the last few years, for example, transient elastography (TE), acoustic radiation force imaging, real-time shear wave elastography (SWE), and so on. Among these technologies, TE has gained increasing attention globally for the diagnosis of liver fibrosis[4,5]. Although TE features low cost, practical use and high accuracy, its limitations still cannot be ignored - it still fails to accurately distinguish moderate liver fibrosis, different degrees of liver inflammation also affect the detection accuracy, inter-observer discrepancies in results are common, and it cannot be used for the examination of patients with ascites[6,7]. Recently, SWE, developed by Supersonic Imagine[8], has boasted good prospects for clinical application[9,10]. However, the designer of SWE has not provided exact valid measurements (VMs) for its application in the clinical evaluation of liver stiffness. The VMs reported in the recent literature are inconsistent (commonly seen are 5, 4 and 3 VMs), and some studies fail to mention it at all[11-13]. Although a number of studies have recommended that the VMs of TE operation can be used for reference by the user of SWE[12], we cannot blindly copy the TE standard practice in view of the following reasons. First, the effective VMs of TE are still controversial - although most studies suggest that the effective VMs of TE should reach more than 10, some studies have shown that the number of measurements had no impact on the diagnostic performance of TE, and accurate diagnostic performance can be achieved through only 3 VMs[14]. Second, SWE and TE have different principles and parameters: TE is a one-dimensional imaging modality in which the detection area cannot be adjusted, while SWE uses two-dimensional real-time imaging and the detection area can be changed as needed, meaning that operators using SWE easily obtain satisfactory imaging. To the best of our knowledge, whether the liver stiffness measurement (LSM) obtained from different VMs of SWE detection has internal consistency and whether different VMs of SWE detection have equivalent performance in the evaluation of liver stiffness have not be studied until now.
Thus, we aimed to evaluate the impact of different VMs on the evaluation of liver stiffness by real-time SWE, and investigate a sufficient number of VMs to assess liver fibrosis without any significant loss of accuracy in patients with chronic hepatitis B.
From April 2010 to April 2012, one hundred and twelve patients with chronic hepatitis B seeking treatment in the Third Affiliated Hospital of Sun Yat-sen University were enrolled. This included 87 males and 25 females, aged 19-62 years, with an average age of 38.43 ± 10.64 years. All subjects received ultrasound-guided liver biopsy to determine the pathological diagnostic stage and underwent LSM by SWE on the second day after liver biopsy. This study was approved by the Ethics Committee of the Third Affiliated Hospital of Sun Yet-sen University, and all participants signed the written informed consent.
Instruments: SWE studies were performed using the AixplorerTM ultrasound system (SuperSonic Imagine S.A., Aix-en-Provence, France) and an SC6-1 convex array probe at a frequency of 6 MHz was employed.
Examination methods: The subject took a supine position and lifted their right upper limb. The detection site was fixed 1.5-2 cm beneath the right liver capsule and away from intrahepatic vessels and the gallbladder. After the elasticity imaging mode was selected, the subject held their breath for 3-5 s for imaging. When the target area was located, the operator launched the SWE sequence measurement. After the colour images were frozen, the circular quantitative sampling frame with a diameter of 30 mm was initiated to measure the elastic modulus of the liver in the region of interest (ROI). Then, the system automatically calculated the elastic modulus mean (in kPa) within the ROI. All patients were continuously assessed for 10 VMs by a doctor with SWE operating experience. The elastic modulus obtained from each valid measurement was recorded, then 1 VM, 2 VMs, 3 VMs and 5 VMs were extracted randomly from all 10 VMs; the means of elastic modulus by using 1 VM, 2 VMs, 3 VMs, 5 VMs and all 10 VMs were calculated.
Histological assessment: The right liver was chosen as the puncture site. An 18G automatic biopsy gun was used under ultrasound guidance to perform biopsy of the liver tissue. The liver biopsy specimens of all subjects were sent to the Department of Pathology, the Third Affiliated Hospital of Sun Yat-sen University for pathological diagnosis. Fibrosis was staged on a five-point scale from 0 to 4 according to the METAVIR scoring system[15] (F0, absent; F1, enlarged fibrotic portal tract; F2, periportal or initial portal-portal septa but intact architecture; F3, architectural distortion but no obvious cirrhosis; and F4, cirrhosis).
SPSS13.0 statistical software (SPSS Inc., Chicago, IL) was employed for statistical analyses of the information collected. Measurement data are expressed as mean ± SD. Comparison of the elastic modulus means of the liver obtained from different VMs of detection at each pathological stage was subjected to one-way ANOVA; the correlation between the elastic modulus means of the liver obtained from different VMs of detection at each pathological stage was analysed using the intraclass correlation coefficient[16] (ICC, which reflects the consistency of the measurements: ICC < 0.2 indicates poor consistency, 0.2-0.4 indicates general consistency, 0.4-0.75 indicates good consistency, and ≥ 0.75 indicates very good consistency). A receiver operating characteristic (ROC) curve was employed to determine the diagnostic performance of different VMs of detection; the areas under the ROC curve of different groups were compared. Detection level was set at α = 0.05.
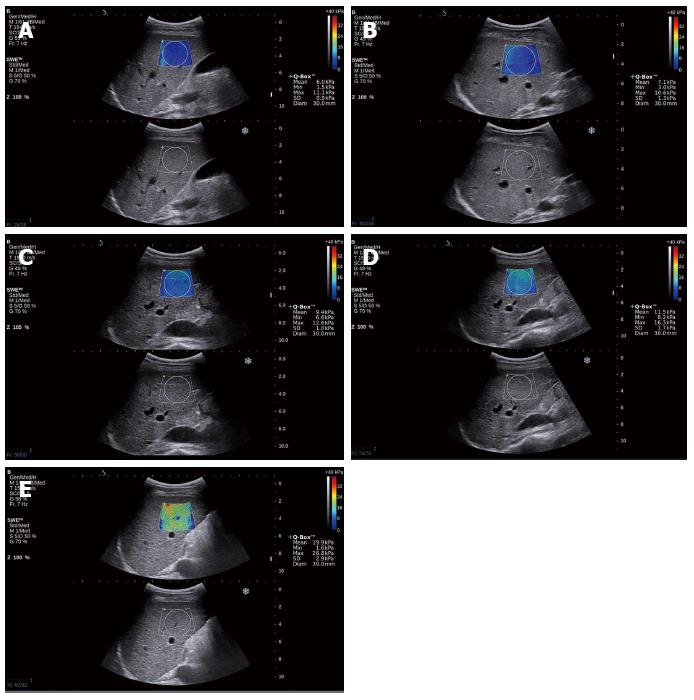
All of the cases could obtain a satisfactory elasticity map, as shown in Figure 1. Figure 1 show the elasticity mapping for patients who were classified as predicted fibrosis levels F1, F2, F3 and F4, respectively.
The liver stiffness values obtained from 1 VM, 2 VMs, 3 VMs, 5 VMs and all 10 VMs for stage F0 were 6.95 ± 2.01 kPa, 6.87 ± 1.83 kPa, 6.90 ± 1.88 kPa, 6.95 ± 1.93 kPa and 7.15 ± 1.89 kPa, respectively. For stage F1, the results were 7.12 ± 1.72 kPa, 7.24 ± 1.72 kPa, 7.21 ± 1.74 kPa, 7.10 ± 1.78 kPa and 7.04 ± 1.70 kPa, respectively. For stage F2, these were 9.37 ± 3.87 kPa, 9.18 ± 3.68 kPa, 9.19 ± 3.81 kPa, 9.18 ± 3.81 kPa and 9.19±3.53 kPa, respectively. For stage F3, the results were 11.91 ± 3.88 kPa, 11.78 ± 4.04 kPa, 11.83 ± 4.07 kPa, 11.94 ± 4.17 kPa and 12.00 ± 4.02 kPa, respectively. For stage F4, the results were 19.30 ± 7.63 kPa, 19.40 ± 7.36 kPa, 19.54 ± 7.43 kPa, 19.73 ± 7.21 kPa and 20.25 ± 7.22 kPa, respectively.
At the five different pathological stages of liver fibrosis from F0 to F4, the elastic modulus values of the liver obtained from 1 VM, 2 VMs, 3 VMs, 5 VMs and all 10 VMs of SWE at each stage showed no statistical differences (P = 0.996, 0.990, 1.000, 1.000, 0.995), as shown in Table 1 and Figure 2.
| Stage | n | 1 VM (kPa) | 2 VMs (kPa) | 3 VMs (kPa) | 5 VMs (kPa) | 10 VMs (kPa) | F | P |
| F0 | 13 | 6.95 ± 2.01 | 6.87 ± 1.83 | 6.90 ± 1.88 | 6.95 ± 1.93 | 7.15 ± 1.89 | 0.043 | 0.996 |
| F1 | 33 | 7.12 ± 1.72 | 7.24 ± 1.72 | 7.21 ± 1.74 | 7.10 ± 1.78 | 7.04 ± 1.70 | 0.075 | 0.99 |
| F2 | 24 | 9.37 ± 3.87 | 9.18 ± 3.68 | 9.19 ± 3.81 | 9.18 ± 3.81 | 9.19 ± 3.53 | 0.012 | 1 |
| F3 | 21 | 11.91 ± 3.88 | 11.78 ± 4.04 | 11.83 ± 4.07 | 11.94 ± 4.17 | 12.00 ± 4.02 | 0.01 | 1 |
| F4 | 21 | 19.30 ± 7.63 | 19.40 ± 7.36 | 19.54 ± 7.43 | 19.73 ± 7.21 | 20.25 ± 7.22 | 0.054 | 0.995 |
At the five different pathological stages of liver fibrosis from F0 to F4, the ICCs among elastic values of the liver obtained from the five different groups of VMs of SWE at each stage were all > 0.75 (Table 2).
| Stage | n | ICC | 95%CI |
| F0 | 13 | 0.995 | 0.988-0.998 |
| F1 | 33 | 0.993 | 0.988-0.996 |
| F2 | 24 | 0.996 | 0.993-0.998 |
| F3 | 21 | 0.994 | 0.989-0.997 |
| F4 | 21 | 0.996 | 0.992-0.998 |
| Overall | 112 | 0.998 | 0.997-0.998 |
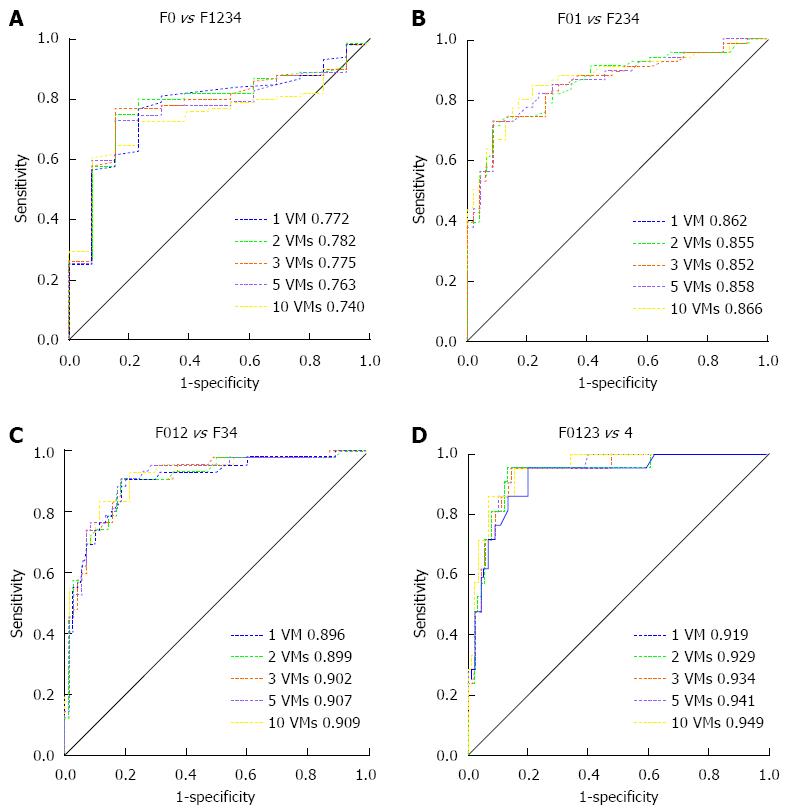
Liver stiffness values obtained from 1 VM, 2 VMs, 3 VMs, 5 VMs and all 10 VMs of SWE were used to determine the liver fibrosis stage and draw the ROC curve, as shown in Figure 3. Areas under the ROC curve (AUROC) at each stage in the group of five different VMs are shown in Table 3. The pairwise comparison of AUROC for each group showed no statistically significant differences (P > 0.05 for all) (Table 4).
| Stage | 1 VM | 2 VMs | 3 VMs | 5 VMs | All 10 VMs | |||||
| ROC | 95%CI | ROC | 95%CI | ROC | 95%CI | ROC | 95%CI | ROC | 95%CI | |
| F0-1234 | 0.772 (0.683-0.846) | 0.782 (0.694-0.854) | 0.775 (0.687-0.849) | 0.763 (0.674-0.839) | 0.740 (0.649-0.818) | |||||
| F01-234 | 0.862 (0.784-0.920) | 0.855 (0.775-0.914) | 0.852 (0.772-0.912) | 0.858 (0.779-0.916) | 0.866 (0.789-0.923) | |||||
| F012-34 | 0.896 (0.824-0.945) | 0.899 (0.827-0.948) | 0.902 (0.831-0.950) | 0.907 (0.838-0.954) | 0.909 (0.840-0.955) | |||||
| F0123-4 | 0.919 (0.852-0.962) | 0.929 (0.865-0.969) | 0.934 (0.871-0.972) | 0.941 (0.880-0.977) | 0.949 (0.891-0.982) | |||||
| Different VMs | F0-1234 | F01-234 | F012-34 | F0123-4 | ||||
| Difference | P | Difference | P | Difference | P | Difference | P | |
| 1 VM vs 2 VMs | 0.001 | 0.618 | 0.008 | 0.323 | 0.003 | 0.703 | 0.010 | 0.267 |
| 1 VM vs 3 VMs | 0.003 | 0.917 | 0.011 | 0.321 | 0.006 | 0.57 | 0.015 | 0.171 |
| 1 VM vs 5 VMs | 0.009 | 0.800 | 0.005 | 0.682 | 0.012 | 0.297 | 0.022 | 0.068 |
| 1 VM vs 10 VMs | 0.032 | 0.365 | 0.004 | 0.787 | 0.013 | 0.379 | 0.030 | 0.089 |
| 2 VMs vs 3 VMs | 0.006 | 0.785 | 0.003 | 0.617 | 0.003 | 0.595 | 0.004 | 0.477 |
| 2 VMs vs 5 VMs | 0.018 | 0.420 | 0.003 | 0.704 | 0.009 | 0.208 | 0.012 | 0.152 |
| 2 VMs vs 10 VMs | 0.042 | 0.152 | 0.012 | 0.415 | 0.010 | 0.475 | 0.020 | 0.212 |
| 3 VMs vs 5 VMs | 0.012 | 0.487 | 0.006 | 0.227 | 0.005 | 0.208 | 0.007 | 0.152 |
| 3 VMs vs 10 VMs | 0.035 | 0.073 | 0.015 | 0.245 | 0.007 | 0.609 | 0.015 | 0.302 |
| 5 VMs vs 10 VMs | 0.023 | 0.451 | 0.009 | 0.461 | 0.002 | 0.900 | 0.008 | 0.538 |
LSM by elastography, such as TE, is now widely recognised in some countries for the assessment of liver fibrosis in chronic liver disease. According to the usual definition, only LSM with more than 10 VMs is generally considered reliable[17,18]. However, the effective VMs of TE are still controversial[14,19]. In a study by Kettaneh et al[20], the AUROCs for the diagnosis of significant fibrosis or cirrhosis barely differed across LSE median values obtained from the first 3, 5 and 10 VMs; they inferred that patients will benefit from this procedure with no significant loss in performance if only 5 valid shots are requested. In another study, Jang et al[14] also showed that three VMs may be sufficient to assess liver fibrosis using LSM without any significant loss of accuracy in patients with CHC and patients with CHB. Moreover, VMs of SWE reported in the literature were variable. Ferraioli et al[12] showed that real-time SWE is more accurate than TE in assessing significant fibrosis (F > 2), using a group of 121 patients with CHC, in which four consecutive SWE measurements were used. Bavu et al[10] reproduced five consecutive measurements for 113 patients to test their LSM, and also suggested that SWE is superior to TE for the assessment of mild and intermediate fibrosis. SWE, as a novel technique, is applied in clinic; it is necessary to determine how many VMs are appropriate for the LSM of SWE so that users can abide by the unified standards and facilitate their communications.
Our study showed that the 1 VM, 2 VMs, 3 VMs, 5 VMs and all 10 VMs groups reported no statistical differences in the elastic modulus means at each stage from F0 to F4 (P > 0.05 for all). In addition, analysis of ICCs showed that ICCs among the five groups at each stage were all up to 0.99, thus indicating very good consistency (ICC greater than 0.75). In our study, satisfactory images were obtained from all patients monitored by the visual two dimensional ultrasound, which uses the 30 mm diameter circular quantitative sampling frame containing a homogeneous colour-coded map. As every circular frame is made up of numerous pixels and each pixel corresponds to a tissue elasticity measurement acquiring an elastic modulus value, the even elastic modulus value of the local liver tissue in the circular frame is eventually calculated to assess liver stiffness. This may explain why the elastic modulus value of 1 VM is close to those of other VMs and has good repeatability. Compared with SWE, TE is a one-dimensional imaging method, meaning that many measurements are needed to obtain highly successful measurements to calculate liver stiffness.
Our study showed that the results obtained from the 1 VM, 2 VMs, 3 VMs, 5 VMs and 10 VMs groups of detection were highly consistent; therefore, it may be speculated that, in the clinical application of SWE to evaluate the degree of liver fibrosis, stable elastic modulus mean data can be achieved through only 1 VM of detection, and its performance is equivalent to that of 2, 3, 5 and 10 VMs. This is because SWE detection requires the patients to hold their breath for 3-5 s each time to obtain stable images in order to generate a quantitative liver elastic modulus. The smaller the number of detections, the easier it is for patients to accept SWE detection. In addition, less detection can also prevent repeated work to simplify the process and improve efficiency.
The elastic modulus means obtained from these different VMs of detection were used to draw an ROC curve, and the AUROCs in the five groups at each stage were very close, showing no statistical differences (P > 0.05 for all). We also found that liver stiffness values increased with the degree of liver fibrosis, and that all values accurately predicted cirrhosis. In comparison with previous studies, we found that LSM predicted cirrhosis less accurately than significant fibrosis when comparing F0-F1. However, the relatively small sample of F0 might explain this finding.
This study has some limitations. First, the population used to generate the results with real-time SWE is limited, particularly for F0. Second, the detection site, which was fixed at 1.5-2 cm beneath the right liver capsule, confined the detection range to some extent, which might lead to selection bias. As a result, further studies based on different detection depths and areas are needed. Finally, we only focused on patients with CHB. Therefore, a larger study, possibly an international multi-centre trial including multiple kinds of chronic liver disease patients, should be carried out to confirm our results.
In conclusion, our preliminary study shows that only one VM may be sufficient to assess the stage of liver fibrosis using LSM by SWE without any significant loss of diagnostic accuracy in patients with CHB. However, this needs to be verified by other researchers in further practice, so that this study can provide a relevant basis and reference for the clinical application of SWE and a simplified working process.
Early and accurate determination of the degree of liver fibrosis in patients with chronic liver disease and effective therapy can significantly improve the prognosis of patients, which is of clinical significance. The current “gold standard” for the clinical diagnosis of liver fibrosis still has certain risks and limitations. There is an urgent clinical need for the non-invasive diagnosis of liver fibrosis.
Shear wave elastography (SWE) boasts good prospects for clinical application. However, the designer of SWE has not given exact valid measurements (VMs) for its application in the clinical evaluation of liver stiffness.
This preliminary study showed that one VM may be enough to assess the stage of liver fibrosis using liver stiffness measurement by SWE without any significant loss of diagnostic accuracy in patients with chronic hepatitis B.
This study may provide relevant basis and reference for the clinical application of SWE and simplify the working process.
The authors studied enough valid measurements to assess liver fibrosis in chronic hepatitis B patients. The manuscript is well written and structured. The results are interesting and may solve the problem of the clinical question.
P- Reviewer: Bauer P, Habr-Gama A S- Editor: Wang JL L- Editor: Wang TQ E- Editor: Ma S
| 1. | Schuppan D, Kim YO. Evolving therapies for liver fibrosis. J Clin Invest. 2013;123:1887-1901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 490] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 2. | Bedossa P, Dargère D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1193] [Cited by in RCA: 1398] [Article Influence: 63.5] [Reference Citation Analysis (0)] |
| 3. | Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, Pyrsopoulos NT, Feng ZZ, Reddy KR, Schiff ER. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614-2618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1504] [Cited by in RCA: 1569] [Article Influence: 68.2] [Reference Citation Analysis (0)] |
| 4. | Shi KQ, Fan YC, Pan ZZ, Lin XF, Liu WY, Chen YP, Zheng MH. Transient elastography: a meta-analysis of diagnostic accuracy in evaluation of portal hypertension in chronic liver disease. Liver Int. 2013;33:62-71. [PubMed] |
| 5. | Fitzpatrick E, Quaglia A, Vimalesvaran S, Basso MS, Dhawan A. Transient elastography is a useful noninvasive tool for the evaluation of fibrosis in paediatric chronic liver disease. J Pediatr Gastroenterol Nutr. 2013;56:72-76. [PubMed] |
| 6. | Foucher J, Castéra L, Bernard PH, Adhoute X, Laharie D, Bertet J, Couzigou P, de Lédinghen V. Prevalence and factors associated with failure of liver stiffness measurement using FibroScan in a prospective study of 2114 examinations. Eur J Gastroenterol Hepatol. 2006;18:411-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 271] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 7. | Kim SU, Seo YS, Cheong JY, Kim MY, Kim JK, Um SH, Cho SW, Paik SK, Lee KS, Han KH. Factors that affect the diagnostic accuracy of liver fibrosis measurement by Fibroscan in patients with chronic hepatitis B. Aliment Pharmacol Ther. 2010;32:498-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Bercoff J, Tanter M, Fink M. Supersonic shear imaging: a new technique for soft tissue elasticity mapping. IEEE Trans Ultrason Ferroelectr Freq Control. 2004;51:396-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1835] [Cited by in RCA: 1514] [Article Influence: 72.1] [Reference Citation Analysis (0)] |
| 9. | Muller M, Gennisson JL, Deffieux T, Tanter M, Fink M. Quantitative viscoelasticity mapping of human liver using supersonic shear imaging: preliminary in vivo feasibility study. Ultrasound Med Biol. 2009;35:219-229. [PubMed] |
| 10. | Bavu E, Gennisson JL, Couade M, Bercoff J, Mallet V, Fink M, Badel A, Vallet-Pichard A, Nalpas B, Tanter M. Noninvasive in vivo liver fibrosis evaluation using supersonic shear imaging: a clinical study on 113 hepatitis C virus patients. Ultrasound Med Biol. 2011;37:1361-1373. [PubMed] |
| 11. | Ling W, Lu Q, Quan J, Ma L, Luo Y. Assessment of impact factors on shear wave based liver stiffness measurement. Eur J Radiol. 2013;82:335-341. [PubMed] |
| 12. | Ferraioli G, Tinelli C, Dal Bello B, Zicchetti M, Filice G, Filice C. Accuracy of real-time shear wave elastography for assessing liver fibrosis in chronic hepatitis C: a pilot study. Hepatology. 2012;56:2125-2133. [PubMed] |
| 13. | Grenier N, Poulain S, Lepreux S, Gennisson JL, Dallaudière B, Lebras Y, Bavu E, Servais A, Meas-Yedid V, Piccoli M. Quantitative elastography of renal transplants using supersonic shear imaging: a pilot study. Eur Radiol. 2012;22:2138-2146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 14. | Jang HW, Kim SU, Park JY, Ahn SH, Han KH, Chon CY, Park YN, Choi EH, Kim do Y. How many valid measurements are necessary to assess liver fibrosis using FibroScan® in patients with chronic viral hepatitis? An analysis of subjects with at least 10 valid measurements. Yonsei Med J. 2012;53:337-345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2860] [Cited by in RCA: 3082] [Article Influence: 106.3] [Reference Citation Analysis (0)] |
| 16. | Wu S, Crespi CM, Wong WK. Comparison of methods for estimating the intraclass correlation coefficient for binary responses in cancer prevention cluster randomized trials. Contemp Clin Trials. 2012;33:869-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 173] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 17. | Castéra L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, Darriet M, Couzigou P, De Lédinghen V. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1796] [Cited by in RCA: 1848] [Article Influence: 92.4] [Reference Citation Analysis (0)] |
| 18. | Ziol M, Handra-Luca A, Kettaneh A, Christidis C, Mal F, Kazemi F, de Lédinghen V, Marcellin P, Dhumeaux D, Trinchet JC. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology. 2005;41:48-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1090] [Cited by in RCA: 1095] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 19. | Pournik O, Alavian SM, Eslami S. Can we accurately assess liver fibrosis with Fibroscan® using fewer valid measurements? Yonsei Med J. 2013;54:541-542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 20. | Kettaneh A, Marcellin P, Douvin C, Poupon R, Ziol M, Beaugrand M, de Lédinghen V. Features associated with success rate and performance of FibroScan measurements for the diagnosis of cirrhosis in HCV patients: a prospective study of 935 patients. J Hepatol. 2007;46:628-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 192] [Article Influence: 10.7] [Reference Citation Analysis (0)] |