Published online Jul 14, 2014. doi: 10.3748/wjg.v20.i26.8740
Revised: March 1, 2014
Accepted: April 5, 2014
Published online: July 14, 2014
Processing time: 173 Days and 20.1 Hours
To our knowledge, patients with immunoglobulin G4-related sclerosing cholangitis (IgG4-SC) associated with autoimmune hemolytic anemia (AIHA) have not been reported previously. Many patients with IgG4-SC have autoimmune pancreatitis (AIP) and respond to steroid treatment. However, isolated cases of IgG4-SC are difficult to diagnose. We describe our experience with a patient who had IgG4-SC without AIP in whom the presence of AIHA led to diagnosis. The patient was a 73-year-old man who was being treated for dementia. Liver dysfunction was diagnosed on blood tests at another hospital. Imaging studies suggested the presence of carcinoma of the hepatic hilus and primary sclerosing cholangitis, but a rapidly progressing anemia developed simultaneously. After the diagnosis of AIHA, steroid treatment was begun, and the biliary stricture improved. IgG4-SC without AIP was thus diagnosed.
Core tip: Patients with immunoglobulin G4-related sclerosing cholangitis (IgG4-SC) associated with autoimmune hemolytic anemia (AIHA) have not been reported previously. Many patients with IgG4-SC have autoimmune pancreatitis (AIP) and respond to steroid treatment. However, isolated cases of IgG4-SC are difficult to diagnose. We describe our experience with a patient who had IgG4-SC without AIP in whom the presence of AIHA led to diagnosis.
- Citation: Masutani H, Okuwaki K, Kida M, Yamauchi H, Imaizumi H, Miyazawa S, Iwai T, Takezawa M, Koizumi W. First case of IgG4-related sclerosing cholangitis associated with autoimmune hemolytic anemia. World J Gastroenterol 2014; 20(26): 8740-8744
- URL: https://www.wjgnet.com/1007-9327/full/v20/i26/8740.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i26.8740
Immunoglobulin G4-related sclerosing cholangitis (IgG4-SC) was first reported in Japan and refers to sclerosing cholangitis of unknown cause, characterized by increased serum IgG4 levels and fibrosis associated with marked infiltration of local lesions by lymphocytes and IgG4-positive plasma cells[1]. Recently, “Clinical Diagnostic Criteria of IgG4-related Sclerosing Cholangitis 2012” have been reported[2]. Many patients with IgG4-SC have autoimmune pancreatitis (AIP) and respond to steroid treatment[3]. It can be difficult to differentially diagnose IgG4-SC from conditions such as primary sclerosing cholangitis (PSC) and biliary cancer, particularly in patients who have IgG4-SC alone. We describe our experience with a patient who had IgG4-SC without AIP in whom the presence of autoimmune hemolytic anemia (AIHA) led to diagnosis. To our knowledge, patients with IgG4-SC and AIHA have not been reported previously, suggesting that such cases are extremely rare.
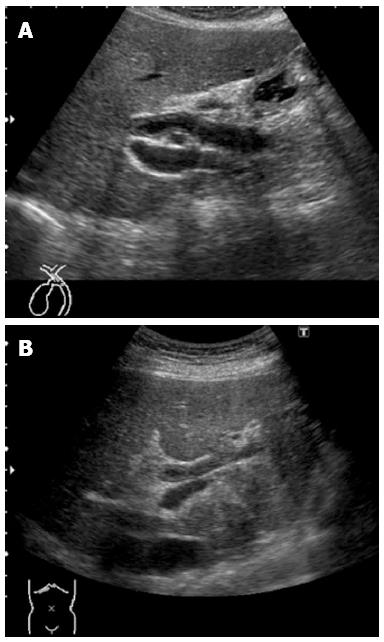
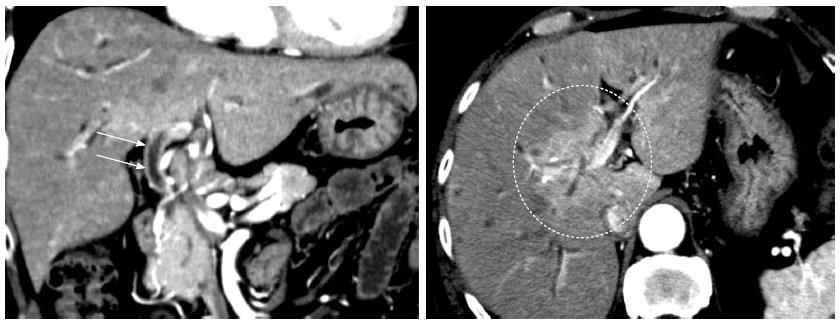
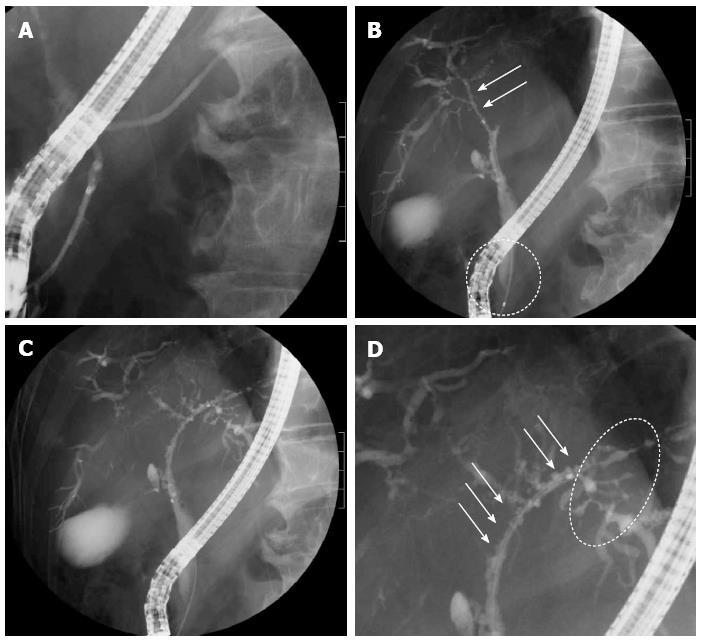
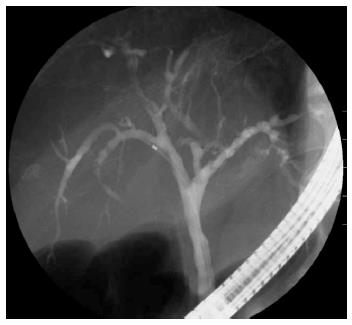
The patient was a 73-year-old man who was receiving treatment for dementia. Liver dysfunction was diagnosed on blood tests performed at another hospital. The family history was not relevant to the current disorder. The patient was not a smoker and did not drink alcohol and had no physical abnormalities or symptoms. The results of blood tests are shown in Table 1. The serum aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, and γ-glutamyl transpeptidase levels were elevated, but there was no evidence of jaundice. The IgG (18.0 g/L) and IgG4 (2.3 g/L) levels were also high. Abdominal ultrasonography showed wall thickening in the region extending from the common bile duct to the intrahepatic bile duct (Figure 1). Contrast-enhanced, early-phase computed tomography showed wall thickening with contrast enhancement in the region from the upper biliary tract to the intrahepatic bile duct and strong contrast enhancement in the hepatic parenchyma in the portal region (Figure 2). Endoscopic retrograde cholangiopancreatography (ERCP) revealed no abnormalities of the pancreatic ducts. Imaging studies of the bile ducts showed dilation after a confluent stricture, with no strictures of the lower common bile duct. However, findings consistent with a “band-like stricture” and “beaded appearance,” frequently associated with PSC (Figure 3), were evident. Initially, hilar cholangiocarcinoma and PSC were suspected. Cytologic examination of bile specimens obtained at the time of ERCP and biliary brush cytology showed no clinically significant findings of malignancy. The patient was therefore observed while receiving ursodeoxycholic acid, and liver dysfunction transiently improved. However, obstructive cholangitis developed after about 3 mo, and a biliary stent was placed to drain the biliary tract. Subsequently, jaundice worsened with a high direct bilirubin level, and anemia progressed (Table 1). Although the patient had jaundice with a very high direct bilirubin level, he had a positive direct Coombs test with a high reticulocyte count, a low haptoglobin level, and an elevated lactate dehydrogenase level. AIHA was thus diagnosed. Prednisolone (1 mg/kg per day, 60 mg) was administered, and the anemia as well as the jaundice gradually improved (Table 1). ERCP images obtained 1 mo after starting steroid therapy are shown in Figure 4. The biliary stricture at the portal region had markedly improved. The biliary images, elevated serum IgG4 level, and response to steroid treatment met the diagnostic criteria for a probable diagnosis of IG4-SC according to the “Clinical Diagnostic Criteria of IgG4-related Sclerosing Cholangitis 2012,” and IgG4-SC was thus diagnosed.
| Initial presentation | At time of cholangitis | After steroid treatment | |
| White blood cells (× 109/L) | 5.8 | 19.5 | 7.2 |
| Red blood cells (× 1012/L) | 3.97 | 0.94 | 3.91 |
| Hemoglobin (g/L) | 129 | 41 | 127 |
| Hematocrit | 38% | 11.5% | 39.2% |
| Platelets (× 109/L) | 223 | 319 | 212 |
| Reticulocytes | 19‰ | 320‰ | 15‰ |
| Haptoglobin (mg/L) | - | 100 | - |
| Total bilirubin (μmol/L) | 7 | 728 | 9 |
| Direct bilirubin (μmol/L) | 3 | 616 | 3 |
| Aspartate aminotransferase (IU/L) | 63 | 144 | 16 |
| Alanine aminotransferase (IU/L) | 149 | 110 | 12 |
| Alkaline phosphatase (IU/L) | 1056 | 1151 | 131 |
| γ-Glutamyl transpeptidase (IU/L) | 784 | 302 | 16 |
| Lactate dehydrogenase (IU/L) | 205 | 1397 | 184 |
| C-reactive protein (nmol/L) | 32 | 137 | < 10 |
| IgG (g/L) (NR 8.7-17.0) | 18 | 16.6 | 11.4 |
| IgG4 (g/L) (NR 0.048-1.05) | 2.3 | - | 1.0 |
| Carbohydrate antigen 19-9 (U/mL) | 15.2 | 94.2 | 22.8 |
A literature search of the PubMed/MEDLINE and Embase databases indicated that cases of IgG4-SC associated with AIHA have not been reported previously. Many patients with IgG4-SC have AIP and respond to steroid therapy. In patients who have IgG4-SC alone, it is particularly difficult to differentiate IgG4-SC from PSC and biliary cancer, making the diagnosis challenging. We described our experience with a patient who had IgG4-SC without AIP in whom the presence of AIHA led to diagnosis. Clinically, IgG4-SC is characterized by jaundice leading to diagnosis as well as stricture of the lower bile duct on imaging studies of the biliary tract. Our patient initially presented with liver dysfunction, with no evidence of jaundice on blood tests, and had no findings suggesting AIP or stricture of the lower bile duct on imaging studies. Blood tests at initial presentation showed an elevated serum IgG4 concentration (Table 1). There was a time lag of about 1 wk until the results of specialized tests became available. IgG4-SC is characterized by elevated serum IgG4 levels, but elevated levels may also be found in PSC. Boonstra et al[4] reported that the IgG4/IgG1 ratio is useful for differentiating IgG4-SC from PSC. However, the IgG4/IgG1 ratio was unavailable because we did not measure serum IgG1 levels. Cholangiographic images in our patient were consistent with type 4 IgG4-SC according to the classification proposed by Nakazawa et al[5,6] and are thus very rarely encountered. These atypical clinical and imaging findings made diagnosis very difficult.
AIHA is a disease that responds to steroid treatment, but can be associated with connective-tissue diseases such as systemic lupus erythematosus, autoimmune lymphoproliferative syndrome, common variable immune deficiency, and hematologic diseases such as chronic lymphocytic leukemia[7]. Several patients with PSC and AIHA have been reported on[8-11], but to our knowledge patients with IgG4-SC associated with AIHA have not been documented previously. In AIHA, IgG antibodies bound to erythrocyte membranes might be identified by IgG Fc receptors of phagocytes and phagocytized. IgG Fc receptors of phagocytes are specific for IgG1 and IgG3 and essentially show no activity for IgG2 or IgG4. Phagocytes have receptors for complement C3b, whereas IgG4 has been reported to lack complement activation activity[12-14]. These findings suggest that IgG4 does not contribute to the development of AIHA. Our patient probably had one of the many potential combinations of autoimmune disorders. In other words, congenital or acquired factors may have caused autoimmune disorders in our patient, resulting in the simultaneous development of IgG4-SC and AIHA.
In our patient, difficulty was encountered in the diagnosis of biliary stricture seen mainly in the portal region, and treatment was required for a rapidly progressing anemia. There was no response to transfusion therapy. Because the patient had a positive direct Coombs test, and the results of blood tests supported a diagnosis of hemolysis, AIHA was diagnosed. Steroid therapy was started to treat AIHA, and the biliary lesions improved relatively promptly, indicating a diagnosis of IgG4-SC. At the time of this writing, 18 mo after the onset of the current disorder, the patient is receiving 3 mg of prednisolone on consecutive days, with no recurrence.
The patient presented with no symptoms, and liver dysfunction was diagnosed on blood tests.
The presence of autoimmune hemolytic anemia (AIHA) led to the diagnosis of immunoglobulin G4-related sclerosing cholangitis (IgG4-SC) in a patient without autoimmune pancreatitis (AIP).
It can be difficult to differentially diagnose IgG4-SC from conditions such as primary sclerosing cholangitis (PSC) and biliary cancer, particularly in patients who have IgG4-SC alone.
The serum IgG, IgG4, aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, and γ-glutamyl transpeptidase levels were elevated, but there was no evidence of jaundice.
Endoscopic retrograde cholangiopancreatography (ERCP) revealed no abnormalities of the pancreatic ducts, and imaging studies of the bile ducts showed dilation after a confluent stricture, with no strictures of the lower common bile duct.
Cytologic examination of bile specimens obtained at the time of ERCP and biliary brush cytology showed no clinically significant findings of malignancy.
Prednisolone (1 mg/kg per day, 60 mg) was administered, and the anemia as well as the jaundice gradually improved.
In patients who have IgG4-SC alone, it is particularly difficult to differentiate IgG4-SC from PSC and biliary cancer, making the diagnosis challenging. IgG4/IgG1 ratio might be useful for distinguish PSC from IgG4-SC as reported by Boonstra et al in Hepatology.
IgG4-SC was first reported in Japan and refers to sclerosing cholangitis of unknown cause, characterized by increased serum IgG4 levels and fibrosis associated with marked infiltration of local lesions by lymphocytes and IgG4-positive plasma cells.
In the patient, the presence of AIHA led to the diagnosis of IgG4-SC unassociated with AIP.
To our knowledge, patients with IgG4-SC and AIHA have not been reported previously, suggesting that such cases are extremely rare. In patients who have IgG4-SC alone, it is particularly difficult to differentiate IgG4-SC from PSC and biliary cancer, making the diagnosis challenging. Boonstra et al reported that the IgG4/IgG1 ratio is useful for differentiating IgG4-SC from PSC. However, the IgG4/IgG1 ratio was unavailable because we did not measure serum IgG1 levels. The authors described their experience with a patient who had IgG4-SC without AIP in whom the presence of AIHA led to diagnosis.
P- Reviewers: Balzano G, Gong ZJ, Hoff DAL, Yazisiz V S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN
| 1. | Kamisawa T, Funata N, Hayashi Y, Eishi Y, Koike M, Tsuruta K, Okamoto A, Egawa N, Nakajima H. A new clinicopathological entity of IgG4-related autoimmune disease. J Gastroenterol. 2003;38:982-984. [PubMed] |
| 2. | Ohara H, Okazaki K, Tsubouchi H, Inui K, Kawa S, Kamisawa T, Tazuma S, Uchida K, Hirano K, Yoshida H. Clinical diagnostic criteria of IgG4-related sclerosing cholangitis 2012. J Hepatobiliary Pancreat Sci. 2012;19:536-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 251] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 3. | Ghazale A, Chari ST, Zhang L, Smyrk TC, Takahashi N, Levy MJ, Topazian MD, Clain JE, Pearson RK, Petersen BT. Immunoglobulin G4-associated cholangitis: clinical profile and response to therapy. Gastroenterology. 2008;134:706-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 667] [Cited by in RCA: 585] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 4. | Boonstra K, Culver EL, de Buy Wenniger LM, van Heerde MJ, van Erpecum KJ, Poen AC, van Nieuwkerk KM, Spanier BW, Witteman BJ, Tuynman HA. Serum immunoglobulin G4 and immunoglobulin G1 for distinguishing immunoglobulin G4-associated cholangitis from primary sclerosing cholangitis. Hepatology. 2014;59:1954-1963. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 125] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 5. | Nakazawa T, Naitoh I, Hayashi K, Miyabe K, Simizu S, Joh T. Diagnosis of IgG4-related sclerosing cholangitis. World J Gastroenterol. 2013;19:7661-7670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 78] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 6. | Nakazawa T, Ohara H, Sano H, Ando T, Joh T. Schematic classification of sclerosing cholangitis with autoimmune pancreatitis by cholangiography. Pancreas. 2006;32:229. [PubMed] |
| 7. | Lechner K, Jäger U. How I treat autoimmune hemolytic anemias in adults. Blood. 2010;116:1831-1838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 215] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 8. | Holm T, Kildahl-Andersen O, Valle PC. Primary sclerosing cholangitis with autoimmune hemolytic anemia and pulmonary infiltrations. Scand J Gastroenterol. 1998;33:669-672. [PubMed] |
| 9. | Eilam O, Goldin E, Shouval D, Gimon T, Brautbar C. Sclerosing cholangitis associated with Crohn’s disease and autoimmune haemolytic anaemia. Postgrad Med J. 1993;69:656-658. [PubMed] |
| 10. | Moeller DD. Sclerosing cholangitis associated with autoimmune hemolytic anemia and hyperthyroidism. Am J Gastroenterol. 1985;80:122-125. [PubMed] |
| 11. | Pineau BC, Pattee LP, McGuire S, Sekar A, Scully LJ. Unusual presentation of primary sclerosing cholangitis. Can J Gastroenterol. 1997;11:45-48. [PubMed] |
| 12. | von dem Borne AE, Beckers D, van der Meulen FW, Engelfriet CP. IgG4 autoantibodies against erythrocytes, without increased haemolysis: a case report. Br J Haematol. 1977;37:137-144. [PubMed] |
| 13. | Engelfriet CP, Borne AE, Beckers D, Van Loghem JJ. Autoimmune haemolytic anaemia: serological and immunochemical characteristics of the autoantibodies; mechanisms of cell destruction. Ser Haematol. 1974;7:328-347. [PubMed] |
| 14. | Komine M. [Autoimmune hemolytic anemia]. Rinsho Ketsueki. 1992;33:897-901. [PubMed] |