Published online Jun 21, 2014. doi: 10.3748/wjg.v20.i23.7298
Revised: December 19, 2013
Accepted: April 30, 2014
Published online: June 21, 2014
Processing time: 236 Days and 22.4 Hours
It has been reported that host defense responses, such as phagocytic function of neutrophils and natural killer (NK) cell activity of lymphocytes, are impaired in cirrhotic patients. This review will concentrate on the impairment of innate immune responses in decompensated cirrhotic patients and the effect of the treatment by branched-chain amino acids (BCAA) on innate immune responses. We already reported that phagocytic function of neutrophils was significantly improved by 3-mo BCAA supplementation. In addition, the changes of NK activity were also significant at 3 mo of supplementation compared with before supplementation. Also, Fisher’s ratios were reported to be significantly increased at 3 mo of BCAA supplementation compared with those before oral supplementation. Therefore, administration of BCAA could reduce the risk of bacterial and viral infection in patients with decompensated cirrhosis by restoring impaired innate immune responses of the host. In addition, it was also revealed that BCAA oral supplementation could reduce the risk of development of hepatocellular carcinoma in cirrhotic patients. The mechanisms of the effects will also be discussed in this review article.
Core tip: This review will discuss the recent research on impairment of innate immune responses in cirrhotic patients and the treatment by branched-chain amino acids (BCAA). It was revealed that BCAA oral supplementation could improve not only nutrition status but phagocytic function of neutrophils and natural killer activity of lymphocytes in cirrhotic patients. Therefore, BCAA supplementation might reduce the risk of bacterial and viral infection in patients with decompensated cirrhosis. Additionally, it was also revealed that BCAA oral supplementation could reduce the risk of development of hepatocellular carcinoma in cirrhotic patients. The mechanisms of the effects of BCAA described above will also be discussed.
- Citation: Nakamura I. Impairment of innate immune responses in cirrhotic patients and treatment by branched-chain amino acids. World J Gastroenterol 2014; 20(23): 7298-7305
- URL: https://www.wjgnet.com/1007-9327/full/v20/i23/7298.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i23.7298
Innate immunity is the first defense mechanism of host against pathogens such as bacteria and viruses. Patients with insufficient innate immunity may have increased risk of bacterial and viral infection. Patients with liver cirrhosis, especially those with decompensated cirrhosis, are liable to suffer from bacterial infection such as bacteremia and spontaneous bacterial peritonitis (SBP), which could be lethal to the patients. The incidence of bacterial infections in cirrhotic patients is almost 4-7 times greater than that of general hospital population[1,2]. SBP occurs in 10%-25% of hospitalized cirrhotic patients and its mortality rate is 17%-50%[3]. The high incidence of bacterial infections in patients with cirrhosis has prompted an assessment of defects in their immune defense against microorganisms. The functional studies of peripheral blood neutrophils have shown defective phagocytic activity and intracellular killing activity in patients with cirrhosis[4-9]. In addition, natural killer (NK) cells also constitute the first line of host defense against invading pathogens. They usually become activated in an early phase of viral infection. Effective clearance of an acute viral infection requires the coordinated function of multiple arms of the immune systems, including innate immune systems mediated by NK cells and cytokines such as interferon, as well as adaptive immune responses. It was reported that not only phagocytic function of neutrophils but NK activity of lymphocytes were impaired in cirrhotic patients[10-14].
So far, there are few therapies for restoration of activity of immune responses of innate immunity in patients with liver cirrhosis. Branched-chain amino acid (BCAA) supplementation has been previously shown to improve serum albumin levels and Fisher’s ratios in those patients[15-17]. In addition, BCAA was also shown to improve impaired glucose tolerance of the cirrhotic patients with creatinine height index (CHI) greater than 80 by Urata et al[18]. It was reported that BCAA oral supplementation might reduce the incidence of events comprising the primary endpoint (which was a composite of death by any cause, development of liver cancer, rupture of esophageal varices, or progress of hepatic failure[19]. In addition, recent studies have revealed that BCAA promotes albumin synthesis in rat hepatocyte through activation of mammalian target of rapamycin (mTOR) signal transduction system[20]. Much is still unknown on the effects of BCAA supplementation on reactions of innate immunity. This review will concentrate on the impairment of innate immune responses in decompensated cirrhotic patients and the effect of the treatment by BCAA on innate immune responses.
It was reported that alterations of the immune system are common in patients with end-stage liver disease and associated with an increased risk of infection and death[1-3,21-24]. Bacterial infection involving such as urinary tract, ascites, blood, respiratory tract is a severe complication of decompensated cirrhosis. Additionally, it might induce longer hospital stay and increased mortality[21]. It was previously reported that infections in patients with cirrhosis increase mortality 4-fold; 30% of patients die within 1 mo after infection and another 30% die by 1 year[22]. In patients with cirrhosis and severe sepsis, high production of proinflammatory cytokines seems to play a role in the worsening of liver function and the development of organ failures such as shock, renal failure, acute lung injury or acute respiratory distress syndrome, coagulopathy, or hepatic encephalopathy[23].
Functional abnormalities of neutrophils and macrophages[4-9], NK cells[10-14], and the complement system[25] contribute to impaired innate immune responses. On Neutrophils, it was reported that there was a defect of neutrophil phagocytosis and a defect of intracellular killing of bacteria in cirrhotic patients. In addition, it was revealed that these neutrophil defects are caused by both reduced production of superoxide and defects of degranulation[4]. In addition, the function of macrophage Fc gamma receptors was reported to be impaired in patients with cirrhosis, and this impairment probably contributes to the high incidence of bacterial infections among such patients[6]. Neutrophil migration and phagocytosis were reported to be decreased in cirrhotic patients with previous episodes of bacterial infection compared with non-infected patients. In addition, expression of complement receptor type III (CR3) in circulating neutrophils was significantly higher in cirrhotic patients. These data suggest that deficient neutrophil recruitment to the infection site and impaired phagocytic activity may contribute to bacterial infections in cirrhotic patients with advanced liver disease[5]. Polymorphonuclear cells (PMNs) obtained from cirrhotic patients were reported to be less effective than those from controls in producing O2- after stimulation with opsonized zymosan, while they were more effective in producing NO, NO synthase activity was higher in leukocytes from cirrhotic patients than in controls[8]. It was also reported that the plasma of cirrhotic patients induced neutrophil phagocytic dysfunction and the degree of the impairment was greater in cirrhotic patients with more severe disease[9]. The study also clarified that dysfunction of phagocytic function of neutrophils was associated with increased expression of toll-like receptors 2 and 4. Stable cirrhosis is characterized by neutrophil phagocytic dysfunction which may be subtle and only revealed in inflamed peripheral tissues where excessive inflammatory mediators continue to be released (Table 1).
| Impairment of phagocytic activity of neutrophils |
| Impairment of intracellular killing activity of neutrophils |
| Impairment of migration of neutrophils |
| Impairment of natural killer cell activity |
| Impairment of function of macrophage Fc gamma receptors |
| Impairment of opsonisation activity |
| Impairment of hemolytic complement function |
NK cell activity was revealed to be significantly decreased in cirrhotic patients compared with normal controls and that in patients with other, non-malignant diseases, supporting the notion that immune-surveillance mechanisms may be affected in these patients[10-12]. Chuang et al[12] reported that cirrhotic patients with Child Pugh’s C grade of severity of liver disease had lower NK cell activity. The depression of NK cell activity in cirrhotic patients was inversely correlated with prothrombin time ratios. And NK cell activity in cirrhotic patients with hepatic encephalopathy was lower than that in patients without hepatic encephalopathy. Thus, the diminished NK cell activity in cirrhotic patients might be related to the severity of liver damage[12]. Reduction of NK cell activity might occur partially due to lower frequency of NK cell in peripheral blood[13]. However, the mechanisms of diminished NK cell activity was not clarified so far.
Also, acquired deficiencies of certain complement proteins and impaired opsonisation activity was reported to be implicated in the pathogenesis of the increased susceptibility to infections of patients with cirrhosis. Low serum C3 concentrations and decreased haemolytic complement function predispose to infection and increased mortality in patients with cirrhosis[25].
On the adaptive immune response in cirrhotic patients, there was evidence to suggest that the response was defective[26,27]. Generally speaking, impairment of adaptive immune responses can be due to either impairment of antigen presentation of professional cells or to reduction of T cell responses. T cell such as cytotoxic T lymphocyte (CTL) and helper T cell (Th) recognize a peptide-MHC complex on antigen presenting cell (Table 2). In cirrhotic patients, levels of interleukin-10 (IL-10) in blood became high due to increase of endotoxin and tumor necrosis factor-alpha (TNF-α)[28-30]. IL-10 could reduce cytokine responses of T cells, reduction of MHC class II expression on antigen-presenting cells and suppression of co-stimulatory signals[31-34].
| Down regulation of major histocompatibility complex class II on monocyte/macrophages |
| Impairment of maturation and function of myeloid dendritic cells |
| Inhibition of T cell proliferation and T cell-mediated cytokine production |
| Inhibition of T-cell co stimulatory pathways |
| Impairment of tumor necrosis factor-alpha production of CD4+ and CD8+ cells |
| Increased fraction of CD4+ CD25+ cells |
| Increase in circulating anti-inflammatory cytokines |
In addition, there was a report on association of T cell responses in vitro and markers of bacterial translocation, serum IL-10, monocyte HLA-DR expression and T cell subsets in cirrhotic patients[24]. Advanced liver disease predisposes to bacterial translocation and endotoxaemia which can contribute to elevated circulating levels of IL-10 and down-regulation of MHC class II on antigen-presenting cells. Peter et al[24] evaluated antigen-specific T-cell responses toward common viral antigens in order to investigate defects in cellular immunity in cirrhosis. Compared to healthy controls, patients with cirrhosis had higher circulating levels of LBP and IL-10, an expansion of peripheral blood CD14+ monocytes with low HLA-DR expression and an increased fraction of CD25-positive CD4+ and CD8+ T cells. These findings were reported to be most pronounced in cirrhotic patients with systemic inflammation. Furthermore, TNF-α production in responding T cells was attenuated in patients with a high frequency of CD14+ HLA-DR- monocytes. The results of the study suggested that bacterial translocation, endotoxaemia, inflammation and T cell activation in cirrhosis are accompanied by an increase in circulating anti-inflammatory cytokines, reduced monocytic MHC class II expression and attenuated cytokine production in T cells.
BCAA comprise three essential amino acids: leucine (Leu), isoleucine (Ile), and valine (Val). And chemical formulas of Leu, Ile, and Val are HO2CCH(NH2)CH2CH(CH3)2, HO2CCH(NH2)CH(CH3)CH2CH3, and HO2CCH(NH2)CH(CH3)2 respectively.
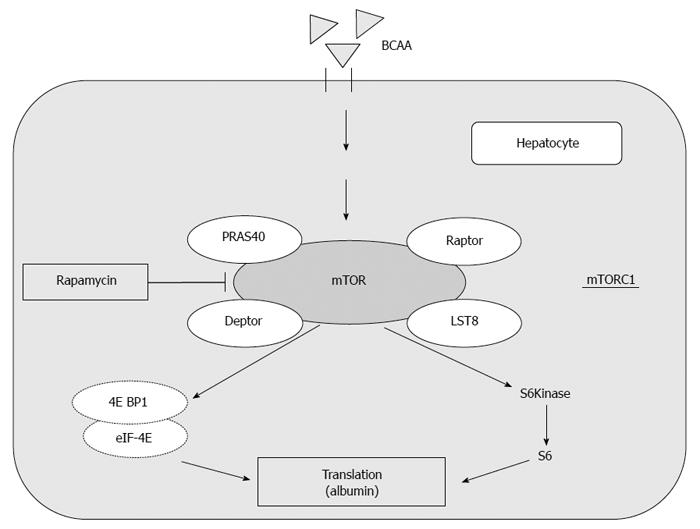
They are often used as supplemental therapy to improve protein malnutrition in patients with liver cirrhosis. Long-term oral supplementation with BCAA granules to cirrhotic patients improved their nutrition status (i.e., hypoalbuminemia)[19,35]. In addition to the role of acting as nutrient substrates, recent studies have demonstrated that BCAA also serve as physiologically active substances. BCAA have been shown to have pharmacological effects, such as induction of protein synthesis[36] and promotion of glucose uptake in skeletal muscle[37]. In rat primary hepatocytes, albumin synthesis is significantly increased by BCAA administration, which is dependent on activation of the mammalian target of rapamycin (mTOR), mainly induced by leucine (Figure 1)[20].
mTOR integrates the input from upstream pathways, including insulin, growth factors (such as IGF-1 and IGF-2), and amino acids[38]. In addition, mTOR also senses cellular nutrient, oxygen, and energy levels[39]. The mTOR pathway is dysregulated in human diseases, such as diabetes, obesity, depression, and certain cancers[40]. mTOR is the catalytic subunit of two molecular complexes: mTORC1 and mTORC2[41]. mTOR complex 1 (mTORC1) is composed of mTOR, regulatory-associated protein of mTOR (Raptor), mammalian lethal with SEC13 protein 8 (LST8) and the recently identified partners PRAS40 and DEPTOR (Figure 1)[42,43]. This complex is characterized by the classic features of mTOR by functioning as a nutrient/energy/redox sensor and controlling protein synthesis[38,42]. The activity of this complex is stimulated by insulin, growth factors, serum, phosphatidic acid, amino acids (particularly leucine), and oxidative stress[42,44].
In this paragraph, several reports on promotion of albumin synthesis by BCAA through activation of the mammalian target of rapamycin (mTOR) signal transduction system were introduced (Figure 1)[20].
We reported on restoration of innate immune responses such as phagocytic function of neutrophils and NK cell activity[45,46]. In the reports, patients with decompensated cirrhosis received 12 g BCAA daily for 3 mo. Phagocytic function of neutrophils and NK cell activity of lymphocytes as well as Fisher’s ratio were determined before and at 1 and 3 mo of BCAA supplementation. For quantification of phagocytic function, fluorescent intensities of cells in the neutrophil region in the cytogram were determined by flow cytometry after incubation of whole blood with fluorescent microspheres. NK cell activity was estimated by 51Cr release assay using K-562 cell line as target cells. Fisher’s ratio was reported to be significantly increased at 1 mo of BCAA supplementation compared with that before oral supplementation and also at 3 mo of BCAA supplementation compared with that before oral supplementation. In addition, the phagocytic function of neutrophils was reported to be significantly increased at 3 mo of supplementation compared with that before BCAA supplementation. It was also reported on the effects of BCAA supplementation on NK activity of lymphocytes. NK cell activity were also significantly improved at 3 mo of supplementation compared with that before BCAA supplementation.
In the chapter of discussion in our report, the several points were discussed as follows[46]. There were few therapies to restore the reactions of innate immunity such as phagocytic function of neutrophils and NK activity of lymphocytes. The mechanisms of the improvement by BCAA supplementation were not completely elucidated. It has been reported that BCAA supplementation promotes albumin synthesis through activation of the mTOR signal transduction system[20]. In addition, not only synthesis of albumin but HGF synthesis was enhanced by BCAA supplementation. Therefore, production of a factor which could stimulate neutrophil function of phagocytosis such as tuftsin[47,48] might be increased by BCAA by mTOR signal transduction system. Alternatively, improvement of poor nutritional status in cirrhotic patient by BCAA might restore phagocytic function of neutrophils and NK activity of lymphocytes by indirect mechanisms. In the study, phagocytic function of neutrophils and NK activity of lymphocytes were significantly restored before serum albumin level became statistically improved. That might imply a clue to clarify the mechanisms of effect on improvement of reactions of innate immunity by BCAA oral supplementation. To estimate the effects of BCAA supplementation on the innate host responses in cirrhotic patients, further studies on intrahepatic neutrophils, Kupffer cells and pit cells might be valuable.
A multicenter, randomized, and nutrient intake-controlled trial on the comparative effects of BCAA, conducted in 646 patients with decompensated cirrhosis, showed that the incidence of events comprising the primary endpoint (which was a composite of death by any cause, development of liver cancer, rupture of esophageal varices, or progress of hepatic failure) significantly decreased in the BCAA supplementation group as compared with the control group[19]. Marchesini et al[35] also reported that long-term oral supplementation of BCAA granules to cirrhotic patients were reported to improve not only their nutrition status (i.e., hypoalbuminemia) but their event-free survival. In addition, Muto et al[49] reported that close association exists between insulin resistance due to hyperinsulinemia and BCAA, and that this association contributes to the progression of hepatocellular carcinoma (HCC) in cirrhotic patients. It was also reported that the risk for liver cancer was significantly reduced by oral BCAA supplementation in the patients with a BMI of 25 or higher. Oral supplemental treatment with BCAA might reduce the risk of liver cancer in cirrhotic patients[49]. Recent studies have revealed that BCAA supplemental therapy to patients with liver cirrhosis improves their insulin resistance and hyperinsulinemia[50,51], which can account for the reduced risk of HCC. BCAA is supposed to prevent insulin resistance through improving glucose tolerance by promoting insulin-independent glucose uptake by skeletal muscle[52].
There have been only a few reports to date regarding the suppression of liver cancer progression by BCAA. Murata et al[53] showed that isoleucine prevents tumor growth in a mouse liver metastatic model of colon cancer through inhibition of vascular endothelial growth factor (VEGF). Yoshiji et al[54] reported that BCAA exerts a chemopreventive effect against HCC, which is associated with the suppression of VEGF expression and hepatic neovascularization in obese diabetic rats. Both of these reports suggest an anti-angiogenesis activity of BCAA or Isoleucine through suppression of VEGF expression.
In the study by Miuma et al[55], they analyzed the expression of vascular VEGF in HepG2 cells under high-insulin culture conditions, and examined the effect of BCAA on VEGF expression. VEGF secretion was significantly increased by 200 nmol/L of insulin under BCAA deficient conditions, but it was repressed by the addition of BCAA. BCAA activated the mTOR pathway and increase HIF-1a expression under high-insulin culture conditions, however quantitative PCR analysis showed that insulin-induced expression of VEGF mRNAs decreased 2 h after the addition of BCAA. The half-lives of VEGF mRNAs were shortened in the presence of BCAA compared to the absence of BCAA. Therefore, the results of the study suggested that BCAA regulate VEGF expression mainly at the post-transcriptional level in patients who have hyperinsulinemia and are in the process of developing HCC. They also examined which of the Valine, Leucine, and Isoleucine components of BCAA were essential for VEGF mRNA degradation. All three BCAA components were revealed to be required for acceleration of insulin-induced VEGF mRNA degradation.
Recently, another study on the mechanisms of reduction of the risk of HCC development by BCAA was reported[56]. Hagiwara et al[56] reported the result of the study to investigate the effects of BCAA on insulin-induced proliferation of hepatic tumor cells and determine the underlying mechanisms. BCAA was reported to suppress insulin-induced cell proliferation of H4IIE, HepG2 cells. They demonstrated that BCAA inhibited PI3K/Akt pathway not only by promoting negative feedback loop from mammalian target of rapamycin complex 1 (mTORC1)/S6K1 to PI3K/Akt pathway, but also by suppressing mTORC2 kinase activity toward Akt. Their findings suggested that BCAA supplementation may be useful to suppress liver cancer progression by inhibiting insulin-induced PI3K/Akt and subsequent anti-apoptotic pathway, indicating the importance of BCAA supplementation to the obese patients with advanced liver disease.
Furthermore, NK cells were previously revealed to play important roles not only in the defense against viral infection but in tumor surveillance[57,58]. Therefore, the restoration of NK cell activity by BCAA supplementation might partly contribute to the reduction of the risk of HCC in cirrhotic patients.
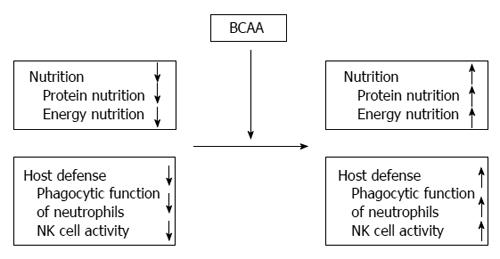
BCAA oral supplementation could improve not only nutrition status (both protein nutrition and energy nutrition ) but phagocytic function of neutrophils and NK activity of lymphocytes in cirrhotic patients (Figure 2). BCAA supplementation might reduce the risk of bacterial and viral infection in patients with decompensated cirrhosis. In addition, administration of BCAA could improve glucose intolerance and hyperinsulinemia in cirrhotic patients. It has also been reported that the risk of developing HCC could be significantly reduced following long-term administration of BCAA in obese cirrhotic patients with diabetes mellitus.
P- Reviewers: Liu Y, Yokota S S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN
| 1. | Rimola A, Soto R, Bory F, Arroyo V, Piera C, Rodes J. Reticuloendothelial system phagocytic activity in cirrhosis and its relation to bacterial infections and prognosis. Hepatology. 1984;4:53-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 313] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 2. | Barnes PF, Arevalo C, Chan LS, Wong SF, Reynolds TB. A prospective evaluation of bacteremic patients with chronic liver disease. Hepatology. 1988;8:1099-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 44] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Hoefs JC. Spontaneous bacterial peritonitis: prevention and therapy. Hepatology. 1990;12:776-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Rajkovic IA, Williams R. Abnormalities of neutrophil phagocytosis, intracellular killing and metabolic activity in alcoholic cirrhosis and hepatitis. Hepatology. 1986;6:252-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 188] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Fiuza C, Salcedo M, Clemente G, Tellado JM. In vivo neutrophil dysfunction in cirrhotic patients with advanced liver disease. J Infect Dis. 2000;182:526-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 157] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 6. | Gomez F, Ruiz P, Schreiber AD. Impaired function of macrophage Fc gamma receptors and bacterial infection in alcoholic cirrhosis. N Engl J Med. 1994;331:1122-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 115] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Bruns T, Peter J, Hagel S, Herrmann A, Stallmach A. The augmented neutrophil respiratory burst in response to Escherichia coli is reduced in liver cirrhosis during infection. Clin Exp Immunol. 2011;164:346-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Masini E, Mugnai L, Foschi M, Laffi G, Gentilini P, Mannaioni PF. Changes in the production of nitric oxide and superoxide by inflammatory cells in liver cirrhosis. Int Arch Allergy Immunol. 1995;107:197-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Tritto G, Bechlis Z, Stadlbauer V, Davies N, Francés R, Shah N, Mookerjee RP, Such J, Jalan R. Evidence of neutrophil functional defect despite inflammation in stable cirrhosis. J Hepatol. 2011;55:574-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 147] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 10. | Nakamura T, Morizane T, Watanabe T, Tsuchimoto K, Inagaki Y, Kumagai N, Tsuchiya M. Decreased natural killer activity in patients with liver cirrhosis. Int J Cancer. 1983;32:573-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Laso FJ, Madruga JI, Girón JA, López A, Ciudad J, San Miguel JF, Alvarez-Mon M, Orfao A. Decreased natural killer cytotoxic activity in chronic alcoholism is associated with alcohol liver disease but not active ethanol consumption. Hepatology. 1997;25:1096-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 81] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Chuang WL, Liu HW, Chang WY, Chen SC, Hsieh MY, Wang LY. Natural killer cell activity in patients with liver cirrhosis relative to severity of liver damage. Dig Dis Sci. 1991;36:299-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Meier UC, Owen RE, Taylor E, Worth A, Naoumov N, Willberg C, Tang K, Newton P, Pellegrino P, Williams I. Shared alterations in NK cell frequency, phenotype, and function in chronic human immunodeficiency virus and hepatitis C virus infections. J Virol. 2005;79:12365-12374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 141] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 14. | Pár G, Rukavina D, Podack ER, Horányi M, Szekeres-Barthó J, Hegedüs G, Paál M, Szereday L, Mózsik G, Pár A. Decrease in CD3-negative-CD8dim(+) and Vdelta2/Vgamma9 TcR+ peripheral blood lymphocyte counts, low perforin expression and the impairment of natural killer cell activity is associated with chronic hepatitis C virus infection. J Hepatol. 2002;37:514-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 78] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Yoshida T, Muto Y, Moriwaki H, Yamato M. Effect of long-term oral supplementation with branched-chain amino acid granules on the prognosis of liver cirrhosis. Gastroenterol Jpn. 1989;24:692-698. [PubMed] |
| 16. | Bianchi GP, Marchesini G, Zoli M, Abbiati R, Ferrario E, Fabbri A, Pisi E. Oral BCAA supplementation in cirrhosis with chronic encephalopathy: effects on prolactin and estradiol levels. Hepatogastroenterology. 1992;39:443-446. [PubMed] |
| 17. | Habu D, Nishiguchi S, Nakatani S, Kawamura E, Lee C, Enomoto M, Tamori A, Takeda T, Tanaka T, Shiomi S. Effect of oral supplementation with branched-chain amino acid granules on serum albumin level in the early stage of cirrhosis: a randomized pilot trial. Hepatol Res. 2003;25:312-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Urata Y, Okita K, Korenaga K, Uchida K, Yamasaki T, Sakaida I. The effect of supplementation with branched-chain amino acids in patients with liver cirrhosis. Hepatol Res. 2007;37:510-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Muto Y, Sato S, Watanabe A, Moriwaki H, Suzuki K, Kato A, Kato M, Nakamura T, Higuchi K, Nishiguchi S. Effects of oral branched-chain amino acid granules on event-free survival in patients with liver cirrhosis. Clin Gastroenterol Hepatol. 2005;3:705-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 364] [Article Influence: 18.2] [Reference Citation Analysis (1)] |
| 20. | Ijichi C, Matsumura T, Tsuji T, Eto Y. Branched-chain amino acids promote albumin synthesis in rat primary hepatocytes through the mTOR signal transduction system. Biochem Biophys Res Commun. 2003;303:59-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 90] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 21. | Borzio M, Salerno F, Piantoni L, Cazzaniga M, Angeli P, Bissoli F, Boccia S, Colloredo-Mels G, Corigliano P, Fornaciari G. Bacterial infection in patients with advanced cirrhosis: a multicentre prospective study. Dig Liver Dis. 2001;33:41-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 297] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 22. | Arvaniti V, D’Amico G, Fede G, Manousou P, Tsochatzis E, Pleguezuelo M, Burroughs AK. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 2010;139:1246-1256, 1256.e1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 720] [Cited by in RCA: 835] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 23. | Gustot T, Durand F, Lebrec D, Vincent JL, Moreau R. Severe sepsis in cirrhosis. Hepatology. 2009;50:2022-2033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 324] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 24. | Peter J, Frey O, Stallmach A, Bruns T. Attenuated antigen-specific T cell responses in cirrhosis are accompanied by elevated serum interleukin-10 levels and down-regulation of HLA-DR on monocytes. BMC Gastroenterol. 2013;13:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Homann C, Varming K, Høgåsen K, Mollnes TE, Graudal N, Thomsen AC, Garred P. Acquired C3 deficiency in patients with alcoholic cirrhosis predisposes to infection and increased mortality. Gut. 1997;40:544-549. [PubMed] |
| 26. | Rožnovský L, Orságová I, Petroušová L, Tvrdík J, Kabieszová L, Rydlo M, Lochman I, Kloudová A. [Low response rate to a vaccination against hepatitis B in patients with end-stage liver disease]. Klin Mikrobiol Infekc Lek. 2010;16:179-181. [PubMed] |
| 27. | Cheong HJ, Song JY, Park JW, Yeon JE, Byun KS, Lee CH, Cho HI, Kim TG, Kim WJ. Humoral and cellular immune responses to influenza vaccine in patients with advanced cirrhosis. Vaccine. 2006;24:2417-2422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | González-Navajas JM, Bellot P, Francés R, Zapater P, Muñoz C, García-Pagán JC, Pascual S, Pérez-Mateo M, Bosch J, Such J. Presence of bacterial-DNA in cirrhosis identifies a subgroup of patients with marked inflammatory response not related to endotoxin. J Hepatol. 2008;48:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Emmanuilidis K, Weighardt H, Maier S, Gerauer K, Fleischmann T, Zheng XX, Hancock WW, Holzmann B, Heidecke CD. Critical role of Kupffer cell-derived IL-10 for host defense in septic peritonitis. J Immunol. 2001;167:3919-3927. [PubMed] |
| 30. | Barsig J, Küsters S, Vogt K, Volk HD, Tiegs G, Wendel A. Lipopolysaccharide-induced interleukin-10 in mice: role of endogenous tumor necrosis factor-alpha. Eur J Immunol. 1995;25:2888-2893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 100] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 31. | Moore KW, O’Garra A, de Waal Malefyt R, Vieira P, Mosmann TR. Interleukin-10. Annu Rev Immunol. 1993;11:165-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1743] [Cited by in RCA: 1731] [Article Influence: 54.1] [Reference Citation Analysis (0)] |
| 32. | Akdis CA, Blaser K. Mechanisms of interleukin-10-mediated immune suppression. Immunology. 2001;103:131-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 262] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 33. | Berry PA, Antoniades CG, Carey I, McPhail MJ, Hussain MJ, Davies ET, Wendon JA, Vergani D. Severity of the compensatory anti-inflammatory response determined by monocyte HLA-DR expression may assist outcome prediction in cirrhosis. Intensive Care Med. 2011;37:453-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 34. | Berres ML, Schnyder B, Yagmur E, Inglis B, Stanzel S, Tischendorf JJ, Koch A, Winograd R, Trautwein C, Wasmuth HE. Longitudinal monocyte human leukocyte antigen-DR expression is a prognostic marker in critically ill patients with decompensated liver cirrhosis. Liver Int. 2009;29:536-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 35. | Marchesini G, Marzocchi R, Noia M, Bianchi G. Branched-chain amino acid supplementation in patients with liver diseases. J Nutr. 2005;135:1596S-1601S. [PubMed] |
| 36. | Anthony JC, Yoshizawa F, Anthony TG, Vary TC, Jefferson LS, Kimball SR. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr. 2000;130:2413-2419. [PubMed] |
| 37. | Nishitani S, Matsumura T, Fujitani S, Sonaka I, Miura Y, Yagasaki K. Leucine promotes glucose uptake in skeletal muscles of rats. Biochem Biophys Res Commun. 2002;299:693-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 116] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 38. | Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926-1945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3080] [Cited by in RCA: 3271] [Article Influence: 155.8] [Reference Citation Analysis (0)] |
| 39. | Tokunaga C, Yoshino K, Yonezawa K. mTOR integrates amino acid- and energy-sensing pathways. Biochem Biophys Res Commun. 2004;313:443-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 208] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 40. | Beevers CS, Li F, Liu L, Huang S. Curcumin inhibits the mammalian target of rapamycin-mediated signaling pathways in cancer cells. Int J Cancer. 2006;119:757-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 183] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 41. | Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4260] [Cited by in RCA: 4432] [Article Influence: 233.3] [Reference Citation Analysis (0)] |
| 42. | Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2250] [Cited by in RCA: 2316] [Article Influence: 100.7] [Reference Citation Analysis (0)] |
| 43. | Kim DH, Sarbassov DD, Ali SM, Latek RR, Guntur KV, Erdjument-Bromage H, Tempst P, Sabatini DM. GbetaL, a positive regulator of the rapamycin-sensitive pathway required for the nutrient-sensitive interaction between raptor and mTOR. Mol Cell. 2003;11:895-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 710] [Cited by in RCA: 731] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 44. | Fang Y, Vilella-Bach M, Bachmann R, Flanigan A, Chen J. Phosphatidic acid-mediated mitogenic activation of mTOR signaling. Science. 2001;294:1942-1945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 807] [Cited by in RCA: 824] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 45. | Nakamura I, Ochiai K, Imawari M. Phagocytic function of neutrophils of patients with decompensated liver cirrhosis is restored by oral supplementation of branched-chain amino acids. Hepatol Res. 2004;29:207-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 46. | Nakamura I, Ochiai K, Imai Y, Moriyasu F, Imawari M. Restoration of innate host defense responses by oral supplementation of branched-chain amino acids in decompensated cirrhotic patients. Hepatol Res. 2007;37:1062-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 47. | Najjar VA, Nishioka K. “Tuftsin”: a natural phagocytosis stimulating peptide. Nature. 1970;228:672-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 239] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 48. | Trevisani F, Castelli E, Foschi FG, Parazza M, Loggi E, Bertelli M, Melotti C, Domenicali M, Zoli G, Bernardi M. Impaired tuftsin activity in cirrhosis: relationship with splenic function and clinical outcome. Gut. 2002;50:707-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 49. | Muto Y, Sato S, Watanabe A, Moriwaki H, Suzuki K, Kato A, Kato M, Nakamura T, Higuchi K, Nishiguchi S. Overweight and obesity increase the risk for liver cancer in patients with liver cirrhosis and long-term oral supplementation with branched-chain amino acid granules inhibits liver carcinogenesis in heavier patients with liver cirrhosis. Hepatol Res. 2006;35:204-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 50. | Kawaguchi T, Taniguchi E, Itou M, Sumie S, Oriishi T, Matsuoka H, Nagao Y, Sata M. Branched-chain amino acids improve insulin resistance in patients with hepatitis C virus-related liver disease: report of two cases. Liver Int. 2007;27:1287-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 51. | Kawaguchi T, Nagao Y, Matsuoka H, Ide T, Sata M. Branched-chain amino acid-enriched supplementation improves insulin resistance in patients with chronic liver disease. Int J Mol Med. 2008;22:105-112. [PubMed] |
| 52. | Nishitani S, Takehana K, Fujitani S, Sonaka I. Branched-chain amino acids improve glucose metabolism in rats with liver cirrhosis. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1292-G1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 125] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 53. | Murata K, Moriyama M. Isoleucine, an essential amino acid, prevents liver metastases of colon cancer by antiangiogenesis. Cancer Res. 2007;67:3263-3268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 54. | Yoshiji H, Noguchi R, Kaji K, Ikenaka Y, Shirai Y, Namisaki T, Kitade M, Tsujimoto T, Kawaratani H, Fukui H. Attenuation of insulin-resistance-based hepatocarcinogenesis and angiogenesis by combined treatment with branched-chain amino acids and angiotensin-converting enzyme inhibitor in obese diabetic rats. J Gastroenterol. 2010;45:443-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 55. | Miuma S, Ichikawa T, Arima K, Takeshita S, Muraoka T, Matsuzaki T, Ootani M, Shibata H, Akiyama M, Ozawa E. Branched-chain amino acid deficiency stabilizes insulin-induced vascular endothelial growth factor mRNA in hepatocellular carcinoma cells. J Cell Biochem. 2012;113:3113-3121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 56. | Hagiwara A, Nishiyama M, Ishizaki S. Branched-chain amino acids prevent insulin-induced hepatic tumor cell proliferation by inducing apoptosis through mTORC1 and mTORC2-dependent mechanisms. J Cell Physiol. 2012;227:2097-2105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 57. | Wu J, Lanier LL. Natural killer cells and cancer. Adv Cancer Res. 2003;90:127-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 300] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 58. | Koziel MJ. NK cells: natural born killers in the conflict between humans and HCV. Hepatology. 2006;43:395-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |