Published online Jun 14, 2014. doi: 10.3748/wjg.v20.i22.6884
Revised: March 21, 2014
Accepted: April 15, 2014
Published online: June 14, 2014
Processing time: 194 Days and 16.5 Hours
AIM: To investigate the effect of mesothelin in the remodeling of the endocrine pancreas in neonatal rats.
METHODS: Overexpression or downregulation of mesothelin expression in INS-1 cells was carried out to investigate the effect of mesothelin during cell proliferation and cell apoptosis in vitro. Adenovirus-mediated RNA interference was performed to block mesothelin in vivo to directly assess the role of mesothelin in the remodeling of the endocrine pancreas in neonatal rats.
RESULTS: Exogenous overexpression of mesothelin promoted cell proliferation, cell colony formation and enhanced cell resistance to apoptosis of INS-1 cells. Down-regulation of mesothelin made no difference in cell proliferation and apoptosis compared with that in the control group. After an injection of adenovirus-mesothelin, a significantly increased number of small islets appeared, and the expression of PCNA was decreased on day 7 and day 14 compared with the Ad-EGFP group.
CONCLUSION: Mesothelin was able to promote β cell proliferation in the remodeling stage of neonatal rats. Mesothelin may have an important role in the remodeling of the endocrine pancreas in neonatal rats.
Core tip: Mesothelin may promote cell proliferation and affect the number of small islets during the remodeling of the endocrine pancreas in neonatal rats.
- Citation: Yin DD, You LH, Yuan QX, Liang XD, Wang N, Wang LT, Yuan L, Wang KM, De W. Mesothelin promotes cell proliferation in the remodeling of neonatal rat pancreas. World J Gastroenterol 2014; 20(22): 6884-6896
- URL: https://www.wjgnet.com/1007-9327/full/v20/i22/6884.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i22.6884
The islets of Langerhans are scattered throughout the pancreas and contribute to only 1% of the total pancreatic cell mass. At approximate embryonic day 16.5 (E16.5), some of the endocrine cells aggregate to form the islets of Langerhans. The islets are not fully formed until they undergo further remodeling and maturation for 2-3 wk after birth[1]. The 2-3 wk period after birth has been defined as the period during which the remodeling of the endocrine pancreas occurs in neonatal rats. Many major developmental changes occur during the pancreatic remodeling stage, including β-cell apoptosis, replication and neogenesis. Islet formation in the neonatal pancreas may occur as a result of the fission of elongated structures composed of β cells and surrounding α cells, following by contiguous proliferation and the branching of endocrine cells into cord-like structures in the newborn mouse pancreas[2]. We hypothesized that cell adhesion and migration molecules may play an important role during the pancreatic remodeling stage.
According to our previous microarray results, a series of molecules, including matrix metalloproteinase (MMPs), integrins and cadherin, which are associated with islet cell adhesion and migration, were highly expressed in the newborn rat pancreas[3]. MMPs have a crucial role in pancreas development, and the reduction of MMP activity results in aberrant islet formation[4-6]. Integrins have also been implicated in endocrine and islet formation[7]. Cadherins regulate the aggregation of pancreatic β cells in vivo[8]. Here, we focused on mesothelin, one of the genes that was highly expressed in late gestation on our previous microarrays.
The rat mesothelin gene contains an 1878-bp open reading frame encoded by 16 exons that occupies 6 kb of rat chromosome 10. Mesothelin, a differentiation antigen of mesothelial cells, is a 40-kDa glycosylphosphatidylinositol (GPI)-linked glycoprotein. It is synthesized as a precursor with a molecular mass of 69 kDa that is then proteolytically processed into an N-terminal secreted form that is 32 kDa and a membrane-bound form that is 40 kDa. Mesothelin is highly expressed in various cancer types, including ovarian cancer, breast cancer and pancreatic adenocarcinoma[9-13]. Mesothelin mediates the transfer of ovarian cancer to the peritoneum in combination with CA125 ovarian cancer antigen[14-16]. In vitro, the over-expression of mesothelin in pancreatic cancer cells and breast cancer cells significantly increased tumor proliferation and migration, suggesting that mesothelin may be a malignant factor and could be a suitable target for a therapeutic vaccine for pancreatic cancer and breast cancer[17,18]. We previously detected the expression of mesothelin and demonstrated that mesothelin is localized in islet β cells. However, the effect of mesothelin during the pancreatic remodeling stage of neonatal rats has not been previously reported.
To investigate the effect of mesothelin during cell proliferation and cell apoptosis, INS-1 cells were treated with pEGFP-N3/mesothelin or pcDNA/miR-mesothelin 1275 to over-express or down-regulate mesothelin, respectively. Furthermore, adenovirus-mediated RNA interference was performed to block the mesothelin gene in the neonatal rat pancreas to directly assess the role of mesothelin during pancreatic development in the neonatal rat.
The INS-1 cell line (rat insulinoma cell) was obtained from the Chinese Academy of Science Committee on Type Culture Collection cell bank. INS-1 cells were grown in regular RPMI medium (11.1 mmol/L glucose) supplemented with 10% heat-treated fetal calf serum (Invitrogen), 100 IU/mL penicillin, 100 μg/mL streptomycin, 2 mmol/L L-glutamine, 1 mmol/L sodium pyruvate, 50 μmol/L β-mercaptoethanol, and 10 mmol/L HEPES (pH 7.4).
Mesothelin (GenBank accession no.: NM 031658) was amplified by polymerase chain reaction (PCR) from rat pancreas cDNA using the following primers: Mesothelin-forward 5’-CCCAAGCTTATGGCCTTGCCAACAGCCC-3’; Mesothelin-reverse, 5’-CGGGATCCTCAGCTCAGTCTTAAAGCT-3’. The amplified mesothelin fragment was digested with the restriction enzymes BamH I and III and ligated into the pEGFP-N3 mammalian expression vector (Clonetech). The sequence of the cloned mesothelin plasmids was verified by DNA sequencing. The mesothelin expression plasmids were transfected into INS-1 cells using Lipofectamine 2000 (Invitrogen), according to the manufacturer’s instructions. INS-1 cells stably expressing mesothelin were selected in the presence of 800 mg/mL G418 (Sigma).
To create pcDNA6.2-GW/EmGFP-miR-mesothelin301 (si-301) and pcDNA6.2-GW/EmGFP-miR-mesothelin1275 (si-1275) encoding rat mesothelin interference RNA, single-strand oligonucleotides were designed using BLOCK-iT RNA Designer (Invitrogen). The following primers were used: mesothelin301-forward 5’-TGCTGTAGGCCAGATACCTCATCACAGTTTTGGCCACTGACTGACTGTGATGATATCTGGCCTA-3’; mesothelin301-reverse 5’-CCTGTAGGCCAGATATCATCACAGTCAGTCAGTGGCCAAAACTGTGATGAGGTATCTGGCCTAC-3’ and mesothelin1275-forward 5’-TGCTGTTAAGCAGAGTATTCACTGTGGTTTTGGCCACTGACTGACCACAGTGAACTCTGCTTAA-3’; mesothelin1275-reverse 5’-CCTGTTAAGCAGAGTTCACTGTGGTCAGTCAGTGGCCAAAACCACAGTGAATACTCTGCTTAAC-3’. The oligonucleotides were annealed and cloned into a pcDNA6.2-GW/EmGFP-miR vector (Invitrogen) using a BLOCK-iT Pol II miR RNAi Expression Kit (Invitrogen), according to the manufacturer’s instructions. The mesothelin interference plasmids, pcDNA6.2-GW/EmGFP-miR-mesothelin301 and pcDNA6.2-GW/EmGFP-miR-mesothelin1275, were transfected into the INS-1 cell line using lipofectamine 2000 (Invitrogen), according to the manufacturer’s instructions.
Total RNA was extracted from rat pancreas tissues or cultured cells with TRIzol reagent (Invitrogen). For qPCR, RNA was reverse transcribed to cDNA from 1 μg of total RNA using a reverse transcription kit (Takara). Real-time PCR analyses were conducted with Power SYBR Green (Takara). All protocols were carried out according to the manufacturer’s instructions. The results were normalized to the expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) or 18S ribosomal RNA (18S). The primer sequences for qPCR were as follows: mesothelin-forward: 5’-GTTTGCTGGTAGTGTTGGATGA-3’; mesothelin-reverse: 5’-GCAGGCTTTCTGTTCTGTGTC-3’; GAPDH-forward: 5’-GACTCATGACCACAGTCCATGC-3’; GAPDH-reverse: 5’-AGAGGCAGGGATGATGTTCTG-3’; 18S-forward: 5’-AAACGGCTACCACATCCAAG-3’; 18S-reverse: 5’- TTGCCCTCCAATGGATCCT-3’. qPCR and data collection were carried out on ABI 7500 PCR system.
The pancreata were homogenized in a detergent lysis buffer. A total of 50 μg of the samples were loaded into each lane of 12% SDS-PAGE gels and transferred to nitrocellulose membranes (Bio-Rad). Membranes were incubated with mouse anti-β-actin (sc-8432, Santa Cruz) and goat anti-mesothelin (sc-27001, Santa Cruz) overnight at 4 °C. The specific protein-antibody complex was detected using HRP-linked goat anti-mouse IgG conjugates (sc-2055, Santa Cruz) or HRP-conjugated rabbit anti-goat IgG (sc-2004, Santa Cruz). The signal was detected by chemiluminescence using an ECL kit (Amersham Life Science). Densitometric quantification of bands at subsaturating levels was carried out using Syngenetool gel analysis software (Syngene).
INS-1 cells transfected with pEGFP-mesothelin or pEGFP-N3 were trypsinized. First, 0.5% agarose in growth medium was added to a six-well plate and allowed to solidify. Then, 4000 cells per well were plated in triplicate in 0.3% agarose onto the bottom agarose. The cells were then incubated at 37 °C in a 5% CO2 atmosphere for 20 d. Fresh growth medium (0.5 mL/well) was added after 1 wk of incubation. At the end of incubation, colonies were stained with 0.005% crystal violet for 1 h and photographed. Colonies were counted using the Image J imaging software developed at the NIH.
INS-1 cells stably expressing mesothelin were seeded in 96-well plates (2 × 104 cells/well) and serum starved (0% fetal bovine serum) for 24 h. For the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, cell growth was assessed 0 h, 12 h, 24 h and 48 h after being releasing from starvation. Twenty microliters of MTT reagent was added to each well and incubated at 37 °C for 4 h. Absorbance was recorded at 490 nm with an ELx-800 universal microplate reader (Bio-Tek Instruments).
The percentage of cells in the G0/G1, S and G2/M phases was determined by flow cytometric analysis following propidium iodide (PI; Invitrogen) staining. For PI staining, cells were seeded at a concentration of 1 × 106 cells/well. Following treatment, cells were harvested using trypsin followed by washing twice with phosphate buffered saline (PBS). Harvested cells were then resuspended and fixed with ice-cold 75% ethanol (750-1000 μL). Samples were then stored at -20 °C overnight. Fixed cells were centrifuged at 1000 ×g for 5 min and resuspended in PBS buffer containing 40 μg/mL PI and 100 μg/mL RNase (Qiagen, United Kingdom) for 30 min at room temperature in the dark. Samples were analyzed using a FACS flow cytometer (BD Biosciences) and winMDI software.
For the cell apoptosis assay, apoptotic cells were evaluated in vitro by Annexin-V-fluorescein isothiocyanate (FITC) and propidium iodide according to the manufacturer’s protocol. Stained cells were then analyzed with a FACS flow cytometer (BD Biosciences).
Recombinant Ad-EGFP and Adenovirus-Mesothelin RNAi were generated using the Ad Max system (Microbix Biosystems). Pregnant Sprague Dawley rats (Animal Center of Nanjing Medical University) were kept under conventional conditions and provided with a 12:12 h light-dark cycle. The litters were reduced to 12 pups at birth. Seven days after birth, four of the pups in each litter were included in the mesothelin group and injected with Ad-mesothelin RNAi solution (5 × 109 pfu in 250 μL of lactated Ringer’s solution) via the intraductal route, as described in Doiron et al[19]. Another group of four rats was included in the Ad-EGFP group and injected with Ad-EGFP by the same method, and the remaining rats were included in the sham group and received a sham operation. The rats were sacrificed at various times after the injection. Blood glucose was measured with a One Touch Ultra blood glucose meter (Life Scan) in blood obtained by lancing the tail vein. Body weight was recorded every two days after treatment. All animal and tissue sample experiments were performed in accordance with the guidelines of the National Institutes of Health and approved by the Research Ethics Committee of Nanjing Medical University.
Tissues were fixed in 4% paraformaldehyde for 24-36 h, followed by a standard protocol of dehydration and paraffin embedding. Sections (5-μm) were cut and mounted on glass slides (Fisher Scientific). For the double fluorescence immunohistochemical localization of insulin/glucagons, a rabbit anti-insulin polyclonal antibody (1:100, sc-9168, Santa Cruz) was applied and then revealed by FITC-labeled anti-Rabbit IgG (1:400, AP123J, Chemicon). A mouse anti-glucagon (1:100, G-2654, Sigma) antibody was applied and revealed using goat anti-mouse IgG-TRITC (1:400, sc-2010, Santa Cruz). The islet number and size were measured as described in Liang et al[20]. The expression of the cell proliferation marker PCNA was examined by immunohistochemistry using an anti-PCNA antibody (1:200; Santa Cruz, United States). A secondary antibody (goat anti-rabbit IgG; 1:500; Bioworld, United States) was applied. The final detection step was carried out using 3,3’-diaminobenzidine (DAB; Sigma-Aldrich Corp, United States) as the chromogen. Sections were lightly counterstained with hematoxylin and mounted. All of the sections were placed in Gel Mount Aqueous Mounting Medium (G0918, Sigma) with a cover glass and were examined under an Olympus BX51 microscope (Olympus Optical, Tokyo, Japan).
The Students t test (2-tailed) and one-way ANOVA were conducted to analyze the in vivo and in vitro data by SPSS 16.0 software. P values less than 0.05 were considered statistically significant.
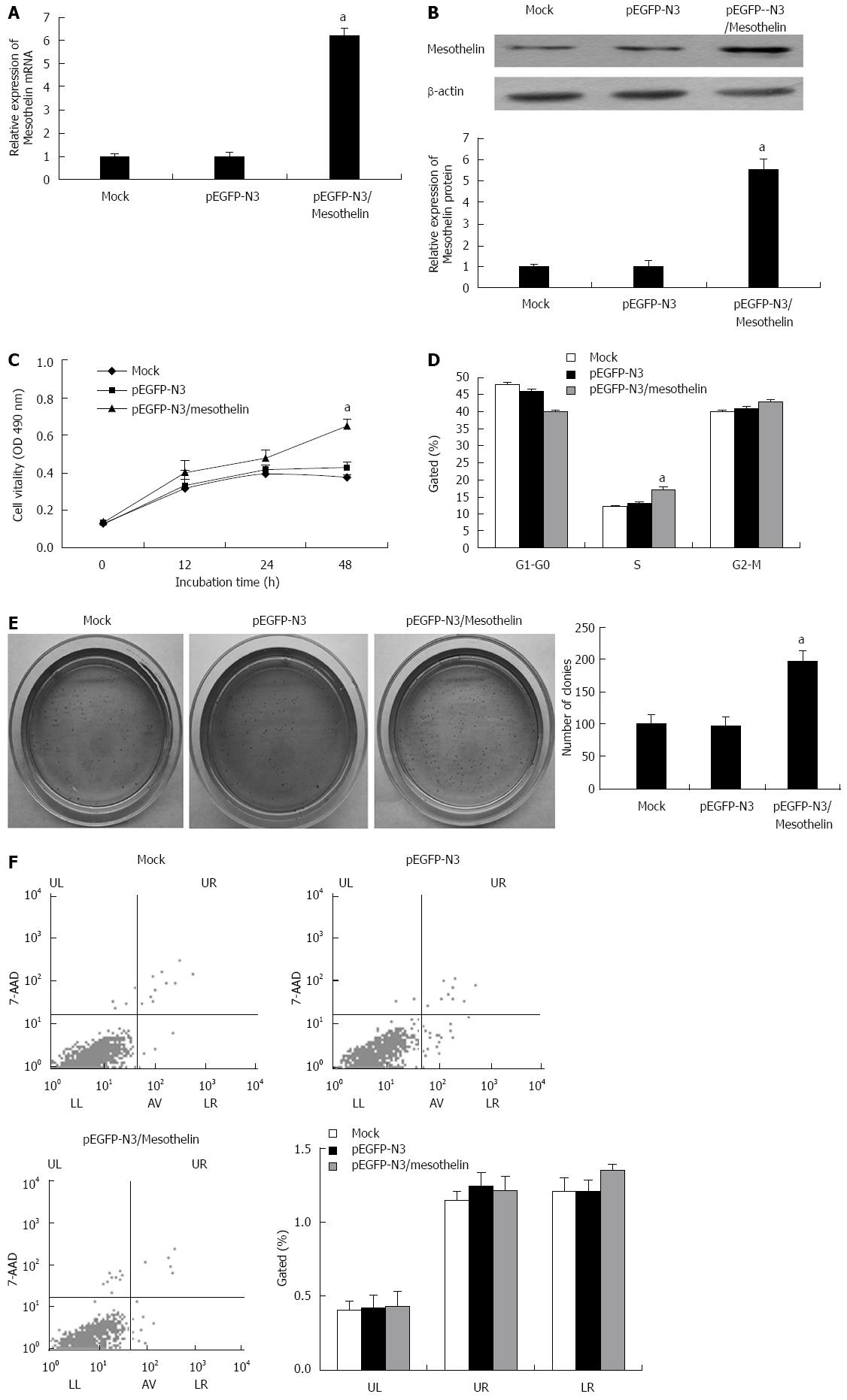
To study the potential functions of mesothelin in β cells, INS-1 cells were transfected with pEGFP-N3/mesothelin or the empty pEGFP-N3 vector. After 4 wk of selection with G418, stably overexpressed mesothelin INS-1 cells were obtained. qPCR and western blot analysis were performed. The mRNA expression of mesothelin in the pEGFP-N3/mesothelin group was up-regulated by 6-fold and protein expression increased by 5.5-fold compared with the mock transfection. Mesothelin over-expression in a representative pEGFP-N3/mesothelin cell line is shown in Figure 1A and B.
MTT was performed to examining the cell viability of pEGFP-N3/mesothelin cells. After 48 h of culture, pEGFP-N3/mesothelin cells exhibited significantly greater viability than their respective vector control cells (Figure 1C). To investigate the effects of mesothelin on cell cycle distributions, flow cytometric analysis was performed to detect cell cycle changes in pEGFP-N3/mesothelin cells. As depicted in Figure 1D, the percentage of INS-1/mesothelin cells in S phase was increased when compared with mock cells. It has been reported that mesothelin can promote anchorage-independent growth in human breast carcinoma. Accordingly, we examined the viability of pEGFP-N3/mesothelin cells under anchorage-independent conditions in soft agar. After 20 d of culture, pEGFP-N3/mesothelin cells formed a significantly greater number of colonies compared with pEGFP-N3 and mock-transfected cells (Figure 1E). To determine whether the over-expression of mesothelin prevents apoptosis in INS-1 cells, we performed flow cytometric analysis. The results showed no significant difference in the cell apoptosis ratio between pEGFP-N3/mesothelin, pEGFP-N3 and mock cells (Figure 1F). Taken together, the over-expression of mesothelin in INS-1 cells is associated with increased cell proliferation.
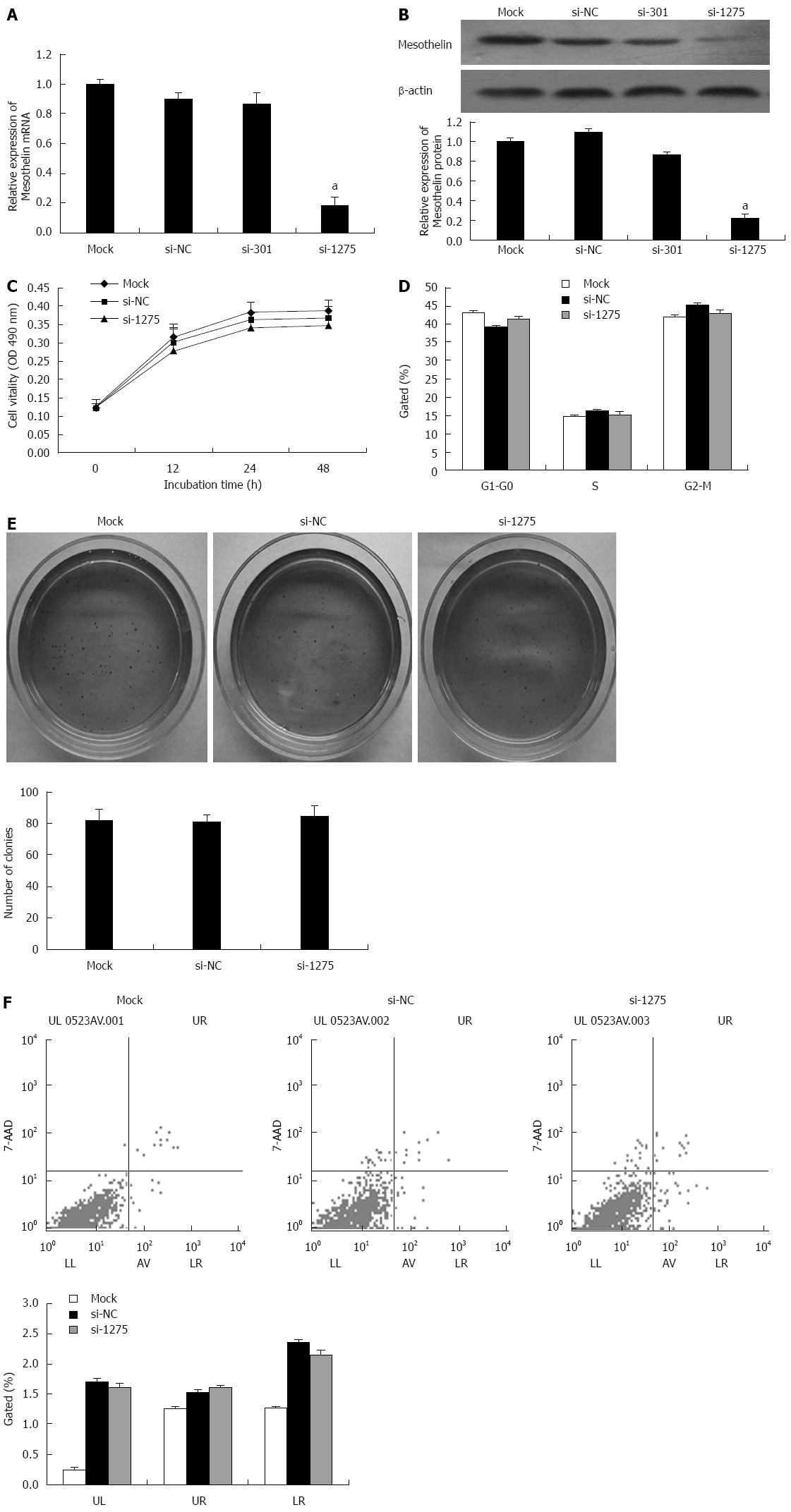
Two specific interference sequences were designed to inhibit mesothelin. PcDNA6.2-GW/EmGFP-miR-mesothelin301 (si-301) and were effective at silencing rat mesothelin gene. qPCR and western blot analysis were performed. Mesothelin mRNA in the si-1275 group was reduced by 80%, and protein expression was reduced by 60% compared with that in si-NC, thereby confirming si-1275 to be more effective for rat mesothelin gene inhibition (Figure 2A and B).
According to the results of the MTT assay, we found that cells transiently transfected with si-1275 showed no significant reduction in cell viability compared to cells transfected with the mock construct or the si-NC controls (Figure 2C). Flow cytometry was carried out to analyze the cell cycle of INS-1 cells after transfection with si-1275 or si-NC, and there were no difference between the two groups (Figure 2D). Soft agar colony formation assays showed no significant difference between the mock, si-NC and si-1275 groups (Figure 2E). Furthermore, flow cytometry detected no change in the apoptosis ratio in si-1275 cells when compared with control cells (Figure 2F). However, the apoptosis ratio of the si-1275 group increased when compared with the mock condition, but there was no significant difference between the si-1275 group and the si-NC group. All of these data indicated that the decrease in mesothelin level does not influence INS-1 proliferation or apoptosis. This result may be because the INS-1 cells we adopted have low mesothelin levels.
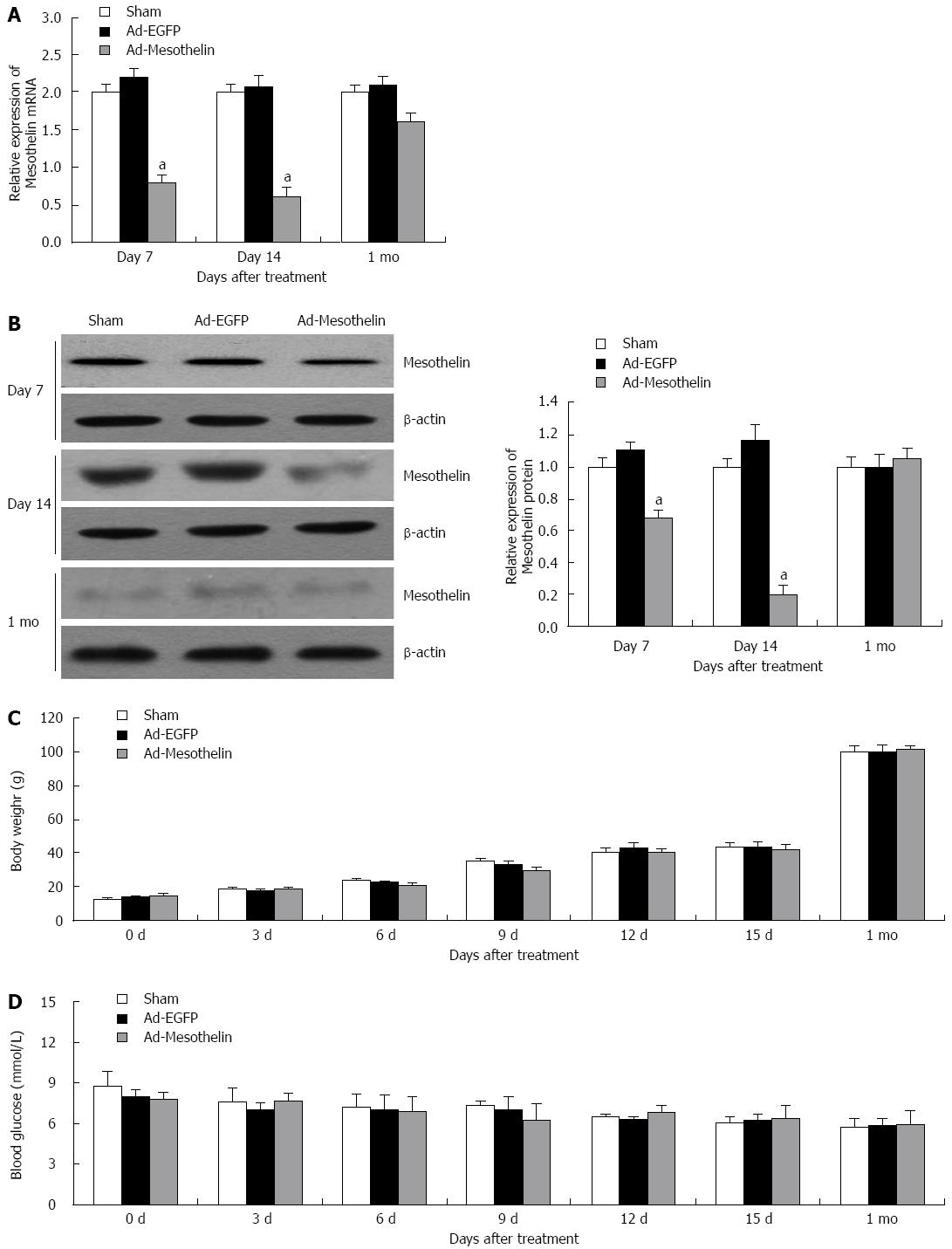
To investigate the effect of mesothelin during neonatal pancreatic development, Ad-mesothelin was injected into rats on postnatal day 7 (P 7) to block the expression of mesothelin in vivo. To detect the interference efficiency, qPCR and western blotting were performed. Mesothelin mRNA and protein were significantly reduced on day 7 (Figure 3A and B), and the expression of mesothelin in the interference group was still lower than that of the Ad-EGFP group at day 14; however, the expression of mesothelin was restored to the level of the Ad-EGFP group after 1 mo, both at the mRNA and protein level. After injecting the virus solution at P7, we examined the bodies and blood of the rats every three days (Figure 3C and D), and no significant change in the rat body weight or blood sugar was found.
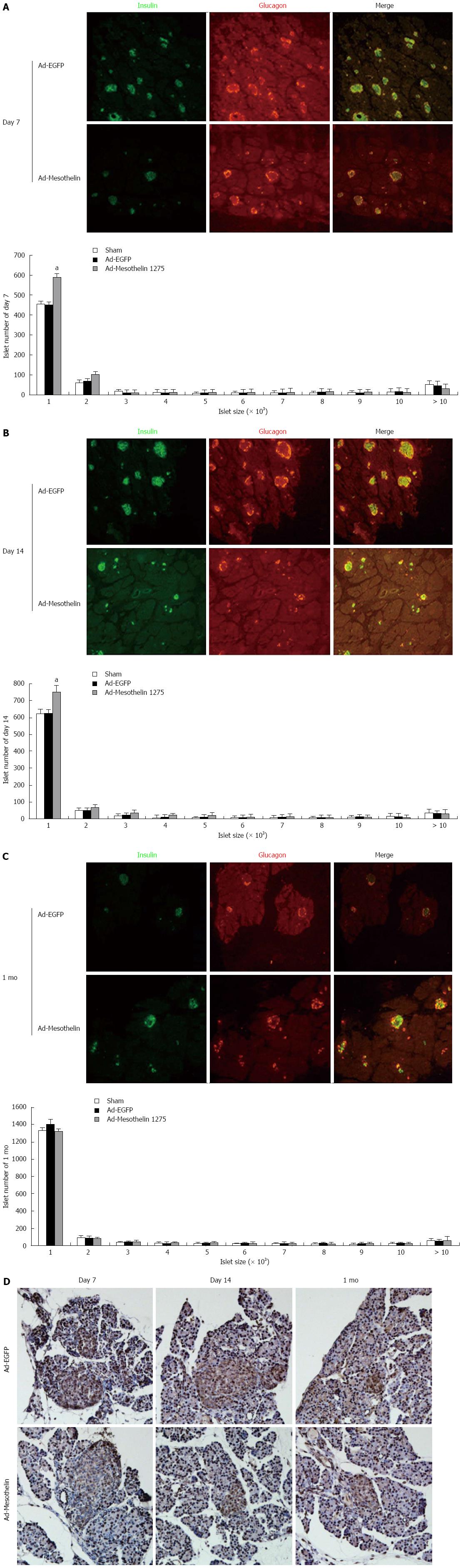
To further examine the role of mesothelin during morphogenesis in neonatal rat islets, we detected insulin and glucagon expression by double-labeling immunofluorescence. Image J was used to measure the number and size distribution of islets according to the fluorescent images. The overall distribution of islets (including small clusters of β cells) on the day 7, day 14 and 1 mo pancreas is shown in a histogram (Figure 4A-C). The number of small islets in the Ad-mesothelin group was greater than that in the Ad-EGFP group on day 7 and day 14 (Figure 4A and B). The total numbers of islets increased in the Ad-mesothelin group compared to the Ad-EGFP group on day 7 and day 14.
Then, we suspected decreased cell proliferation in pancreas tissues by immunohistochemical staining of PCNA protein. PCNA is a marker of cell proliferation. As shown in Figure 4D, PCNA expression in islets was decreased at 7 d and 14 d in the Ad-mesothelin group compared with the Ad-EGFP group. Down-regulation of mesothelin in vivo decreased cell proliferation; therefore, the number of small islets was increased. These results indicated that the increased number of small islets may be related to the expression of mesothelin. Mesothelin may have an important role in the remodeling of the endocrine pancreas in neonatal rats.
It has been established that mesothelin is a malignant factor and a target for therapeutic vaccines in several cancers[17]. However, the role of mesothelin in neonatal rat pancreatic development is not clear. In this article, we demonstrated that the over-expression of mesothelin promotes cell proliferation in vitro. Meanwhile, the reduction of mesothelin in pancreatic β cells led to decreased cell proliferation and the increase of small islets in vivo.
Mesothelin is one of the glycoproteins that is attached to the cell surface by GPI. GPI-linked proteins have a wide variety of functions in different cells. Some are receptors involved in cell signaling; others are involved in cellular recognition and migration. Mesothelin overexpression was reported to be present in 60 resected primary pancreatic adenocarcinomas but not in the adjacent normal pancreas or benign pancreatic ducts[21,22]. Moreover, mesothelin mediates the transfer of ovarian cancer to the peritoneum in combination with CA125 ovarian cancer antigen. In vitro, overexpressed mesothelin in pancreatic cancer cells significantly increases tumor proliferation and migration. Mesothelin-induced pancreatic cancer cell proliferation involves the alteration of cyclin E via the activation of transcription protein 3[23]. However, the details of the biological function of mesothelin during pancreatic development of the neonatal rat are not clear.
Mesothelin over-expression has been reported to promote anchorage-independent growth in breast cancer[18]. The viability of pEGFP-N3/mesothelin cells under anchorage-independent conditions in soft agar was examined. MTT was also performed to examine the viability of pEGFP-N3/mesothelin cells. Otherwise, down-regulation of mesothelin did not influence the cell proliferation and apoptosis established by a MTT assay, cell colony formation assay and flow cytometry assay. These findings may be due to the low expression of mesothelin in INS-1 cells.
As shown in Figure 3C and D, no significant change in the rat body weight or blood glucose level was observed between the Ad-mesothelin group and Ad-EGFP group after injection. We considered the following reasons for these results: (1) mesothelin is a surface adhesion protein that is not involved in the secretion function of β cells, so the inhibition did not affect insulin release and blood glucose; (2) small islets are superior to large islets in function. It has been reported that small islets release three times more insulin under basal conditions than large islets during static incubation. During exposure to high glucose conditions, small islets release four times more insulin than islet equivalents of large islets[24]; and (3) the downturn of mesothelin protein may induce a compensatory up-regulation of islet structure and function from other proteins.
It has been well established that intercellular interactions within the islets of Langerhans are important for the development and functional competence of the islet. Cell-to-cell interactions are important for the development and maintenance of the β cell phenotype. Pancreatic β cells express a number of cell migration molecules, including mesothelin which is described as a cell migration molecule[8,24-26]. The in vivo reduction of mesothelin induced an amount of small islets on day 7 and day 14 that was comparable with that in the Ad-EGFP group. This finding indicated that mesothelin likely plays an important role in cell to cell interactions and in maintaining primary islet architecture.
Bera et al[27] demonstrated that mesothelin is not essential for growth or reproduction in mice after mesothelin protein is blocked. These results have some similarities with ours, but they do not show the effects of mesothelin on the islets. Methods to promote the formation of mature pancreatic islet β-cells in vitro or in vivo is still a research focus. In summary, we demonstrated that mesothelin can promote cell proliferation and affect the number of small islets, and we have therefore established the importance of mesothelin during the rat pancreatic remodeling stage.
Knowledge of the effect of mesothelin on cell proliferation will be useful in understanding its potential role in the remodeling endocrine pancreas of neonatal rats. However, no study has investigated the role of mesothelin in the remodeling of the endocrine pancreas in neonatal rats.
The authors demonstrated that mesothelin may have an important role in the remodeling of endocrine pancreas in neonatal rats.
The study demonstrated that mesothelin could promote cell proliferation and downregulation of mesothelin could lead to a reduction in cell proliferation and an increase in the number of small islets in the remodeling of endocrine pancreas in neonatal rats.
The work has important implications in the future as it shows the ability of mesothelin as a mediator stimulating the proliferation of pancreatic cells and their possible use in the future. It would be interesting to establish the relationship between mesothelin, damaged normal cells, and local proliferation of cells but also migration of progenitor cells from bone marrow. This mechanism would put the stamp seal help. The work not only stands out for its findings but also by the detailed methodology.
P- Reviewers: Cao DF, Kanai M, Mesples A S- Editor: Ma YJ L- Editor: O’Neill M E- Editor: Ma S
| 1. | Scaglia L, Cahill CJ, Finegood DT, Bonner-Weir S. Apoptosis participates in the remodeling of the endocrine pancreas in the neonatal rat. Endocrinology. 1997;138:1736-1741. [PubMed] |
| 2. | Miller K, Kim A, Kilimnik G, Jo J, Moka U, Periwal V, Hara M. Islet formation during the neonatal development in mice. PLoS One. 2009;4:e7739. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Hou LQ, Wang YH, Liu LJ, Guo J, Teng LP, Cao LH, Shi H, Yuan L, De W. Expression and localization of mesothelin in developing rat pancreas. Dev Growth Differ. 2008;50:531-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | van der Zee E, Jansen I, Hoeben K, Beertsen W, Everts V. EGF and IL-1 alpha modulate the release of collagenase, gelatinase and TIMP-1 as well as the release of calcium by rabbit calvarial bone explants. J Periodontal Res. 1998;33:65-72. [PubMed] |
| 5. | Miettinen PJ, Huotari M, Koivisto T, Ustinov J, Palgi J, Rasilainen S, Lehtonen E, Keski-Oja J, Otonkoski T. Impaired migration and delayed differentiation of pancreatic islet cells in mice lacking EGF-receptors. Development. 2000;127:2617-2627. [PubMed] |
| 6. | Miralles F, Battelino T, Czernichow P, Scharfmann R. TGF-beta plays a key role in morphogenesis of the pancreatic islets of Langerhans by controlling the activity of the matrix metalloproteinase MMP-2. J Cell Biol. 1998;143:827-836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 141] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 7. | Cirulli V, Beattie GM, Klier G, Ellisman M, Ricordi C, Quaranta V, Frasier F, Ishii JK, Hayek A, Salomon DR. Expression and function of alpha(v)beta(3) and alpha(v)beta(5) integrins in the developing pancreas: roles in the adhesion and migration of putative endocrine progenitor cells. J Cell Biol. 2000;150:1445-1460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 129] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 8. | Dahl U, Sjødin A, Semb H. Cadherins regulate aggregation of pancreatic beta-cells in vivo. Development. 1996;122:2895-2902. [PubMed] |
| 9. | Glass JP, Parasher G, Arias-Pulido H, Donohue R, Prossnitz ER, Cerilli LA. Mesothelin and GPR30 staining among a spectrum of pancreatic epithelial neoplasms. Int J Surg Pathol. 2011;19:588-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Iacobuzio-Donahue CA, Ashfaq R, Maitra A, Adsay NV, Shen-Ong GL, Berg K, Hollingsworth MA, Cameron JL, Yeo CJ, Kern SE. Highly expressed genes in pancreatic ductal adenocarcinomas: a comprehensive characterization and comparison of the transcription profiles obtained from three major technologies. Cancer Res. 2003;63:8614-8622. [PubMed] |
| 11. | Rump A, Morikawa Y, Tanaka M, Minami S, Umesaki N, Takeuchi M, Miyajima A. Binding of ovarian cancer antigen CA125/MUC16 to mesothelin mediates cell adhesion. J Biol Chem. 2004;279:9190-9198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 430] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 12. | Hellstrom I, Hellstrom KE. fTwo novel biomarkers, mesothelin and HE4, for diagnosis of ovarian carcinoma. Expert Opin Med Diagn. 2011;5:227-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Scholler N, Fu N, Yang Y, Ye Z, Goodman GE, Hellström KE, Hellström I. Soluble member(s) of the mesothelin/megakaryocyte potentiating factor family are detectable in sera from patients with ovarian carcinoma. Proc Natl Acad Sci USA. 1999;96:11531-11536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 242] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 14. | Scholler N, Garvik B, Hayden-Ledbetter M, Kline T, Urban N. Development of a CA125-mesothelin cell adhesion assay as a screening tool for biologics discovery. Cancer Lett. 2007;247:130-136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Scholler N, Urban N. CA125 in ovarian cancer. Biomark Med. 2007;1:513-523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 149] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 16. | Bergan L, Gross JA, Nevin B, Urban N, Scholler N. Development and in vitro validation of anti-mesothelin biobodies that prevent CA125/Mesothelin-dependent cell attachment. Cancer Lett. 2007;255:263-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Li M, Bharadwaj U, Zhang R, Zhang S, Mu H, Fisher WE, Brunicardi FC, Chen C, Yao Q. Mesothelin is a malignant factor and therapeutic vaccine target for pancreatic cancer. Mol Cancer Ther. 2008;7:286-296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 128] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 18. | Uehara N, Matsuoka Y, Tsubura A. Mesothelin promotes anchorage-independent growth and prevents anoikis via extracellular signal-regulated kinase signaling pathway in human breast cancer cells. Mol Cancer Res. 2008;6:186-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Doiron B, Hu W, Norton L, DeFronzo RA. Lentivirus shRNA Grb10 targeting the pancreas induces apoptosis and improved glucose tolerance due to decreased plasma glucagon levels. Diabetologia. 2012;55:719-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Liang XD, Guo YY, Sun M, Ding Y, Wang N, Yuan L, De W. Streptozotocin-induced expression of Ngn3 and Pax4 in neonatal rat pancreatic α-cells. World J Gastroenterol. 2011;17:2812-2820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 21. | Argani P, Iacobuzio-Donahue C, Ryu B, Rosty C, Goggins M, Wilentz RE, Murugesan SR, Leach SD, Jaffee E, Yeo CJ. Mesothelin is overexpressed in the vast majority of ductal adenocarcinomas of the pancreas: identification of a new pancreatic cancer marker by serial analysis of gene expression (SAGE). Clin Cancer Res. 2001;7:3862-3868. [PubMed] |
| 22. | Hassan R, Laszik ZG, Lerner M, Raffeld M, Postier R, Brackett D. Mesothelin is overexpressed in pancreaticobiliary adenocarcinomas but not in normal pancreas and chronic pancreatitis. Am J Clin Pathol. 2005;124:838-845. [PubMed] |
| 23. | Bharadwaj U, Li M, Chen C, Yao Q. Mesothelin-induced pancreatic cancer cell proliferation involves alteration of cyclin E via activation of signal transducer and activator of transcription protein 3. Mol Cancer Res. 2008;6:1755-1765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 24. | MacGregor RR, Williams SJ, Tong PY, Kover K, Moore WV, Stehno-Bittel L. Small rat islets are superior to large islets in in vitro function and in transplantation outcomes. Am J Physiol Endocrinol Metab. 2006;290:E771-E779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 139] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 25. | Gillian AM, Brion JP, Breen KC. Expression of the neural cell adhesion molecule (NCAM) in Alzheimer’s disease. Neurodegeneration. 1994;3:283-291. [PubMed] |
| 26. | Rouiller DG, Cirulli V, Halban PA. Uvomorulin mediates calcium-dependent aggregation of islet cells, whereas calcium-independent cell adhesion molecules distinguish between islet cell types. Dev Biol. 1991;148:233-242. [PubMed] |
| 27. | Bera TK, Pastan I. Mesothelin is not required for normal mouse development or reproduction. Mol Cell Biol. 2000;20:2902-2906. [PubMed] |