Published online Jun 14, 2014. doi: 10.3748/wjg.v20.i22.6707
Revised: January 13, 2014
Accepted: April 1, 2014
Published online: June 14, 2014
Processing time: 264 Days and 17.8 Hours
The hepatitis C virus (HCV) causes an acute infection that is frequently asymptomatic, but a spontaneous eradication of HCV infection occurs only in one-third of patients. The remaining two-thirds develop a chronic infection that, in most cases, shows an indolent course and a slow progression to the more advanced stages of the illness. Nearly a quarter of cases with chronic hepatitis C (CHC) develop liver cirrhosis with or without hepatocellular carcinoma. The indolent course of the illness may be troubled by the occurrence of a hepatic flare, i.e., a spontaneous acute exacerbation of CHC due to changes in the immune response, immunosuppression and subsequent restoration, and is characterized by an increase in serum aminotransferase values, a frequent deterioration in liver fibrosis and necroinflammation but also a high frequency of sustained viral response to pegylated interferon plus ribavirin treatment. A substantial increase in serum aminotransferase values during the clinical course of CHC may also be a consequence of a superinfection by other hepatotropic viruses, namely hepatitis B virus (HBV), HBV plus hepatitis D virus, hepatitis E virus, cytomegalovirus, particularly in geographical areas with high endemicity levels. The etiology of a hepatic flare in patients with CHC should always be defined to optimize follow-up procedures and clinical and therapeutic decisions.
Core tip: Patients with chronic hepatitis C virus infection may experience hepatic flares due to an acute exacerbation of this disease that is frequently characterized by a significant increase in liver fibrosis and necroinflammation but by a high rate of sustained viral response to Peg-interferon plus ribavirin treatment. A hepatic flare may also be due to superinfection by other hepatotropic viruses, drug injury or a concomitant autoimmune disease. The aim of this review is to be of some help in identifying the cause of the flare in a single patient in order to optimize the follow-up and clinical and therapeutic decisions.
-
Citation: Sagnelli E, Sagnelli C, Pisaturo M, Coppola N. Hepatic flares in chronic hepatitis C: Spontaneous exacerbation
vs hepatotropic viruses superinfection. World J Gastroenterol 2014; 20(22): 6707-6715 - URL: https://www.wjgnet.com/1007-9327/full/v20/i22/6707.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i22.6707
Hepatitis C virus (HCV) infection is a major global health problem with approximately 3% of the world’s population infected[1,2]. HCV is a small enveloped positive-strand RNA virus of the genus hepacivirus of the flaviviridae family[3]. Phylogenetic analysis of HCV isolates has enabled the viral classification into six major genotypes (from 1 to 6) and more than 100 subtypes[3].
HCV transmission follows percutaneous exposure to human blood, but more recently increasing evidence has highlighted the role of unsafe sexual intercourse[4-6], particularly in human immunodeficiency virus-positive males who have sex with males[1,7-9]. Worthy of note is the changing impact of various risk factors for HCV transmission in the last 20 years in several countries, consequent to variations in social and economic conditions and to the widespread use of invasive medical procedures, particularly in developing countries[1,4,5,8,10,11].
HCV causes an acute infection that is frequently asymptomatic, but in its symptomatic form it is characterized by nausea, malaise and jaundice. A spontaneous eradication of HCV infection occurs in 15%-30% of patients, whereas in the remaining percentage the infection becomes chronic[12-14].
Thus, the outcome of the infection is viral clearance or viral persistence, depending on certain, not fully recognized, host (sex, age at the time of infection, initial immune response, and more recently IL28B genotype)[15-20] and viral characteristics (HCV genotypes, subtypes, quasispecies)[21-23]. Today, chronic HCV infection is a leading cause of end-stage liver disease including liver cirrhosis and hepatocellular carcinoma (HCC)[24-27]. For patients with HCV genotype 2 or 3, treatment is still based on a 24-wk administration of pegylated interferon (Peg-IFN) given once a week in combination with a daily oral dose of 800 mg of ribavirin, whereas for those with HCV genotype 1 a combination treatment with Peg-IFN, ribavirin and a protease inhibitor has been more recently introduced[28-31]. These treatment schedules allow a sustained virological response in nearly 80% of patients with HCV genotype 2 or 3 and in 65%-75% of those with HCV genotype 1[28,29,32,33]. At present interferon-free combination therapies with directly acting antivirals are under investigation, with excellent preliminary results[34-38].
The natural history of chronic hepatitis C (CHC) may include the development of a spontaneous hepatic flare, i.e., an acute exacerbation of CHC (ae-CHC), characterized by a substantial increase in the serum aminotransferase levels (usually 5 times or more the baseline values)[39-43], a frequent deterioration in liver fibrosis and necroinflammation of 2 or more scores in the Ishak scoring system but also a higher rate of sustained viral response (SVR) to Peg-INF plus ribavirin treatment[43]. Hepatic flares in patients with chronic hepatitis C may also be a consequence of the restoration of the immune system after pharmacological or spontaneous immunosuppression. In geographical areas where other viral infections of the liver are highly endemic, CHC patients may also develop hepatic flares due to hepatitis A virus (HAV), hepatitis B virus (HBV) or HBV plus hepatitis delta virus (HDV), hepatitis E virus (HEV) or cytomegalovirus (CMV) superinfection, particularly in groups of subjects at a greater risk of acquiring these viruses.
The aim of this review article is to analyze the pathogenesis, clinical course, difficulties in correctly diagnosing and possible treatment of flares of various origins, on the basis of our personal experience and published data.
This is a clinical event characterized by a substantial increase in the serum aminotransferase values compared to the previous levels, associated or not with an increase in serum bilirubin and with other symptoms characteristic of acute hepatitis[39-48]. This acute exacerbation can occur spontaneously[15-19,21-25] or due to the restoration of a deficient immune system, a clinical condition frequently observed in patients with onco-hematological diseases[49-64]. (Table 1).
| Ref. | Type of study | Patients (flare/total) | Patients with flare | Etiology of flare |
| Sheen et al[39] | Prospective | 78/194 | 40% | Spontaneous ae-CHC |
| Tsuji et al[47] | Prospective | 28/120 | 23.30% | Spontaneous ae-CHC |
| Rumi et al[41] | Prospective | 39/206 | 19% | Spontaneous ae-CHC |
| Ferri et al[64] | Prospective | 1/31 | 3.20% | ae-CHC due to anti-TNF alfa |
| Pitini et al[53] | Prospective | 10/10 | / | ae-CHC due to anti-CD20 |
| Coppola et al[50] | Prospective longitudinal | 7/7 | / | ae-CHC due to anti-CD20 |
| Grebely et al[85] | Prospective | 5/136 | 3% | HCV-superinfection |
| Sagnelli et al[43] | Cross-sectional | 82/82 | / | Spontaneous ae-CHC |
| Coppola et al[48] | Cross-sectional | 57/57 | / | Spontaneous ae-CHC |
| Sagnelli et al[67] | Cross-sectional | 8/8 | / | HAV superinfection |
| Biliotti et al[80] | Cross-sectional | 14/14 | / | HBV superinfection |
| Sagnelli et al[81] | Cross-sectional | 29/29 | / | HBV superinfection |
| Pritchard[65] | Case report | 1/1 | / | ae-CHC due to anti-TNF alfa |
| Deterding et al[84] | Case report | 1/1 | 7% | HBV/HDV superinfection |
| Sagnelli et al[86] | Case report | 1/1 | / | HCV-superinfection |
| Accapezzato et al[90] | Case report | 1/1 | / | HCV-superinfection |
| Hiraga et al[40] | Retrospective | 22/1760 | 1.25% | Spontaneous ae-CHC |
| Ennishi et al[51] | Retrospective | 36/131 | 27.40% | ae-CHC due to anti-CD20 |
| Mahale et al[62] | Retrospective | 33/308 | 11% | ae-CHC related to chemotherapy |
| Li et al[66] | Retrospective | 1/8 | 12.50% | ae-CHC due to anti-TNF alfa |
| Marignani et al[52] | Retrospective | 2/3 | 66.60% | ae-CHC due to anti-CD20 |
Regarding spontaneous ae-CHC, most information comes from the studies carried out by Sheen et al[39], Rumi et al[41] and Sagnelli et al[43]. In 1996 Sheen et al[39] first described this clinical event observed during a follow-up of 5 or more years of 194 patients with CHC. This Author reported a hepatic flare in 78 (40.2%) patients, with an incidence of 11.9% per year.
Rumi et al[41] followed up 206 patients (106 with genotype 1 and 100 with genotype 2) for 71 mo (range 24-144) and observed spontaneous hepatic flares in 31 patients with genotype 2c and in 8 patients with genotype 1b (with a rate of 55.6 per 1000 persons/year for genotype 2c vs 15.0 for genotype 1b, P = 0.001). A second liver biopsy was available for a few patients, and those who experienced a hepatic flare more frequently showed a more than 2-point increase in the fibrosis score in the second biopsy, according to the Ishak scoring system (63% vs 19%, P = 0.003).
In 2013, Sagnelli et al[43] extensively analyzed the clinical presentation and clinical course of ae-CHC, and for the first time described the response to pegylated interferon plus ribavirin. In this study, 82 patients with a spontaneous symptomatic exacerbation of chronic HCV infection were pair-matched by age, sex and HCV genotype with 82 patients with chronic HCV infection who had not shown signs or symptoms of an acute exacerbation for at least 10 years. HCV genotype 2 was detected in 46.4% and genotype 1 in 43.9% of cases, and IL-28B CC genotype was more frequent in patients with ae-CHC than in those without (40.2% vs 24.4%, P < 0.05). A second liver biopsy was available for 23 patients with ae-CHC and for 31 patients without, the first performed at enrolment and the second during the follow-up. These patients were naïve for anti-HCV therapy. A deterioration in liver fibrosis > 2 scores (Ishak) was observed in 78.3% of patients with ae-CHC and in 35.5% of those without (P < 0.005); a deterioration in necroinflammation > 2 scores occurred in 60.9% of patients with ae-CHC and in 9.6% of those without (P < 0.005). In this study 32 (46.4%) patients with ae-CHC and 38 without (52%) received pegylated interferon plus ribavirin, with an SVR in 81.2% of the patients in the first group and in 60.5% of those in the second, a difference not significant to the statistical analysis but of substantial clinical importance.
Other authors have described episodes of ae-CHC: Tsuji et al[47] reported ae-CHC in 27.4% of 120 patients with CHC with persistently normal alanine aminotransferase levels; Coppola et al[48] described 57 consecutive HCV-RNA-positive patients with a hepatic flare hospitalized because of the severity of symptoms and found a high prevalence of cases with HCV genotype 2a, whereas Hiraga et al[40] reported a prevalence of less than 1.5% of flares in a follow-up study of 1760 patients with CHC.
To conclude on this point, patients with a spontaneous ae-CHC frequently show HCV genotype 2, IL28-B CC genotype and an unfavorable outcome. The more rapid progression to liver cirrhosis and the risk of developing HCC strongly warrant an early initiation of anti-HCV therapy, also considering that about 80% of subjects obtain an SVR with standard Peg-IFN plus ribavirin treatment. The high frequency of HCV genotype 2 and of IL28-B CC genotype and the reactivation of a cell-mediated immune response favoring HCV clearance may be among the reasons for this favorable response to treatment.
Hepatic flares in chronic HCV infection may also occur during interferon-based antiviral treatment, but with no increase in HCV viral load in the case of drug-related toxicity[54,55] and interferon-induced autoimmune disorders[56-60] and with viral rebound in the case of treatment failure. A hepatic flare in patients who have achieved a sustained viral response with interferon-based treatment can be considered rare since in a 4-year follow-up study of more than 1300 such patients this event never occurred[61].
Some authors have recently demonstrated HCV reactivation during or after drug-induced immunosuppression, but the knowledge of ae-CHC in these immunosuppressed patients is scanty[49] (Table 1). Coppola et al[50] described an increase in HCV RNA of at least 1.5 log IU/mL in plasma and of at least 1.1 log IU/mL in peripheral blood mononuclear cells of 7 patients receiving rituximab and corticosteroid-based chemotherapy; these patients developed a hepatic flare 3-5 mo after treatment was discontinued. In a retrospective study Ennishi et al[51] described a hepatic flare in 27% of 131 patients with non-Hodgkin’s lymphoma (NHL) and HCV infection treated with rituximab and prednisone-based chemotherapy. Marignani et al[52] described 3 patients with NHL and HCV infection treated with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP) therapy, two of whom experienced HCV reactivation and a subsequent hepatic flare after chemotherapy was discontinued. Pitini et al[53] described HCV reactivation with a hepatic flare in 10 anti-HCV-positive patients treated with R-CHOP for NHL.
In a retrospective study on 308 patients with cancer and HCV infection, Mahale et al[62] described a hepatic flare in 11% of cases, 73% in those with hematological malignancies and 29% in those with solid cancer (P < 0.001); in the multivariate analysis, the underlying hematological malignancy (P = 0.02, OR = 3.2, 95%CI: 1.2-8.7) and the use of rituximab (P = 0.004, OR = 4.2, 95%CI: 1.6-10.9) were the only independent predictors of ae-CHC.
Also the relationship between anti-TNF-alfa agents and HCV infection needs further investigation. These biological agents are effective therapy for a wide spectrum of chronic inflammatory diseases such as psoriasis, rheumatoid arthritis and inflammatory bowel disease and have been demonstrated to be safe in CHC[63]. In fact, Ferri et al[64] reported that of 31 patients with rheumatoid arthritis and HCV infection treated with anti-TNF-alfa agents only one developed a hepatic flare but without an elevation in the HCV viral load. Pritchard[65] described a patient with rheumatoid arthritis treated with the anti-TNF-alfa agent etanercept who experienced a 3-fold increase in the serum aminotransferase levels together with an increase in HCV viral load and who reverted to the previous values once etanercept treatment was discontinued. Li et al[66] also described a patient who developed a hepatic flare under etanercept and showed remission once this drug was replaced with infliximab.
To conclude on this point, in patients with an onco-hematological disease, rituximab-based chemotherapy, and to a lesser degree rituximab-sparing chemotherapy, favors HCV replication in hepatocytes, which, once the treatment is discontinued and the immunological conditions restored, may be a target for enhanced cell-mediated activity. In these patients, ae-CHC may have a further unfavorable clinical impact since the hepatic flare frequently requires the discontinuation of life-saving chemotherapy. Thus, the HCV load and serum aminotransferases should be closely monitored in onco-hematological patients receiving chemotherapy, particularly if therapy is rituximab-based and/or includes high-dose corticosteroids.
In patients with chronic CHC a hepatic flare may also be the clinical manifestation of HAV, HBV, HBV plus HDV, HEV or CMV superinfection (Table 1) or of lifestyle factors such as alcohol intake or the consumption of hepatotoxic drugs.
HAV superinfection in patients with CHC has been documented by several authors[67-74]. Sagnelli et al[67] described 21 patients with chronic hepatitis who developed hepatitis A, 13 with an underlying chronic HBV infection and 8 with a chronic HCV infection. In all cases HAV always had a self-limiting clinical course, associated with a marked inhibition of HBV and HCV genomes; no patient had clinical, laboratory or ultrasound evidence of liver cirrhosis in this study and the underlying chronic hepatitis showed a regular clinical course. These data are in contrast with those from a previous study reporting a high rate of fulminant hepatitis due to acute hepatitis A occurring in patients with pre-existing chronic hepatitis[68], but are in complete agreement with those reported in numerous other studies[69-74].
Several studies have been published on HBV superinfection in HCV chronic carriers and an inhibition of the HCV genome, temporary or persistent, has always been documented[75-83]. Biliotti et al[80] in 2008 described 14 patients with an underlying chronic HCV infection who developed acute hepatitis B of mild clinical course that suppressed HCV replication. To this regard Sagnelli et al[81] in 2009 described the clinical and virological impact of hepatitis B virus superinfection in 29 patients with chronic HCV infection and compared the data obtained with those from a control group of 29 normal subjects who developed acute hepatitis B in the same period, pair-matched by age, sex, and risk factors for the acquisition of HBV infection. Acute hepatitis B showed a severe course more frequently in patients with a pre-existing chronic HCV infection (34.5% vs 6.9%, P < 0.05). HCV RNA was undetectable in all patients during HBV superinfection: one-third eradicated the HCV chronic infection and two-thirds became HCV-RNA-positive during a follow-up of 4-6 years; HCV clearance was more frequent in patients with acute hepatitis B with a severe course (83.3% vs 22.2%, P < 0.05). Some published case reports are in good agreement with these data[75-77].
Hepatic flares due to superinfections by other viruses such as HDV, heterologous HCV strains, HEV and CMV in patients with chronic hepatitis C have been poorly investigated.
There is little information on the outcome of chronic HCV infection after HBV/HDV superinfection. To this regard Deterding et al[84] described a patient with chronic hepatitis C who cleared HCV infection during an acute self-limiting HBV/HDV superinfection.
Patients with chronic HCV infection may also develop a superinfection by a heterologous HCV strain if they continue to be at risk of acquiring HCV infection[85-90]. Grebely et al[85] described 6 patients with chronic HCV infection and a history of intravenous drug addiction who became superinfected with a different HCV strain; 5 of these 6 developed a new episode of acute hepatitis C. In a patient with chronic hepatitis C and a history of intravenous drug addiction, Sagnelli et al[86] documented a second episode of HCV infection associated with a hepatic flare and with the replacement of HCV subgenotype 2c with subgenotype 2b.
Accapezzato et al[90] described a patient with chronic hepatitis C who developed a second episode of acute hepatitis C after colonoscopy, and the replacement of HCV genotype 1b with genotype 4. Thus, this might suggest re-determining the HCV subgenotype in the case of a hepatic flare in patients with CHC who continue to be at risk of acquiring HCV infection.
HEV infection is endemic in Southeast Asia and some studies have suggested that HEV superinfection in patients with an underlying chronic liver disease can cause severe hepatic decompensation[91-94]. This event should be considered for a differential diagnosis even in geographical areas where HEV infection is infrequent[95].
Cytomegalovirus can also cause liver damage and hepatic flares in the context of a systemic infection[96,97]. Reactivation of CMV infection is a frequent complication after solid-organ transplantation[99], but it may occur also in immunocompetent patients[98-105] and, consequently, it should be considered among the possible causes of hepatic flares in patients with CHC.
Concluding on this point, HAV or HBV superinfection in patients with a pre-existing chronic HCV infection can cause acute viral hepatitis that may induce a strong inhibition of HCV replication and lead in some cases to a long-term HCV eradication. Viral superinfection, however, may be life-threatening in HCV chronic carriers at an advanced stage of the disease. Therefore, efforts should be made to extend HAV and/or HBV vaccination to patients with CHC, a practice recommended by several international and national healthcare institutions but which remains poorly applied[28,29]. The data on the clinical impact of HEV and CMV superinfection in patients with underlying chronic hepatitis C are scanty, but there is enough evidence to conclude that these two viruses should be considered in the differential diagnosis of flares.
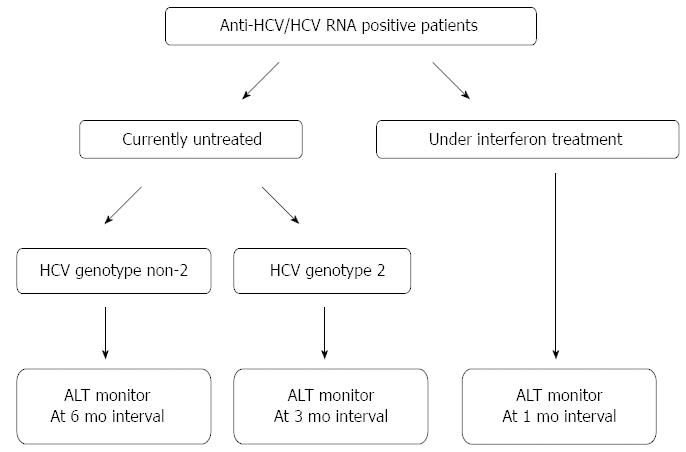
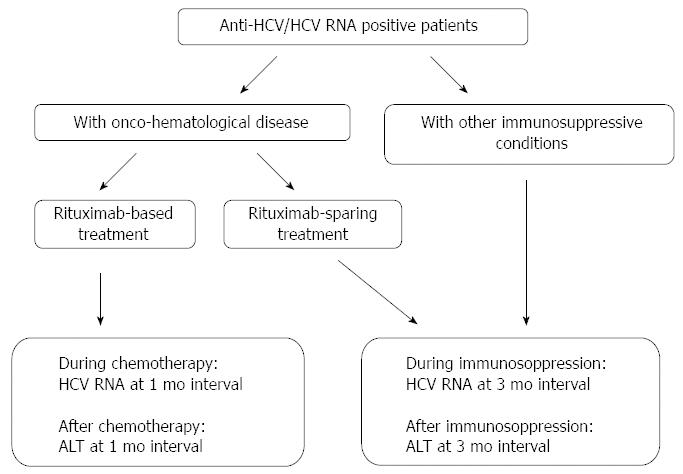
The hepatic flare is a frequent and well-known event that may occur during the clinical course of CHC and may be due to different causes and have different outcomes. Apart from IL28B-CC aplotype and HCV genotype 2, the risk factors for the development of a hepatic flare are still somewhat obscure, but a possible role of the metabolic disorders and endocrine derangements, such as liver steatosis, diabetes and insulin resistance should be investigated. The clinician treating a patient with chronic HCV infection should monitor for hepatic flares during the follow-up since an increase in the serum aminotransferase values may be asymptomatic. To this regard, suggested monitoring schedules for immunocompetent and immunocompromised HCV patients are shown in Figures 1 and 2, respectively. When a hepatic flare occurs, the cause of this event or the etiologic agent should be identified and, when possible, appropriate treatment administered. The influence of the flares on the course of the underlying CHC should be monitored in order that the best clinical and therapeutic decisions be made.
P- Reviewers: Lonardo A, Narciso-Schiavon JL S- Editor: Gou SX L- Editor: A E- Editor: Zhang DN
| 1. | Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1927] [Cited by in RCA: 1931] [Article Influence: 96.6] [Reference Citation Analysis (0)] |
| 2. | Guidotti LG, Chisari FV. Immunobiology and pathogenesis of viral hepatitis. Annu Rev Pathol. 2006;1:23-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 595] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 3. | Moradpour D, Penin F, Rice CM. Replication of hepatitis C virus. Nat Rev Microbiol. 2007;5:453-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 973] [Cited by in RCA: 1005] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 4. | Götz HM, van Doornum G, Niesters HG, den Hollander JG, Thio HB, de Zwart O. A cluster of acute hepatitis C virus infection among men who have sex with men--results from contact tracing and public health implications. AIDS. 2005;19:969-974. [PubMed] |
| 5. | van de Laar TJ, van der Bij AK, Prins M, Bruisten SM, Brinkman K, Ruys TA, van der Meer JT, de Vries HJ, Mulder JW, van Agtmael M. Increase in HCV incidence among men who have sex with men in Amsterdam most likely caused by sexual transmission. J Infect Dis. 2007;196:230-238. [PubMed] |
| 6. | Danta M, Brown D, Bhagani S, Pybus OG, Sabin CA, Nelson M, Fisher M, Johnson AM, Dusheiko GM. Recent epidemic of acute hepatitis C virus in HIV-positive men who have sex with men linked to high-risk sexual behaviours. AIDS. 2007;21:983-991. [PubMed] |
| 7. | Bottieau E, Apers L, Van Esbroeck M, Vandenbruaene M, Florence E. Hepatitis C virus infection in HIV-infected men who have sex with men: sustained rising incidence in Antwerp, Belgium, 2001-2009. Euro Surveill. 2010;15:19673. [PubMed] |
| 8. | Santantonio T, Medda E, Ferrari C, Fabris P, Cariti G, Massari M, Babudieri S, Toti M, Francavilla R, Ancarani F. Risk factors and outcome among a large patient cohort with community-acquired acute hepatitis C in Italy. Clin Infect Dis. 2006;43:1154-1159. [PubMed] |
| 9. | Filippini P, Coppola N, Scolastico C, Rossi G, Onofrio M, Sagnelli E, Piccinino F. Does HIV infection favor the sexual transmission of hepatitis C? Sex Transm Dis. 2001;28:725-729. [PubMed] |
| 10. | Gentile I, De Stefano A, Di Flumeri G, Buonomo AR, Carlomagno C, Morisco F, De Placido S, Borgia G. Concomitant interferon-alpha and chemotherapy in hepatitis C and colorectal cancer: a case report. In Vivo. 2013;27:527-529. [PubMed] |
| 11. | Gentile I, Di Flumeri G, Scarica S, Frangiosa A, Foggia M, Reynaud L, Borgia G. Acute hepatitis C in patients undergoing hemodialysis: experience with high-dose interferon therapy. Minerva Urol Nefrol. 2013;65:83-84. [PubMed] |
| 12. | Orland JR, Wright TL, Cooper S. Acute hepatitis C. Hepatology. 2001;33:321-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 137] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 13. | Wang CC, Krantz E, Klarquist J, Krows M, McBride L, Scott EP, Shaw-Stiffel T, Weston SJ, Thiede H, Wald A. Acute hepatitis C in a contemporary US cohort: modes of acquisition and factors influencing viral clearance. J Infect Dis. 2007;196:1474-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 147] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 14. | Deutsch M, Papadopoulos N, Hadziyannis ES, Koskinas J. Clinical characteristics, spontaneous clearance and treatment outcome of acute hepatitis C: a single tertiary center experience. Saudi J Gastroenterol. 2013;19:81-85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Rehermann B. Hepatitis C virus versus innate and adaptive immune responses: a tale of coevolution and coexistence. J Clin Invest. 2009;119:1745-1754. [PubMed] |
| 16. | Post J, Ratnarajah S, Lloyd AR. Immunological determinants of the outcomes from primary hepatitis C infection. Cell Mol Life Sci. 2009;66:733-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Thomas DL, Thio CL, Martin MP, Qi Y, Ge D, O’Huigin C, Kidd J, Kidd K, Khakoo SI, Alexander G. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009;461:798-801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1706] [Cited by in RCA: 1687] [Article Influence: 105.4] [Reference Citation Analysis (0)] |
| 18. | Rauch A, Gaudieri S, Thio C, Bochud PY. Host genetic determinants of spontaneous hepatitis C clearance. Pharmacogenomics. 2009;10:1819-1837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Coppola N, Marrone A, Pisaturo M, Starace M, Signoriello G, Gentile I, Adinolfi LE, Sagnelli E, Zampino R. Role of interleukin 28-B in the spontaneous and treatment-related clearance of HCV infection in patients with chronic HBV/HCV dual infection. Eur J Clin Microbiol Infect Dis. 2014;33:559-567. [PubMed] |
| 20. | Fortunato G, Calcagno G, Bresciamorra V, Salvatore E, Filla A, Capone S, Liguori R, Borelli S, Gentile I, Borrelli F. Multiple sclerosis and hepatitis C virus infection are associated with single nucleotide polymorphisms in interferon pathway genes. J Interferon Cytokine Res. 2008;28:141-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Ray SC, Wang YM, Laeyendecker O, Ticehurst JR, Villano SA, Thomas DL. Acute hepatitis C virus structural gene sequences as predictors of persistent viremia: hypervariable region 1 as a decoy. J Virol. 1999;73:2938-2946. [PubMed] |
| 22. | Farci P, Shimoda A, Coiana A, Diaz G, Peddis G, Melpolder JC, Strazzera A, Chien DY, Munoz SJ, Balestrieri A. The outcome of acute hepatitis C predicted by the evolution of the viral quasispecies. Science. 2000;288:339-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 680] [Cited by in RCA: 648] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 23. | Harris HE, Eldridge KP, Harbour S, Alexander G, Teo CG, Ramsay ME. Does the clinical outcome of hepatitis C infection vary with the infecting hepatitis C virus type? J Viral Hepat. 2007;14:213-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Afdhal NH. The natural history of hepatitis C. Semin Liver Dis. 2004;24 Suppl 2:3-8. [PubMed] |
| 25. | Alberti A, Chemello L, Benvegnù L. Natural history of hepatitis C. J Hepatol. 1999;31 Suppl 1:17-24. [PubMed] |
| 26. | Gentile I, Coppola N, Pasquale G, Liuzzi R, D’Armiento M, Di Lorenzo ME, Capoluongo N, Buonomo AR, Sagnelli E, Morisco F. A Simple Noninvasive Score Based on Routine Parameters can Predict Liver Cirrhosis in Patients With Chronic Hepatitis C. Hepat Mon. 2013;13:e8352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Coppola N, Gentile I, Pasquale G, Buonomo AR, Capoluongo N, D’Armiento M, Borgia G, Sagnelli E. Anti-HBc positivity was associated with histological cirrhosis in patients with chronic hepatitis C. Ann Hepatol. 2013;13:20-26. [PubMed] |
| 28. | Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335-1374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2320] [Cited by in RCA: 2240] [Article Influence: 140.0] [Reference Citation Analysis (1)] |
| 29. | Italian Association for the Study of the Liver; Italian Society of Infectious, Tropical Diseases; Italian Society for the Study of Sexually Transmitted Diseases. Practice guidelines for the treatment of hepatitis C: recommendations from an AISF/SIMIT/SIMAST Expert Opinion Meeting. Dig Liver Dis. 2010;42:81-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 30. | Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL, Häussinger D, Diago M, Carosi G, Dhumeaux D. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975-982. [PubMed] |
| 31. | Gentile I, Viola C, Borgia F, Castaldo G, Borgia G. Telaprevir: a promising protease inhibitor for the treatment of hepatitis C virus infection. Curr Med Chem. 2009;16:1115-1121. [PubMed] |
| 32. | Coppola N, Pisaturo M, Tonziello G, Sagnelli C, Sagnelli E, Angelillo IF. Efficacy of Pegylated interferon α-2a and α-2b in patients with genotype 1 chronic hepatitis C: a meta-analysis. BMC Infect Dis. 2012;12:357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 33. | Hadziyannis SJ, Sette H, Morgan TR, Balan V, Diago M, Marcellin P, Ramadori G, Bodenheimer H, Bernstein D, Rizzetto M. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004;140:346-355. [PubMed] |
| 34. | Younossi ZM, Stepanova M, Henry L, Gane E, Jacobson IM, Lawitz E, Nelson D, Gerber L, Nader F, Hunt S. Effects of Sofosbuvir-Based Treatment, With and Without Interferon, on Outcome and Productivity of Patients With Chronic Hepatitis C. Clin Gastroenterol Hepatol. 2013;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 35. | Asselah T. Sofosbuvir for the treatment of hepatitis C virus. Expert Opin Pharmacother. 2014;15:121-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 36. | Gentile I, Borgia F, Coppola N, Buonomo AR, Castaldo G, Borgia G. Daclatasvir: the first of a new class of drugs targeted against hepatitis C virus NS5A. Curr Med Chem. 2014;21:1391-1404. [PubMed] |
| 37. | Gentile I, Borgia F, Zappulo E, Buonomo AR, Spera AM, Castaldo G, Borgia G. Efficacy and Safety of Sofosbuvir in Treatment of Chronic Hepatitis C: The Dawn of the a New Era. Rev Recent Clin Trials. 2013;Epub ahead of print. [PubMed] |
| 38. | Gentile I, Borgia F, Buonomo AR, Castaldo G, Borgia G. A novel promising therapeutic option against hepatitis C virus: an oral nucleotide NS5B polymerase inhibitor sofosbuvir. Curr Med Chem. 2013;20:3733-3742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 39. | Sheen IS, Liaw YF, Lin DY, Chu CM. Acute exacerbations in chronic hepatitis C: a clinicopathological and prognostic study. J Hepatol. 1996;24:525-531. [PubMed] |
| 40. | Hiraga N, Suzuki F, Akuta N, Suzuki Y, Sezaki H, Hosaka T, Someya T, Kobayashi M, Saitoh S, Arase Y. Clinical and virological characteristics of untreated patients with chronic hepatitis C who develop serum alanine aminotransferase flare-up. J Med Virol. 2005;75:240-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 41. | Rumi MG, De Filippi F, La Vecchia C, Donato MF, Gallus S, Del Ninno E, Colombo M. Hepatitis C reactivation in patients with chronic infection with genotypes 1b and 2c: a retrospective cohort study of 206 untreated patients. Gut. 2005;54:402-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 51] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 42. | Sagnelli E, Coppola N, Marrocco C, Coviello G, Battaglia M, Messina V, Rossi G, Sagnelli C, Scolastico C, Filippini P. Diagnosis of hepatitis C virus related acute hepatitis by serial determination of IgM anti-HCV titres. J Hepatol. 2005;42:646-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 43. | Sagnelli E, Pisaturo M, Stanzione M, Messina V, Alessio L, Sagnelli C, Starace M, Pasquale G, Coppola N. Clinical presentation, outcome, and response to therapy among patients with acute exacerbation of chronic hepatitis C. Clin Gastroenterol Hepatol. 2013;11:1174-1180.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 44. | Coppola N, Pisapia R, Marrocco C, Martini S, Vatiero LM, Messina V, Tonziello G, Sagnelli C, Filippini P, Piccinino F. Anti-HCV IgG avidity index in acute hepatitis C. J Clin Virol. 2007;40:110-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 45. | Coppola N, Pisapia R, Tonziello G, Masiello A, Martini S, Pisaturo M, Messina V, Sagnelli C, Macera M, Signoriello G. Improvement in the aetiological diagnosis of acute hepatitis C: a diagnostic protocol based on the anti-HCV-IgM titre and IgG Avidity Index. J Clin Virol. 2009;46:222-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 46. | Sagnelli E, Tonziello G, Pisaturo M, Sagnelli C, Coppola N. Clinical applications of antibody avidity and immunoglobulin M testing in acute HCV infection. Antivir Ther. 2012;17:1453-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 47. | Tsuji K, Yamasaki K, Yamanishi M, Kawakami M, Shirahama S. Risk of alanine aminotransferase flare-up among asymptomatic hepatitis C virus RNA carriers: a 10-year follow-up study. J Gastroenterol Hepatol. 2001;16:536-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 48. | Coppola N, Vatiero LM, Sagnelli E. HCV genotype 2 as a risk factor for reactivation of chronic HCV infection. Gut. 2005;54:1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 49. | Sagnelli E, Pisaturo M, Sagnelli C, Coppola N. Rituximab-based treatment, HCV replication, and hepatic flares. Clin Dev Immunol. 2012;2012:945950. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 50. | Coppola N, Pisaturo M, Guastafierro S, Tonziello G, Sica A, Iodice V, Sagnelli C, Ferrara MG, Sagnelli E. Increased hepatitis C viral load and reactivation of liver disease in HCV RNA-positive patients with onco-haematological disease undergoing chemotherapy. Dig Liver Dis. 2012;44:49-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 51. | Ennishi D, Maeda Y, Niitsu N, Kojima M, Izutsu K, Takizawa J, Kusumoto S, Okamoto M, Yokoyama M, Takamatsu Y. Hepatic toxicity and prognosis in hepatitis C virus-infected patients with diffuse large B-cell lymphoma treated with rituximab-containing chemotherapy regimens: a Japanese multicenter analysis. Blood. 2010;116:5119-5125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 99] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 52. | Marignani M, Mangone M, Cox MC, Angeletti S, Veggia B, Ferrari A, di Fonzo M, Begini P, Gigante E, Laverde G. HCV-positive status and hepatitis flares in patients with B-cell non-Hodgkin’s lymphoma treated with rituximab-containing regimens. Dig Liver Dis. 2011;43:139-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 53. | Pitini V, Sturniolo G, Arrigo C, Leonardi S, Pino S, Altavilla G. HCV genotype 2 as a risk factor for reactivation in patients with B-cell lymphoma undergoing rituximab combination chemotherapy. Br J Haematol. 2010;150:116-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 54. | Thurairajah PH, Thorburn D, Hubscher S, White A, Lai WK, O’Donnell K, Mutimer D. Incidence and characterization of serum transaminases elevations in pegylated interferon and ribavirin treated patients with chronic hepatitis C. Aliment Pharmacol Ther. 2007;25:1293-1300. [PubMed] |
| 55. | Caroleo B, Gallelli L, Staltari O, De Sarro G, Guadagnino V. Serum transaminase elevations during pegylated interferon treatment of chronic HCV hepatitis probably induced by polyethylene glycol. Intervirology. 2008;51:407-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 56. | Papo T, Marcellin P, Bernuau J, Durand F, Poynard T, Benhamou JP. Autoimmune chronic hepatitis exacerbated by alpha-interferon. Ann Intern Med. 1992;116:51-53. [PubMed] |
| 57. | García-Buey L, García-Monzón C, Rodriguez S, Borque MJ, García-Sánchez A, Iglesias R, DeCastro M, Mateos FG, Vicario JL, Balas A. Latent autoimmune hepatitis triggered during interferon therapy in patients with chronic hepatitis C. Gastroenterology. 1995;108:1770-1777. [PubMed] |
| 58. | Todros L, Saracco G, Durazzo M, Abate ML, Touscoz G, Scaglione L, Verme G, Rizzetto M. Efficacy and safety of interferon alfa therapy in chronic hepatitis C with autoantibodies to liver-kidney microsomes. Hepatology. 1995;22:1374-1378. [PubMed] |
| 59. | Bayraktar Y, Bayraktar M, Gurakar A, Hassanein TI, Van Thiel DH. A comparison of the prevalence of autoantibodies in individuals with chronic hepatitis C and those with autoimmune hepatitis: the role of interferon in the development of autoimmune diseases. Hepatogastroenterology. 1997;44:417-425. [PubMed] |
| 60. | Kogure T, Ueno Y, Fukushima K, Nagasaki F, Inoue J, Kakazu E, Matsuda Y, Kido O, Nakagome Y, Kimura O. Fulminant hepatic failure in a case of autoimmune hepatitis in hepatitis C during peg-interferon-alpha 2b plus ribavirin treatment. World J Gastroenterol. 2007;13:4394-4397. [PubMed] |
| 61. | Swain MG, Lai MY, Shiffman ML, Cooksley WG, Zeuzem S, Dieterich DT, Abergel A, Pessôa MG, Lin A, Tietz A. A sustained virologic response is durable in patients with chronic hepatitis C treated with peginterferon alfa-2a and ribavirin. Gastroenterology. 2010;139:1593-1601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 235] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 62. | Mahale P, Kontoyiannis DP, Chemaly RF, Jiang Y, Hwang JP, Davila M, Torres HA. Acute exacerbation and reactivation of chronic hepatitis C virus infection in cancer patients. J Hepatol. 2012;57:1177-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 63. | Brunasso AM, Puntoni M, Gulia A, Massone C. Safety of anti-tumour necrosis factor agents in patients with chronic hepatitis C infection: a systematic review. Rheumatology (Oxford). 2011;50:1700-1711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 64. | Ferri C, Ferraccioli G, Ferrari D, Galeazzi M, Lapadula G, Montecucco C, Triolo G, Valentini G, Valesini G. Safety of anti-tumor necrosis factor-alpha therapy in patients with rheumatoid arthritis and chronic hepatitis C virus infection. J Rheumatol. 2008;35:1944-1949. [PubMed] |
| 65. | Pritchard C. Etanercept and hepatitis C. J Clin Rheumatol. 1999;5:179. [PubMed] |
| 66. | Li S, Kaur PP, Chan V, Berney S. Use of tumor necrosis factor-alpha (TNF-alpha) antagonists infliximab, etanercept, and adalimumab in patients with concurrent rheumatoid arthritis and hepatitis B or hepatitis C: a retrospective record review of 11 patients. Clin Rheumatol. 2009;28:787-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 67. | Sagnelli E, Coppola N, Pisaturo M, Pisapia R, Onofrio M, Sagnelli C, Catuogno A, Scolastico C, Piccinino F, Filippini P. Clinical and virological improvement of hepatitis B virus-related or hepatitis C virus-related chronic hepatitis with concomitant hepatitis A virus infection. Clin Infect Dis. 2006;42:1536-1543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 68. | Vento S, Garofano T, Renzini C, Cainelli F, Casali F, Ghironzi G, Ferraro T, Concia E. Fulminant hepatitis associated with hepatitis A virus superinfection in patients with chronic hepatitis C. N Engl J Med. 1998;338:286-290. [PubMed] |
| 69. | Mele A, Tosti ME, Stroffolini T. Hepatitis associated with hepatitis A superinfection in patients with chronic hepatitis C. N Engl J Med. 1998;338:1771; author reply 1772-1773. [PubMed] |
| 70. | Battegay M, Naef M, Bucher HC. Hepatitis associated with hepatitis A superinfection in patients with chronic hepatitis C. N Engl J Med. 1998;338:1771-172; author reply 1772-1773. [PubMed] |
| 71. | Leino T, Pebody R, Leinikki P. Hepatitis associated with hepatitis A superinfection in patients with chronic hepatitis C. N Engl J Med. 1998;338:1772; author reply 1772-1773. [PubMed] |
| 72. | Martins EB. Hepatitis associated with hepatitis A superinfection in patients with chronic hepatitis C. N Engl J Med. 1998;338:1772; author reply 1772-1773. [PubMed] |
| 73. | Stroffolini T, Almasio PL, Di Stefano R, Andreone P, Di Gaetano G, Fattovich G, Gaeta GB, Morisco F, Smedile A, Tripi S. Anti-hepatitis A virus seroprevalence and seroconversion in a cohort of patients with chronic viral hepatitis. Dig Liver Dis. 2002;34:656-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 74. | Cacopardo B, Nunnari G, Nigro L. Clearance of HCV RNA following acute hepatitis A superinfection. Dig Liver Dis. 2009;41:371-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 75. | Gruener NH, Jung MC, Ulsenheimer A, Gerlach TJ, Diepolder HM, Schirren CA, Hoffmann R, Wächtler M, Backmund M, Pape GR. Hepatitis C virus eradication associated with hepatitis B virus superinfection and development of a hepatitis B virus specific T cell response. J Hepatol. 2002;37:866-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 76. | Guido M, Rugge M, Colombari R, Cecchetto A, Scarpa A, Cadrobbi P. Prompt hepatitis C virus suppression following hepatitis B virus superinfection in chronic untreated hepatitis C. Ital J Gastroenterol Hepatol. 1998;30:414-417. [PubMed] |
| 77. | Liaw YF, Yeh CT, Tsai SL. Impact of acute hepatitis B virus superinfection on chronic hepatitis C virus infection. Am J Gastroenterol. 2000;95:2978-2980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 78. | Sagnelli E, Coppola N, Messina V, Di Caprio D, Marrocco C, Marotta A, Onofrio M, Scolastico C, Filippini P. HBV superinfection in hepatitis C virus chronic carriers, viral interaction, and clinical course. Hepatology. 2002;36:1285-1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 101] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 79. | Wietzke P, Schott P, Braun F, Mihm S, Ramadori G. Clearance of HCV RNA in a chronic hepatitis C virus-infected patient during acute hepatitis B virus superinfection. Liver. 1999;19:348-353. [PubMed] |
| 80. | Biliotti E, Kondili LA, Furlan C, Ferretti G, Zacharia S, De Angelis M, Guidi S, Gusman N, Taliani G. Acute hepatitis B in patients with or without underlying chronic HCV infection. J Infect. 2008;57:152-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 81. | Sagnelli E, Coppola N, Pisaturo M, Masiello A, Tonziello G, Sagnelli C, Messina V, Filippini P. HBV superinfection in HCV chronic carriers: a disease that is frequently severe but associated with the eradication of HCV. Hepatology. 2009;49:1090-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 82. | Coppola N, Marrocco C, Di Caprio D, Coviello G, Scolastico C, Filippini P, Sagnelli E. Acute hepatitis B and C virus coinfection: a virological and clinical study of 3 cases. Clin Infect Dis. 2003;36:528-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 83. | Sagnelli E, Coppola N, Marrocco C, Onofrio M, Sagnelli C, Coviello G, Scolastico C, Filippini P. Hepatitis C virus superinfection in hepatitis B virus chronic carriers: a reciprocal viral interaction and a variable clinical course. J Clin Virol. 2006;35:317-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 84. | Deterding K, Pothakamuri SV, Schlaphoff V, Hadem J, Metzler F, Bahr MJ, Manns MP, Cornberg M, Wedemeyer H. Clearance of chronic HCV infection during acute delta hepatitis. Infection. 2009;37:159-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 85. | Grebely J, Pham ST, Matthews GV, Petoumenos K, Bull RA, Yeung B, Rawlinson W, Kaldor J, Lloyd A, Hellard M. Hepatitis C virus reinfection and superinfection among treated and untreated participants with recent infection. Hepatology. 2012;55:1058-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 86. | Sagnelli E, Argentini C, Genovese D, Pisaturo M, Coppola N, Taffon S, Sagnelli C, Rapicetta M. Virological and epitope evolution of HCV infection from acute hepatitis C to subsequent episodes of HCV-related acute liver cell necrosis. Infection. 2009;37:344-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 87. | Lai ME, Mazzoleni AP, Argiolu F, De Virgilis S, Balestrieri A, Purcell RH, Cao A, Farci P. Hepatitis C virus in multiple episodes of acute hepatitis in polytransfused thalassaemic children. Lancet. 1994;343:388-390. [PubMed] |
| 88. | Proust B, Dubois F, Bacq Y, Le Pogam S, Rogez S, Levillain R, Goudeau A. Two successive hepatitis C virus infections in an intravenous drug user. J Clin Microbiol. 2000;38:3125-3127. [PubMed] |
| 89. | Dove L, Phung Y, Bzowej N, Kim M, Monto A, Wright TL. Viral evolution of hepatitis C in injection drug users. J Viral Hepat. 2005;12:574-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 90. | Accapezzato D, Fravolini F, Casciaro MA, Paroli M. Hepatitis C flare due to superinfection by genotype 4 in an HCV genotype 1b chronic carrier. Eur J Gastroenterol Hepatol. 2002;14:879-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 91. | Hamid SS, Atiq M, Shehzad F, Yasmeen A, Nissa T, Salam A, Siddiqui A, Jafri W. Hepatitis E virus superinfection in patients with chronic liver disease. Hepatology. 2002;36:474-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 152] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 92. | Monga R, Garg S, Tyagi P, Kumar N. Superimposed acute hepatitis E infection in patients with chronic liver disease. Indian J Gastroenterol. 2004;23:50-52. [PubMed] |
| 93. | Kc S, Sharma D, Basnet BK, Mishra AK. Effect of acute hepatitis E infection in patients with liver cirrhosis. JNMA J Nepal Med Assoc. 2009;48:226-229. [PubMed] |
| 94. | Radha Krishna Y, Saraswat VA, Das K, Himanshu G, Yachha SK, Aggarwal R, Choudhuri G. Clinical features and predictors of outcome in acute hepatitis A and hepatitis E virus hepatitis on cirrhosis. Liver Int. 2009;29:392-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 95. | Bayram A, Eksi F, Mehli M, Sözen E. Prevalence of hepatitis E virus antibodies in patients with chronic hepatitis B and chronic hepatitis C. Intervirology. 2007;50:281-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 96. | Sissons JG, Carmichael AJ. Clinical aspects and management of cytomegalovirus infection. J Infect. 2002;44:78-83. [PubMed] |
| 97. | Sinzger C, Bissinger AL, Viebahn R, Oettle H, Radke C, Schmidt CA, Jahn G. Hepatocytes are permissive for human cytomegalovirus infection in human liver cell culture and In vivo. J Infect Dis. 1999;180:976-986. [PubMed] |
| 98. | Steininger C. Clinical relevance of cytomegalovirus infection in patients with disorders of the immune system. Clin Microbiol Infect. 2007;13:953-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 90] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 99. | Ten Napel HH, Houthoff HJ, The TH. Cytomegalovirus hepatitis in normal and immune compromised hosts. Liver. 1984;4:184-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 92] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 100. | Bentata-Pessayre M, Beaugrand M, Callard P, Brun-Vesinet F, Krivitsky A, Mornet P, Delzant G. [Granulomatous hepatitis in cytomegalovirus infection in healthy adults]. Ann Med Interne (Paris). 1987;138:353-357. [PubMed] |
| 101. | Mannstadt C, Stein G, Färber I. [Clinical aspects of acute cytomegalovirus infection in adults without immune deficiency]. Z Gesamte Inn Med. 1991;46:107-109. [PubMed] |
| 102. | Diego Lorenzo A, de González-Asanza C, García Durán F, Piqueras B, Romero M, Salcedo M, Masud K, Cos E, Clemente G. [Cytomegalovirus infection as a cause of granulomatous hepatitis in an immunocompetent patient]. Rev Esp Enferm Dig. 1996;88:295-297. [PubMed] |
| 103. | Kunno A, Abe M, Yamada M, Murakami K. Clinical and histological features of cytomegalovirus hepatitis in previously healthy adults. Liver. 1997;17:129-132. [PubMed] |
| 104. | de la Serna-Higuera C, González-García M, Milicua JM, Muñoz V. [Cytomegalovirus granulomatous hepatitis in an immunocompetent patient]. Gastroenterol Hepatol. 1999;22:230-231. [PubMed] |
| 105. | Vujacich C, Vidiella G, Barcelona L, Sturba E, Stamboulian D. [Cytomegalovirus infection with hepatic involvement in immunocompetent adults]. Medicina (B Aires). 2006;66:206-210. [PubMed] |