Published online Jun 7, 2014. doi: 10.3748/wjg.v20.i21.6691
Revised: February 20, 2014
Accepted: March 19, 2014
Published online: June 7, 2014
Processing time: 235 Days and 20.1 Hours
Reversible posterior leukoencephalopathy syndrome (RPLS) is a rare brain-capillary leak syndrome, characterized by clinical symptoms of headache, visual loss, seizures and altered mental functioning. This syndrome is usually reversible and is associated with hypertension, nephropathy, and use of immunosuppressive medication and cytotoxic agents. We describe two rare cases of RPLS occurring in colorectal cancer, both of which presented with coma, that we believe can be directly attributed to bevacizumab, a monoclonal antibody that inhibits the angiogenesis of tumours by specifically blocking vascular endothelial growth factor. We analysed the clinical features, risk factors and outcomes of RPLS in these two patients, and although no typical finding was identified on imaging examination, we found that inadequate blood pressure control was one of the risk factors leading to RPLS and that supportive treatment including intensive blood pressure control improved outcomes. Due to the increasing use of bevacizumab in colorectal cancer, clinicians should be aware of this potential complication.
Core tip: This is the first report of reversible posterior leukoencephalopathy syndrome (RPLS) induced by bevacizumab in China. RPLS is a rare complication of bevacizumab, but may present with life-threatening symptoms such as coma. Sudden blood pressure increase is the most common risk factor, and early recognition and prompt control of blood pressure may make this complication reversible.
- Citation: Wang W, Zhao LR, Lin XQ, Feng F. Reversible posterior leukoencephalopathy syndrome induced by bevacizumab plus chemotherapy in colorectal cancer. World J Gastroenterol 2014; 20(21): 6691-6697
- URL: https://www.wjgnet.com/1007-9327/full/v20/i21/6691.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i21.6691
Reversible posterior leukoencephalopathy syndrome (RPLS),first described by Hinchey et al[1] in 1996,is also known as posterior reversible encephalopathy syndrome. RPLS is an underappreciated syndrome characterized by clinical symptoms of headache, altered mental functioning, visual loss, and seizures, and radiological findings by magnetic resonance imaging (MRI) of subcortical oedema predominantly in the posterior cerebral white matter. In most cases both the symptoms and radiological features of RPLS are reversible. The precise pathophysiology of RPLS remains uncertain. Deficiency in cerebrovascular auto-regulation is a favoured hypothesis because the syndrome is associated with hypertensive encephalopathy.
Bevacizumab, an angiogenesis inhibitor, was approved in the United States in February 2004 for the treatment of newly diagnosed metastatic colorectal cancer, usually combined with intravenous fluorouracil chemotherapy. Bevacizumab is the first-line treatment for metastatic colorectal cancers. It specifically binds to the vascular endothelial growth factor (VEGF) in peripheral blood and significantly improves the efficacy of the combined chemotherapy drugs[2,3]. The major adverse effects of bevacizumab include hypertension, proteinuria, thrombotic events and delayed wound healing, largely due to its anti-angiogenic effect. Although this combination regimen has recently been associated with RPLS, RPLS-associated coma is not common. Here we report the first known series of cases in China of RPLS-associated coma in the context of bevacizumab combination with chemotherapy, and provide a review of the relevant literature.
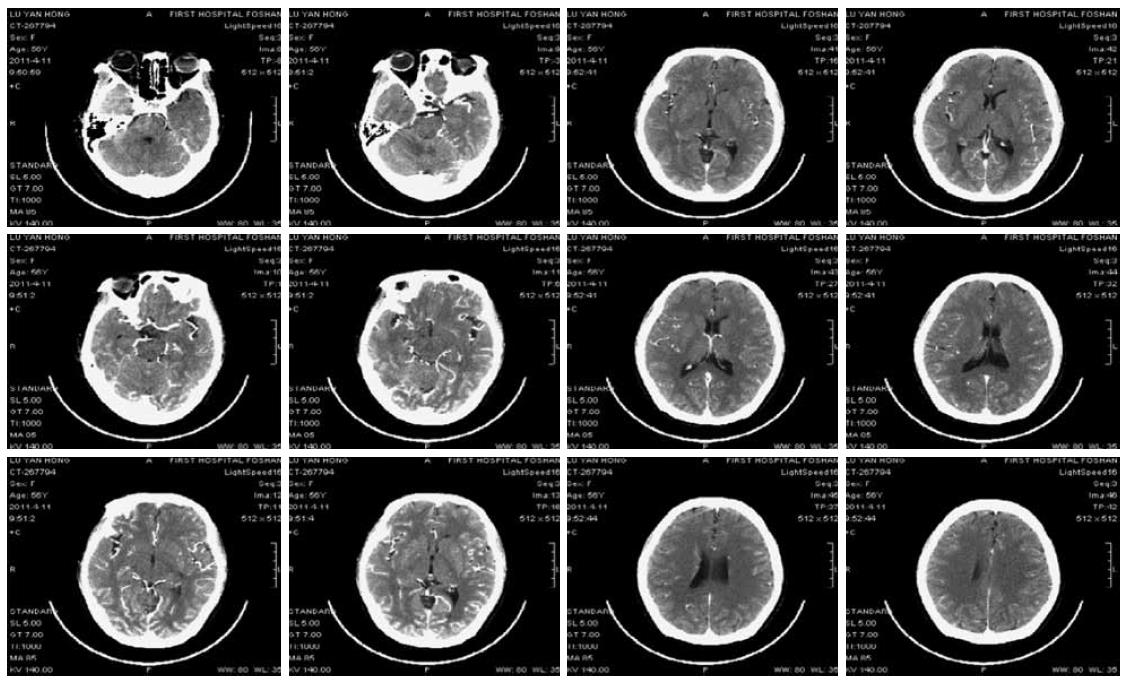
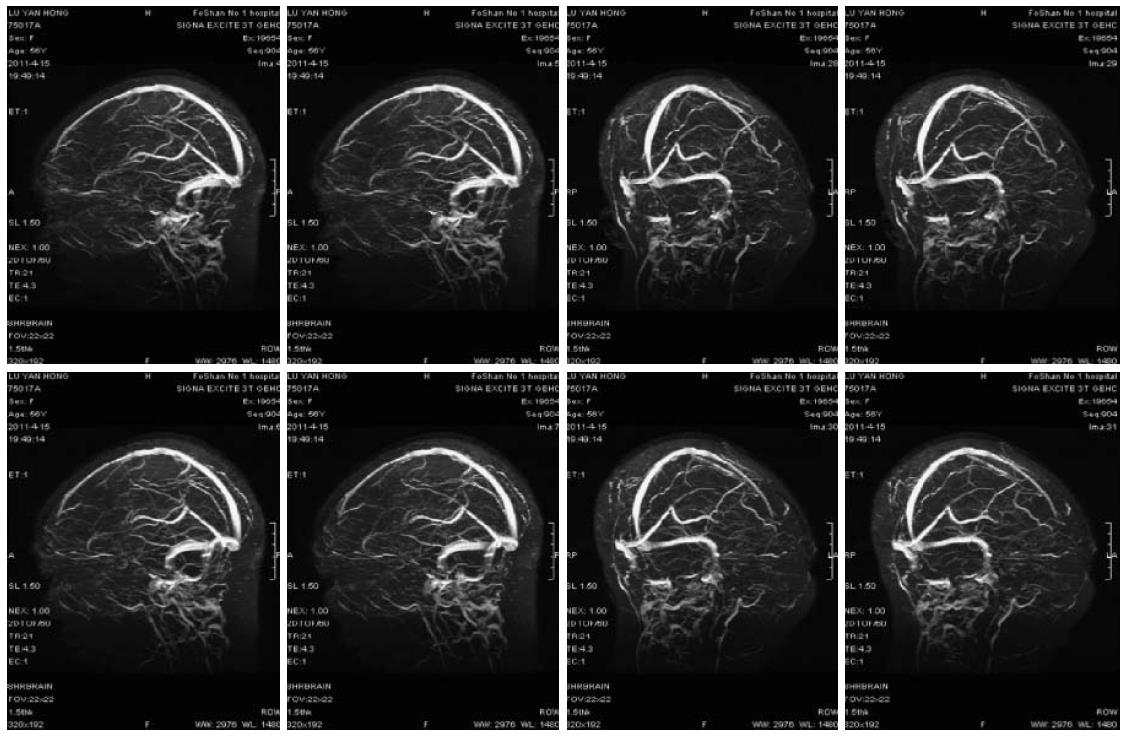
A 56-year-old female patient was diagnosed as having Stage IV rectal cancer and developed retroperitoneal and left supraclavicular lymph node metastasis in March 2011, as confirmed by PET-CT scan and colonoscopy. She was treated with three cycles of “FOLFIRI + bevacizumab” regimen (bevacizumab 300 mg IV infusion on D1, irinotecan 270 mg IV infusion on D1, leucovorin 0.3 IV infusion on D1, 5-FU 0.6 IV bolus on D1, 5-FU 3.6 continuous IV 46 h, repeated every 2 wk). The patient had no history of hypertension. Her blood pressure and urine protein were normal during treatment. The third cycle of chemotherapy was given on April 9th, 2011. The patient developed coma and convulsion of the limbs at 6:00 AM on April 11th, and her blood pressure was 126/81 mmHg at that point. She had a normal neurological examination. Infusion of 5-FU was withdrawn immediately. The patient was treated intravenously to reduce intracranial hypertension and was given medication for sedation. Continuous blood pressure monitoring recorded that blood pressure was stable at around 105-165/63-92 mmHg. Eight hours after coma an enhanced computed tomography (CT) scan of the brain showed mild brain atrophy (Figure 1). After 12 h of coma, she recovered with some verbal response but was still aphasic. Her limbs demonstrated some involuntary movements. The administration was withdrawn. On the morning of April 14th, she showed complete recovery. She could answer questions correctly and walk around. Enhanced MRI scan and venography (MRV) on April 16th showed (Figure 2) mottled lesions in the left parietal lobe, which was a possible microhaemorrhage, but no other abnormal findings. The patient was discharged on April 18th. Subsequent follow-ups documented partial response to treatment. Given the toxicity of the previous treatment, her subsequent regimen was switched to palliative chemotherapy alone without monoclonal antibody.
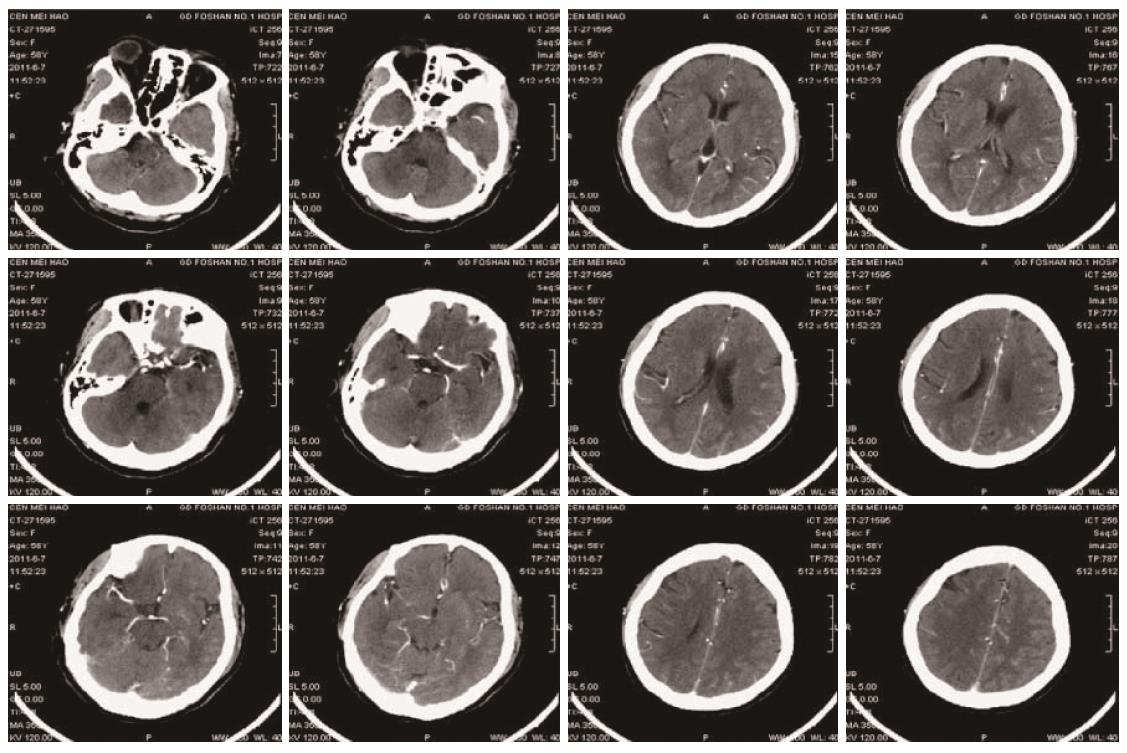
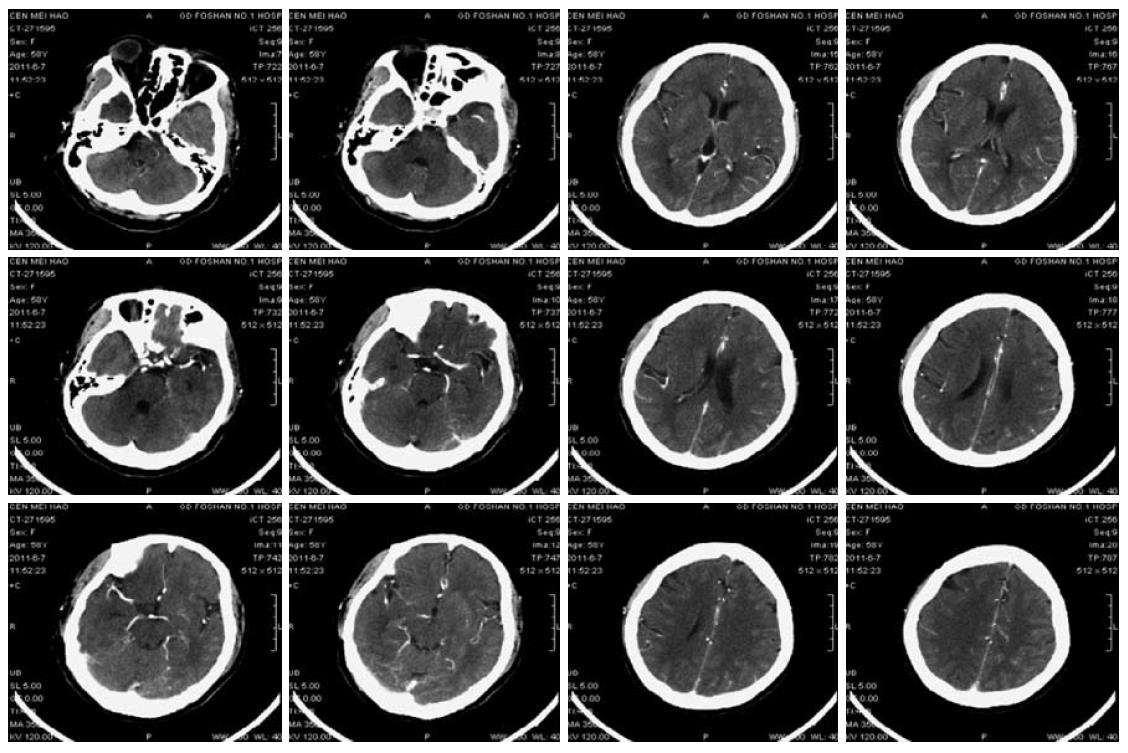
A 58-year-old female was admitted with abdominal pain for more than 1 mo. She was previously diagnosed as having Stage IV carcinoma of the descending colon in January 2011 with extensive metastasis, including mesenteric, mesenteric root, retroperitoneal, bilateral hilar, mediastinal and bilateral supra- and infra-clavicular lymph nodes based on enteroscopy, lymph node puncture and biopsy, and PET-CT scan. She received eight cycles of “mFOLFOX6 + bevacizumab” combination regimen (bevacizumab 300 mg IV infusion on D1, oxaliplatin 150 mg IV infusion on D1, leucovorin 0.68 IV bolus on D1, 5-FU 0.68 IV bolus on D1, 5-FU 4.0 CIV 46 h, repeated every 2 weeks). The last dose of chemotherapy was administered on May 21st, 2011. The patient had a history of hypertension and bronchitis for 10 years. No records were available about her medication. Blood pressure monitoring and urine protein were normal on admission. On the morning of June 4th, the patient presented severe headache and dizziness. Her blood pressure was 225/135 mmHg. Other vital signs were normal. She was treated intravenously with uradil hydrochloride as well as oral amlodipine, hydrochlorothiazide and spironolactone, but the symptom was not relieved. Continuous blood pressure monitoring showed blood pressure around 127-191/80-165 mmHg. The patient then developed coma, restlessness, muscle weakness of the left limbs, and pathological signs in the left lower limb on June 7th. Right cerebral infarction was suspected. CT scan and CTA (CT angiography) of the brain (Figure 3) showed mild cerebral atherosclerosis; narrowing in the A1 segment of the right anterior cerebral artery and the intracranial segment of the right vertebral artery, but appropriate distal blood supply; mild brain atrophy and a small lacunar infarction in the bilateral region of the basal ganglia. Symptomatic treatment was maintained. On the morning of June 8th, her blood pressure was 152/82 mmHg. She recovered consciousness and the muscle strength of her limbs and could eat food. On June 9th, the patient experienced mild dizziness but blood pressure was normal. The intravenous antihypertensive drug was discontinued. The results of a CT scan and CTA of the brain were similar to those obtained previously (Figure 4). The patient then received oral medications to maintain normal blood pressure. She was discharged from hospital on June 15th. Follow-up examination showed that her tumour partially responded to the chemotherapy. The patient is currently undergoing regular follow-up.
The two cases described above were diagnosed as RPLS according to current clinical guidelines. To the best of our knowledge these are the first series of cases in China of RPLS-associated coma induced by bevacizumab combined with chemotherapy. RPLS is a rare brain-capillary leak syndrome, and is associated with hypertension, nephropathy, and use of immunosuppressive medication and cytotoxic agents (e.g., cyclosporin A, tacrolimus). The clinical manifestations include rapidly progressing intracranial hypertension, seizure, visual disorder, disturbance of consciousness, and mental disorder. Coma is rarely seen in RPLS. The imaging findings of RPLS are characteristic of reversible extensive oedema in the white matter of bilateral posterior cerebral hemispheres, frontal lobe, region of basal ganglia, brain stem, cerebellum, and cerebral cortex. The diagnosis of RPLS primarily depends on MRI, however typical imaging findings may not always be present.
Although the pathogenesis of RPLS is not yet fully understood two common theories have been described. The first one argues that a sudden increase in blood pressure could lead to the dysfunction of cerebral vascular autoregulation, including deficiency of vasodilative prostaglandin release and cerebral vascular endothelial dysfunction. Even a mild rise in blood pressure, if acute, especially in the presence of an underlying endothelial dysfunction, may result in breakdown of the blood-brain barrier, pathological vasodilation, and capillary leakage, leading to extravasation of fluids into the brain parenchyma. As a result, vasospasm and brain hypoperfusion, activation of the coagulation system and fluid effusion eventually take place. For this reason RPLS is also known as “hypertensive encephalopathy”. The posterior cerebral circulation is more susceptible to such injury, probably due to the presence of fewer adrenergic nerves in the posterior cerebral circulation system, which makes the blood vessels more sensitive to sudden changes in blood pressure[4].
The second theory states that toxic substances such as immunosuppressive agents could directly lead to transient impairment of the blood-brain barrier by injuring the vascular endothelium. Reconditioning vasoconstriction or microthrombosis will result in occlusion of the cerebral artery, cerebral ischaemia/hypoxia and vasogenic oedema[5].
Bevacizumab is a monoclonal antibody that inhibits the angiogenesis of tumours by specifically blocking vascular endothelial growth factor (VEGF). Bevacizumab is commonly used in combination with various chemotherapy regimens to provide additional survival benefits to patients with metastatic colorectal cancer[6]. Currently, the NCCN guideline recommends bevacizumab in combination with chemotherapy as the standard treatment regimen for metastatic colorectal cancer. The two patients in this report received bevacizumab as well as chemotherapy agents, including oxaliplatin, irinotecan, 5-FU, etc. It has been reported that 5-FU can cause a rare kind of encephalopathy, known as multifocal inflammatory leukoencephalopathy, which usually occurs from 6 wk to 5 mo after 5-FU treatment. However, the clinical and imaging features differ from those of RPLS. In our cases leukoencephalopathy can be excluded[7]. To date, neither RPLS nor other relevant encephalopathies have been reported to be induced by irinotecan or oxaliplatin. In March 2006, Professor Glusker et al[8] of Stanford University reported the first case of bevacizumab-induced typical RLPS in the New England Journal of Medicine. Furthermore, the United States Food and Drug Administration updated the safety information of bevacizumab on September 25, 2006, indicating that healthcare professionals should pay attention to RPLS, a rare adverse reaction during bevacizumab treatment. Hypertension is the most frequently reported adverse reaction during bevacizumab treatment. Bevacizumab may cause RPLS via the following possible mechanisms: sudden blood pressure rise during bevacizumab treatment causes dysfunction of cerebral vascular autoregulation. Moreover, bevacizumab can disrupt the blood-brain barrier by extensively impairing the endothelium. When blood pressure in the systemic circulation increases, the above changes can cause vasogenic oedema and eventually RPLS[9,10].
RPLS may occur at any time during bevacizumab treatment. However, in most cases it develops within the half-life (about 20 d) of bevacizumab[8,11,12]. Typical RPLS-related adverse symptoms have been observed in colorectal and renal cancers treated with bevacizumab combination chemotherapy[12,13]. RPLS usually occurs during the first seven cycles of bevacizumab treatment. The interval between the administration of bevacizumab and onset of RPLS ranges from 16 h to 11 d. The first patient in our report developed RPLS on day 2 of the third cycle of bevacizumab therapy. The second patient developed RPLS on day 17 after eight cycles of treatment. These are consistent with previous reports.
Poor blood pressure control is the most important risk factor for RPLS. Most cases of RPLS are associated with increased blood pressure. The second patient in this report developed RPLS when her blood pressure was not controlled appropriately. The first patient also experienced increased blood pressure before she developed RPLS. Generally, if grade 2 or higher hypertension (according to NCI-CTC, grade 2 hypertension is defined as diastolic blood pressure increase > 20 mmHg, or > 150/100 mmHg if previously normal blood pressure) is documented, it is recommended that the offending agent should be withdrawn as soon as possible and blood pressure should be controlled[14].
Fortunately RPLS is reversible. Immediate diagnosis, proper blood pressure control and withdrawal of the implicated drugs will enable recovery of the clinical and imaging findings. Although some patients may develop progressive neurological symptoms, these will generally improve or resolve within several days. Instant and effective blood pressure control is the primary objective of managing RPLS. If malignant hypertension is present, the diastolic blood pressure must be reduced at a steady speed to below < 100 mmHg within several hours. Blood pressure control is recommended for even mild hypertension. Intravenous antihypertensive agents, e.g., sodium nitroprusside and nicardipine, are recommended for rapid onset. Such intravenous therapy can also maintain adequate cerebral perfusion pressure.
It is not clear whether it is safe for patients who have experienced RPLS to continue bevacizumab, although discontinuation of bevacizumab is recommended. Since it became available on the market 6 years ago, five cases of bevacizumab-induced RLPS have been reported worldwide[15], while no similar case has ever been reported in China since it entered the Chinese market in 2010. Indeed, only two out of 30 cases developed RLPS induced by bevacizumab in combination with chemotherapy.
The lack of typical imaging or thrombotic changes in the central nervous system makes early recognition of RPLS crucial. Whenever coma is present during bevacizumab treatment, RPLS should be considered, especially when complicated with hypertension. Moreover, bevacizumab combination with chemotherapy should be carefully used in patients with a history of hypertension, and blood pressure should be monitored closely during bevacizumab therapy. Precaution and timely management are vital to prevent coma. RLPS is a reversible complication if handled appropriately.
In conclusion, these are the first cases of coma of RPLS induced by bevacizumab combination chemotherapy reported in China. Although usually reversible, RPLS is a serious and potentially life-threatening syndrome and its association with hypertension in the setting of bevacizumab combination chemotherapy should be recognized. In addition, a history of hypertension should be addressed prior to the combination regimen. If RPLS develops, a less toxic regimen should be considered to prevent possible effects on future cognitive function.
Two colorectal cancer patients treated with bevacizumab plus chemotherapy presented with the rare complication of reversible posterior leukoencephalopathy syndrome (RPLS).
Reversible clinical symptoms of coma after treatment with bevacizumab.
Multifocal inflammatory leukoencephalopathy, hypertensive encephalopathy, encephalitis, demyelinating diseases.
No typical finding was identified on imaging examination.
Rectal cancer and colon cancer.
The two patients received “FOLFIRI + bevacizumab” and “mFOLFOX6 + bevacizumab” anticancer treatment separately.
Bevacizumab combination with chemotherapy should be carefully used in patients with a history of hypertension, and blood pressure should be monitored closely during bevacizumab therapy.
RPLS is a syndrome characterized by clinical symptoms of headache, altered mental functioning, visual loss and seizures, and is associated with hypertension, nephropathy, and use of immunosuppressive medication and cytotoxic agents.
RPLS is a rare complication of bevacizumab, and may present with life-threatening symptoms such as coma; however, early recognition and prompt control of blood pressure may make this complication reversible.
This article demonstrates a rare complication of bevacizumab in colorectal cancer, and given the increasing use of bevacizumab, clinicians should be aware of this potential complication.
P- Reviewers: Bandoh S, Cummings JE, Ling Y S- Editor: Wang JL L- Editor: A E- Editor: Liu XM
| 1. | Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A, Pessin MS, Lamy C, Mas JL, Caplan LR. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334:494-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2250] [Cited by in RCA: 2153] [Article Influence: 74.2] [Reference Citation Analysis (0)] |
| 2. | Saltz LB, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2302] [Cited by in RCA: 2270] [Article Influence: 133.5] [Reference Citation Analysis (0)] |
| 3. | Fuchs CS, Marshall J, Barrueco J. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: updated results from the BICC-C study. J Clin Oncol. 2008;26:689-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 184] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 4. | Edvinsson L, Owman C, Sjöberg NO. Autonomic nerves, mast cells, and amine receptors in human brain vessels. A histochemical and pharmacological study. Brain Res. 1976;115:377-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 333] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 5. | Petrovic BD, Nemeth AJ, McComb EN, Walker MT. Posterior reversible encephalopathy syndrome and venous thrombosis. Radiol Clin North Am. 2011;49:63-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Rocha JA. Bevacizumab in combination with irinotecan, 5-fluorouracil and leucovorin given as first-line treatment of metastatic colorectal cancer. Anticancer Drugs. 2011;22 Suppl 2:S9-S13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Hook CC, Kimmel DW, Kvols LK, Scheithauer BW, Forsyth PA, Rubin J, Moertel CG, Rodriguez M. Multifocal inflammatory leukoencephalopathy with 5-fluorouracil and levamisole. Ann Neurol. 1992;31:262-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 120] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Glusker P, Recht L, Lane B. Reversible posterior leukoencephalopathy syndrome and bevacizumab. N Engl J Med. 2006;354:980-982; discussion 980-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 257] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 9. | Hurwitz H, Saini S. Bevacizumab in the treatment of metastatic colorectal cancer: safety profile and management of adverse events. Semin Oncol. 2006;33:S26-S34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 130] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 10. | Weis SM, Cheresh DA. Pathophysiological consequences of VEGF-induced vascular permeability. Nature. 2005;437:497-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 623] [Cited by in RCA: 685] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 11. | Peter S, Hausmann N, Schuster A, Boehm HF. Reversible posterior leukoencephalopathy syndrome and intravenous bevacizumab. Clin Experiment Ophthalmol. 2008;36:94-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Koopman M, Muller EW, Punt CJ. Reversible posterior leukoencephalopathy syndrome caused by bevacizumab: report of a case. Dis Colon Rectum. 2008;51:1425-1426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Allen JA, Adlakha A, Bergethon PR. Reversible posterior leukoencephalopathy syndrome after bevacizumab/FOLFIRI regimen for metastatic colon cancer. Arch Neurol. 2006;63:1475-1478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 105] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 14. | Tam CS, Galanos J, Seymour JF, Pitman AG, Stark RJ, Prince HM. Reversible posterior leukoencephalopathy syndrome complicating cytotoxic chemotherapy for hematologic malignancies. Am J Hematol. 2004;77:72-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 89] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 15. | Shord SS, Bressler LR, Tierney LA, Cuellar S, George A. Understanding and managing the possible adverse effects associated with bevacizumab. Am J Health Syst Pharm. 2009;66:999-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (1)] |