Published online Jun 7, 2014. doi: 10.3748/wjg.v20.i21.6586
Revised: February 18, 2014
Accepted: March 7, 2014
Published online: June 7, 2014
Processing time: 195 Days and 17.2 Hours
AIM: To evaluate the depth of invasion of small, early colorectal cancers (ECCs) using conventional endoscopic features.
METHODS: From January 2005 to September 2011, colonoscopy cohort showed that a total of 72 patients with small colorectal cancers with the size less than 20 mm underwent colonoscopy at the Yonsei University College of Medicine, Seoul, South Korea. Among them, 8 patients were excluded due to incomplete medical records. Finally, a total of 64 ECCs with submucosa (SM) invasion and size less than 20 mm were included. One hundred fifty-two adenomas with size less than 20 mm were included as controls. Nine endoscopic features, including seven morphological findings (i.e., loss of lobulation, excavation, demarcated and depressed areas, stalk swelling, fullness, fold convergence, and bleeding ulcers), pit patterns, and non-lifting signs, were evaluated retrospectively. All endoscopic features were evaluated by two experienced endoscopists who have each performed over 1000 colonoscopies annually for more than five years without knowledge of the histology.
RESULTS: Among the morphological findings, the size of deep submucosal cancers was bigger than that of superficial lesions (16.9 mm vs 12.3 mm, P < 0.001). Also, demarcated depressed areas, stalk swelling, and fullness were more common in deep SM cancers than in superficial tumors (demarcated depressed areas: 52.0% vs 15.7%, P < 0.001; stalk swelling: 100% vs 4.2%, P < 0.001; fullness: 25.0% vs 0%, P = 0.001). Among deep SM cancers, 96% of polyps showed invasive pit patterns, whereas 19.4% of superficial tumors showed invasive pit patterns (P < 0.001). A positive non-lifting sign was more common in deep SM cancers (85.0% vs 28.6%, P < 0.001). Diagnostic accuracy of invasive morphology, invasive pit patterns, and non-lifting signs for deep SM cancers were 71%, 82%, and 75%, respectively.
CONCLUSION: Conventional endoscopic findings were insufficient to discriminate small, deep SM cancers from superficial SM cancers by white light, standard colonoscopy.
Core tip: This present study was designed to evaluate the depth of invasion of small, early colorectal cancers using conventional endoscopic features. This study exhibited that invasive pit patterns were a more accurate finding than morphological features or non- lifting signs to discriminate small, deep submucosa (SM) cancers from superficial SM cancers by a white light, standard colonoscopy. However, it also showed that conventional endoscopic findings, such as morphological features, non-lifting sign, and invasive pit patterns are insufficient to discriminate deep SM cancers to determine therapeutic strategy under white light standard colonoscopy.
- Citation: Park W, Kim B, Park SJ, Cheon JH, Kim TI, Kim WH, Hong SP. Conventional endoscopic features are not sufficient to differentiate small, early colorectal cancer. World J Gastroenterol 2014; 20(21): 6586-6593
- URL: https://www.wjgnet.com/1007-9327/full/v20/i21/6586.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i21.6586
Endoscopic management for early colorectal neoplasm has been accepted as an effective method to treat or prevent colorectal cancer. Currently, colorectal neoplasms either confined to the mucosa or after invasion to submucosa (SM) with a size less than 1000 μm are considered good candidates for endoscopic treatment[1-3]. Because 6%-12% of lymph node metastases have been reported in deep SM cancers, these lesions are not indicated for endoscopic treatment[4]. Therefore, it is crucial to precisely evaluate the depth of invasion in advanced colorectal neoplasm for adequate therapeutic treatment[5].
Size is one of the important indicators of the depth of invasion and for the choice of an adequate treatment for advanced colorectal neoplasms. A previous study reported that 7.4%-14% of colorectal polyps larger than 20 mm were submucosal carcinoma[4], thus endoscopists must treat large colorectal neoplasms endoscopically to avoid an incomplete resection. However, during screening colonoscopy, most colorectal polyps are detected as small polyps less than 20 mm in size and can be resected by a simple polypectomy during the procedure[4,6-10]. Although a previous report showed that only 0.07%-5.80% of polyps less than 20 mm in size are submucosal carcinoma[4], recent advances in colonoscopy technology enable the frequent detection of small advanced colorectal neoplasms. However, it is difficult to determine whether those small polyps are invasive carcinoma prior to histologic evaluation. Generally, endoscopists remove these lesions using a simple polypectomy and fail to achieve complete resection.
Recent innovative technology has allowed endoscopists to differentiate advanced colorectal neoplasms during a colonoscopy. Magnifying chromoendoscopy or narrow-band imaging (NBI) has been widely studied to assess depth of invasion[11-13]. However, most of studies have been focused on large colorectal neoplasms which are candidates for advanced endoscopic techniques, such as endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD)[1-3]. Therefore, the present study evaluated conventional endoscopic findings, including morphological features, pit patterns, and no-lifting signs, to assess depth of invasion in advanced colorectal neoplasms less than 20 mm in size using a standard colonoscope for accurate diagnoses and treatment.
From January 2005 to September 2011, colonoscopy cohort showed that a total of 72 patients with small colorectal cancers with the size less than 20 mm underwent a colonoscopy at the Yonsei University College of Medicine, Seoul, South Korea. Among them, eight patients were excluded from the present study due to incomplete medical records. Finally, a total of 64 small colorectal cancers with SM invasion were included; 25 (39%) deep submucosal cancers, and 39 (61%) superficial submucosal cancers. All lesions were histologically confirmed to be adenocarcinomas. 39 superficial submucosal cancers and 152 adenomas with high-grade dysplasia less than 20 mm were included as control.
Colonoscopy was performed after bowel preparation with 4 L polyethylene glycol solution (Colyte; Taejun, Seoul, South Korea or Colyte-F or Colonlyte; Dreampharma, Seoul, South Korea) by three experienced gastroenterologists. All colonoscopies were performed with a standard colonoscope (CF Q240L, CF Q240I, CF H260AI, CF Q260AI, or PCF Q260AI; Olympus Optical Co, Ltd, Tokyo, Japan). The shape, size, number, location, and histology of small advanced colorectal neoplasms were evaluated. The shape of small colorectal neoplasms was classified as either pedunculated or non-pedunculated (sessile or flat/depressed) type. Location was divided into the right colon (including the cecum, ascending colon, transverse colon, or splenic flexure) or left colon (including the descending colon, sigmoid colon, or rectum). Polyp size was estimated using a 7 mm diameter open-biopsy forceps.
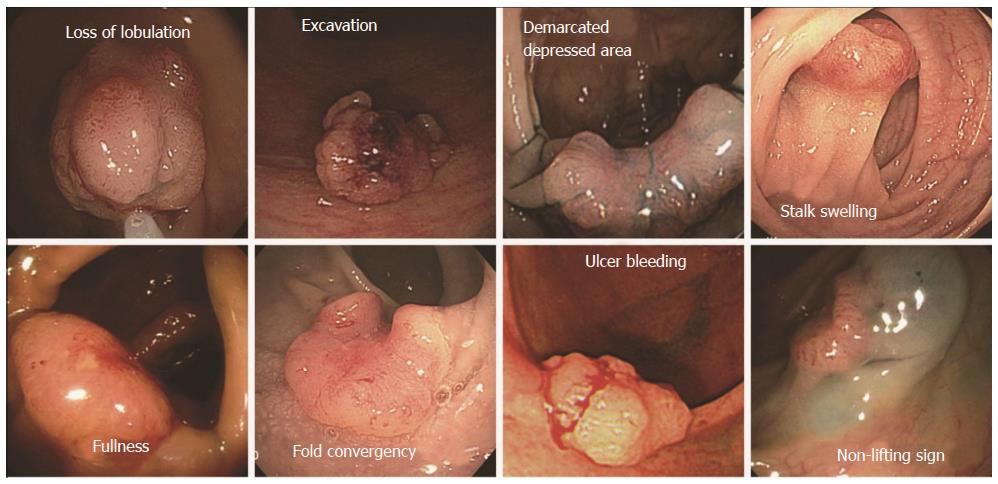
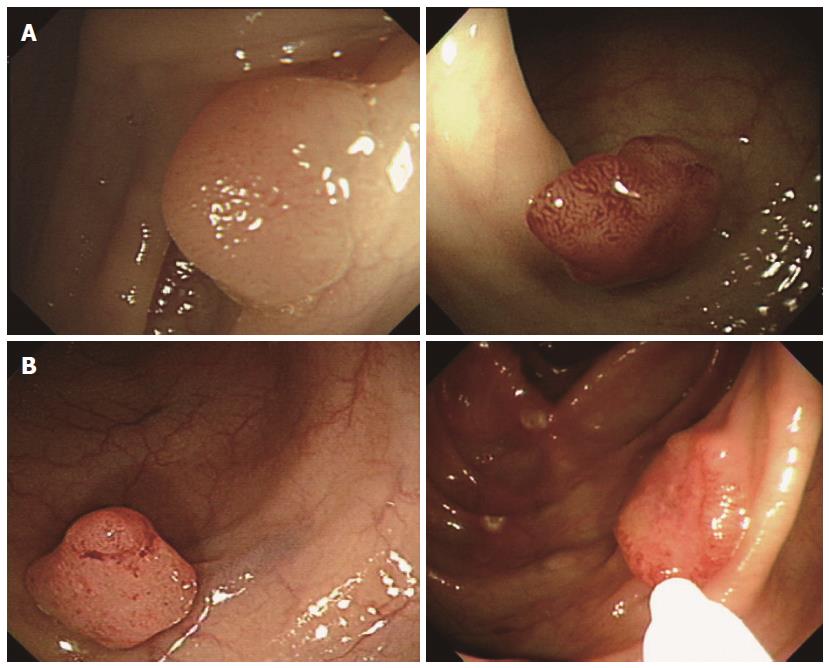
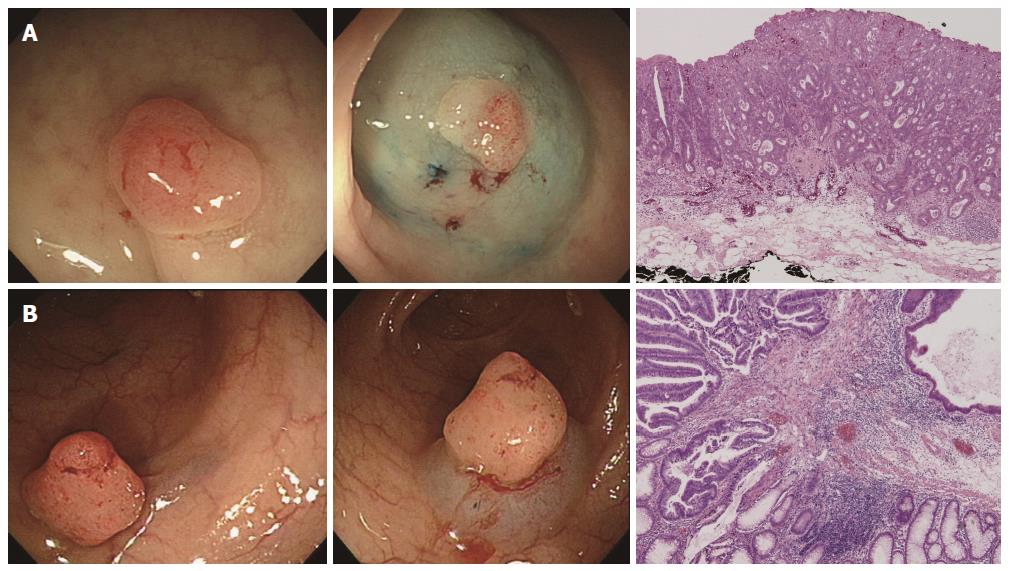
We investigated nine endoscopic findings of the colorectal neoplasms, including seven morphological features (i.e., loss of lobulation, excavation, demarcated depressed areas, stalk swelling, fullness, fold convergence, and bleeding ulcers), pit patterns, and non-lifting signs from the previously published literature[14]. The definitions of the nine endoscopic findings were as follows (Figure 1). (1) loss of lobulation: loss of normal lobulation; (2) excavation: a crumbled, damaged area of the tumor that prevents observation of the surface structure; (3) demarcated depressed areas: depressed demarcations on the surface of the tumor; (4) stalk swelling: a thickened and expanded stalk; (5) fullness: a bursting appearance due to expansive growth of the tumor; (6) fold convergence: fold convergence towards the tumor; (7) bleeding ulcer; (8) Pit pattern: Sub-classified as invasive or non-invasive (Figures 2 and 3)[12]. Non-invasive pattern: normal mucosa, star-shaped crypts (Kudo’s type I or II), or regular crypts with or without demarcated areas or irregular pits without demarcated areas (Kudo’s type IIIS, IIIL, or IV); Invasive pattern: irregular and distorted crypts in demarcated areas (Kudo’s type VN and VI); and (9) Non-lifting sign: SM injection was performed at a point approximately 2 mm from the edge of the lesion using a 23-gauge needle. A saline solution containing epinephrine (0.01 mg/mL) and 0.8% indigo carmine was injected into the submucosal layer to lift the lesion off the muscle layer. A non-lifting sign was defined as positive when the surrounding mucosa, but not the lesion, was elevated and negative when the lesion itself was elevated[15].
All endoscopic features were evaluated retrospectively by two experienced endoscopists who have each performed over 1000 colonoscopies annually for more than five years without knowledge of the histology. Final endoscopic features were determined after agreement between the two endoscopists.
Histopathological diagnoses were based on the Vienna classification by a highly experienced pathologist[16]. A microscope with a built-in ruler was used to determine the depth of SM invasion. Superficial SM cancer was defined as invasion less than 1000 μm and deep SM cancer was defined as invasion greater than 1000 μm.
The primary outcome of the present study was to evaluate the different endoscopic findings between small, deep SM cancer and small, superficial SM cancers. Patients’ baseline characteristics were analyzed using descriptive statistics. The differences of categorical variables were analyzed by Fisher’s exact test. Continuous variables were analyzed by the Student’s t test. The morphological features were analyzed according to shape. Stalk swelling was assessed for only the pedunculated type; loss of lobulation and excavation were assessed for both pedunculated and sessile types; fullness and fold convergence were assessed for the flat/depressed type; demarcated depressed areas were assessed for all three types. Continuous variables are expressed as the means ± SD. P values of less than 0.05 were considered statistically significant. Statistical analyses were carried out using SPSS 18.0 (SPSS, Chicago, IL, United States) and SAS 9.2 (SAS Institute Inc., Cary, NC, United States).
Baseline characteristics and endoscopic findings between SM cancers and adenomas were described at the Table 1. The size of SM cancers was bigger than that of adenomas (15.91 mm vs. 11.47 mm, P < 0.001) and the superficial (flat) shape was frequently observed in SM cancer than adenomas (45.3% vs 13.2%, P < 0.001). Also, demarcated depressed areas, stalk swelling, and fullness were more common in SM cancers than adenomas (demarcated depressed areas: 46.9% vs 8.6%, P < 0.001; stalk swelling: 77.8% vs 0%, P < 0.001; fullness: 10.9% vs 0%, P = 0.001). Other morphological features (i.e., loss of lobulation, excavation, fold convergence, and bleeding ulcers) were not statistically different between the two groups. Among SM cancers, 95.3% of polyps showed invasive pit patterns, while 0% of adenomas showed invasive pit patterns (P < 0.001). A positive non-lifting sign was more common in SM cancers than in adenomas (45.3% vs 11.9%, P < 0.001).
| Deep submucosal cancer (n = 25) | Superficial submucosal cancer (n = 39) | P value | |
| Age (mean ± SD, yr) | 58.4 ± 8.5 | 62.4 ± 9.8 | 0.097 |
| Sex | 0.241 | ||
| Male | 13 (52.0) | 26 (66.7) | |
| Female | 12 (48.0) | 13 (33.3) | |
| Size (mm) | 16.88 ± 3.28 | 15.28 ± 3.64 | 0.080 |
| Shape | |||
| Sessile | 8 (32.0) | 18 (46.2) | 0.397 |
| Pedunculated | 5 (20.0) | 4 (10.3) | |
| Superfical (Flat) | 12 (48.0) | 17 (43.6) | |
| Location | 0.581 | ||
| Right colon | 8 (32.0) | 10 (25.6) | |
| Left colon | 17 (68.0) | 29 (74.4) | |
| Endoscopic finding | |||
| Morphological features | |||
| Any of them | 22 (88.0) | 27 (69.2) | 0.084 |
| None of them | 3 (12.0) | 12 (30.8) | |
| (P, S) Loss of lobulation | 3/13 (23.1) | 7/22 (31.8) | 0.709 |
| (P, S) Excavation | 0/13 (0.0) | 1/22 (4.5) | 1.000 |
| (All) Demarcated depressed area | 13/25 (52.0) | 17/39 (43.6) | 0.511 |
| (P) Stalk swelling | 5/5 (100.0) | 2/4 (50.0) | 0.167 |
| (F) Fullness | 3/12 (25.0) | 0/17 (0.0) | 0.060 |
| (F) Fold convergency | 1/12 (8.3) | 0/17 (0.0) | 0.414 |
| (All) Ulcer bleeding | 1/25 (4.0) | 1/39 (2.6) | 1.000 |
| Pit pattern | 1.000 | ||
| Non-invasive | 1 (4.0) | 2 (5.1) | |
| Invasive | 24 (96.0) | 37 (94.9) | |
| Non-lifting sign | 0.010 | ||
| Positive | 17 (85.0) | 17 (48.6) | |
| Negative | 3 (15.0) | 18 (51.4) |
Baseline characteristics of 64 submucosal cancer patients were as follows. Among 64 SM cancers, 6 cases (9%) were 10 mm or less in size, 33 cases (52%) were 11-15 mm in size, and 25 cases (39%) were 16-19 mm in size. Deep SM cancers were larger than superficial SM cancers, but it was not statistically significant (16.88 mm vs 15.28 mm, P = 0.080). The flat and sessile types were more common in two groups than pedunculated type. The distributions of cancers were similar in the two groups, more common in left colon (68.0% in deep SM, and 74.4% in superficial sm). Non-lifting sign was more common in deep SM cancers than in superficial SM cancers (85.0% vs 48.6%, P = 0.010).
Baseline characteristics and endoscopic findings according to the depth of invasion were seen at the Table 2. When comparing endoscopic findings between deep SM cancers and superficial tumors, the size of deep submucosal cancers was bigger than that of superficial lesions (16.9 mm vs 12.3 mm, P < 0.001). Also, demarcated depressed areas, stalk swelling, and fullness were more common in deep SM cancers than superficial tumors (demarcated depressed areas: 52.0% vs 15.7%, P < 0.001; stalk swelling: 100% vs 4.2%, P < 0.001; fullness: 25.0% vs 0%, P = 0.001; Table 2). Other morphological features (i.e., loss of lobulation, excavation, fold convergence, and bleeding ulcers) were not statistically different between the two groups. Among deep SM cancers, 96% of polyps showed invasive pit patterns, while 19.4% of superficial tumors showed invasive pit patterns (P < 0.001). A positive non-lifting sign was more common in deep SM cancers than in superficial tumors (85.0% vs 28.6%, P < 0.001).
| Deep submucosal cancer (n = 25) | Superficial tumors (n = 191) | P value | |
| Age (mean ± SD, yr) | 58.4 ± 8.5 | 61.0 ± 9.4 | 0.191 |
| Sex | 0.179 | ||
| Male | 13 (52.0) | 128 (67.0) | |
| Female | 12 (48.0) | 63 (33.0) | |
| Size (mm) | 16.88 ± 3.28 | 12.25 ± 4.93 | < 0.001 |
| Shape | |||
| Sessile | 8 (32.0) | 106 (55.5) | 0.005 |
| Pedunculated | 5 (20.0) | 48 (25.1) | |
| Superfical (Flat) | 12 (48.0) | 37 (19.4) | |
| Location | 1.000 | ||
| Right colon | 8 (32.0) | 57 (29.8) | |
| Left colon | 17 (68.0) | 134 (70.2) | |
| Endoscopic finding | |||
| Morphological features | |||
| Any of them | 22 (88.0) | 59 (30.9) | < 0.001 |
| None of them | 3 (12.0) | 132 (69.1) | |
| (P, S) Loss of lobulation | 3/13 (23.1) | 27/154 (17.5) | 0.705 |
| (P, S) Excavation | 0/13 (0.0) | 3/154 (1.9) | 1.000 |
| (All) Demarcated depressed area | 13/25 (52.0) | 30/191 (15.7) | < 0.001 |
| (P) Stalk swelling | 5/5 (100.0) | 2/48 (4.2) | < 0.001 |
| (F) Fullness | 3/12 (25.0) | 0/37 (0.0) | 0.001 |
| (F) Fold convergency | 1/12 (8.3) | 0/37 (0.0) | 0.310 |
| (All) Ulcer bleeding | 1/24 (4.0) | 2/189 (1.0) | 0.245 |
| Pit pattern | < 0.001 | ||
| Non-invasive | 1 (4.0) | 154 (80.6) | < 0.001 |
| Invasive | 24 (96.0) | 37 (19.4) | |
| Non-lifting sign | < 0.001 | ||
| Positive | 17 (85.0) | 22 (28.6) | |
| Negative | 3 (15.0) | 55 (71.4) |
When comparing deep SM cancers with superficial SM tumors, the sensitivity, specificity, PPV, and NPV of any of the invasive morphology were 88%, 69%, 27% and 98%, respectively (Table 3). The sensitivity, specificity, PPV, and NPV of invasive pit patterns were 96%, 81%, 46%, and 95%, respectively. The sensitivity, specificity, PPV, and NPV of non-lifting signs were 85%, 73%, 46% and 95% in sessile and flat polyps, respectively. The diagnostic accuracy of the presence of morphological features, invasive pit patterns, and non-lifting sign were 71%, 82% and 75%, respectively.
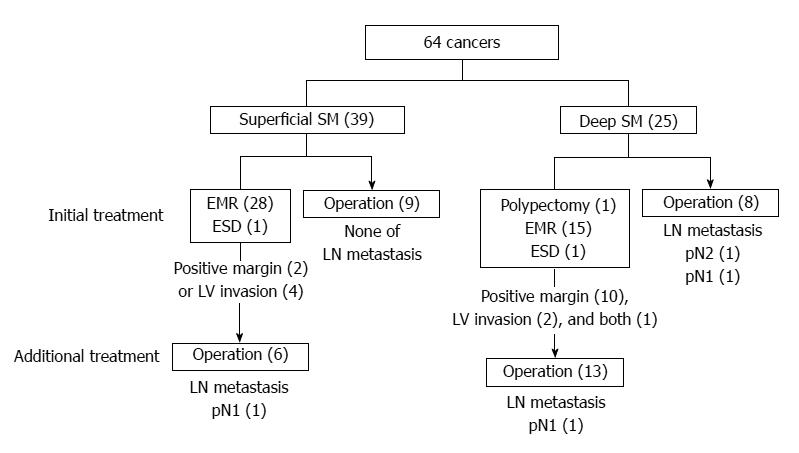
Among 39 superficial SM cancers, 30 cases were initially treated with EMR (28 cases) or ESD (2 cases) and nine cases were treated with surgery (Figure 4). Among 25 deep SM cancers, 17 cases were initially treated with endoscopic techniques (polypectomy, 1 case; EMR, 15 cases; ESD, 1 case). Subsequently, 13 cases received further surgical treatment, and one case showed lymph node metastasis. Eight cases with deep SM cancers were initially treated with surgery.
A recent study showed that endoscopic treatment of SM cancers was safe and feasible with favorable long-term efficacy when the following conditions are satisfied: a lesion is determined histopathologically to be well differentiated; invasion of the SM layer is less than 1000 μm (superficial SM cancer); and the lesion is negative for both lymphovascular invasion and sprouting[17]. Thus, it is important to estimate whether the depth of SM invasion is less than 1000 μm to determine appropriate treatment. However, the present study showed that conventional endoscopic features were insufficient to differentiate deep SM cancers less than 20 mm in size resulting in a diagnostic accuracy of 79%. Among 25 patients with deep SM cancers, 68% were initially under-treatmented.
During colonoscopy, endoscopists usually assess colorectal polyps according to morphological findings and choose a treatment. The present study evaluated seven morphological features of polyps and found that demarcated depression, fullness, and stalk swelling were typical findings of small deep SM cancers. A previous study, which included polyps larger than 20 mm in size, showed that a loss of lobulation was also a typical endoscopic finding[14], but this observation was not confirmed by the present study. The diagnostic accuracy of the presence of any of the invasive morphological features was 71%, meaning that the morphological characteristics themselves are insufficient to assess depth of invasion of small colorectal neoplasms. In a previous study, Uno et al[15] reported the clinical usefulness of non-lifting signs to predict depth of invasion for colorectal neoplasms. Adenomas or superficial SM cancers are readily lifted by SM injection, thus the non-lifting signs are clinically used to determine the therapeutic strategy for advanced colorectal neoplasm. A previous study showed that the accuracy of non-lifting signs for deep SM cancers were 94.8%[18]. However, this does not seem to be applied to small polyps, as 15% of patients with deep SM cancers showed negative non-lifting signs and were treated with EMR. The present study revealed that the non-lifting signs were limited to predict deep SM invasion in polyps less than 20 mm in size. In this study, NPVs for SM deep cancers of invasive morphology, invasive pit pattern and non-lifting sign are over 95%. Surely, High NPVs useful for determination of treatment strategy Nevertheless, the diagnostic accuracy for SM deep cancers was not sufficient and it leads to initial under-treatment.
Recently magnifying chromoendoscopy has been used to assess depth of invasion of colorectal polyps, overcoming the limitations of morphological features[19]. Pit pattern classification of colorectal neoplasm, initially proposed by Kudo and modified by Kudo and Tsuruta, is reported to be related to the histologic characteristics of the lesions[20]. A previous study demonstrated that invasive pit patterns are able to differentiate superficial SM cancers from deep SM cancers with the diagnostic accuracy of 98.8%[12]. Under magnifying chromoendoscopy, Kudo’s classification type V pit pattern is usually considered to be invasive to the SM, and type VN is strongly suggestive of deep SM cancer[21]. Therefore, it is crucial to discriminate between type VI and type VN patterns to assess precisely the depth of invasion of colorectal polyps. Under non-magnifying colonoscopy, it is difficult to discriminate type VN; for that reason, the present study showed a low diagnostic accuracy of pit patterns for deep SM cancers. However, it is unrealistic for clinics to apply this method as magnifying chromoendoscopy is not a conventional or universal method for screening or simple surveillance colonoscopy.
The present study has several limitations. First, because of the retrospective study design, there were some cases with poor qualified photos which made it difficult to precisely evaluate all of the endoscopic features. Second, pit patterns were evaluated after the conventional endoscopic diagnoses, suggesting an influence by the morphological features of polyps. Finally, pit patterns were evaluated by only standard colonoscopy; thus, it was impossible to discriminate the type VN pit pattern, which is strongly suggestive of deep SM cancer. Therefore, the results of the present study should not be simply compared to those of previous studies using magnifying chromoendoscopy in terms of clinical usefulness. From a different point of view, the present study is more realistic because magnifying chromoendoscopy is not usually used in screening or surveillance colonoscopy.
In conclusion, although the prevalence of SM invasion is low in small colorectal polyps, the present study showed that conventional endoscopic findings, such as morphological features, non-lifting sign, and invasive pit patterns, are insufficient to discriminate deep SM cancers to determine therapeutic strategy under white light standard colonoscopy. Further studies are mandatory to evaluate precisely the depth of invasion in small colorectal polyps, using magnifying chromoendoscopy or NBI.
At present, colorectal neoplasms either confined to the mucosa or after invasion to submucosa (SM) with a size less than 1000 μm are considered good candidates for endoscopic treatment. In contrast, because 6%-12% of lymph node metastases have been reported in deep SM cancers, these lesions are not indicated for endoscopic treatment. Therefore, it is crucial to precisely evaluate the depth of invasion in advanced colorectal neoplasm for adequate therapeutic treatment.
This present study was designed to evaluate the depth of invasion of small, early colorectal cancers using conventional endoscopic features. This study exhibited that invasive pit pattern was more accurate finding than morphological features or non- lifting sign to discriminate small, deep SM cancers from superficial tumors by white light, standard colonoscopy. In addition, it is insufficient to discriminate deep SM cancers to determine therapeutic strategy under white light standard colonoscopy only. Recent innovative technology has allowed endoscopists to differentiate advanced colorectal neoplasms during colonoscopy. Especially, magnifying chromoendoscopy or narrow-band imaging has been widely studied to assess depth of invasion. Nevertheless, these techniques have some barriers and unrealistic to apply to the clinic for the daily practice. Therefore, future research should aim to develop the practical method or technology to evaluate the precise depth of invasion of small colorectal neoplasms.
This study identified the limitation of white light standard colonoscopy in the depth of invasion evaluation of small colorectal neoplasms. Furthermore, this study suggests the further studies to develop the method or technology to evaluate the precise depth of invasion of small colorectal neoplasms.
This study could be helpful to establish the therapeutic plan when the small polyps detected in usual colonoscopy using white light standard endoscopy.
It is insufficient to discriminate deep SM cancers to determine therapeutic strategy under white light standard colonoscopy only. These results are interesting and these findings arouse the necessity for the further studies to develop the practical method or technology to evaluate the precise depth of invasion of small colorectal neoplasms.
P- Reviewers: Doughan S, Kim YJ, Tamai N S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
| 1. | Repici A, Pellicano R, Strangio G, Danese S, Fagoonee S, Malesci A. Endoscopic mucosal resection for early colorectal neoplasia: pathologic basis, procedures, and outcomes. Dis Colon Rectum. 2009;52:1502-1515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 86] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 2. | Bergmann U, Beger HG. Endoscopic mucosal resection for advanced non-polypoid colorectal adenoma and early stage carcinoma. Surg Endosc. 2003;17:475-479. [PubMed] |
| 3. | Moss A, Bourke MJ, Williams SJ, Hourigan LF, Brown G, Tam W, Singh R, Zanati S, Chen RY, Byth K. Endoscopic mucosal resection outcomes and prediction of submucosal cancer from advanced colonic mucosal neoplasia. Gastroenterology. 2011;140:1909-1918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 439] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 4. | Kudo Se, Lambert R, Allen JI, Fujii H, Fujii T, Kashida H, Matsuda T, Mori M, Saito H, Shimoda T. Nonpolypoid neoplastic lesions of the colorectal mucosa. Gastrointest Endosc. 2008;68:S3-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 364] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 5. | Kitajima K, Fujimori T, Fujii S, Takeda J, Ohkura Y, Kawamata H, Kumamoto T, Ishiguro S, Kato Y, Shimoda T. Correlations between lymph node metastasis and depth of submucosal invasion in submucosal invasive colorectal carcinoma: a Japanese collaborative study. J Gastroenterol. 2004;39:534-543. [PubMed] |
| 6. | Williams CB, Saunders BP, Talbot IC. Endoscopic management of polypoid early colon cancer. World J Surg. 2000;24:1047-1051. [PubMed] |
| 7. | Kunii Y, Kamano T, Tomiki Y, Hirai S, Kasamaki S, Sakamoto K. Advanced colorectal carcinomas measuring 20 mm or less exhibit markedly higher invasiveness despite their size. Dig Dis Sci. 2004;49:1899-1905. [PubMed] |
| 8. | Matsui T, Yao T, Iwashita A. Natural history of early colorectal cancer. World J Surg. 2000;24:1022-1028. [PubMed] |
| 9. | Matsuda T, Saito Y, Fujii T, Uraoka T, Nakajima T, Kobayashi N, Emura F, Ono A, Shimoda T, Ikematsu H. Size does not determine the grade of malignancy of early invasive colorectal cancer. World J Gastroenterol. 2009;15:2708-2713. [PubMed] |
| 10. | Henriksson ML, Edin S, Dahlin AM, Oldenborg PA, Öberg Å, Van Guelpen B, Rutegård J, Stenling R, Palmqvist R. Colorectal cancer cells activate adjacent fibroblasts resulting in FGF1/FGFR3 signaling and increased invasion. Am J Pathol. 2011;178:1387-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 111] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 11. | Hurlstone DP, Cross SS, Adam I, Shorthouse AJ, Brown S, Sanders DS, Lobo AJ. Endoscopic morphological anticipation of submucosal invasion in flat and depressed colorectal lesions: clinical implications and subtype analysis of the kudo type V pit pattern using high-magnification-chromoscopic colonoscopy. Colorectal Dis. 2004;6:369-375. [PubMed] |
| 12. | Matsuda T, Fujii T, Saito Y, Nakajima T, Uraoka T, Kobayashi N, Ikehara H, Ikematsu H, Fu KI, Emura F. Efficacy of the invasive/non-invasive pattern by magnifying chromoendoscopy to estimate the depth of invasion of early colorectal neoplasms. Am J Gastroenterol. 2008;103:2700-2706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 261] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 13. | Hurlstone DP, Cross SS, Drew K, Adam I, Shorthouse AJ, Brown S, Sanders DS, Lobo AJ. An evaluation of colorectal endoscopic mucosal resection using high-magnification chromoscopic colonoscopy: a prospective study of 1000 colonoscopies. Endoscopy. 2004;36:491-498. [PubMed] |
| 14. | Ikehara H, Saito Y, Matsuda T, Uraoka T, Murakami Y. Diagnosis of depth of invasion for early colorectal cancer using magnifying colonoscopy. J Gastroenterol Hepatol. 2010;25:905-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Uno Y, Munakata A. The non-lifting sign of invasive colon cancer. Gastrointest Endosc. 1994;40:485-489. [PubMed] |
| 16. | Schlemper RJ, Riddell RH, Kato Y, Borchard F, Cooper HS, Dawsey SM, Dixon MF, Fenoglio-Preiser CM, Fléjou JF, Geboes K. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47:251-255. [PubMed] |
| 17. | Ferrara F, Luigiano C, Ghersi S, Fabbri C, Bassi M, Landi P, Polifemo AM, Billi P, Cennamo V, Consolo P. Efficacy, safety and outcomes of ‘inject and cut’ endoscopic mucosal resection for large sessile and flat colorectal polyps. Digestion. 2010;82:213-220. [PubMed] |
| 18. | Kobayashi N, Saito Y, Sano Y, Uragami N, Michita T, Nasu J, Matsuda T, Fu KI, Fujii T, Fujimori T. Determining the treatment strategy for colorectal neoplastic lesions: endoscopic assessment or the non-lifting sign for diagnosing invasion depth? Endoscopy. 2007;39:701-705. [PubMed] |
| 19. | Tanaka S, Kaltenbach T, Chayama K, Soetikno R. High-magnification colonoscopy (with videos). Gastrointest Endosc. 2006;64:604-613. [PubMed] |
| 20. | Kanao H, Tanaka S, Oka S, Kaneko I, Yoshida S, Arihiro K, Yoshihara M, Chayama K. Clinical significance of type V(I) pit pattern subclassification in determining the depth of invasion of colorectal neoplasms. World J Gastroenterol. 2008;14:211-217. [PubMed] |
| 21. | Kudo SE, Takemura O, Ohtsuka K. Flat and depressed types of early colorectal cancers: from East to West. Gastrointest Endosc Clin N Am. 2008;18:581-593, xi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |