Published online Jun 7, 2014. doi: 10.3748/wjg.v20.i21.6425
Revised: January 12, 2014
Accepted: February 17, 2014
Published online: June 7, 2014
Processing time: 196 Days and 2.1 Hours
The technique of endoscopic submucosal dissection (ESD) is now a well-known endoscopic therapy for early gastric cancer. ESD was introduced to resect large specimens of early gastric cancer in a single piece. ESD can provide precision of histologic diagnosis and can also reduce the recurrence rate. However, the drawback of ESD is its technical difficulty, and, consequently, it is associated with a high rate of complications, the need for advanced endoscopic techniques, and a lengthy procedure time. Various advances in the devices and techniques used for ESD have contributed to overcoming these drawbacks.
Core tip: Endoscopic submucosal dissection (ESD) enables en bloc resection of early gastric cancer, facilitating detailed histopathological evaluation. ESD needs to be performed by highly skilled endoscopists in order to prevent procedural complications. To overcome this disadvantage of ESD, there have been various advances in the knives and other accessories used for this procedure.
- Citation: Kume K. Endoscopic therapy for early gastric cancer: Standard techniques and recent advances in ESD. World J Gastroenterol 2014; 20(21): 6425-6432
- URL: https://www.wjgnet.com/1007-9327/full/v20/i21/6425.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i21.6425
Endoscopic submucosal dissection (ESD) enables en bloc resection of early gastric cancer, facilitating detailed histopathological evaluation[1]. ESD needs to be performed by highly skilled endoscopists in order to prevent procedural complications[2,3]. To overcome this disadvantage of ESD, there have been various advances in the knives and other accessories used for this procedure. I previously reported a review of endoscopic therapy for early gastric cancer in 2009[4]. The current review of ESD will provide an overview of standard techniques, devices and recent advances in the procedure since 2010.
Standard ESD requires special cutting knives, such as a needle knife (KD-1L-1, Olympus, Tokyo, Japan)[5], an insulation-tipped electrosurgical (IT) knife (KD-610L/KD-611L, Olympus)[1,2,6-9], a hook knife (KD-620LR/KD-620QR, Olympus)[10,11], a flex knife (KD-630L, Olympus)[12], a dual knife (KD-650L/KD-650Q, Olympus), a flush knife (DK2618JB/DK2618JN, Fujifilm, Saitama, Japan)[13], a triangle-tip (TT) knife (KD-640L, Olympus)[14], a mucosectome (DP-D2518/DP-D2622, Pentax, Tokyo, Japan)[15], a grasping type scissor forceps (DP2618DT, Fujifilm)[16,17], SB knife (MD-47706/MD-47704 and MD-47703, Sumitomo Bakelite, Akita, Japan)[18], a Fork knife (Kachu Technology, Seoul, South Korea)[19] and a Cap knife (Create Medic, Yokohama, Japan)[20].
Standard ESD is performed with a standard, single accessory-channel endoscope. Typical procedural steps include marking, incision and submucosal dissection with simultaneous hemostasis. After making several marking dots outside the lesion, various submucosal solutions, including a normal saline and epinephrine mixture, glycerol mixture and hyaluronic acid, are injected. A circumferential incision into the mucosa is made using one of the special cutting knives. Direct dissection of the submucosal layer is carried out with one of the specified knives until complete removal is achieved. During ESD, we perform endoscopic hemostasis either with the knife itself or with a hemostatic forceps whenever active bleeding is noticed. After the ESD, we perform preventive endoscopic hemostasis for any oozing or exposed vessel. High-frequency generators (Erbotom ICC 200 or VIO 300D; ERBE, Tuebingen, Germany) are used for marking, incision of the gastric mucosa, gastric submucosal dissection and endoscopic hemostasis.
Absence of lymph node metastasis in the stomach is considered a prerequisite for ESD for early gastric cancer. A large series of patients with well-differentiated intestinal mucosal cancer without ulcers and no size limit, with ulcers less than 30 mm in size, patients with submucosal cancer limited to sm1 infiltration (< 500 μm deep in the submucosa, starting from the muscularis mucosae) that was less than 30 mm in diameter, and patients with undifferentiated cancer without ulcers or with ulcer size less than 20 mm were found to satisfy this criterion[21]. Therefore, ESD is indicated in such patients.
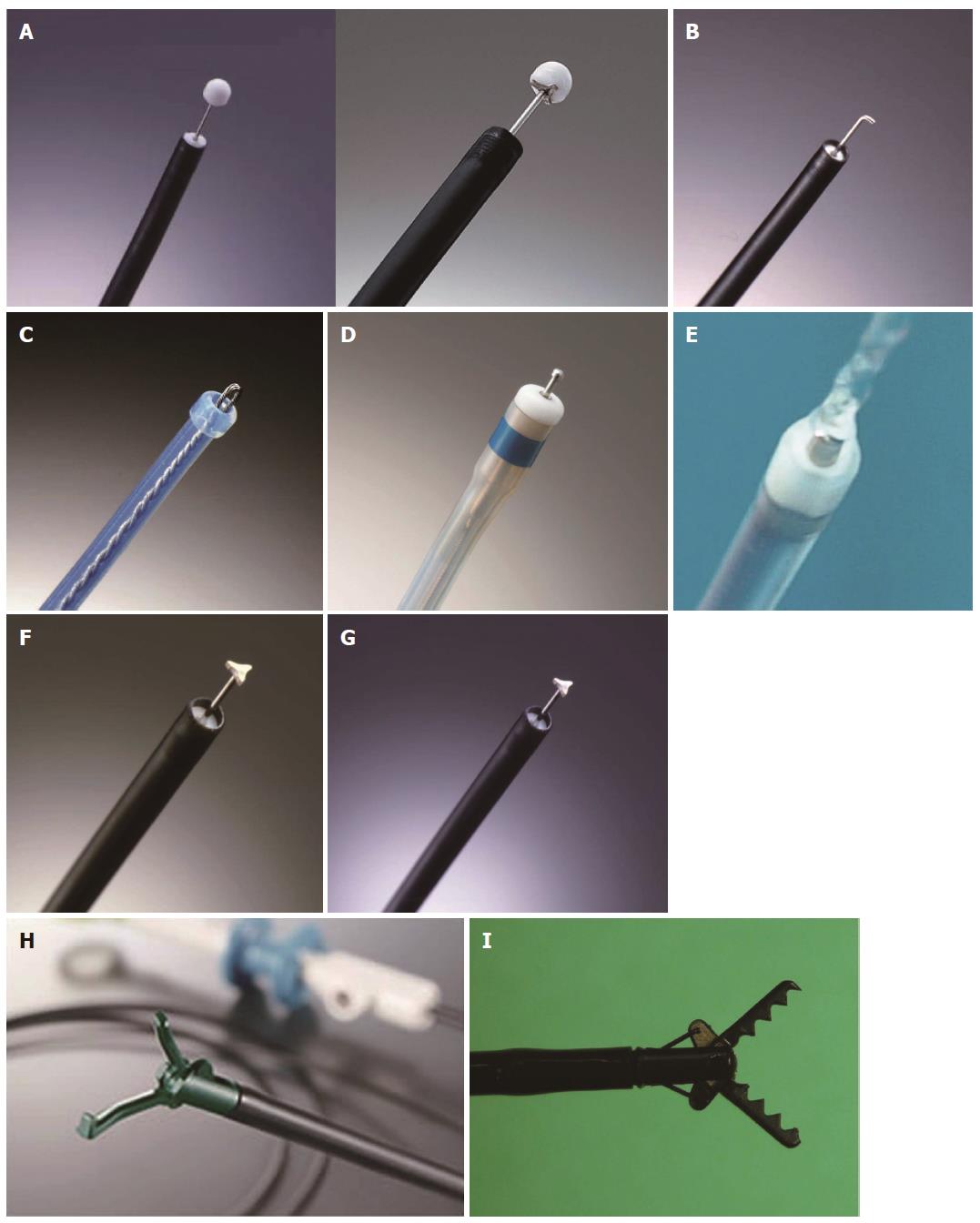
The IT knife consists of a small ceramic ball attached to the tip of a high-frequency needle knife. The ceramic ball functions as an insulator for the tip of the needle knife, so that incision and dissection of the mucosa and submucosa can be performed safely. The insulator helps to prevent perforation due to accidental cutting of the muscularis propria. A specialized feature of the IT knife is that the portion between the insulator tip and the sheath is used for incision, sweeping off the tissue with the blade portion of the knife instead of the tip. This feature makes a pull-cut, which limits the direction of the incision; however, a straightforward incision is difficult while looking directly at the incision line or submucosa (Figure 1A).
The IT knife 2 is an improved version of the IT knife, with a small metallic plate mounted inside the ceramic tip, facilitating procedures in the traverse direction.
The top of the hook-type knife is right-angled and is 1 mm in size. Compared to the use of a needle knife, use of this knife is associated with greater safety because the submucosal tissue is hooked and pulled before the incision. This knife has a rotating function so that the operator can select the optimal direction of the hook (Figure 1B).
The point of the flex knife is rounded and has a twisted wire that serves as a snare. The sheath is soft and flexible. This knife is less likely to cause perforation when it reaches the muscular layer, as its tip is rounded, and the entire knife is soft and flexible. As the tip of the sheath is thick and functions as a stopper, operators can control the depth of the incision very easily (Figure 1C).
This knife has a small ball-like process on the top, which prevents it from slipping (unlike the Needle Knife). When the tip is retracted, only 0.3 mm of the ball-like process protrudes, increasing the ease of creating markings on the lesion. The thicker end of the sheath prevents inadvertent tissue perforation (Figure 1D).
The Flush knife is a characteristic knife with a needle 0.4 mm in diameter, and five projecting parts 1, 1.5, 2, 2.5 and 3 mm in length. The knife clamp at the tip of the sheath is made of ceramic for heat protection. The outer sheath is 2.6 mm in diameter, and water emission is possible through the lumen of the sheath by connecting a water pump to it. The water jet is swiftly activated by pressing the foot pedal of the conduction pump. The conductor of the sheath lumen is insulated in order to prevent electric current dispersion (Figure 1E).
The TT knife has evolved along with the process of ESD, which began with the IT knife. The triangular tip of the knife can be used for either cutting or coagulating, and has been designed to operate in any direction (Figure 1F).
The mucosectome is composed of a flexible plastic shaft and cutting wire (5 mm long in the standard device and 2.5 mm long in the Mucosectome 2). By operation of the handle, the top of this device turns freely, which enables the cutting wire to face in the proper direction. The plastic shaft moves the muscular layer aside, and the cutting wire moves the mucosal layer away from the submucosa during ESD, so that the procedure itself can be performed safely (Figure 1G).
Each step of ESD (circumferential incision, submucosal excision, hemostatic treatment) can be achieved by the following three operations: (1) grasping the target tissue (fixation); (2) lifting up the grasped tissue (separation of the grasped tissue from the underlying muscle layer); and (3) cutting the grasped tissue (or coagulating the blood vessel) using an electrosurgical current. These operations are simple and as easy as the bite biopsy technique (Figure 1H).
These forceps have a claw and curved scissors to prevent unnecessary injury to the normal muscle layer. The SB knife line includes the following types of knives: standard type (7 mm knife), short type (6 mm knife) and thin type (SB knife Jr.; 3.5 mm knife). The use of specially designed transparent hoods (SB hoods) is recommended when these knives are used[18] (Figure 1I).
The author developed a novel partial (one-third) transparent hood that facilitates endoscopic hemostatic procedures while simultaneously allowing irrigation at the site of bleeding[22]. The one-third partial hood is easily placed on the tip of the endoscope, although the hood has to be fitted to the right side of the endoscope. The hood-knife was fabricated by drilling an extra side hole, in addition to the hole of the irrigation tube at the cap portion of the transparent end hood[23]. A snare forceps was glued to the exterior surface over the hole and was attached using short tubes at the inside of the cap. Based on this prototype, the irrigation cap-knife [the cap-knife attachment (Type KUME) with a fixed snare] was developed[20] (Figure 1J).
ESD procedures using the hood-knife are performed as follows. After the tumor is separated from the surrounding normal mucosa by complete incision around the lesion using the IT knife, the endoscope is removed and the hood-knife is placed on the tip and fixed with tape. A grasping forceps is passed through the accessory channel and the lesion is pushed away from the muscle layer. In submucosal exfoliation, the hood-knife is only slid by the coagulation current on the muscle layer.
The Fork knife has two interchangeable knives, a fixed flexible snare and a forked knife, which form a single working unit, and an inlet for material injection or saline irrigation during the procedure. The knives can be changed during the procedure by using two switches, the fork knob and core knob, located on the center of the body.
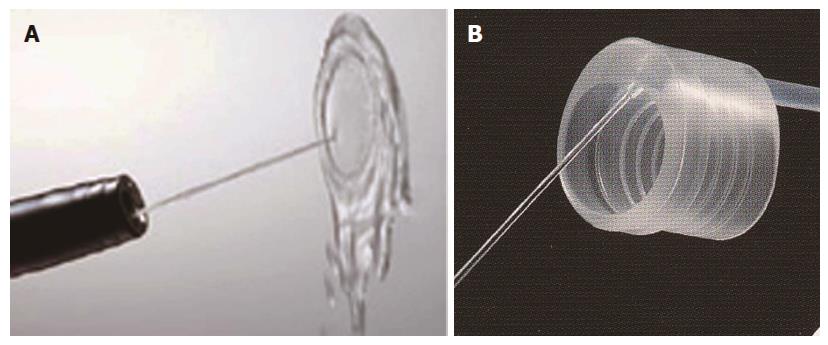
By washing the surgical field with a water jet, the source of bleeding can be immediately identified and coagulated, although it can be difficult to identify the bleeding source in a small number of cases with erupting venous bleeding (Figure 2A).
The author developed an end hood that facilitates endoscopic hemostatic procedures while simultaneously allowing irrigation of the hemorrhage site. The end hood piece was fabricated by drilling a side hole in the cap portion of a conventional transparent hood, and the irrigation tube was glued to the exterior surface of the hole[22,24]. The fabricated transparent hood was placed at the tip of the endoscope. Based on this prototype, the irrigation hood [the irrigation cap (Type KUME)] was developed (Create Medic) (Figure 2B).
A transparent hood facilitates better visualization of the operating field. In particular, good visualization of the submucosal tissue with the aid of a small-caliber-tip transparent hood makes the cutting procedures easy and safe[25] (Figures 2C and 3).
There are certain tumor locations where it is difficult to perform EMR using the conventional scope, such as the lesser curvature or posterior wall of the gastric body, and the cardia. To facilitate EMR of tumors at these locations, a two-channel scope with two independently curving segments, that is, a multibending scope (the ‘‘M-scope’’), was developed[26]. The M-scope consists of a distal flexible segment that can bend in any of the four major directions, and a proximal flexible segment that can bend in two directions. Combined operation of the segments allows the operator to obtain a variety of visual fields, to randomly approach or recede from the lesions, and to obtain an en face view.
The multibending double-channel therapeutic endoscope (the ‘‘R-scope’’) was designed for lifting lesions and for improved dissection by the incorporation of two movable channels[27,28]. The R-scope has two movable instrument channels: one moves vertically and the other swings horizontally. During the operation, the two instruments can be manipulated with a knob and lever that surround the angulation control knobs of the R-scope.
The magnetic anchor (Pentax) consists of three parts: a hand-made magnetic weight made of magnetic stainless steel, microforceps, and a connecting thread. The weight is designed to facilitate gastric ESD by use of an extracorporeal hands-free electromagnet, whereby magnetic forces allow suitable countertraction for submucosal dissection[29].
A small snare is introduced into the gastric lumen through a percutaneous gastric port (2-mm diameter), to grasp and pull the lesion away from the muscularis propria, thus facilitating resection[30].
In ESD using an external grasping forceps, oral traction applied with the external forceps can elevate the lesion and make the submucosal layer on the aboral side wider and more visible, thereby facilitating submucosal dissection under direct vision[31].
In ESD using an external grasping forceps through the EndoLifter (LA-201, 202, Olympus), traction applied with the external forceps can elevate the lesion and make the submucosal layer wider and more visible, thereby facilitating submucosal dissection under direct vision (Figure 4).
There are two types of solutions for submucosal injection: an isotonic solution (normal saline, hyaluronic acid) and a hypertonic solution (hypertonic saline, glucose, Glyceol®)[25,32-35]. The advantage of hypertonic solutions is better mucosal elevation and hemostatic effect than normal saline. However, a hypertonic solution is more likely to damage tissue in the resected sample, post-resection ulcer or the surrounding mucosa, compared with an isotonic solution.
Hyaluronic acid solution (MucoUp, Johnson and Johnson, Tokyo, Japan) makes a better, long-lasting submucosal cushion without tissue damage, as compared to other available solutions[25,32].
Water jet-assisted knife: The water jet-assisted knife (ERBEJET, Erbe Elektromedizin GmbH, Tuebingen, Germany) with an outer diameter of 2.1 mm allows injection or hydrodissection without a needle, with a preselected effect setting through a standard working channel of the endoscope. Without switching instruments, the operator can alternatingly use the tool for marking the targeted lesion, circumferential cutting, dissection, and coagulation by radiofrequency application[36].
Thulium laser: The thulium 2-μm wavelength laser system (RevoLix; LISA Laser Products OHG, Katlenburg-Lindau, Germany) is used during ESD procedures for marking, mucosal incision and submucosal dissection. The original device, which consists of a 550-μm flexible silica with metallic attachment, is inserted through the working channel of the endoscope instead of endoscopy knives[37].
Master and Slave Transluminal Endoscopic Robot: The Master and Slave Transluminal Endoscopic Robot is deployed through a two-channel therapeutic endoscope. By remote operation of the two-armed master interface, the endoscopist can intuitively control the slave endoscopic tools at the distal end of the endoscope[38].
Double-endoscope: The use of two endoscopes for ESD provides a good field of vision and allows countertraction to be applied to the lesion, clearly facilitating submucosal dissection. Submucosal dissection is performed with the main scope. Countertraction is applied to the lesion with the use of the grasping forceps, introduced through the forceps channel of the sub-scope[39].
Yo-yo technique: The yo-yo technique is a traction method. The endoscopic snare is mobilized through the patient’s nose independently of the endoscope. The partially resected specimen can be pushed or pulled according to the snare movements, exposing the dissection plane and the distal luminal area, respectively, and facilitating submucosal dissection under direct vision[40].
Carbon dioxide insufflation: Compared with air insufflation, CO2 insufflation during ESD reduces the volume of residual gas in the digestive tract (air vs CO2 = 1047 mL vs 643 mL, P < 0.001), but not the 100-mm visual analog scale score for abdominal pain and distension after the procedure[41].
Local steroid injection: Local steroid injection (triamcinolone acetonide 50 mg/5 mL) into the floor of a post-ESD artificial ulcer promotes the formation of granulation tissue at an early stage of the healing process, leading to regeneration of gastric mucosa without mucosal convergence or gastric deformity[42].
Antithrombotic drugs: Continuous aspirin use increases the risk of bleeding after ESD. It is reported that post-ESD bleeding occurred in 4.1% (21/514) of patients, and was more frequent in continuous aspirin users (4/19, 21.1%) than in those who never used aspirin (15/439, 3.4%, P = 0.006) and those with interrupted aspirin use (2/56, 3.6%, P = 0.033)[43]. Thus, aspirin use should be discontinued in patients with a low risk for thromboembolic disease who undergo ESD, to minimize bleeding complications.
Interruption of antithrombotic drug therapy may be adequate for preventing early post-ESD bleeding; however, reinitiating antithrombotic drug therapy is a significant independent risk factor for delayed post-ESD bleeding[44].
Deep vein thrombosis: ESD procedures carry a moderate risk for venous thromboembolism. D-dimer measurements were higher in patients with DVT than in patients without deep vein thrombosis (DVT). Kusunoki et al[45] reported that according to receiver operating characteristic curve analysis, the resulting cut-off value of the D-dimer level on the day after ESD was 1.9-μg/mL for ESD patients, with superior association to pre-ESD or immediate post-ESD levels as compared to. High D-dimer levels the day after ESD and the presence of comorbidities are associated with DVT development.
Second-look endoscopy after ESD: A second-look endoscopy after ESD may contribute little to the prevention of delayed bleeding[46].
In this article, I have described some basic knowledge and highlighted the progresses in ESD. It should be emphasized that the performance of ESD, which involves invasive endoscopic procedures, requires a high level of technical skill and sufficient knowledge.
P- Reviewers: Caboclo JLF, Nunobe S, Smith SM, Wu WJ S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
| 1. | Muto M, Miyamoto S, Hosokawa A, Doi T, Ohtsu A, Yoshida S, Endo Y, Hosokawa K, Saito D, Shim CS. Endoscopic mucosal resection in the stomach using the insulated-tip needle-knife. Endoscopy. 2005;37:178-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | Oka S, Tanaka S, Kaneko I, Mouri R, Hirata M, Kawamura T, Yoshihara M, Chayama K. Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest Endosc. 2006;64:877-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 526] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 3. | Ono H, Kondo H, Gotoda T, Shirao K, Yamaguchi H, Saito D, Hosokawa K, Shimoda T, Yoshida S. Endoscopic mucosal resection for treatment of early gastric cancer. Gut. 2001;48:225-229. [PubMed] |
| 4. | Kume K. Endoscopic mucosal resection and endoscopic submucosal dissection for early gastric cancer: Current and original devices. World J Gastrointest Endosc. 2009;1:21-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 5. | Hirao M, Masuda K, Asanuma T, Naka H, Noda K, Matsuura K, Yamaguchi O, Ueda N. Endoscopic resection of early gastric cancer and other tumors with local injection of hypertonic saline-epinephrine. Gastrointest Endosc. 1988;34:264-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 266] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 6. | Ohkuwa M, Hosokawa K, Boku N, Ohtu A, Tajiri H, Yoshida S. New endoscopic treatment for intramucosal gastric tumors using an insulated-tip diathermic knife. Endoscopy. 2001;33:221-226. [PubMed] |
| 7. | Miyamoto S, Muto M, Hamamoto Y, Boku N, Ohtsu A, Baba S, Yoshida M, Ohkuwa M, Hosokawa K, Tajiri H. A new technique for endoscopic mucosal resection with an insulated-tip electrosurgical knife improves the completeness of resection of intramucosal gastric neoplasms. Gastrointest Endosc. 2002;55:576-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 183] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 8. | Rösch T, Sarbia M, Schumacher B, Deinert K, Frimberger E, Toermer T, Stolte M, Neuhaus H. Attempted endoscopic en bloc resection of mucosal and submucosal tumors using insulated-tip knives: a pilot series. Endoscopy. 2004;36:788-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 185] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 9. | Gotoda T. A large endoscopic resection by endoscopic submucosal dissection procedure for early gastric cancer. Clin Gastroenterol Hepatol. 2005;3:S71-S73. [PubMed] |
| 10. | Oyama T, Kikuchi Y. Aggressive endoscopic mucosal resection in the upper GI tract-hook knife EMR method. Minim Invasive Ther Allied Technol. 2002;11:291-295. |
| 11. | Oyama T, Tomori A, Hotta K, Morita S, Kominato K, Tanaka M, Miyata Y. Endoscopic submucosal dissection of early esophageal cancer. Clin Gastroenterol Hepatol. 2005;3:S67-S70. [PubMed] |
| 12. | Yahagi N, Fujishiro M, Kakushima N, Kobayashi K, Hashimoto T, Oka M, Iguchi M, Enomoto S, Ichinose M, Niwa H. Endoscopic submucosal dissection for early gastric cancer using the tip of an electro-sergical snare (thin type). Dig Endosc. 2004;16:34-38. |
| 13. | Toyonaga T, Nishino E, Dozaiku T, Ueda C, Hirooka T. Management to prevent bleeding during endoscopic submucosal dissection using the flush knife for gastric tumor. Dig Endosc. 2007;19:S14-S18. |
| 14. | Inoue H, Sato Y, Kazawa T, Sugaya S, Usui S, Satodate H, Kudo S. Endoscopic submucosal dissection-using atriangletipped knife. Sto Int. 2004;39:53-56. |
| 15. | Kawahara Y, Takenaka R, Okada H. Risk management to prevent perforation during endoscopic submucosal dissection. Dig Endosc. 2007;19:S9-S13. |
| 16. | Akahoshi K, Akahane H, Murata A, Akiba H, Oya M. Endoscopic submucosal dissection using a novel grasping type scissors forceps. Endoscopy. 2007;39:1103-1105. [PubMed] |
| 17. | Akahoshi K, Akahane H. A new breakthrough: ESD using a newly developed grasping type scissor forceps for early gastrointestinal tract neoplasms. World J Gastrointest Endosc. 2010;2:90-96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Homma K, Otaki Y, Sugawara M, Kobayashi M. Efficacy of novel SB knife Jr examined in a multicenter study on colorectal endoscopic submucosal dissection. Dig Endosc. 2012;24:117-120. [DOI] [Full Text] |
| 19. | Kim HG, Cho JY, Bok GH, Cho WY, Kim WJ, Hong SJ, Ko BM, Kim JO, Lee JS, Lee MS. A novel device for endoscopic submucosal dissection, the Fork knife. World J Gastroenterol. 2008;14:6726-6732. [PubMed] |
| 20. | Kume K, Yamasaki M, Yoshikawa I, Otsuki M. Grasping-forceps-assisted endoscopic submucosal dissection using a novel irrigation cap-knife for large superficial early gastric cancer. Endoscopy. 2007;39:566-569. [PubMed] |
| 21. | Gotoda T. Endoscopic resection of early gastric cancer. Gastric Cancer. 2007;10:1-11. [PubMed] |
| 22. | Kume K, Yamasaki M, Yamasaki T, Yoshikawa I, Otsuki M. Endoscopic hemostatic treatment under irrigation for upper-GI hemorrhage: a comparison of one third and total circumference transparent end hoods. Gastrointest Endosc. 2004;59:712-716. [PubMed] |
| 23. | Kume K, Yamasaki M, Kanda K, Yoshikawa I, Otsuki M. Endoscopic submucosal dissection using a novel irrigation hood-knife. Endoscopy. 2005;37:1030-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Kume K, Yoshikawa I, Otsuki M. Endoscopic treatment of upper GI hemorrhage with a novel irrigating hood attached to the endoscope. Gastrointest Endosc. 2003;57:732-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Yamamoto H, Kawata H, Sunada K, Sasaki A, Nakazawa K, Miyata T, Sekine Y, Yano T, Satoh K, Ido K. Successful en-bloc resection of large superficial tumors in the stomach and colon using sodium hyaluronate and small-caliber-tip transparent hood. Endoscopy. 2003;35:690-694. [PubMed] |
| 26. | Isshi K, Tajiri H, Fujisaki J, Mochizuki K, Matsuda K, Nakamura Y, Saito N, Narimiya N. The effectiveness of a new multibending scope for endoscopic mucosal resection. Endoscopy. 2004;36:294-297. [PubMed] |
| 27. | Yonezawa J, Kaise M, Sumiyama K, Goda K, Arakawa H, Tajiri H. A novel double-channel therapeutic endoscope (“R-scope”) facilitates endoscopic submucosal dissection of superficial gastric neoplasms. Endoscopy. 2006;38:1011-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 28. | Neuhaus H, Costamagna G, Devière J, Fockens P, Ponchon T, Rösch T. Endoscopic submucosal dissection (ESD) of early neoplastic gastric lesions using a new double-channel endoscope (the “R-scope”). Endoscopy. 2006;38:1016-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 99] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 29. | Gotoda T, Oda I, Tamakawa K, Ueda H, Kobayashi T, Kakizoe T. Prospective clinical trial of magnetic-anchor-guided endoscopic submucosal dissection for large early gastric cancer (with videos). Gastrointest Endosc. 2009;69:10-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 117] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 30. | Kondo H, Gotoda T, Ono H, Oda I, Kozu T, Fujishiro M, Saito D, Yoshida S. Percutaneous traction-assisted EMR by using an insulation-tipped electrosurgical knife for early stage gastric cancer. Gastrointest Endosc. 2004;59:284-288. [PubMed] |
| 31. | Imaeda H, Iwao Y, Ogata H, Ichikawa H, Mori M, Hosoe N, Masaoka T, Nakashita M, Suzuki H, Inoue N. A new technique for endoscopic submucosal dissection for early gastric cancer using an external grasping forceps. Endoscopy. 2006;38:1007-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 77] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 32. | Yamamoto H, Yube T, Isoda N, Sato Y, Sekine Y, Higashizawa T, Ido K, Kimura K, Kanai N. A novel method of endoscopic mucosal resection using sodium hyaluronate. Gastrointest Endosc. 1999;50:251-256. [PubMed] |
| 33. | Fujishiro M, Yahagi N, Nakamura M, Kakushima N, Kodashima S, Ono S, Kobayashi K, Hashimoto T, Yamamichi N, Tateishi A. Successful outcomes of a novel endoscopic treatment for GI tumors: endoscopic submucosal dissection with a mixture of high-molecular-weight hyaluronic acid, glycerin, and sugar. Gastrointest Endosc. 2006;63:243-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 200] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 34. | Uraoka T, Fujii T, Saito Y, Sumiyoshi T, Emura F, Bhandari P, Matsuda T, Fu KI, Saito D. Effectiveness of glycerol as a submucosal injection for EMR. Gastrointest Endosc. 2005;61:736-740. [PubMed] |
| 35. | Akahoshi K, Yoshinaga S, Fujimaru T, Kondoh A, Higuchi N, Furuno T, Oya M. Endoscopic resection with hypertonic saline-solution-epinephrine injection plus band ligation for large pedunculated or semipedunculated gastric polyp. Gastrointest Endosc. 2006;63:312-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Schumacher B, Charton JP, Nordmann T, Vieth M, Enderle M, Neuhaus H. Endoscopic submucosal dissection of early gastric neoplasia with a water jet-assisted knife: a Western, single-center experience. Gastrointest Endosc. 2012;75:1166-1174. [RCA] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 37. | Cho JH, Cho JY, Kim MY, Jeon SR, Lee TH, Kim HG, Jim SY, Hong SJ. Endoscopic submucosal dissection using a thulium laser: preliminary results of a new method for treatment of gastric epithelial neoplasia. Endoscopy. 2013;45:725-728. [RCA] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 38. | Phee SJ, Reddy N, Chiu PW, Rebala P, Rao GV, Wang Z, Sun Z, Wong JY, Ho KY. Robot-assisted endoscopic submucosal dissection is effective in treating patients with early-stage gastric neoplasia. Clin Gastroenterol Hepatol. 2012;10:1117-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 39. | Higuchi K, Tanabe S, Azuma M, Sasaki T, Katada C, Ishido K, Naruke A, Mikami T, Koizumi W. Double-endoscope endoscopic submucosal dissection for the treatment of early gastric cancer accompanied by an ulcer scar (with video). Gastrointest Endosc. 2013;78:266-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 40. | Baldaque-Silva F, Vilas-Boas F, Velosa M, Macedo G. Endoscopic submucosal dissection of gastric lesions using the “yo-yo technique”. Endoscopy. 2013;45:218-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 41. | Maeda Y, Hirasawa D, Fujita N, Obana T, Sugawara T, Ohira T, Harada Y, Yamagata T, Suzuki K, Koike Y. A prospective, randomized, double-blind, controlled trial on the efficacy of carbon dioxide insufflation in gastric endoscopic submucosal dissection. Endoscopy. 2013;45:335-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 42. | Mori H, Rafiq K, Kobara H, Fujihara S, Nishiyama N, Kobayashi M, Himoto T, Haba R, Hagiike M, Izuishi K. Local steroid injection into the artificial ulcer created by endoscopic submucosal dissection for gastric cancer: prevention of gastric deformity. Endoscopy. 2012;44:641-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 43. | Cho SJ, Choi IJ, Kim CG, Lee JY, Nam BH, Kwak MH, Kim HJ, Ryu KW, Lee JH, Kim YW. Aspirin use and bleeding risk after endoscopic submucosal dissection in patients with gastric neoplasms. Endoscopy. 2012;44:114-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 44. | Koh R, Hirasawa K, Yahara S, Oka H, Sugimori K, Morimoto M, Numata K, Kokawa A, Sasaki T, Nozawa A. Antithrombotic drugs are risk factors for delayed postoperative bleeding after endoscopic submucosal dissection for gastric neoplasms. Gastrointest Endosc. 2013;78:476-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 45. | Kusunoki M, Miyake K, Shindo T, Ueki N, Kawagoe T, Gudis K, Futagami S, Tsukui T, Takagi I, Hosaka J. The incidence of deep vein thrombosis in Japanese patients undergoing endoscopic submucosal dissection. Gastrointest Endosc. 2011;74:798-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 46. | Goto O, Fujishiro M, Kodashima S, Ono S, Niimi K, Hirano K, Yamamichi N, Koike K. A second-look endoscopy after endoscopic submucosal dissection for gastric epithelial neoplasm may be unnecessary: a retrospective analysis of postendoscopic submucosal dissection bleeding. Gastrointest Endosc. 2010;71:241-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |