Published online May 28, 2014. doi: 10.3748/wjg.v20.i20.6055
Revised: February 20, 2014
Accepted: April 15, 2014
Published online: May 28, 2014
Processing time: 241 Days and 0.6 Hours
Colorectal cancer (CRC) is one of the leading causes of cancer and cancer-related mortality worldwide. The disease has been traditionally a major health problem in industrial countries, however the CRC rates are increasing in the developing countries that are undergoing economic growth. Several environmental risk factors, mainly changes in diet and life style, have been suggested to underlie the rise of CRC in these populations. Diet and lifestyle impinge on nuclear receptors, on the intestinal microbiota and on crucial molecular pathways that are implicated in intestinal carcinogenesis. In this respect, the epidemiological transition in several regions of the world offers a unique opportunity to better understand CRC carcinogenesis by studying the disease phenotypes and their environmental and molecular associations in different populations. The data from these studies may have important implications for the global prevention and treatment of CRC.
Core tip: This highlight addresses the rise of colorectal cancer (CRC) in the developing countries. We review the epidemiological data on the growing CRC burden in these countries, discuss the role of changing environmental risk factors, and examine preventive strategies that could contribute to control the spread of CRC. The molecular pathways of CRC and the roles of nuclear receptors and of the intestinal microbiota are discussed in the light of the current epidemiological transition.
- Citation: Bishehsari F, Mahdavinia M, Vacca M, Malekzadeh R, Mariani-Costantini R. Epidemiological transition of colorectal cancer in developing countries: Environmental factors, molecular pathways, and opportunities for prevention. World J Gastroenterol 2014; 20(20): 6055-6072
- URL: https://www.wjgnet.com/1007-9327/full/v20/i20/6055.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i20.6055
Colorectal cancer (CRC) is among the top three most commonly diagnosed cancers in the world, accounting for 8% of all cancer-related deaths annually[1]. CRC still is a major health problem in the more industrialized and developed countries, where the annual age-standardized incidence rates exceed 40, compared to rates below 4 observed in several less-developed countries. The over 10-fold variation in the global CRC rates, along with the rapid rise of CRC risk in the same generation that immigrated from low to high-risk areas, have suggested a strong environmental influence on CRC pathogenesis[2,3]. In the developing countries that are witnessing an economic advancement, the adoption of a Western life style and of dietary habits characterized by higher intake of meat, fat and total calories, along with increasing life expectancy and population growth, herald a remarkable increase in the burden of CRC[4]. This review addresses the global epidemiological characteristics of CRC, with a focus on its trend in the developing countries and on the implications for the epidemiology and the molecular pathogenesis of the disease, as well as for preventive strategies. We also discuss the emerging roles of nuclear receptors and of the gut microbiota as mediators between the environment and CRC tumorigenesis. All this can have potential for designing novel preventive and therapeutic strategies.
The designation of the “developing countries” in this manuscript is based on the United Nation’s definition and encompasses any country that is not part of the more developed countries, i.e., all of Europe plus Northern America, Australia/New Zealand and Japan [World Health Organization (WHO) Databank Statistical Information System. Geneva: World Health Organization; Year. Available at: http://www.who.int/whosis. 2010. Last accessed 2/16/2010]. This grouping is only for the purpose of epidemiological comparisons and does not express any judgment about the stage reached by a particular region or country in the development process.
CRC is the third most commonly diagnosed cancer in males, the second in females, and is the third cause of cancer-related mortality in both sexes worldwide[1]. In 2008, more than 1.2 million new CRC cases and 608700 deaths have occurred. The CRC rate varies among different regions; the rates were traditionally higher in the developed and industrial countries, whereas the less-developed countries had lower rates. Nevertheless, CRC incidence is dramatically increasing in many developing countries. The rates in the former Eastern European Communist Bloc, that recently underwent a major economic transition, have already reached or exceeded those of the industrial countries of the former Western bloc[5]. Likewise, reports from Eastern Asian regions, such as Hong Kong, Taiwan, urban China, Singapore, and Thailand, indicate a rapid rise in CRC incidence, close to the rates reported in Western populations[6,7].
A rise in CRC incidence has also been observed in Western Asian countries that were historically considered to have very low rates of the disease. For instance, epidemiological studies in Iran have shown that the CRC rate, although still relatively low, has increased significantly over the past three decades[8]. Similar trends have been reported in other populations in the region, including Saudi Arabia, Jordan, Yemen and Egypt[9-11].
Notwithstanding the rise of CRC in almost all developing countries, the acceleration rates may differ among populations. For example, in India, where an increase in the rates of CRC over the past decades has been reported, the steep is steadier and less rapid compared to other developing countries in East Asia[12].
The increase in CRC incidence in developing countries, that are often equipped with fewer resources, are paralleled by an increase in the mortality rates, as indicated by studies from South America and Eastern Europe[4].
The uptrend in CRC rates is not explainable by the effect of screening programs, as such programs are either limited or only newly implemented in these regions[5,13]. In addition, the rise in CRC is usually more prominent in the younger populations, who are not subjected to screening programs. In fact, a high proportion of early-onset CRCs has been reported from countries with new epidemiological transition[14,15]. For example, almost 20% of the CRC cases recorded in Iran occur at or below age 40, in comparison to 2%-8% reported in the developed countries for the same age subset[16]. The higher proportions of CRC in young patients can be related not only to the age-structure of these populations, but, more importantly, to the relatively lower rates of the disease in the older individuals. In other words, in these historically low-risk regions for CRC, where the rates in the older population sectors has remained low, CRC incidence has increased significantly in the newer generations. As the young population with an accelerated rate of CRC becomes older, the incidence of the disease is expected to grow further also in the older subset[8,16]. Hence, it is predicted that the incidence of CRC will dramatically increase over the next decade, nearing a doubling of the current rates, with most of the new cases occurring in developing countries[12,17].
The rise of CRC in developing countries is attributed to environmental changes, prompted by the economic transition[5,18]. Environmental factors, such as dietary patterns, obesity, smoking and heavy alcohol consumption, are considered to affect CRC risk[19].
The effect of dietary habit and lifestyle on CRC has been highlighted by migration studies, that demonstrate a rise in CRC rates in originally low-risk ethnic groups when migrated to high-risk areas[2,20,21]. Sedentary lifestyle and a “Western” diet, rich in fat and meat and usually poor in unrefined cereals and fiber, are suggested to increase the incidence of CRC[22-24]. A decrease in physical activity and a trend towards consumption of a more Westernized diet have been ubiquitously reported in countries of Central-West Asia and North Africa[8,25]. A similar shift in lifestyle and dietary patterns occurred in the Eastern European countries during the transition from planned to open market economies, which led to increased availability of food, consumption of refined products, and increased obesity[5]. Likewise, in the East Asian countries, dietary changes and obesity have preceded and paralleled the increase in CRC incidence[26-28].
Obesity, and the metabolic syndrome, characterized by glucose intolerance, and dyslipidemia, have been shown to be associated with higher risk of both colonic adenoma and CRC[29-32]. Rising rates of obesity and metabolic syndrome are increasingly reported from developing countries, with the improvement in economic status and the rapid urbanization[33-36].
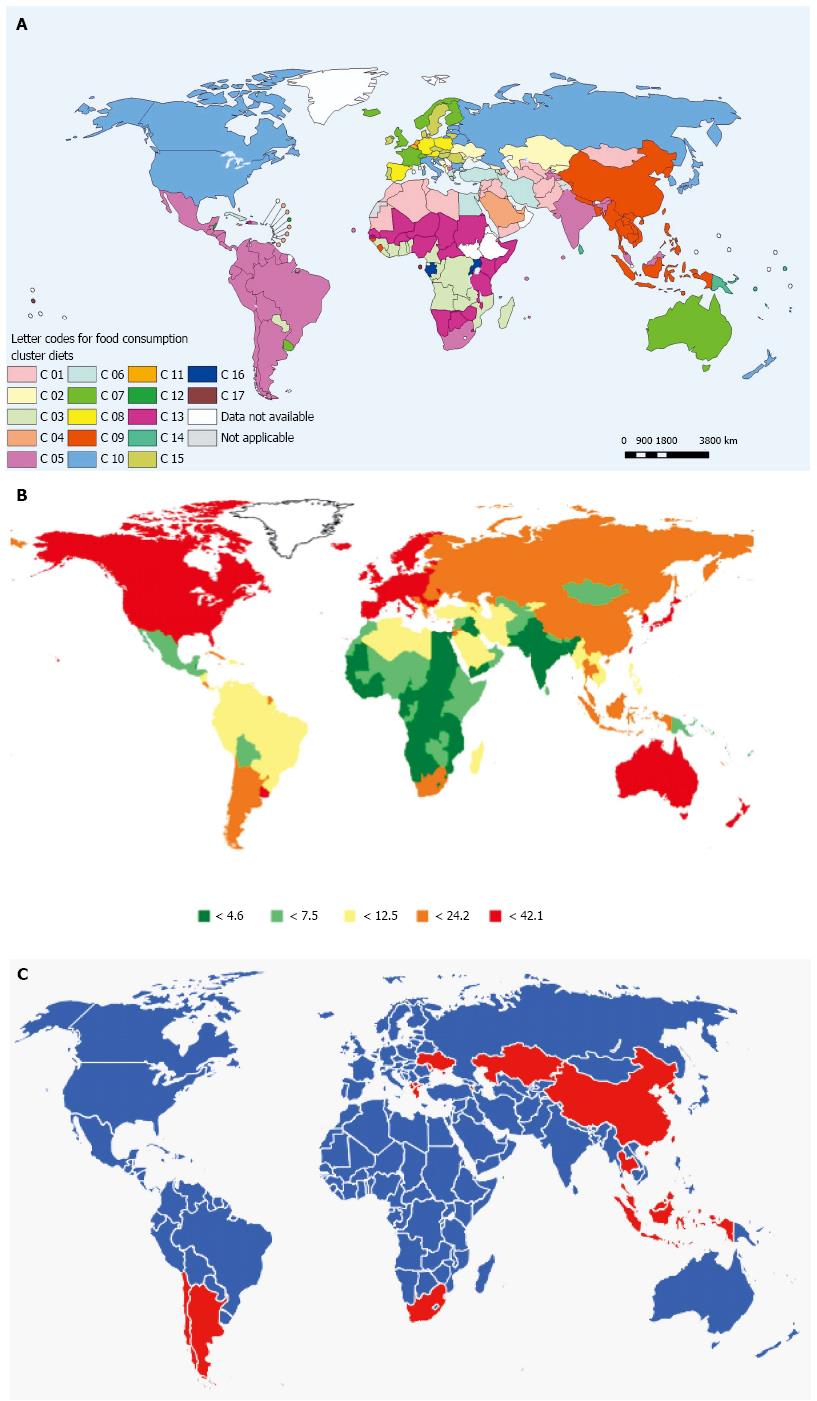
A prominent role of the environment/diet on the risk of CRC is also suggested by the age-structure of the disease in the countries under epidemiological transition. As discussed above, these countries typically witness a higher proportion of young CRC cases, while the rates are still relatively low in the older individuals. The higher CRC incidence in the younger subgroups of these populations suggests a recent change in the environmental risk factors, that affected individuals who shared these exposures during childhood and younger adulthood[16]. The proposed association of dietary profile and CRC is outlined in Figure 1, that compares the pattern of regional food consumption in the world, as reported by the WHO, with the global spread of CRC, based on the data from Globocan 2008[37]. There is a concordance in most regions of the world between the dietary pattern, mainly categorized by the fraction of meat and animal fat in the consumed food, and the CRC rate.
Most CRC cases are considered sporadic, and only a small proportion is due to known genetic syndromes. This points to a strong role of the environment in CRC development[38]. However genetic factors may modify the effects of the environment in the predisposition to the disease. This is suggested by the unequal CRC risk in different ethnic groups in the same region. For example, although the incidence of CRC is increasing in all ethnic groups in Singapore and Malay, the rates are higher in Chinese vs Malay individuals[4,39]. Disparities in the CRC rates are also seen between the Jewish and the Arab population in Israel[40]. In addition to still unknown genetic factors, ethnicity-related environmental exposures could contribute to differences in the CRC rates between distinct ethnic groups within the same geographic region or country.
Despite the overall correlation between CRC and diet highlighted by the epidemiological evidence, the results of case-control and cohort studies have been controversial[19]. Numerous reviews and meta-analyses have been conducted to test the association of single dietary compounds and CRC risk. The World Cancer Research Fund concluded that, in addition to obesity and lack of exercise, there is convincing evidence for high meat consumption to affect CRC risk[23]. The evidence for the effect of red meat on CRC risk was also considered convincing in a recent review by an expert panel[41]. Disparities among dietary studies may partly reflect the fact that the effect of each dietary component is confounded by other components in the food[42]. In this respect, associations between dietary patterns and disease could be achieved by applying a global analytical approach, such as cluster and factor analyses, to classify individuals in clusters with a global similarity in dietary habits[43,44]. By applying this approach to a large prospective cohort, Kesse et al[45] found an increased risk of adenomas and of high-risk adenomas with the Western diet, high in fats, animal products and snacks and low in foods of vegetable origin. The highest CRC risk was observed in the group with high consumption of meat. Similar findings have been reported by other researchers[46]. Direct or indirect effects of dietary factors on colonic and intestinal tumorigenesis were also demonstrated in laboratory and animal investigations[47]. Overall, these studies support the strong influence of the environment, including diet, on CRC predisposition.
The molecular alterations underlying CRC development have been extensively studied over the past two decades. Our current understanding of the molecular landscape of CRC mainly originates from studies conducted on tumors from Western populations. The tumors from developing countries have not been analyzed as extensively, but the available data show that the major molecular pathways correspond to those involved in “Western” CRC. Nevertheless the mutational spectra appear to be often distinctive, reflecting different environmental and/or genetic factors[48]. Our group showed that CRCs from Iran, as those from high-risk areas, harbor genetic alterations in major genes such as K-RAS and p53, however the spectra of the mutations are quite distinct and could be related to different environmental exposures[49]. Similar findings have been reported from other countries that are in epidemiological transition, but still have relatively low CRC incidence[50,51]. As reviewed above, several lines of evidence support the role of environmental factors, such as diet, on CRC, however, the relationships between dietary risk factors and genetic alterations is as yet incompletely understood.
Furthermore, epigenetic alterations that are central to the process of genomic imprinting, such as histone modifications and DNA methylation, as well as changes in non-coding RNAs, mainly involved in post-transcriptional regulation, can affect the gene expression patterns independent of or in combination with inherited or somatic changes in the DNA sequence.
DNA methylation, i.e., conversion of cytosine to 5-methylcytosine, normally occurs at isolated CpG dinucleotides, while the regions enriched in CpGs, so called CpG islands, mainly situated at or near gene promoters, are generally hypomethylated[52]. Abnormal methylation of CpG islands may turn off gene expression and can lead to inactivation of tumor-suppressor genes in the process of carcinogenesis[52]. For example, hypermethylation of MLH1 (a major mismatch repair gene), accounts for more than 60% of non-hereditary microsatellite unstable CRCs, and can be found in more than one fifth of all CRC cases[53-55]. Methylation of other genes involved in CRC, such as APC, MGMT, GATA-4 and GATA-5, and FXR have also been described[56,57].
On the other end, genetic instability could also be caused by global genomic hypomethylation[58]. Studies have identified number of different genes hypomethylated in CRC, such as CARD 14, CCDC116, TIAM1, and MAEL, that can have a variety of effects on signaling, e.g., by nuclear factor-kappa B (NF-κB), or other cellular functions relevant to CRC carcinogenesis including, but not limited to, cell adhesion, cell cycle control, cell migration and differentiation[59].
These epigenetic alterations can be strongly affected by dietary habits, because several dietary components can alter the methylation profile of the genome. Polyphenols, abundant in green tea, promote demethylation, and may reactivate tumor suppressor genes that were inactivated by promoter methylation[60]. Effects on methylation pattern have similarly been reported for other potentially protective natural compounds, such as quercetin, folate, and selenium[61-63]. For example, a selenium-low diet altered the methylation pattern of colonic DNA in rat models, causing activation of tumor suppressor gene, such as the rat homolog of VHL, while a selenium-rich diet reversed the abnormal methylation profile[64].
Hypermethylation can also affect the expression of microRNAs (miRNAs). MiRNAs are short non-coding RNAs of about 19-28 nucleotides that affect gene expression and/or mRNA translation by binding the untranslated regions of target genes[65]. Numerous miRNAs targeting tumor-suppressive and oncogenic pathways have been found to be altered in CRC (reviewed in[66]) and it has been found that their expression can be affected by diet[67,68]. High-risk diets seem to cause downregulation of tumor suppressive miRNAs, and progression of CRC[67]. Decrease in the tumorigenesis process upon use of dietary factors that are generally thought to lower the risk of CRC, was associated with normalization of the deregulated miRNA pattern[68,69]. For example, the level of the let-7 miRNA family was found to increase with dietary vegetable consumption in rat colon tumors induced by a heterocyclic amine from cooked meat, leading to the normalization of cancer-related proteins, such as c-myc and p53[68].
Histones modification is another mode of epigenetic alteration that affects gene expression by regulating chromatin structure and activity[70]. Acetylation and methylation of histones, among other modifications, modulate the gene expression pattern during cell differentiation and can result in activation of oncogenes and inactivation of tumor suppressor genes in CRC[70]. In-vitro and in-vivo experiments have proposed histone modification, e.g., histone deacetylases inhibition and histone hyperacetylation, as a mechanism implicated in the anti-carcinogenic effect of some dietary components, such as short-chained fatty acids, garlic, vegetable metabolites and other organic compounds[62,71-73]. Some of these dietary components act via nuclear receptors, that can be a target of therapeutic or preventive strategies for CRC (see below). Epigenetic alterations can be the target of other environmental risk factors as well[74].
Other mechanisms suggested to mediate the effect of diet on CRC include cytotoxic and mutagenic effects of food metabolites or their by-products on colorectal epithelium. Moreover, food metabolites can contribute to CRC carcinogenesis through oxidative stress, immune regulation, and alterations in the mucosal inflammatory milieu[75-80].
The responsiveness to the environment, particularly diet, and the potential reversibility, has made epigenetics a promising target for dietary interventions in the chemoprevention of CRC[59,81]. In order to achieve this goal, the epigenetic aberrations that occur early in the disease and that are modifiable by dietary agents need to be better characterized[82]. Furthermore, it is predicted that a major contribution to the chemoprevention of CRC will derive from pharmacological interventions targeting nuclear receptors that control the metabolic pathways involved in normal enterocyte proliferation and differentiation and in CRC tumorigenesis.
Nuclear receptors (NRs) are transcription factors (48 in humans, 49 in rodents) able to transduce extracellular signals into fast and coherent changes in gene expression[83]. These transcription factors are thus key players in the coordination of organism physiology[83]. Some NRs bind extracellular lipophilic molecules (e.g., hormones, vitamins, dietary lipids, bile acids, etc.)[83]. In the absence of ligand, NRs are bound to transcriptional co-repressor complexes that cause chromatin condensation and gene silencing. After ligand binding, a change in the three-dimensional conformation of the NR results in the recruitment of tissue-specific co-regulators that activate the gene transcription machinery[83]. For some NRs, called “orphans”, the endogenous ligands are still unknown, while other NRs, designated “true orphans”, are ligand-independent[83].
Previous studies have provided evidence that lipid-sensing NRs modulate enterocyte physiology (metabolism, proliferation, differentiation, and death) and that their localization pattern along the crypt-villus axis predicts the modulation of their expression in tumors[84,85]. For example, the vitamin D receptor, the farnesoid X receptor α (FXRα, bile acid -BA- sensor), the liver X receptor α (LXRα, the oxysterol sensor), and the retinoid X receptor α (RXRα), that are expressed mainly in the differentiated compartment of the intestinal mucosa (villus/epitelium), are suppressed in neoplasms[57,85-88].
FXRα is the master transcriptional regulator in bile acids (BA) metabolism. BA play an important role in intestinal homeostasis, and may have a dual function of either promoting or inhibiting cell differentiation and death, depending on the bile acids composition[89] and gut microbiota[57], that are modifiable by diet. One of the main functions of FXR is to promote the detoxification of the enterocytes from hydrophobic BA[90], thus reducing BA-induced oxidative DNA damage and inflammation in the colorectal epithelium[91]. In fact, when FXR is absent in the intestine, there is a promotion of Wnt signaling, with expansion of the basal proliferative compartment, and a concomitant reduction in the apical differentiated apoptosis-competent compartment[92]. The role of FXR in protecting from CRC is confirmed by the observation that the loss of FXR in the ApcMin/+ and in the chronic colitis mouse models results in increased intestinal tumorigenesis and tumor progression via promotion of Wnt signaling and up-regulation of Cyclin D1. On the other hand, the activation of FXR triggers proapoptotic programs in both normal and transformed colonocytes[92,93]. Additionally, FXR displays anti-inflammatory properties by interacting with NF-κB signaling[93-95], and loss of FXR function results in enhanced infiltration and production of interleukin 6 and tumor necrosis factor alpha[92,93]. Thus FXR agonists could be effective in preventing CRC and strategies aimed at reactivating FXR expression might be useful in CRC treatment.
Proliferating cells need excess cholesterol, and several studies have shown that LXR activation affects cell proliferation and promotes apoptosis[96-98]. In fact, cholesterol acts as a regulator of cell cycle progression, and cholesterol starvation results in cell cycle arrest in the G2-phase; this effect is reverted by supplying cholesterol[99,100]. The need of coordinate cholesterol metabolism tuning during membrane synthesis, cellular differentiation and proliferation thus candidates LXRs as important in cell proliferation[96,98]. LXRα and LXRβ, acting as obligate heterodimers with RXR, are central actors in lipid homeostasis and respond to physiological concentrations of the cholesterol derivatives oxysterols. In proliferating cells, reduced intracellular concentrations of oxysterols are associated with increased cholesterol synthesis and down regulation of LXR target genes involved in cholesterol catabolism and transport[101]. In the enterocytes, LXRs control the intracellular flow, catabolism, and efflux of cholesterol[102], but also have many other effects, including inhibition of NF-κB signaling[103]. This anti-inflammatory action of LXRs leads to reduced inflammatory processes in chronic gut diseases[104,105]. LXR overexpression in the ApcMin/+ and chronic colitis mouse models results in decreased intestinal tumorigenesis and tumor progression. Additionally, pharmacological activation as well as adenoviral overexpression of LXR blocks the G1 phase, increases caspase-dependent apoptosis, and slows the growth of tumor xenografts in mice by affecting lipid metabolic networks and by increasing cholesterol efflux in the intestine[96,106]. Therefore LXR agonists might become novel therapeutic agents in CRC treatment, applicable to the control of the CRC epidemics in the developing world.
As discussed earlier, obesity and a sedentary lifestyle are associated with increased risk of CRC. Several molecular pathways have been linked to the effects of obesity and physical inactivity on CRC. Hyperinsulinemia, a consequence of an increased calorie intake, induces insulin-like growth factor (IGF)-I, which can promote cell growth and inhibit apoptosis[107]. The insulin pathway promotes proliferation in preneoplastic lesions of ApcMin/+ mice, is upregulated in human CRC tissue, and is associated with aggressive tumor behavior and metastasis[108-110]. Insulin can also induce steroid hormones that are involved in cellular proliferation, and apoptosis[111].
Polypeptide growth factors and cytokines released from adipose tissues, known as adipokines, have abnormal levels in obesity, as a result of systemic upregulation of insulin/IGF-I signaling, steroid hormones and inflammatory mediators[112-115]. High level of leptin and low level of adiponectin have been associated with CRC in mouse models[116-118]. In the physiologic state, the proliferative, survival, and pro-invasive actions of leptin, via pathways such as PI3-kinase/AKT and JAK-STAT, are balanced by the antiproliferative and antiangiogenic effects of adiponectin[119-122]. Moreover, obesity is associated with chronic low-grade inflammation[123]. A growing body of literature is supporting the role of chronic inflammation in promoting CRC[124,125]. The pro-inflammatory state in obesity is characterized by dysregulated release of pro-tumorigenic inflammatory cytokines, such as IL-6, and IL-17, from adipocytes and associated macrophages, as well as by altered mucosal immune composition[126,127].
Other lifestyle factors, such as chronic cigarette smoking and alcohol consumption, contribute to pro-carcinogenic inflammation[74,128,129]. Smoking, in addition to the induction of inflammation and of epigenetic and genetic alterations, can affect other pathways leading to CRC. Smoking-related oxidative stress, a result of increased reactive oxygen species (ROS) levels, and smoke metabolites such as nitrosamines, through effects on the nicotine signaling receptors, can lead to upregulation of MAPK signaling, activation of COX2 and of the matrix metalloproteinase (MMP) pathways. Furthermore accelerated DNA-adduct formation activates base excision repair (BER)[129-132].
Also excessive chronic alcohol consumption can accelerate CRC initiation and progression through multiple cellular mechanisms, including inflammation and epigenetics, as discussed earlier. Moreover, increased ROS generation and NADPH oxidase, combined with alcoholism-related vitamin deficiencies (Bs and A) can affect apoptotic (e.g., PI3K/AKT), proliferative (e.g., ERK1/2) and metastatic (e.g., VEGF, and MMPs) pathways[128,133,134].
Several association studies, at both individual and population level[135-139], have explored the relations between modifiable environmental factors, such as life style and diet, and CRC-related molecular pathways.
Upon discovery of obesity as a risk factor for CRC, many investigators tested the effect of obesity on CRC in relation to obesity-related pathways[114]. An inverse association between adiponectin level and CRC, mainly in men, is supported by several studies, reviewed in a recent meta-analysis[139]. The levels of leptin or soluble leptin receptor have been found to be associated with increased risk of CRC in some studies[140,141].
The association between inflammatory markers and risk of CRC is not consistent. An increased level of IL-6, and soluble tumor necrosis factor receptor 2 in CRC patients, suggested by retrospective studies, has not been confirmed in prospective series[142,143]. This association could depend on specific molecular features of the tumor. For example, in rectal cancer, IL-6 was suggested to modify the effect of dietary components depending on p53 mutation status[144]. Similarly, the role of positive energy status, as defined by higher BMI and physical inactivity, can vary according to the molecular make-up of CRC. Obesity was reported to be associated with higher CRC risk in patients with tumors negative for beta-catenin activation or fatty acid synthase expression[145,146]. These patients showed lower CRC recurrence rates if exercised after diagnosis[147]. The risk of rectal tumors harboring P53 and K-RAS mutations was lower in individuals with high levels of physical activity[136]. In other studies, the mortality for CRC increased with higher BMI, depending on the expression status of anti-proliferative nuclear proteins, such as P21 and P53[148,149].
Other life style-related risk factors, such as cigarette smoking and alcohol intake, have been also studied in relation to molecular subtypes of CRC. Cigarette smoking was found to increase the risk of microsatellite unstable, CIMP-positive, and BRAF mutated CRC[150], while smoking cessation was protective against CIMP-high CRC[151]. These data are in line with the involvement of epigenetic modifications in smoking-related colorectal carcinogenesis. Although one might expect epigenetic fingerprints also from alcoholism, most studies could not find associations between specific molecular subtypes of CRC and alcohol intake[152,153]. The effects of alcohol, however, can vary among individuals depending on genetic polymorphisms in the alcohol metabolism pathways, and this could modify CRC risk[154].
The association between dietary components and CRC-related molecular pathways has been tested in population-based series. The variation among studies could be partly due to heterogeneity among series with regard to tumor location, sex, and other known and unknown potential confounders[155,156].
Earlier studies found differential associations between dietary factors and the P53 pathway in CRC, depending on whether protein expression or mutational analysis was used. Fat-intake was associated with P53-negative CRC using immunohistochemistry[157]. In subsequent larger studies, the Western-style diet, characterized by high consumption of red meat and increased glycemic load, was associated with P53 mutations in CRC[158].
With regard to the K-RAS pathway, cruciferous vegetables were found to be associated with lower chance of occurrence of K-RAS mutations, and monounsaturated fats with likelihood of G->T K-RAS mutation[159].
In rectal cancer, Omega-3 fatty acids were associated with the CpG island methylator phenotype (CIMP+), and refined grains with P53 mutations. Reduced risks of mutations in P53 and/or K-RAS were seen in groups with higher intakes of vegetables and whole grains[136]. Omega-3 fatty acids are agonists of the NRs peroxisome proliferator activated receptors (PPARs), that have been implicated in CRC. PPARγ activation has been shown to inhibit CRC cell proliferation[84] by promotion of cell maturation and inhibition of genes involved in inflammation and in cell growth[84]. Omega-3 fatty acids and other PPARγ agonists could thus serve as new anti-inflammatory and anti-cancer agents. In line with this, also the peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC1α), a coactivator of the PPARs, has been shown to modulate intestinal epithelial cell fate and to inhibit CRC development and growth[160]. Being PGC1α a downstream effector of the AMPK/SIRT pathway[161], the protective role of PGC1α on CRC could account for some of the mechanisms by which physical exercise[162], metformin[163,164], and resveratrol[165,166] protect against CRC.
Overall, despite significant advances in the field of molecular epidemiology, the attributable effects of diet on CRC molecular subtype are not yet clear. Almost all the existing association studies for diet and molecular markers of CRC have been conducted in regions with high rates of CRC, mainly located in Western countries[136,157,167-169]. Nevertheless, the relatively restricted divergence of the Western dietary profiles and the similar epidemiological features of CRC in Western populations may limit the chances of finding associations between dietary factors and molecular pathways[170]. Comparative studies involving populations more dissimilar in dietary pattern and disease epidemiology might help to fill our knowledge gap on how diet influences CRC.
In a study that analyzed the molecular features of CRC, including CpG island methylator phenotype, microsatellite instability, and K-RAS and P53 gene status, in Egypt, Jordan, and Turkey, differences in gene methylation patterns and mutational status were found between CRCs from these countries and Western CRC series. Interestingly the molecular features of the CRCs from Turkey, the country with most rapid rise of CRC rate among the three studied, were most similar to those reported for Western series[171].
Our group, by applying a mathematical approach to the investigation of geographic correlations between p53 mutation patterns, based on the International Agency for Research on Cancer (IARC) P53 database, and food factors, derived from the dataset of the Food and Agriculture Organization of the United Nations (FAO), found relationships between P53 mutation type and site and higher availability of meat, sweeteners and animal fats, i.e., the energy-dense foods that characterize the “Western” diet and that are also linked to overweight and obesity[171]. This P53 mutational “fingerprint” could be explained by differential exposure to nitrosative DNA damage, due to foods that promote metabolic stress and chronic inflammation. However direct evidence for the molecular fingerprint of diet may not be easily achievable, due to complex interactions among dietary factors in the gastrointestinal lumen and in colonic epithelium that could be affected by host genetic susceptibilities as well. In addition, a rapidly growing body of evidence suggests that the gut microbial community modulates the effect of the diet on CRC risk[172].
The number of bacteria in our body exceeds by 10 times that of human cells[173]. A comprehensive analysis of the human intestinal microbiota is now possible thanks to the availability of high-throughput sequencing techniques. The human colon is highly populated with a microbial community reaching as many as 10(13)-10(14) microorganisms. Chronic inflammation can contribute to CRC carcinogenesis[125]. The gastrointestinal (GI) microbiota plays an important role in maintaining the mucosal immune functions and in balancing pro and anti-inflammatory signals[174,175]. The composition of the GI microbiota is affected by environmental factors, such as diet[176]. The “Western diet, exemplified by high intake of animal products and low in unrefined carbohydrates, promotes a pro-inflammatory microbiota that can predispose susceptible individuals to CRC[177]. Therefore, it is prudent to hypothesize that the effect of diet on adenoma and CRC development may be mediated by shifts in the balance between detrimental and non-pathogenic commensal microbiota[178]. Moreover, the microbial community can modify the production of diet-related by-products implicated in CRC carcinogenesis, such as ROS and hydrogen sulfide (H2S), among several others[178]. Other CRC risk factors, such as obesity, can be influenced by the GI microbiota, hence reinforcing the link with CRC development[179]. This is supported by a number of case-control studies that have shown different GI microbial compositions in normal subjects versus adenoma- and CRC-affected patients[180-182]. Some bacterial taxa, such as Bacteroides, Eubacterium, and Bifidobacterium, were shown to be associated with higher risk of CRC, whereas Lactobacillus was associated with low CRC risk[179,182]. Therefore, it is conceivable to propose an association between compositions of the GI microbiota and CRC epidemiology. In other words, the GI microbial communities of the populations with lower risk of CRC could differ from those of the populations with higher risk, and this could reflect or mediate the effects of protective environmental/dietary profiles. In this regard, Nava et al[183] compared stool samples of native Africans to those of African Americans, that have overall CRC rates about 60 times higher than native Africans. Interestingly, a more diverse and numerous population of 7-dehydroxylating colonic bacteria was found in native African stools, reflecting a diet enriched in resistant starch and low in animal products, compared to stools of African Americans, who consume a “Western” diet.
In a more recent study, the same group found higher total bacteria and butyrate-producing groups, along with higher levels of antineoplastic short-chain fatty acids, in the stool samples of native Africans, whereas in the stools of African Americans the genus Bacteroides and microbial genes encoding for carcinogenic secondary bile acid production were more abundant[184]. The protective or detrimental effect of certain dietary components on the colonic mucosa, in conjunction with altered GI microbiota, has been confirmed in animal studies[185,186].
Manipulations of the GI microbiota with probiotics, prebiotics and oral vaccines are currently under intensive research for the primary and secondary prevention of CRC[178].
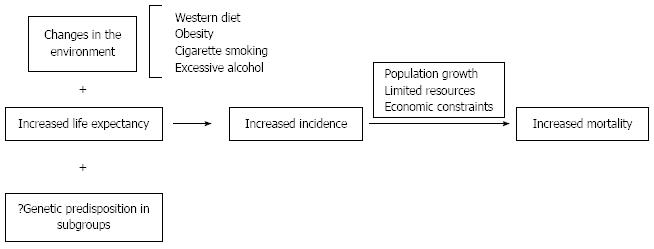
It is estimated that over the next two decades the number of CRC cases will increase dramatically, from 1.2 to 2.2 million worldwide, with most of the increase (62%) in the developing countries[12]. This estimate is mainly based on the population growth and increased life expectancy in these regions. However, as it was discussed earlier, these countries are experiencing a rise in their age-adjusted rates of CRC, due to the adoption a more Westernized lifestyle. As a result, the number of CRC cases in the developing countries could outgrow the current estimates[12,187].
This upcoming burden of CRC will bring about a large demand for patient care in areas of CRC detection, staging, and therapeutic interventions, such as surgery, radiation and chemotherapy. Yet, the resources may not grow in parallel to meet the demand (Figure 2). Therefore, it is crucial to implement preventive strategies to control CRC in populations where incidence rates are still low, rather than wait for the impact of the disease burden in the near future.
Population-based screening aims at the early detection of colorectal neoplasms, amenable for curative treatment, and is shown to be an efficient and cost-effective method to lower the disease mortality in developed countries with high CRC incidence[188]. The screening methods, recommended by multiple guidelines, include stool-based testing to detect early stage CRCs, and endoscopic-based testings, such as flexible sigmoidoscopy and colonoscopy, that allow to detect and remove pre-cancerous lesions/polyps. Screening by any of these methods on a regular basis can effectively reduce CRC risk[189].
The Council of the European Union and the ad-hoc United States task force recommend starting population screening at age 50[190,191]. However, the age for offering screening cannot be absolutely generalized from one population to another. Rather, the age of screening initiation needs to be determined based on local epidemiological data, and on the life expectancy of the population.
Population-based screening is a secondary prevention method and warrants availability of the necessary resources, including nation-wide endoscopic units and gastroenterologists that are limited in many developing countries. This, coupled with a lower incidence of CRC in many of these regions, argues against the cost-effectiveness of nation-wide screening in such countries. Alternatively, the CRC screening could be individualized based on genetic or environmental risk factors. For example, more intense screening could be considered in family members of patients with CRC, or in those with environmental risk factors, such as obesity or smoking, that have higher CRC risk than the general population[192].
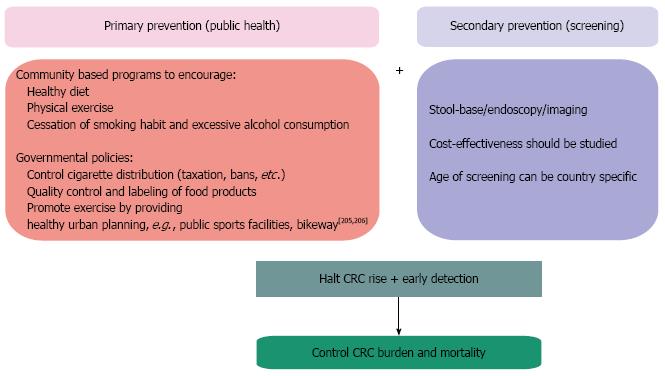
In contrast to secondary prevention, which is offered after exposure to CRC-causing factors, primary prevention focuses on the reduction of carcinogenic exposures to avert CRC development. Changes in environmental factors, including consumption of a “Western” diet, is assumed to be responsible for the increasing disease incidence in developing countries[188].
It has been proposed that consumption of unrefined grains, legumes, and fruits as sources of carbohydrate, and of poultry, fish, and legumes as sources of protein, could lower CRC risk[193]. These were main staple foods in the populations of the less developed countries, where the adoption of a “Western” diet is only a recent event. The modification of the dietary habits of these populations towards their traditional diet could be more achievable than in the Western societies, that embraced the Western diet since the industrial revolution and the development of the food processing industry[194].
Promoting a more “healthy” diet, characterized by consumption of less fat and less red meat and more vegetable food, could be a good strategy for primary CRC prevention[81]. However, the dietary adjustments should be complemented by modifications in other lifestyle habits associated with increased CRC risk. As discussed earlier, obesity, sedentary life, excessive alcohol intake, and smoking are all suggested to increase the risk. Thus, controlling these factors at the population level should be essential in primary CRC prevention (Figure 3).
It is noteworthy that several of the aforementioned environmental factors are also known risk factors for other cancers and chronic diseases that are on the rise in developing countries[195]. Therefore the allocation of appropriate resources towards primary prevention programs targeting the young generations would be an invaluable investment to control the burden of chronic diseases and cancer in the developing world[196]. At first glance, interventions directed to primary prevention seem to be extremely challenging endeavors, as they require well-designed and strong educational support, based on community programs and media, to achieve behavioral changes evident only over extended periods of time. Nevertheless, long-term efforts to implement educational and awareness programs starting at early ages, through schools or other public health programs, have been shown to be both accomplishable and effective, as exemplified by the very significant drop of smokers among United States adults over the past 6 decades[188].
Several studies have shown a decreased risk of CRC in patients taking certain medications, most notably non-steroidal anti-inflammatory agents (NSAIDs); hence this led to a growing interest in the chemoprevention of CRC. NSAIDs, such as aspirin and celecoxib, can reduce risks of adenoma and CRC[197,198]. However their utility as a primary prevention tool for CRC is questionable in the general population, given the possible gastrointestinal, cardiovascular and renal side effects associated with these drugs at high doses in the long-term[199]. The effect of statins on the overall CRC risk, despite the biologically plausible protective mechanisms, may not be as substantial as initially thought[200,201].
Nevertheless, the chemoprevention of CRC is an option that must be carefully considered after balancing the risk and benefits of each drug at the individual level[202]. This would be relevant for CRC prevention also in low-income countries, where simple drugs such as aspirin are readily available and could be prescribed to individuals that have concurrent indications for treatment, such as cardiovascular disease.
As more focus is placed on minimizing the potential toxicity of CRC chemoprophylaxis by combination therapy, this strategy may become even more justifiable, particularly in groups at higher risk because of environmental factors, hereditary predisposition or previous history of colonic adenomas and/or CRC[198,199,203-205].
CRC, historically a cancer typical of the industrial countries, is now among the common newly diagnosed cancers and causes of cancer death globally. The evidence that the disease is significantly increasing in most developing countries heralds an even more remarkable disease burden in the near future. Environmental factors, such as changes in life style and diet, are proposed to play major roles in the current transition of CRC epidemiology. The molecular mechanisms mediating the effects of the environment on CRC pathogenesis are complex and need to be fully characterized, but new targets for therapeutic and preventive interventions, such as nuclear receptors and the intestinal microbiota, are emerging. Candidate pathways, elucidated by in vitro and in vivo studies, and their weight on CRC risk, are yet to be validated in population-based cohorts. Studying populations in transition could help to identify environmental risk factors, affected pathways and possible mediators such as gut microbiota. The data obtained from these studies could have an impact in allowing better detection of at risk individuals, implementation of non-invasive strategies to lower the burden of the disease, and optimization of treatment.
P- Reviewers: Lin SR, Maric I S- Editor: Zhai HH L- Editor: A E- Editor: Ma S
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25545] [Article Influence: 1824.6] [Reference Citation Analysis (7)] |
| 2. | Dunn JE. Cancer epidemiology in populations of the United States--with emphasis on Hawaii and California--and Japan. Cancer Res. 1975;35:3240-3245. [PubMed] |
| 3. | Parkin DM. International variation. Oncogene. 2004;23:6329-6340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 460] [Article Influence: 21.9] [Reference Citation Analysis (1)] |
| 4. | Center MM, Jemal A, Smith RA, Ward E. Worldwide variations in colorectal cancer. CA Cancer J Clin. 2009;59:366-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 786] [Cited by in RCA: 865] [Article Influence: 54.1] [Reference Citation Analysis (0)] |
| 5. | Center MM, Jemal A, Ward E. International trends in colorectal cancer incidence rates. Cancer Epidemiol Biomarkers Prev. 2009;18:1688-1694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 532] [Cited by in RCA: 566] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 6. | Sung JJ, Lau JY, Goh KL, Leung WK. Increasing incidence of colorectal cancer in Asia: implications for screening. Lancet Oncol. 2005;6:871-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 598] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 7. | Khuhaprema T, Srivatanakul P. Colon and rectum cancer in Thailand: an overview. Jpn J Clin Oncol. 2008;38:237-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Malekzadeh R, Bishehsari F, Mahdavinia M, Ansari R. Epidemiology and molecular genetics of colorectal cancer in iran: a review. Arch Iran Med. 2009;12:161-169. [PubMed] |
| 9. | Al-Jaberi TM, Ammari F, Gharieybeh K, Khammash M, Yaghan RJ, Heis H, Al-Omari M, Al-Omari N. Colorectal adenocarcinoma in a defined Jordanian population from 1990 to 1995. Dis Colon Rectum. 1997;40:1089-1094. [PubMed] |
| 10. | Ibrahim EM, Zeeneldin AA, El-Khodary TR, Al-Gahmi AM, Bin Sadiq BM. Past, present and future of colorectal cancer in the Kingdom of Saudi Arabia. Saudi J Gastroenterol. 2008;14:178-182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Basaleem HO, Al-Sakkaf KA. Colorectal cancer among Yemeni patients. Characteristics and trends. Saudi Med J. 2004;25:1002-1005. [PubMed] |
| 12. | Karsa LV, Lignini TA, Patnick J, Lambert R, Sauvaget C. The dimensions of the CRC problem. Best Pract Res Clin Gastroenterol. 2010;24:381-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 13. | Gellad ZF, Provenzale D. Colorectal cancer: national and international perspective on the burden of disease and public health impact. Gastroenterology. 2010;138:2177-2190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 116] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 14. | Isbister WH. Colorectal cancer below age 40 in the Kingdom of Saudi Arabia. Aust N Z J Surg. 1992;62:468-472. [PubMed] |
| 15. | Veruttipong D, Soliman AS, Gilbert SF, Blachley TS, Hablas A, Ramadan M, Rozek LS, Seifeldin IA. Age distribution, polyps and rectal cancer in the Egyptian population-based cancer registry. World J Gastroenterol. 2012;18:3997-4003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Ansari R, Mahdavinia M, Sadjadi A, Nouraie M, Kamangar F, Bishehsari F, Fakheri H, Semnani S, Arshi S, Zahedi MJ. Incidence and age distribution of colorectal cancer in Iran: results of a population-based cancer registry. Cancer Lett. 2006;240:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 98] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990-2020: Global Burden of Disease Study. Lancet. 1997;349:1498-1504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4227] [Cited by in RCA: 4050] [Article Influence: 144.6] [Reference Citation Analysis (2)] |
| 18. | de Kok IM, Wong CS, Chia KS, Sim X, Tan CS, Kiemeney LA, Verkooijen HM. Gender differences in the trend of colorectal cancer incidence in Singapore, 1968-2002. Int J Colorectal Dis. 2008;23:461-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Bishehsari FJ. B. Cancers of the Colon and Rectum; A Multidisciplinary Approach to Diagnosis and Management. United States: Demos Medical Publishing, Inc.; 2013; . |
| 20. | McMichael AJ, Giles GG. Cancer in migrants to Australia: extending the descriptive epidemiological data. Cancer Res. 1988;48:751-756. [PubMed] |
| 21. | Yavari P, Hislop TG, Bajdik C, Sadjadi A, Nouraie M, Babai M, Malekzadeh R. Comparison of cancer incidence in Iran and Iranian immigrants to British Columbia, Canada. Asian Pac J Cancer Prev. 2006;7:86-90. [PubMed] |
| 22. | Potter JD. Colorectal cancer: molecules and populations. J Natl Cancer Inst. 1999;91:916-932. [PubMed] |
| 23. | Wiseman M. The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Proc Nutr Soc. 2008;67:253-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 654] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 24. | Tseng M, DeVillis R. Correlates of the “western” and “prudent” diet patterns in the us. Ann Epidemiol. 2000;10:481-482. [PubMed] |
| 25. | Kelishadi R, Motlagh ME, Roomizadeh P, Abtahi SH, Qorbani M, Taslimi M, Heshmat R, Aminaee T, Ardalan G, Poursafa P. First report on path analysis for cardiometabolic components in a nationally representative sample of pediatric population in the Middle East and North Africa (MENA): the CASPIAN-III Study. Ann Nutr Metab. 2013;62:257-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Minami Y, Nishino Y, Tsubono Y, Tsuji I, Hisamichi S. Increase of colon and rectal cancer incidence rates in Japan: trends in incidence rates in Miyagi Prefecture, 1959-1997. J Epidemiol. 2006;16:240-248. [PubMed] |
| 27. | Matsushita Y, Takahashi Y, Mizoue T, Inoue M, Noda M, Tsugane S. Overweight and obesity trends among Japanese adults: a 10-year follow-up of the JPHC Study. Int J Obes (Lond). 2008;32:1861-1867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 28. | Zhang J, Dhakal IB, Zhao Z, Li L. Trends in mortality from cancers of the breast, colon, prostate, esophagus, and stomach in East Asia: role of nutrition transition. Eur J Cancer Prev. 2012;21:480-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 29. | Matsuo K, Mizoue T, Tanaka K, Tsuji I, Sugawara Y, Sasazuki S, Nagata C, Tamakoshi A, Wakai K, Inoue M. Association between body mass index and the colorectal cancer risk in Japan: pooled analysis of population-based cohort studies in Japan. Ann Oncol. 2012;23:479-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 30. | Kim BC, Shin A, Hong CW, Sohn DK, Han KS, Ryu KH, Park BJ, Nam JH, Park JW, Chang HJ. Association of colorectal adenoma with components of metabolic syndrome. Cancer Causes Control. 2012;23:727-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 31. | Odegaard AO, Koh WP, Yu MC, Yuan JM. Body mass index and risk of colorectal cancer in Chinese Singaporeans: the Singapore Chinese Health Study. Cancer. 2011;117:3841-3849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 32. | Yang MH, Rampal S, Sung J, Choi YH, Son HJ, Lee JH, Kim YH, Chang DK, Rhee PL, Kim JJ. The association of serum lipids with colorectal adenomas. Am J Gastroenterol. 2013;108:833-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 33. | de Onis M, Blössner M. Prevalence and trends of overweight among preschool children in developing countries. Am J Clin Nutr. 2000;72:1032-1039. [PubMed] |
| 34. | Misra A, Khurana L. Obesity and the metabolic syndrome in developing countries. J Clin Endocrinol Metab. 2008;93:S9-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 618] [Cited by in RCA: 651] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 35. | Balkau B, Deanfield JE, Després JP, Bassand JP, Fox KA, Smith SC, Barter P, Tan CE, Van Gaal L, Wittchen HU. International Day for the Evaluation of Abdominal Obesity (IDEA): a study of waist circumference, cardiovascular disease, and diabetes mellitus in 168,000 primary care patients in 63 countries. Circulation. 2007;116:1942-1951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 463] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 36. | Kelishadi R. Childhood overweight, obesity, and the metabolic syndrome in developing countries. Epidemiol Rev. 2007;29:62-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 332] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 37. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11128] [Cited by in RCA: 11837] [Article Influence: 845.5] [Reference Citation Analysis (4)] |
| 38. | Migliore L, Migheli F, Spisni R, Coppedè F. Genetics, cytogenetics, and epigenetics of colorectal cancer. J Biomed Biotechnol. 2011;2011:792362. [PubMed] |
| 39. | Wang H, Seow A, Lee HP. Trends in cancer incidence among Singapore Malays: a low-risk population. Ann Acad Med Singapore. 2004;33:57-62. [PubMed] |
| 40. | Fireman Z, Sandler E, Kopelman Y, Segal A, Sternberg A. Ethnic differences in colorectal cancer among Arab and Jewish neighbors in Israel. Am J Gastroenterol. 2001;96:204-207. [PubMed] |
| 41. | Vargas AJ, Thompson PA. Diet and nutrient factors in colorectal cancer risk. Nutr Clin Pract. 2012;27:613-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 150] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 42. | Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13:3-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2698] [Cited by in RCA: 2978] [Article Influence: 129.5] [Reference Citation Analysis (0)] |
| 43. | Jacobs DR, Steffen LM. Nutrients, foods, and dietary patterns as exposures in research: a framework for food synergy. Am J Clin Nutr. 2003;78:508S-513S. [PubMed] |
| 44. | Slattery ML. Defining dietary consumption: is the sum greater than its parts? Am J Clin Nutr. 2008;88:14-15. [PubMed] |
| 45. | Kesse E, Clavel-Chapelon F, Boutron-Ruault MC. Dietary patterns and risk of colorectal tumors: a cohort of French women of the National Education System (E3N). Am J Epidemiol. 2006;164:1085-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 46. | Flood A, Rastogi T, Wirfält E, Mitrou PN, Reedy J, Subar AF, Kipnis V, Mouw T, Hollenbeck AR, Leitzmann M. Dietary patterns as identified by factor analysis and colorectal cancer among middle-aged Americans. Am J Clin Nutr. 2008;88:176-184. [PubMed] |
| 47. | Lund EK, Belshaw NJ, Elliott GO, Johnson IT. Recent advances in understanding the role of diet and obesity in the development of colorectal cancer. Proc Nutr Soc. 2011;70:194-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 48. | Chan AO, Soliman AS, Zhang Q, Rashid A, Bedeir A, Houlihan PS, Mokhtar N, Al-Masri N, Ozbek U, Yaghan R. Differing DNA methylation patterns and gene mutation frequencies in colorectal carcinomas from Middle Eastern countries. Clin Cancer Res. 2005;11:8281-8287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 49. | Bishehsari F, Mahdavinia M, Malekzadeh R, Verginelli F, Catalano T, Sotoudeh M, Bazan V, Agnese V, Esposito DL, De Lellis L. Patterns of K-ras mutation in colorectal carcinomas from Iran and Italy (a Gruppo Oncologico dell’Italia Meridionale study): influence of microsatellite instability status and country of origin. Ann Oncol. 2006;17 Suppl 7:vii91-vii96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 50. | Al-Allawi NA, Ismaeel AT, Ahmed NY, Merza NS. The frequency and spectrum of K-ras mutations among Iraqi patients with sporadic colorectal carcinoma. Indian J Cancer. 2012;49:163-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 51. | Nieminen TT, Shoman S, Eissa S, Peltomäki P, Abdel-Rahman WM. Distinct genetic and epigenetic signatures of colorectal cancers according to ethnic origin. Cancer Epidemiol Biomarkers Prev. 2012;21:202-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 52. | Curtin K, Slattery ML, Samowitz WS. CpG island methylation in colorectal cancer: past, present and future. Patholog Res Int. 2011;2011:902674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 53. | Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6773] [Cited by in RCA: 6674] [Article Influence: 513.4] [Reference Citation Analysis (0)] |
| 54. | Kuismanen SA, Holmberg MT, Salovaara R, de la Chapelle A, Peltomäki P. Genetic and epigenetic modification of MLH1 accounts for a major share of microsatellite-unstable colorectal cancers. Am J Pathol. 2000;156:1773-1779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 205] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 55. | Li X, Yao X, Wang Y, Hu F, Wang F, Jiang L, Liu Y, Wang D, Sun G, Zhao Y. MLH1 promoter methylation frequency in colorectal cancer patients and related clinicopathological and molecular features. PLoS One. 2013;8:e59064. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 56. | Kim MS, Lee J, Sidransky D. DNA methylation markers in colorectal cancer. Cancer Metastasis Rev. 2010;29:181-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 232] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 57. | Sayin SI, Wahlström A, Felin J, Jäntti S, Marschall HU, Bamberg K, Angelin B, Hyötyläinen T, Orešič M, Bäckhed F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17:225-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1367] [Cited by in RCA: 1684] [Article Influence: 140.3] [Reference Citation Analysis (0)] |
| 58. | Kim YH, Lee HC, Kim SY, Yeom YI, Ryu KJ, Min BH, Kim DH, Son HJ, Rhee PL, Kim JJ. Epigenomic analysis of aberrantly methylated genes in colorectal cancer identifies genes commonly affected by epigenetic alterations. Ann Surg Oncol. 2011;18:2338-2347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 127] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 59. | Schnekenburger M, Diederich M. Epigenetics Offer New Horizons for Colorectal Cancer Prevention. Curr Colorectal Cancer Rep. 2012;8:66-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 60. | Yang CS, Fang M, Lambert JD, Yan P, Huang TH. Reversal of hypermethylation and reactivation of genes by dietary polyphenolic compounds. Nutr Rev. 2008;66 Suppl 1:S18-S20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 61. | Tan S, Wang C, Lu C, Zhao B, Cui Y, Shi X, Ma X. Quercetin is able to demethylate the p16INK4a gene promoter. Chemotherapy. 2009;55:6-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 62. | Lu R, Wang X, Sun DF, Tian XQ, Zhao SL, Chen YX, Fang JY. Folic acid and sodium butyrate prevent tumorigenesis in a mouse model of colorectal cancer. Epigenetics. 2008;3:330-335. [PubMed] |
| 63. | Zeng H, Yan L, Cheng WH, Uthus EO. Dietary selenomethionine increases exon-specific DNA methylation of the p53 gene in rat liver and colon mucosa. J Nutr. 2011;141:1464-1468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 64. | Davis CD, Uthus EO, Finley JW. Dietary selenium and arsenic affect DNA methylation in vitro in Caco-2 cells and in vivo in rat liver and colon. J Nutr. 2000;130:2903-2909. [PubMed] |
| 65. | Lao VV, Grady WM. Epigenetics and colorectal cancer. Nat Rev Gastroenterol Hepatol. 2011;8:686-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 523] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 66. | Rossi S, Di Narzo AF, Mestdagh P, Jacobs B, Bosman FT, Gustavsson B, Majoie B, Roth A, Vandesompele J, Rigoutsos I. microRNAs in colon cancer: a roadmap for discovery. FEBS Lett. 2012;586:3000-3007. [PubMed] |
| 67. | Zhu H, Dougherty U, Robinson V, Mustafi R, Pekow J, Kupfer S, Li YC, Hart J, Goss K, Fichera A. EGFR signals downregulate tumor suppressors miR-143 and miR-145 in Western diet-promoted murine colon cancer: role of G1 regulators. Mol Cancer Res. 2011;9:960-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 68. | Parasramka MA, Dashwood WM, Wang R, Abdelli A, Bailey GS, Williams DE, Ho E, Dashwood RH. MicroRNA profiling of carcinogen-induced rat colon tumors and the influence of dietary spinach. Mol Nutr Food Res. 2012;56:1259-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 69. | Davidson LA, Wang N, Shah MS, Lupton JR, Ivanov I, Chapkin RS. n-3 Polyunsaturated fatty acids modulate carcinogen-directed non-coding microRNA signatures in rat colon. Carcinogenesis. 2009;30:2077-2084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 124] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 70. | Bannister AJ, Kouzarides T. Regulation of chromatin by histone modifications. Cell Res. 2011;21:381-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3359] [Cited by in RCA: 4040] [Article Influence: 288.6] [Reference Citation Analysis (0)] |
| 71. | Dashwood RH, Ho E. Dietary histone deacetylase inhibitors: from cells to mice to man. Semin Cancer Biol. 2007;17:363-369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 205] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 72. | Scharlau D, Borowicki A, Habermann N, Hofmann T, Klenow S, Miene C, Munjal U, Stein K, Glei M. Mechanisms of primary cancer prevention by butyrate and other products formed during gut flora-mediated fermentation of dietary fibre. Mutat Res. 2009;682:39-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 236] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 73. | Nian H, Delage B, Ho E, Dashwood RH. Modulation of histone deacetylase activity by dietary isothiocyanates and allyl sulfides: studies with sulforaphane and garlic organosulfur compounds. Environ Mol Mutagen. 2009;50:213-221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 124] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 74. | Biliński P, Wojtyła A, Kapka-Skrzypczak L, Chwedorowicz R, Cyranka M, Studziński T. Epigenetic regulation in drug addiction. Ann Agric Environ Med. 2012;19:491-496. [PubMed] |
| 75. | Ferguson LR, Philpott M, Karunasinghe N. Dietary cancer and prevention using antimutagens. Toxicology. 2004;198:147-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 140] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 76. | Bruce WR, Giacca A, Medline A. Possible mechanisms relating diet and risk of colon cancer. Cancer Epidemiol Biomarkers Prev. 2000;9:1271-1279. [PubMed] |
| 77. | Kim YS, Milner JA. Dietary modulation of colon cancer risk. J Nutr. 2007;137:2576S-2579S. [PubMed] |
| 78. | Diggs DL, Huderson AC, Harris KL, Myers JN, Banks LD, Rekhadevi PV, Niaz MS, Ramesh A. Polycyclic aromatic hydrocarbons and digestive tract cancers: a perspective. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2011;29:324-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 178] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 79. | Barone M, Lofano K, De Tullio N, Licinio R, Albano F, Di Leo A. Dietary, endocrine, and metabolic factors in the development of colorectal cancer. J Gastrointest Cancer. 2012;43:13-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 80. | Lakatos PL, Kiss LS, Miheller P. Nutritional influences in selected gastrointestinal diseases. Dig Dis. 2011;29:154-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 81. | Gingras D, Béliveau R. Colorectal cancer prevention through dietary and lifestyle modifications. Cancer Microenviron. 2011;4:133-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 82. | Verma M. Cancer control and prevention by nutrition and epigenetic approaches. Antioxid Redox Signal. 2012;17:355-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 83. | Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schütz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835-839. [PubMed] |
| 84. | D’Errico I, Moschetta A. Nuclear receptors, intestinal architecture and colon cancer: an intriguing link. Cell Mol Life Sci. 2008;65:1523-1543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 85. | Modica S, Gofflot F, Murzilli S, D’Orazio A, Salvatore L, Pellegrini F, Nicolucci A, Tognoni G, Copetti M, Valanzano R. The intestinal nuclear receptor signature with epithelial localization patterns and expression modulation in tumors. Gastroenterology. 2010;138:636-48, 648.e1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 86. | Torres J, Bao X, Iuga AC, Chen A, Harpaz N, Ullman T, Cohen BL, Pineton de Chambrun G, Asciutti S, Odin JA. Farnesoid X receptor expression is decreased in colonic mucosa of patients with primary sclerosing cholangitis and colitis-associated neoplasia. Inflamm Bowel Dis. 2013;19:275-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 87. | Wada K, Tanaka H, Maeda K, Inoue T, Noda E, Amano R, Kubo N, Muguruma K, Yamada N, Yashiro M. Vitamin D receptor expression is associated with colon cancer in ulcerative colitis. Oncol Rep. 2009;22:1021-1025. [PubMed] |
| 88. | De Gottardi A, Touri F, Maurer CA, Perez A, Maurhofer O, Ventre G, Bentzen CL, Niesor EJ, Dufour JF. The bile acid nuclear receptor FXR and the bile acid binding protein IBABP are differently expressed in colon cancer. Dig Dis Sci. 2004;49:982-989. [PubMed] |
| 89. | Barrasa JI, Olmo N, Lizarbe MA, Turnay J. Bile acids in the colon, from healthy to cytotoxic molecules. Toxicol In Vitro. 2013;27:964-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 90. | Modica S, Gadaleta RM, Moschetta A. Deciphering the nuclear bile acid receptor FXR paradigm. Nucl Recept Signal. 2010;8:e005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 230] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 91. | Bernstein H, Bernstein C, Payne CM, Dvorakova K, Garewal H. Bile acids as carcinogens in human gastrointestinal cancers. Mutat Res. 2005;589:47-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 458] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 92. | Modica S, Murzilli S, Salvatore L, Schmidt DR, Moschetta A. Nuclear bile acid receptor FXR protects against intestinal tumorigenesis. Cancer Res. 2008;68:9589-9594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 198] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 93. | Maran RR, Thomas A, Roth M, Sheng Z, Esterly N, Pinson D, Gao X, Zhang Y, Ganapathy V, Gonzalez FJ. Farnesoid X receptor deficiency in mice leads to increased intestinal epithelial cell proliferation and tumor development. J Pharmacol Exp Ther. 2009;328:469-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 185] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 94. | Gadaleta RM, van Erpecum KJ, Oldenburg B, Willemsen EC, Renooij W, Murzilli S, Klomp LW, Siersema PD, Schipper ME, Danese S. Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut. 2011;60:463-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 657] [Cited by in RCA: 638] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 95. | Wang YD, Chen WD, Wang M, Yu D, Forman BM, Huang W. Farnesoid X receptor antagonizes nuclear factor kappaB in hepatic inflammatory response. Hepatology. 2008;48:1632-1643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 525] [Cited by in RCA: 497] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 96. | Lo Sasso G, Bovenga F, Murzilli S, Salvatore L, Di Tullio G, Martelli N, D’Orazio A, Rainaldi S, Vacca M, Mangia A. Liver X receptors inhibit proliferation of human colorectal cancer cells and growth of intestinal tumors in mice. Gastroenterology. 2013;144:1497-507, 1507.e1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 97. | Lo Sasso G, Celli N, Caboni M, Murzilli S, Salvatore L, Morgano A, Vacca M, Pagliani T, Parini P, Moschetta A. Down-regulation of the LXR transcriptome provides the requisite cholesterol levels to proliferating hepatocytes. Hepatology. 2010;51:1334-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 98. | Mehrotra A, Kaul D, Joshi K. LXR-α selectively reprogrammes cancer cells to enter into apoptosis. Mol Cell Biochem. 2011;349:41-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 99. | Martínez-Botas J, Suárez Y, Ferruelo AJ, Gómez-Coronado D, Lasuncion MA. Cholesterol starvation decreases p34(cdc2) kinase activity and arrests the cell cycle at G2. FASEB J. 1999;13:1359-1370. [PubMed] |
| 100. | Fernández C, Lobo Md Mdel V, Gómez-Coronado D, Lasunción MA. Cholesterol is essential for mitosis progression and its deficiency induces polyploid cell formation. Exp Cell Res. 2004;300:109-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 110] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 101. | Bensinger SJ, Bradley MN, Joseph SB, Zelcer N, Janssen EM, Hausner MA, Shih R, Parks JS, Edwards PA, Jamieson BD. LXR signaling couples sterol metabolism to proliferation in the acquired immune response. Cell. 2008;134:97-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 602] [Cited by in RCA: 571] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 102. | Vacca M, Degirolamo C, Mariani-Costantini R, Palasciano G, Moschetta A. Lipid-sensing nuclear receptors in the pathophysiology and treatment of the metabolic syndrome. Wiley Interdiscip Rev Syst Biol Med. 2011;3:562-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 103. | Hong C, Tontonoz P. Coordination of inflammation and metabolism by PPAR and LXR nuclear receptors. Curr Opin Genet Dev. 2008;18:461-467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 189] [Cited by in RCA: 185] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 104. | Canavan M, McCarthy C, Larbi NB, Dowling JK, Collins L, O’Sullivan F, Hurley G, Murphy C, Quinlan A, Moloney G. Activation of liver X receptor suppresses the production of the IL-12 family of cytokines by blocking nuclear translocation of NF-κBp50. Innate Immun. 2014;Jan 29; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 105. | Heimerl S, Moehle C, Zahn A, Boettcher A, Stremmel W, Langmann T, Schmitz G. Alterations in intestinal fatty acid metabolism in inflammatory bowel disease. Biochim Biophys Acta. 2006;1762:341-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 106. | Vedin LL, Gustafsson JÅ, Steffensen KR. The oxysterol receptors LXRα and LXRβ suppress proliferation in the colon. Mol Carcinog. 2013;52:835-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 107. | Becker S, Dossus L, Kaaks R. Obesity related hyperinsulinaemia and hyperglycaemia and cancer development. Arch Physiol Biochem. 2009;115:86-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 130] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 108. | Fenton JI, Hord NG, Lavigne JA, Perkins SN, Hursting SD. Leptin, insulin-like growth factor-1, and insulin-like growth factor-2 are mitogens in ApcMin/+ but not Apc+/+ colonic epithelial cell lines. Cancer Epidemiol Biomarkers Prev. 2005;14:1646-1652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 109. | Giovannucci E. Metabolic syndrome, hyperinsulinemia, and colon cancer: a review. Am J Clin Nutr. 2007;86:s836-s842. [PubMed] |
| 110. | Esposito DL, Aru F, Lattanzio R, Morgano A, Abbondanza M, Malekzadeh R, Bishehsari F, Valanzano R, Russo A, Piantelli M. The insulin receptor substrate 1 (IRS1) in intestinal epithelial differentiation and in colorectal cancer. PLoS One. 2012;7:e36190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 111. | Moschos SJ, Mantzoros CS. The role of the IGF system in cancer: from basic to clinical studies and clinical applications. Oncology. 2002;63:317-332. [PubMed] |
| 112. | Maury E, Brichard SM. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol Cell Endocrinol. 2010;314:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 713] [Cited by in RCA: 754] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 113. | de Heredia FP, Gómez-Martínez S, Marcos A. Obesity, inflammation and the immune system. Proc Nutr Soc. 2012;71:332-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 557] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 114. | Bardou M, Barkun AN, Martel M. Obesity and colorectal cancer. Gut. 2013;62:933-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 587] [Article Influence: 48.9] [Reference Citation Analysis (0)] |
| 115. | Vongsuvanh R, George J, Qiao L, van der Poorten D. Visceral adiposity in gastrointestinal and hepatic carcinogenesis. Cancer Lett. 2013;330:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 116. | Endo H, Hosono K, Uchiyama T, Sakai E, Sugiyama M, Takahashi H, Nakajima N, Wada K, Takeda K, Nakagama H. Leptin acts as a growth factor for colorectal tumours at stages subsequent to tumour initiation in murine colon carcinogenesis. Gut. 2011;60:1363-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 117. | Padidar S, Farquharson AJ, Williams LM, Kelaiditi E, Hoggard N, Arthur JR, Drew JE. Leptin up-regulates pro-inflammatory cytokines in discrete cells within mouse colon. J Cell Physiol. 2011;226:2123-2130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 118. | Moon HS, Liu X, Nagel JM, Chamberland JP, Diakopoulos KN, Brinkoetter MT, Hatziapostolou M, Wu Y, Robson SC, Iliopoulos D. Salutary effects of adiponectin on colon cancer: in vivo and in vitro studies in mice. Gut. 2013;62:561-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 119. | Barb D, Williams CJ, Neuwirth AK, Mantzoros CS. Adiponectin in relation to malignancies: a review of existing basic research and clinical evidence. Am J Clin Nutr. 2007;86:s858-s866. [PubMed] |
| 120. | Shehzad A, Iqbal W, Shehzad O, Lee YS. Adiponectin: regulation of its production and its role in human diseases. Hormones (Athens). 2012;11:8-20. [PubMed] |
| 121. | Vansaun MN. Molecular pathways: adiponectin and leptin signaling in cancer. Clin Cancer Res. 2013;19:1926-1932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 175] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 122. | Uddin S, Hussain AR, Khan OS, Al-Kuraya KS. Role of dysregulated expression of leptin and leptin receptors in colorectal carcinogenesis. Tumour Biol. 2014;35:871-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 123. | Yehuda-Shnaidman E, Schwartz B. Mechanisms linking obesity, inflammation and altered metabolism to colon carcinogenesis. Obes Rev. 2012;13:1083-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 124. | Terzić J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101-2114.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1333] [Cited by in RCA: 1512] [Article Influence: 100.8] [Reference Citation Analysis (0)] |
| 125. | Moossavi S, Bishehsari F. Inflammation in sporadic colorectal cancer. Arch Iran Med. 2012;15:166-170. [PubMed] |
| 126. | Gil A, María Aguilera C, Gil-Campos M, Cañete R. Altered signalling and gene expression associated with the immune system and the inflammatory response in obesity. Br J Nutr. 2007;98 Suppl 1:S121-S126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 127. | Federico A, D’Aiuto E, Borriello F, Barra G, Gravina AG, Romano M, De Palma R. Fat: a matter of disturbance for the immune system. World J Gastroenterol. 2010;16:4762-4772. [PubMed] |
| 128. | Oyesanmi O, Snyder D, Sullivan N, Reston J, Treadwell J, Schoelles KM. Alcohol consumption and cancer risk: understanding possible causal mechanisms for breast and colorectal cancers. Evid Rep Technol Assess (Full Rep). 2010;1-151. [PubMed] |
| 129. | Chu KM, Cho CH, Shin VY. Nicotine and gastrointestinal disorders: its role in ulceration and cancer development. Curr Pharm Des. 2013;19:5-10. [PubMed] |
| 130. | Hecht SS. DNA adduct formation from tobacco-specific N-nitrosamines. Mutat Res. 1999;424:127-142. [PubMed] |
| 131. | Ye YN, Wu WK, Shin VY, Cho CH. A mechanistic study of colon cancer growth promoted by cigarette smoke extract. Eur J Pharmacol. 2005;519:52-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 132. | Lodovici M, Bigagli E. Biomarkers of induced active and passive smoking damage. Int J Environ Res Public Health. 2009;6:874-888. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 133. | Forsyth CB, Tang Y, Shaikh M, Zhang L, Keshavarzian A. Alcohol stimulates activation of Snail, epidermal growth factor receptor signaling, and biomarkers of epithelial-mesenchymal transition in colon and breast cancer cells. Alcohol Clin Exp Res. 2010;34:19-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 134. | Haas SL, Ye W, Löhr JM. Alcohol consumption and digestive tract cancer. Curr Opin Clin Nutr Metab Care. 2012;15:457-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 135. | Slattery ML, Lundgreen A, Herrick JS, Caan BJ, Potter JD, Wolff RK. Diet and colorectal cancer: analysis of a candidate pathway using SNPS, haplotypes, and multi-gene assessment. Nutr Cancer. 2011;63:1226-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 136. | Slattery ML, Curtin K, Wolff RK, Herrick JS, Caan BJ, Samowitz W. Diet, physical activity, and body size associations with rectal tumor mutations and epigenetic changes. Cancer Causes Control. 2010;21:1237-1245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 137. | Wang J, Joshi AD, Corral R, Siegmund KD, Marchand LL, Martinez ME, Haile RW, Ahnen DJ, Sandler RS, Lance P. Carcinogen metabolism genes, red meat and poultry intake, and colorectal cancer risk. Int J Cancer. 2012;130:1898-1907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 138. | Hutter CM, Chang-Claude J, Slattery ML, Pflugeisen BM, Lin Y, Duggan D, Nan H, Lemire M, Rangrej J, Figueiredo JC. Characterization of gene-environment interactions for colorectal cancer susceptibility loci. Cancer Res. 2012;72:2036-2044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 141] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 139. | Xu XT, Xu Q, Tong JL, Zhu MM, Huang ML, Ran ZH, Xiao SD. Meta-analysis: circulating adiponectin levels and risk of colorectal cancer and adenoma. J Dig Dis. 2011;12:234-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 140. | Aleksandrova K, Boeing H, Jenab M, Bueno-de-Mesquita HB, Jansen E, van Duijnhoven FJ, Rinaldi S, Fedirko V, Romieu I, Riboli E. Leptin and soluble leptin receptor in risk of colorectal cancer in the European Prospective Investigation into Cancer and Nutrition cohort. Cancer Res. 2012;72:5328-5337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 141. | Gialamas SP, Sergentanis TN, Antonopoulos CN, Dessypris N, Chrousos GP, Petridou ET. Circulating leptin levels and risk of colorectal cancer and adenoma: a case-control study and meta-analysis. Cancer Causes Control. 2013;24:2129-2141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 142. | Chan AT, Ogino S, Giovannucci EL, Fuchs CS. Inflammatory markers are associated with risk of colorectal cancer and chemopreventive response to anti-inflammatory drugs. Gastroenterology. 2011;140:799-808, quiz e11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 143. | Song M, Wu K, Ogino S, Fuchs CS, Giovannucci EL, Chan AT. A prospective study of plasma inflammatory markers and risk of colorectal cancer in men. Br J Cancer. 2013;108:1891-1898. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 144. | Slattery ML, Wolff RK, Herrick J, Caan BJ, Samowitz W. Tumor markers and rectal cancer: support for an inflammation-related pathway. Int J Cancer. 2009;125:1698-1704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 145. | Kuchiba A, Morikawa T, Yamauchi M, Imamura Y, Liao X, Chan AT, Meyerhardt JA, Giovannucci E, Fuchs CS, Ogino S. Body mass index and risk of colorectal cancer according to fatty acid synthase expression in the nurses’ health study. J Natl Cancer Inst. 2012;104:415-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 146. | Morikawa T, Kuchiba A, Lochhead P, Nishihara R, Yamauchi M, Imamura Y, Liao X, Qian ZR, Ng K, Chan AT. Prospective analysis of body mass index, physical activity, and colorectal cancer risk associated with β-catenin (CTNNB1) status. Cancer Res. 2013;73:1600-1610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 147. | Morikawa T, Kuchiba A, Yamauchi M, Meyerhardt JA, Shima K, Nosho K, Chan AT, Giovannucci E, Fuchs CS, Ogino S. Association of CTNNB1 (beta-catenin) alterations, body mass index, and physical activity with survival in patients with colorectal cancer. JAMA. 2011;305:1685-1694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 162] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 148. | Ogino S, Nosho K, Shima K, Baba Y, Irahara N, Kirkner GJ, Hazra A, De Vivo I, Giovannucci EL, Meyerhardt JA. p21 expression in colon cancer and modifying effects of patient age and body mass index on prognosis. Cancer Epidemiol Biomarkers Prev. 2009;18:2513-2521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 149. | Morikawa T, Kuchiba A, Liao X, Imamura Y, Yamauchi M, Qian ZR, Nishihara R, Sato K, Meyerhardt JA, Fuchs CS. Tumor TP53 expression status, body mass index and prognosis in colorectal cancer. Int J Cancer. 2012;131:1169-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 150. | Limsui D, Vierkant RA, Tillmans LS, Wang AH, Weisenberger DJ, Laird PW, Lynch CF, Anderson KE, French AJ, Haile RW. Cigarette smoking and colorectal cancer risk by molecularly defined subtypes. J Natl Cancer Inst. 2010;102:1012-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 239] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 151. | Nishihara R, Morikawa T, Kuchiba A, Lochhead P, Yamauchi M, Liao X, Imamura Y, Nosho K, Shima K, Kawachi I. A prospective study of duration of smoking cessation and colorectal cancer risk by epigenetics-related tumor classification. Am J Epidemiol. 2013;178:84-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 152. | Schernhammer ES, Giovannucci E, Baba Y, Fuchs CS, Ogino S. B vitamins, methionine and alcohol intake and risk of colon cancer in relation to BRAF mutation and CpG island methylator phenotype (CIMP). PLoS One. 2011;6:e21102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 153. | Razzak AA, Oxentenko AS, Vierkant RA, Tillmans LS, Wang AH, Weisenberger DJ, Laird PW, Lynch CF, Anderson KE, French AJ. Alcohol intake and colorectal cancer risk by molecularly defined subtypes in a prospective study of older women. Cancer Prev Res (Phila). 2011;4:2035-2043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 154. | Crous-Bou M, Rennert G, Cuadras D, Salazar R, Cordero D, Saltz Rennert H, Lejbkowicz F, Kopelovich L, Monroe Lipkin S, Bernard Gruber S. Polymorphisms in alcohol metabolism genes ADH1B and ALDH2, alcohol consumption and colorectal cancer. PLoS One. 2013;8:e80158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 155. | Li FY, Lai MD. Colorectal cancer, one entity or three. J Zhejiang Univ Sci B. 2009;10:219-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 184] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 156. | Ogino S, Chan AT, Fuchs CS, Giovannucci E. Molecular pathological epidemiology of colorectal neoplasia: an emerging transdisciplinary and interdisciplinary field. Gut. 2011;60:397-411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 430] [Cited by in RCA: 452] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 157. | Voskuil DW, Kampman E, van Kraats AA, Balder HF, van Muijen GN, Goldbohm RA, van’t Veer P. p53 over-expression and p53 mutations in colon carcinomas: relation to dietary risk factors. Int J Cancer. 1999;81:675-681. [PubMed] |
| 158. | Slattery ML, Curtin K, Ma K, Edwards S, Schaffer D, Anderson K, Samowitz W. Diet activity, and lifestyle associations with p53 mutations in colon tumors. Cancer Epidemiol Biomarkers Prev. 2002;11:541-548. [PubMed] |
| 159. | Slattery ML, Curtin K, Anderson K, Ma KN, Edwards S, Leppert M, Potter J, Schaffer D, Samowitz WS. Associations between dietary intake and Ki-ras mutations in colon tumors: a population-based study. Cancer Res. 2000;60:6935-6941. [PubMed] |
| 160. | D’Errico I, Salvatore L, Murzilli S, Lo Sasso G, Latorre D, Martelli N, Egorova AV, Polishuck R, Madeyski-Bengtson K, Lelliott C. Peroxisome proliferator-activated receptor-gamma coactivator 1-alpha (PGC1alpha) is a metabolic regulator of intestinal epithelial cell fate. Proc Natl Acad Sci USA. 2011;108:6603-6608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 142] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 161. | Feige JN, Auwerx J. Transcriptional coregulators in the control of energy homeostasis. Trends Cell Biol. 2007;17:292-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 252] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 162. | Harriss DJ, Cable NT, George K, Reilly T, Renehan AG, Haboubi N. Physical activity before and after diagnosis of colorectal cancer: disease risk, clinical outcomes, response pathways and biomarkers. Sports Med. 2007;37:947-960. [PubMed] |
| 163. | Decensi A, Puntoni M, Goodwin P, Cazzaniga M, Gennari A, Bonanni B, Gandini S. Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer Prev Res (Phila). 2010;3:1451-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 733] [Cited by in RCA: 686] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 164. | Buzzai M, Jones RG, Amaravadi RK, Lum JJ, DeBerardinis RJ, Zhao F, Viollet B, Thompson CB. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 2007;67:6745-6752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 673] [Cited by in RCA: 723] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 165. | Athar M, Back JH, Kopelovich L, Bickers DR, Kim AL. Multiple molecular targets of resveratrol: Anti-carcinogenic mechanisms. Arch Biochem Biophys. 2009;486:95-102. [PubMed] |
| 166. | Patel KR, Brown VA, Jones DJ, Britton RG, Hemingway D, Miller AS, West KP, Booth TD, Perloff M, Crowell JA. Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer Res. 2010;70:7392-7399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 499] [Cited by in RCA: 448] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 167. | Slattery ML, Curtin K, Ma K, Schaffer D, Potter J, Samowitz W. GSTM-1 and NAT2 and genetic alterations in colon tumors. Cancer Causes Control. 2002;13:527-534. [PubMed] |
| 168. | Le Marchand L, Hankin JH, Pierce LM, Sinha R, Nerurkar PV, Franke AA, Wilkens LR, Kolonel LN, Donlon T, Seifried A. Well-done red meat, metabolic phenotypes and colorectal cancer in Hawaii. Mutat Res. 2002;506-507:205-214. [PubMed] |
| 169. | Voutsinas J, Wilkens LR, Franke A, Vogt TM, Yokochi LA, Decker R, Le Marchand L. Heterocyclic amine intake, smoking, cytochrome P450 1A2 and N-acetylation phenotypes, and risk of colorectal adenoma in a multiethnic population. Gut. 2013;62:416-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 170. | Sempos CT, Liu K, Ernst ND. Food and nutrient exposures: what to consider when evaluating epidemiologic evidence. Am J Clin Nutr. 1999;69:1330S-1338S. [PubMed] |
| 171. | Verginelli F, Bishehsari F, Napolitano F, Mahdavinia M, Cama A, Malekzadeh R, Miele G, Raiconi G, Tagliaferri R, Mariani-Costantini R. Transitions at CpG dinucleotides, geographic clustering of TP53 mutations and food availability patterns in colorectal cancer. PLoS One. 2009;4:e6824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 172. | O’Keefe SJ. Nutrition and colonic health: the critical role of the microbiota. Curr Opin Gastroenterol. 2008;24:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 173. | Vipperla K, O’Keefe SJ. The microbiota and its metabolites in colonic mucosal health and cancer risk. Nutr Clin Pract. 2012;27:624-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 174. | Harrison OJ, Maloy KJ. Innate immune activation in intestinal homeostasis. J Innate Immun. 2011;3:585-593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 175. | Kipanyula MJ, Seke Etet PF, Vecchio L, Farahna M, Nukenine EN, Nwabo Kamdje AH. Signaling pathways bridging microbial-triggered inflammation and cancer. Cell Signal. 2013;25:403-416. [PubMed] |
| 176. | Nyangale EP, Mottram DS, Gibson GR. Gut microbial activity, implications for health and disease: the potential role of metabolite analysis. J Proteome Res. 2012;11:5573-5585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 202] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 177. | Greer JB, O’Keefe SJ. Microbial induction of immunity, inflammation, and cancer. Front Physiol. 2011;1:168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 178. | Azcárate-Peril MA, Sikes M, Bruno-Bárcena JM. The intestinal microbiota, gastrointestinal environment and colorectal cancer: a putative role for probiotics in prevention of colorectal cancer? Am J Physiol Gastrointest Liver Physiol. 2011;301:G401-G424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 173] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 179. | Sobhani I, Amiot A, Le Baleur Y, Levy M, Auriault ML, Van Nhieu JT, Delchier JC. Microbial dysbiosis and colon carcinogenesis: could colon cancer be considered a bacteria-related disease? Therap Adv Gastroenterol. 2013;6:215-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 180. | Kostic AD, Gevers D, Pedamallu CS, Michaud M, Duke F, Earl AM, Ojesina AI, Jung J, Bass AJ, Tabernero J. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012;22:292-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1199] [Cited by in RCA: 1499] [Article Influence: 107.1] [Reference Citation Analysis (0)] |
| 181. | Marchesi JR, Dutilh BE, Hall N, Peters WH, Roelofs R, Boleij A, Tjalsma H. Towards the human colorectal cancer microbiome. PLoS One. 2011;6:e20447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 182. | Shen XJ, Rawls JF, Randall T, Burcal L, Mpande CN, Jenkins N, Jovov B, Abdo Z, Sandler RS, Keku TO. Molecular characterization of mucosal adherent bacteria and associations with colorectal adenomas. Gut Microbes. 2010;1:138-147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 330] [Cited by in RCA: 312] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 183. | Nava GM, Carbonero F, Ou J, Benefiel AC, O’Keefe SJ, Gaskins HR. Hydrogenotrophic microbiota distinguish native Africans from African and European Americans. Environ Microbiol Rep. 2012;4:307-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 184. | Ou J, Carbonero F, Zoetendal EG, DeLany JP, Wang M, Newton K, Gaskins HR, O’Keefe SJ. Diet, microbiota, and microbial metabolites in colon cancer risk in rural Africans and African Americans. Am J Clin Nutr. 2013;98:111-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 463] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 185. | Conlon MA, Kerr CA, McSweeney CS, Dunne RA, Shaw JM, Kang S, Bird AR, Morell MK, Lockett TJ, Molloy PL. Resistant starches protect against colonic DNA damage and alter microbiota and gene expression in rats fed a Western diet. J Nutr. 2012;142:832-840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 186. | Tlaskalová-Hogenová H, Stěpánková R, Kozáková H, Hudcovic T, Vannucci L, Tučková L, Rossmann P, Hrnčíř T, Kverka M, Zákostelská Z. The role of gut microbiota (commensal bacteria) and the mucosal barrier in the pathogenesis of inflammatory and autoimmune diseases and cancer: contribution of germ-free and gnotobiotic animal models of human diseases. Cell Mol Immunol. 2011;8:110-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 500] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 187. | Huxley RR, Ansary-Moghaddam A, Clifton P, Czernichow S, Parr CL, Woodward M. The impact of dietary and lifestyle risk factors on risk of colorectal cancer: a quantitative overview of the epidemiological evidence. Int J Cancer. 2009;125:171-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 457] [Article Influence: 28.6] [Reference Citation Analysis (1)] |
| 188. | Armour BS, Campbell VA, Crews JE, Malarcher A, Maurice E, Richard RA. State-level prevalence of cigarette smoking and treatment advice, by disability status, United States, 2004. Prev Chronic Dis. 2007;4:A86. [PubMed] |
| 189. | Levin B, Lieberman DA, McFarland B, Smith RA, Brooks D, Andrews KS, Dash C, Giardiello FM, Glick S, Levin TR. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58:130-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1169] [Cited by in RCA: 1203] [Article Influence: 70.8] [Reference Citation Analysis (0)] |
| 190. | Segnan N, Patnick J, Karsa L, European Commission. Directorate-General for Health and Consumer Protection., International Agency for Research on Cancer. European guidelines for quality assurance in colorectal cancer screening and diagnosis. 1st ed. Luxembourg: Office for Official Publications of the European Communities 2010; 386. |
| 191. | US Preventive Services Task Force. Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:627-637. [PubMed] |
| 192. | Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected]. Am J Gastroenterol. 2009;104:739-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 981] [Cited by in RCA: 1059] [Article Influence: 66.2] [Reference Citation Analysis (0)] |
| 193. | Chan AT, Giovannucci EL. Primary prevention of colorectal cancer. Gastroenterology. 2010;138:2029-2043.e10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 475] [Cited by in RCA: 443] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 194. | Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, Watkins BA, O’Keefe JH, Brand-Miller J. Origins and evolution of the Western diet: health implications for the 21st century. Am J Clin Nutr. 2005;81:341-354. [PubMed] |
| 195. | Fahed AC, El-Hage-Sleiman AK, Farhat TI, Nemer GM. Diet, genetics, and disease: a focus on the middle East and north Africa region. J Nutr Metab. 2012;2012:109037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 196. | Willett WC, Koplan JP, Nugent R, Dusenbury C, Puska P, Gaziano TA. Prevention of Chronic Disease by Means of Diet and Lifestyle Changes. Disease Control Priorities in Developing Countries. 2nd ed. Washington (DC): World Bank 2006; . |
| 197. | Cook NR, Lee IM, Zhang SM, Moorthy MV, Buring JE. Alternate-day, low-dose aspirin and cancer risk: long-term observational follow-up of a randomized trial. Ann Intern Med. 2013;159:77-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 233] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 198. | Cooper K, Squires H, Carroll C, Papaioannou D, Booth A, Logan RF, Maguire C, Hind D, Tappenden P. Chemoprevention of colorectal cancer: systematic review and economic evaluation. Health Technol Assess. 2010;14:1-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 137] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 199. | Zhou P, Cheng SW, Yang R, Wang B, Liu J. Combination chemoprevention: future direction of colorectal cancer prevention. Eur J Cancer Prev. 2012;21:231-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 200. | Lochhead P, Chan AT. Statins and colorectal cancer. Clin Gastroenterol Hepatol. 2013;11:109-118; quiz e13-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 201. | Jacobs RJ, Kodach LL, Hardwick JC. The potential of statins for individualized colorectal cancer chemoprevention. Curr Drug Targets. 2011;12:1903-1908. [PubMed] |
| 202. | Meyskens FL, Curt GA, Brenner DE, Gordon G, Herberman RB, Finn O, Kelloff GJ, Khleif SN, Sigman CC, Szabo E. Regulatory approval of cancer risk-reducing (chemopreventive) drugs: moving what we have learned into the clinic. Cancer Prev Res (Phila). 2011;4:311-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 203. | Burn J, Mathers JC, Bishop DT. Chemoprevention in Lynch syndrome. Fam Cancer. 2013;12:707-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 204. | Nishihara R, Lochhead P, Kuchiba A, Jung S, Yamauchi M, Liao X, Imamura Y, Qian ZR, Morikawa T, Wang M. Aspirin use and risk of colorectal cancer according to BRAF mutation status. JAMA. 2013;309:2563-2571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 131] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 205. | Kirsten W, Bauman A, Pratt M. Promoting physical activity globally for population health. Promot Educ. 2006;13:90-1, 147-8, 154-5. [PubMed] |
| 206. | Edwards P, Tsouros A. Promoting physical activity and active living in urban environments: the role of local governments. Copenhagen: WHO Regional Office for Europe 2006; 12-40. |