Published online May 7, 2014. doi: 10.3748/wjg.v20.i17.5092
Revised: February 13, 2014
Accepted: March 4, 2014
Published online: May 7, 2014
Processing time: 158 Days and 12.9 Hours
AIM: To investigate the usefulness of chromoendoscopy, using an acetic acid indigocarmine mixture (AIM), for gastric adenoma diagnosed by forceps biopsy.
METHODS: A total of 54 lesions in 45 patients diagnosed as gastric adenoma by forceps biopsy were prospectively enrolled in this study and treated by endoscopic submucosal dissection (ESD) between January 2011 and January 2012. AIM-chromoendoscopy (AIM-CE) was performed followed by ESD. AIM solution was sprinkled and images were recorded every 30 s for 3 min. Clinical characteristics such as tumor size (< 2 cm, ≥ 2 cm), surface color in white light endoscopy (WLE) (whitish, normochromic or reddish), macroscopic appearance (flat or elevated, depressed), and reddish change in AIM-CE were selected as valuables.
RESULTS: En bloc resection was achieved in all 54 cases, with curative resection of fifty two lesions (96.3%). Twenty three lesions (42.6%) were diagnosed as well-differentiated adenocarcinoma and the remaining 31 lesions (57.4%) were gastric adenoma. All adenocarcinoma lesions were well-differentiated tubular adenocarcinomas and were restricted within the mucosal layer. The sensitivity of reddish color change in AIM-CE is significantly higher than that in WLE (vs tumor size ≥ 2 cm, P = 0.016, vs normochromic or reddish surface color, P = 0.046, vs depressed macroscopic type, P = 0.0030). On the other hand, no significant differences were found in the specificity and accuracy. In univariate analysis, normochromic or reddish surface color in WLE (OR = 3.7, 95%CI: 1.2-12, P = 0.022) and reddish change in AIM-CE (OR = 14, 95%CI: 3.8-70, P < 0.001) were significantly related to diagnosis of early gastric cancer (EGC). In multivariate analysis, only reddish change in AIM-CE (OR = 11, 95%CI: 2.3-66, P = 0.0022) was a significant factor associated with diagnosis of EGC.
CONCLUSION: AIM-CE may have potential for screening EGC in patients initially diagnosed as gastric adenoma by forceps biopsy.
Core tip: A novel chromoendoscopy procedure using an acetic acid indigocarmine mixture (AIM) is effective for recognizing the margins of early gastric cancer (EGC). In some cases the color of the EGC area gradually becomes reddish after instillation of the AIM solution. The study shows that the sensitivity of AIM-chromoendoscopy (AIM-CE) in the diagnosis of EGC was significantly higher than that of white light endoscopy, but the specificity and accuracy were not. AIM-CE may have potential for screening EGC in patients initially diagnosed as gastric adenoma by forceps biopsy.
- Citation: Kono Y, Takenaka R, Kawahara Y, Okada H, Hori K, Kawano S, Yamasaki Y, Takemoto K, Miyake T, Fujiki S, Yamamoto K. Chromoendoscopy of gastric adenoma using an acetic acid indigocarmine mixture. World J Gastroenterol 2014; 20(17): 5092-5097
- URL: https://www.wjgnet.com/1007-9327/full/v20/i17/5092.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i17.5092
Histological diagnosis by forceps biopsy before endoscopic resection (ER), such as endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD), is important to determine whether ER should be performed[1]. However, forceps biopsy specimens represent limited local pathological findings of the whole lesion. In fact, disagreement between the histological results of the forceps biopsy and the pathological results of endoscopically resected specimens has been reported[2-7].
The use of narrow band imaging (NBI) and acetic acid with magnifying endoscopy for the diagnosis of gastric cancer has been reported[8-12],but it is difficult to predict a coexisting gastric cancer component in borderline lesions. We previously reported that a novel chromoendoscopy procedure using an acetic acid indigocarmine mixture (AIM) was effective for recognizing the margins of early gastric cancer (EGC)[13], and that the color of the EGC sometimes turned reddish by degrees after instillation of the AIM solution[14]. It has been reported that the characteristics of gastric adenomas, such as size, red color and depressed type, are significant variables that may be seen by white light endoscopy (WLE)[15]. However, there have been no reports of AIM-chromoendoscopy (AIM-CE) for diagnosing a coexisting gastric cancer component in gastric adenomas.
The aim of this study was to evaluate the usefulness of AIM-CE for lesions initially diagnosed gastric adenoma by forceps biopsy.
This was a prospective study carried out at a single endoscopy unit at the Tsuyama Chuo Hospital, following a preliminary study. From January 2011 to January 2012, a total of 54 lesions in 45 patients with an endoscopic diagnosis of gastric adenoma by forceps biopsy were treated by ESD following AIM-CE. All patients were recruited prospectively and each provided written informed consent. The study design was approved by the Tsuyama Chuo Hospital Clinical Ethics Committee on Human Experiments, in accordance with the Helsinki Declaration.
The lesions were initially observed by WLE, after which 20 mL of 0.4% indigo carmine (IC) solution was sprinkled onto the lesions through the accessory channel of the endoscope. Images from WLE and IC observation were recorded using a digital filing system. After washing away the IC solution, 40 mL AIM solution (0.6% acetic acid with 0.4% IC) was sprinkled onto the lesions and images were recorded again every 30 s for 3 min[14]. Before the lesions were treated, clinical variables such as tumor size (< 2 cm, ≥ 2 cm), surface color in WLE (whitish, normochromic or reddish), macroscopic appearance (flat or elevated, depressed), and reddish color change in AIM-CE, were evaluated. Images of WLE and AIM-CE were evaluated by two endoscopists (Kono Y and Takenaka R) who were blinded to the final histology.
ESD was performed using an IT knife 2, needle knife and/or dual knife. The histological diagnosis of biopsy specimens and the curability of ESD were evaluated according to the standards in Japanese Classification of Gastric Cancer[16]. The specimens obtained by forceps biopsy and ESD were reviewed by a single pathologist (Miyake T).
Before this prospective study, we designed a preliminary retrospective study to estimate the beyond-chance agreement. Twenty lesions in 16 patients, previously diagnosed as gastric adenoma by forceps biopsy, were evaluated between two endoscopists’ diagnosis of AIM-CE and the κ-index was calculated. A κ value < 0.4 cases was regarded as representative of poor agreement; 0.41 to 0.60, fair agreement; 0.61 to 0.80, good agreement and > 0.80, excellent agreement.
Fisher’s exact test or χ2 test was used for all categorical variables, and the Mann-Whitney U test was used for continuous valuables. McNemar’s test was used for comparison of diagnostic ability between WLE and AIM-CE. Univariate and multivariate logistic regression analyses were used to determine the significant factors contributing to diagnosis of EGC. Variables found in the univariate analysis to be significantly associated with diagnosis of EGC were included in a multivariable logistic regression analysis. All statistical calculations were carried out using JMP software (for Windows, version 10). P values < 0.05 were considered to be statistically significant in all tests.
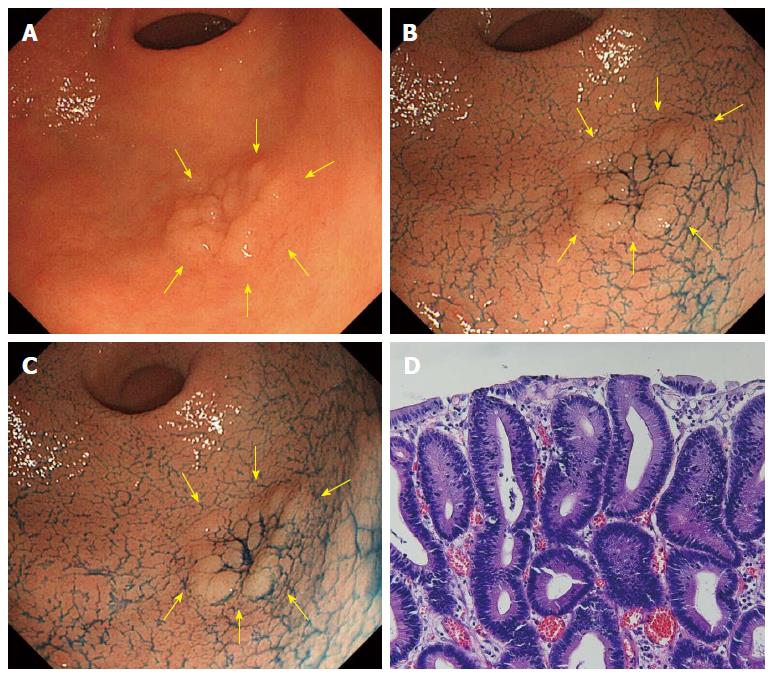
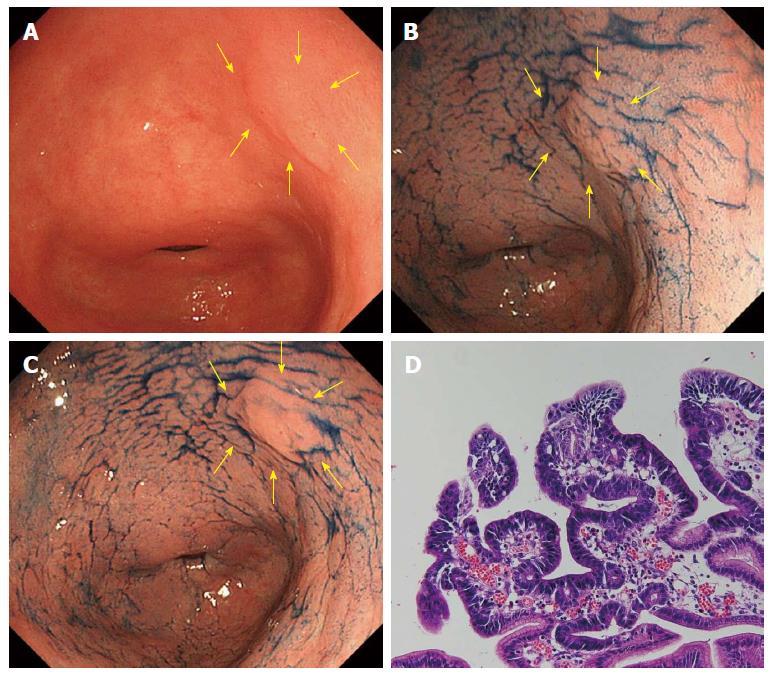
In AIM-CE, the reddish color change was recorded as positive when the surface color was judged to have turned reddish compared to the surrounding mucosa. Figures 1 and 2 show the typical cases of no change and reddish color change in AIM-CE, respectively.
Table 1 shows the clinical characteristics of the 54 cases. The difference of mean ages between the adenocarcinoma group and the adenoma group was not significant (P = 0.85). The gender ratio differed significantly different between the adenocarcinoma group and the adenoma group (P = 0.047)
| Characteristics | Adenoma (n = 31) | Adenocarcinoma(n = 23) | P value |
| Age (mean ± SD), yr | 71 ± 6.8 | 71 ± 7.4 | 0.850 |
| Gender (male/female) | 15/16 | 18/5 | 0.047 |
| Tumor size (mm, mean ± SD) | 12 ± 5.5 | 19 ± 11 | 0.019 |
| Location | |||
| Upper third of the stomach | 1 (3.2) | 3 (13) | 0.330 |
| Middle third of the stomach | 13 (42) | 7 (30) | |
| Lower third of the stomach | 17 (54.8) | 13 (57) | |
| Macroscopic type | |||
| 0-I | 1 (3.2) | 1 (4.3) | 0.840 |
| 0-IIa | 25 (80.7) | 17 (74) | |
| 0-IIc | 5 (16.1) | 5 (21.7) |
All adenocarcinoma lesions were well-differentiated tubular adenocarcinomas and were restricted within the mucosal layer. The adenocarcinoma lesions were significantly larger in diameter than the adenoma lesions (Z = 2.3, P = 0.019, Man-Whitney U test). There were no significant differences between the adenocarcinoma group and the adenoma group in tumor location and macroscopic type (P = 0.33 and P = 0.84, respectively).
Before this prospective study, we designed a preliminary pilot study. There was excellent inter-observer agreement on the findings made by AIM-CE (κ index = 0.90).
Table 2 shows the diagnostic ability of WLE and AIM-CE. The sensitivity of reddish color change in AIM-CE is significantly higher than that of WLE (vs tumor size ≥ 2 cm; P = 0.016, vs normochromic or reddish surface color; P = 0.046, depressed macroscopic type; P = 0.0030). On the other hand, no significant differences were found in the specificity and accuracy.
In the univariate analysis, normochromic or reddish surface color in WLE (OR = 3.7, 95%CI: 1.2-12, P = 0.022) and reddish color change in AIM-CE (OR = 14, 95%CI: 3.8-70, P < 0.001) were significantly related to diagnosis of EGC. In the multivariate analysis, only reddish change in AIM-CE (OR = 11, 95%CI: 2.3-66, P = 0.0022) is a significant factor associated with diagnosis of EGC (Table 3).
| Parameters | Subgroup | Univariate OR(95%CI) | Multivariate OR(95%CI) |
| Tumor size | < 2 cm | 1.0 | 1.0 |
| ≥ 2 cm | 3.1 (0.99-11) | 1.1 (0.25-5.0) | |
| Surface color in WLE | Whitish | 1.0 | 1.0 |
| Normochromic or reddish | 3.74 (1.2-12)a | 1.8 (0.44-7.2) | |
| Macroscopic appearance | Flat or elevated | 1.0 | - |
| Depressed | 1.4 (0.35-5.9) | - | |
| Reddish change in AIM-CE | No | 1.0 | 1.0 |
| Yes | 14 (3.8-70)b | 11 (2.3-66)b |
Gastric adenomas are reportedly associated with synchronous gastric cancers with varying frequencies, ranging from 8% to 59%[17]. Even using forceps biopsy, a definite diagnosis of cancer may be difficult before endoscopic resection. Discrepancies may exist between forceps biopsy samples and resected specimens[17]. In this study, AIM-CE was found to be useful to predict gastric malignancy in the lesions initially diagnosed as gastric adenoma by forceps biopsy. This provides a treatment strategy for such lesions when the surface color of the lesions changes to a reddish color after applying AIM solution.
EMR or ESD allows en bloc resection of the entire mucosal lesion[2-7]. However, application of EMR/ESD for all gastric adenoma lesions may excessively increase time and costs. In addition, we should take care of complications such as bleeding and perforation. If we could predict malignant transformation, EMR/ESD could be selectively performed for high-risk gastric adenoma patients. According to the results of several studies, the concordance rates between endoscopic forceps biopsied samples and post-treatment specimens are 65%-90%[18-23]. In this study, lesions were resected in en bloc fashion by ESD and, because the sample number was lower and the en bloc resection rate was higher, the concordance rate could be lower.
The rate of malignant transformation of gastric adenomas increases with the size of the tumor[17,24,25]. The surface appearance of gastric adenomas also may be an important factor contributing to the diagnosis of carcinoma[17]. In 2005, Kitoh et al[26] reported that two endoscopic findings, focal redness and lack of glossiness, were significant factors associated with gastric cancer. In our study, the adenocarcinoma lesions were significantly greater in diameter than the adenomatous lesions and more frequently had a red color in WLE, as described previously. However, these factors did not contribute to diagnosis of EGC in multivariate analysis. Only AIM-CE contributed to predictive diagnosis of borderline lesions, although it was difficult to predict malignant potential from tumor size, surface color, and macroscopic appearance in WLE.
Although the use of NBI and acetic acid with magnifying endoscopy for the diagnosis of gastric cancer is effective[8-12], there are some clinical problems. First, magnifying endoscopy is available only in a limited number of medical institutions. Second, because magnifying endoscopy is used near the lesion, an accidental touch of the mucosa often causes bleeding, making examinations difficult thereafter. We consider that AIM-CE is a safe, easy and cost-effective method for diagnosis of EGC.
It is not fully understood why the cancerous lesions diagnosed by forceps biopsy as borderline malignancy may turn a reddish color after spraying the AIM solution is sprayed. After spraying the acetic acid solution, the duration of whitening differed between the gastric cancer and noncancerous mucosa: aceto-whitening disappeared earlier from the gastric cancer than the noncancerous mucosa. Furthermore, while non-cancerous mucosa whitens when it comes into contact with acetic acid, cancer cells do not, producing a good contrast between a pinkish cancer lesion and the surrounding non-cancerous tissue[27]. We speculate that adding indigocarmine makes this contrast even clearer. However, in this study, a reddish change in AIM-CE was determined by the subjective judgment of two endoscopists. Therefore, we are planning a quantitative analysis of color change in AIM-CE between the adenomas and cancers diagnosed by ESD.
In conclusion, AIM-CE may have potential for screening EGC in patients initially diagnosed as gastric adenoma by forceps biopsy. If the surface color of the lesions changes to reddish after applying the AIM solution, we should consider an endoscopic treatment such as EMR or ESD.
It is reported that gastric adenomas have a malignant potential, and disagreement between the histological results of the forceps biopsy and the pathological results of endoscopically resected specimens could occur. However, it is difficult to predict a coexisting gastric cancer component before performing endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD).
In white light endoscopy (WLE), the clinical characteristics of gastric adenomas which are related to malignant potential have been investigated, but there have been no reports of acetic acid indigocarmine mixture-chromoendoscopy (AIM-CE) for diagnosing coexisting gastric cancer components in gastric adenomas. The color of the early gastric cancer (EGC) sometimes changes to reddish by degrees after instillation of the AIM solution. The authors aimed to evaluate the usefulness of AIM-CE for lesions initially diagnosed gastric adenoma by forceps biopsy.
The use of NBI and acetic acid with magnifying endoscopy for the diagnosis of EGC is effective, but magnifying endoscopy is often difficult because it needs an endoscopists’ technique. Authors consider that AIM-CE is a safe, easy and cost-effective method for diagnosis of EGC.
They consider AIM-CE may have potential for screening EGC in patients initially diagnosed as gastric adenoma by forceps biopsy. They recommend EMR or ESD if the color of gastric lesions changes reddish in AIM-CE.
For successful treatment (endoscopic mucosal/submucosal resection or laparoscopic gastrectomy) in patients with EGC it is crucial to determinate the lateral extent of the tumor. In this paper the authors evaluated the usefulness of novel chromoendoscopy procedure using AIM-CE for detection of EGC in lesions initially diagnosed as gastric adenoma by forceps biopsy. The procedure was followed by ESD. It was found that the sensitivity of AIM-CE for recognizing the margins of EGC is significantly higher than that of WLE, but there is no difference in specificity and accuracy. The authors conclude that AIM-CE is a safe, easy and cost-effective method for the diagnosis of EGC. The results are interesting and promising for clinical practice as a screening test for EGC.
P- Reviewer: Gerova VA S- Editor: Wen LL L- Editor: A E- Editor: Zhang DN
| 1. | Lee JH, Kim JH, Rhee K, Huh CW, Lee YC, Yoon SO, Youn YH, Park H, Lee SI. Undifferentiated early gastric cancer diagnosed as differentiated histology based on forceps biopsy. Pathol Res Pract. 2013;209:314-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Chávez Rossell M. [Endoscopic treatment of early gastric cancer: from Endoscopic Mucosal Resection (EMR) to Endoscopic Submucosal Dissection (ESD)]. Rev Gastroenterol Peru. 2005;25:76-92. [PubMed] |
| 3. | Gotoda T. A large endoscopic resection by endoscopic submucosal dissection procedure for early gastric cancer. Clin Gastroenterol Hepatol. 2005;3:S71-S73. [PubMed] |
| 4. | Hirasaki S, Tanimizu M, Nasu J, Shinji T, Koide N. Treatment of elderly patients with early gastric cancer by endoscopic submucosal dissection using an insulated-tip diathermic knife. Intern Med. 2005;44:1033-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Kato M. Endoscopic submucosal dissection (ESD) is being accepted as a new procedure of endoscopic treatment of early gastric cancer. Intern Med. 2005;44:85-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Gotoda T, Yamamoto H, Soetikno RM. Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol. 2006;41:929-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 507] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 7. | Imagawa A, Okada H, Kawahara Y, Takenaka R, Kato J, Kawamoto H, Fujiki S, Takata R, Yoshino T, Shiratori Y. Endoscopic submucosal dissection for early gastric cancer: results and degrees of technical difficulty as well as success. Endoscopy. 2006;38:987-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 234] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 8. | Nakayoshi T, Tajiri H, Matsuda K, Kaise M, Ikegami M, Sasaki H. Magnifying endoscopy combined with narrow band imaging system for early gastric cancer: correlation of vascular pattern with histopathology (including video). Endoscopy. 2004;36:1080-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 335] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 9. | Uedo N, Iishi H, Tatsuta M, Yamada T, Ogiyama H, Imanaka K, Sugimoto N, Higashino K, Ishihara R, Narahara H. A novel videoendoscopy system by using autofluorescence and reflectance imaging for diagnosis of esophagogastric cancers. Gastrointest Endosc. 2005;62:521-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 96] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Yagi K, Aruga Y, Nakamura A, Sekine A, Umezu H. The study of dynamic chemical magnifying endoscopy in gastric neoplasia. Gastrointest Endosc. 2005;62:963-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Kuznetsov K, Lambert R, Rey JF. Narrow-band imaging: potential and limitations. Endoscopy. 2006;38:76-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 153] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 12. | Tanaka K, Toyoda H, Kadowaki S, Kosaka R, Shiraishi T, Imoto I, Shiku H, Adachi Y. Features of early gastric cancer and gastric adenoma by enhanced-magnification endoscopy. J Gastroenterol. 2006;41:332-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Kawahara Y, Takenaka R, Okada H, Kawano S, Inoue M, Tsuzuki T, Tanioka D, Hori K, Yamamoto K. Novel chromoendoscopic method using an acetic acid-indigocarmine mixture for diagnostic accuracy in delineating the margin of early gastric cancers. Dig Endosc. 2009;21:14-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Takenaka R, Kawahara Y, Kono Y, Yamasaki Y, Kawai D, Takemoto K, Taira A, Tsugeno H, Fujiki S. Reddish color change in AIM-chromoendoscopy in patients with early gastric cancer. Gastrointest Endosc. 2013;77:AB285. |
| 15. | Jung MK, Jeon SW, Park SY, Cho CM, Tak WY, Kweon YO, Kim SK, Choi YH, Bae HI. Endoscopic characteristics of gastric adenomas suggesting carcinomatous transformation. Surg Endosc. 2008;22:2705-2711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2390] [Cited by in RCA: 2872] [Article Influence: 205.1] [Reference Citation Analysis (0)] |
| 17. | Park DI, Rhee PL, Kim JE, Hyun JG, Kim YH, Son HJ, Kim JJ, Paik SW, Rhee JC, Choi KW. Risk factors suggesting malignant transformation of gastric adenoma: univariate and multivariate analysis. Endoscopy. 2001;33:501-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Habu H, Takeshita K, Sunagawa M, Endo M. Lymph node metastasis in early gastric cancer. Int Surg. 1986;71:244-247. [PubMed] |
| 19. | Hakim NS, Sarr MG, van Heerden JA. Does endoscopy really help the surgeon evaluate gastric cancer? Can J Surg. 1989;32:175-177. [PubMed] |
| 20. | Ichiyoshi Y, Toda T, Minamisono Y, Nagasaki S, Yakeishi Y, Sugimachi K. Recurrence in early gastric cancer. Surgery. 1990;107:489-495. [PubMed] |
| 21. | Palli D, Bianchi S, Cipriani F, Duca P, Amorosi A, Avellini C, Russo A, Saragoni A, Todde P, Valdes E. Reproducibility of histologic classification of gastric cancer. Br J Cancer. 1991;63:765-768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Hansson LE, Lindgren A, Nyrén O. Can endoscopic biopsy specimens be used for reliable Laurén classification of gastric cancer? Scand J Gastroenterol. 1996;31:711-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Namieno T, Koito K, Higashi T, Shimamura T, Yamashita K, Sato N, Kondo Y. Assessing the suitability of gastric carcinoma for limited resection: histologic differentiation of endoscopic biopsy. World J Surg. 1998;22:865-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Ming SC, Goldman H. Gastric polyps; a histogenetic classification and its relation to carcinoma. Cancer. 1965;18:721-726. [PubMed] |
| 25. | Tomasulo J. Gastric polyps. Histologic types and their relationship to gastric carcinoma. Cancer. 1971;27:1346-1355. [PubMed] |
| 26. | Kitoh T, Yanai H, Matsubara Y, Nakamura Y, Okamoto T, Hirano A, Yoshida T, Okita K. Endoscopic findings potentially predictive of gastric cancer in borderline lesions diagnosed by forceps biopsy. Hepatogastroenterology. 2005;52:404-408. [PubMed] |
| 27. | Lambert R, Rey JF, Sankaranarayanan R. Magnification and chromoscopy with the acetic acid test. Endoscopy. 2003;35:437-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 64] [Article Influence: 2.9] [Reference Citation Analysis (0)] |