Published online Apr 28, 2014. doi: 10.3748/wjg.v20.i16.4817
Revised: February 9, 2014
Accepted: March 4, 2014
Published online: April 28, 2014
Processing time: 149 Days and 8.5 Hours
Gastrointestinal stromal tumors (GISTs) usually develop in the stomach and small intestine and only rarely occur at the ampulla of Vater, with only 11 cases reported in the literature. We report a case of a GIST of the ampulla of Vater. A 36-year-old, previously healthy man presented with a loss of consciousness lasting a few minutes. A gastroduodenal endoscopy revealed a submucosal tumor with central ulceration at the ampulla of Vater. The enhanced computed tomography scan revealed a smooth-outlined hypervascular solid mass (24 mm × 30 mm) in the second part of the duodenum. Neither lymphadenopathy nor metastasis was observed. Magnetic resonance cholangiopancreatography and endoscopic retrograde cholangiopancreatography showed normal bile and pancreatic ducts. Biopsies were collected from the ulcerative lesion, and the tumor was diagnosed as a GIST. A submucosal tumor with central ulceration may be a characteristic form of GISTs of the ampulla of Vater, and biopsy studies are useful for the diagnosing such tumors. The patient underwent pancreatoduodenectomy, and the operative specimen revealed a 2.2-cm GIST with 1 mitosis per 50 high-power fields. The gold standard for treatment of GISTs is surgical resection without rupture of a capsule. If technically possible, local resection may be considered. However, when the location of the lesion presents challenges, a pancreatoduodenectomy should be performed for GIST of the ampulla of Vater.
Core tip: Gastrointestinal stromal tumor (GIST) usually develops in the stomach and small intestine, and GIST of the ampulla of Vater is extremely rare, with only 11 cases reported in the literature. We report a case of GIST of the ampulla of Vater in a 36-year-old, previously healthy man who presented with a brief loss of consciousness. A gastroduodenal endoscopy revealed a submucosal tumor with central ulceration at the ampulla of Vater. Biopsies were collected from the ulcerative lesion, and the tumor was diagnosed as a GIST. The patient underwent pancreatoduodenectomy. The operative specimen revealed a 2.2-cm GIST with 1 mitosis per 50 high-power fields.
- Citation: Kobayashi M, Hirata N, Nakaji S, Shiratori T, Fujii H, Ishii E. Gastrointestinal stromal tumor of the ampulla of Vater: A case report. World J Gastroenterol 2014; 20(16): 4817-4821
- URL: https://www.wjgnet.com/1007-9327/full/v20/i16/4817.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i16.4817
The ampulla of Vater can exhibit a variety of neoplasms, such as carcinoma, adenoma, neuroendocrine tumor, gangliocytic paraganglioma and gastrointestinal stromal tumor (GIST). GISTs are mesenchymal tumors of the gastrointestinal tract that express the tyrosine kinase receptor and originate from the interstitial cells of Cajal. The majority of GISTs are located in the stomach (60%-70%) and the small intestine (20%-25%), with only 4% occurring in the duodenum[1,2]. Cases of GIST affecting the ampulla of Vater are extremely rare, with only eleven cases described in the literature[1-11]. We report a case of GIST of the ampulla of Vater that was discovered because of loss of consciousness.
A 36-year-old, previously healthy man presented with loss of consciousness lasting a few minutes. On admission, his blood pressure was 116/59 mmHg, heart rate was 85 beats/min, and temperature was 36.5 °C. A physical examination revealed mild anemia of the palpebral conjunctivae. The peripheral blood cell count indicated anemia with a hematocrit of 24.5%. The liver and renal function tests were normal. The levels of serum tumor markers, including carcinoembryonic antigen and carbohydrate antigen 19-9, were within normal limits.
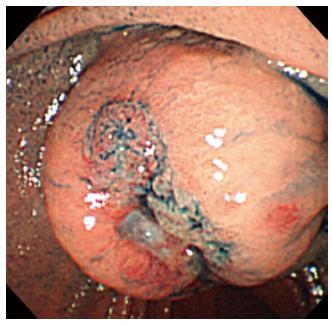
A gastroduodenal endoscopy showed a submucosal tumor with central ulceration at the ampulla of Vater (Figure 1). Because there were small amounts of blood around the tumor, we considered that the patient had lost consciousness because of the bleeding from the tumor, which had already ceased spontaneously.
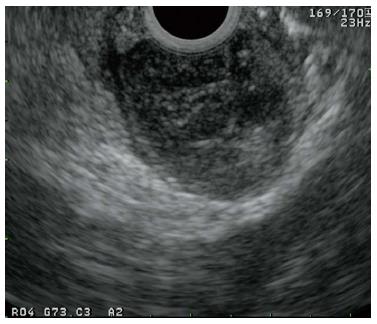
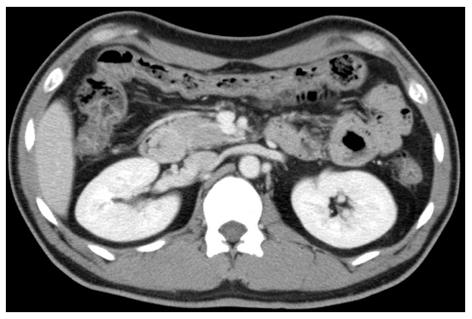
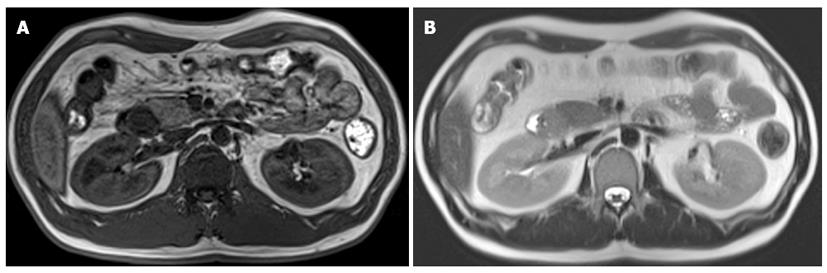
Endoscopic ultrasonography demonstrated a round, low-echoic mass originating from the muscularis propria (Figure 2). The enhanced computed tomography (CT) scan revealed a smooth-outlined hypervascular solid mass (24 mm × 30 mm) in the second part of the duodenum. Neither lymphadenopathy nor metastasis was observed (Figure 3). We additionally performed magnetic resonance imaging (MRI) and endoscopic retrograde cholangiopancreatography (ERCP) to obtain more information about the tumor, especially the relative position of the mass and the biliopancreatic duct because the tumor was located at the ampulla of Vater. On MRI, the mass showed low signal intensity on T1-weighted images and high signal intensity on T2-weighted images (Figure 4). Magnetic resonance cholangiopancreatography and ERCP showed normal bile and pancreatic ducts.
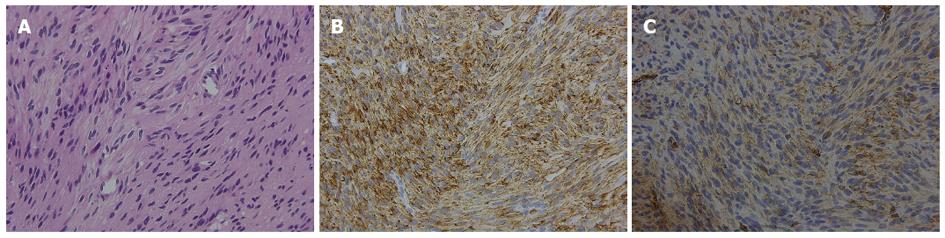
Biopsies were collected from the ulcerating lesion. The microscopic examination revealed a spindle-cell neoplasm, with the tumor cells positive for c-kit and CD34 (Figure 5). Thus, the tumor was diagnosed as a GIST.
The patient then underwent pancreatoduodenectomy. The operative specimen revealed a 2.2-cm GIST with 1 mitosis per 50 high-power fields, which classified the patient in the low-risk group, according to the National Institutes of Health (NIH) consensus criteria for risk stratification of GISTs. There was no lymph node metastasis. The patient was discharged 18 days after an uneventful postoperative course and has been doing well, with no recurrence, during the one-and-a-half years since the operation.
GISTs are mesenchymal tumors of the gastrointestinal tract that express the tyrosine kinase receptor and originate from the interstitial cells of Cajal. The majority of GISTs are located in the stomach (60%-70%) and the small intestine (20%-25%), and only 4% of GISTs occur in the duodenum[1,2]. GIST of the ampulla of Vater is extremely rare. There are only eleven cases described in the literature according to a Medline search (Table 1) using the key words “ampulla of Vater” and “gastrointestinal stromal tumor”. The patients in the published reports were seven men and four women between 37 and 83 years of age. Our case was the youngest among these. Almost all cases exhibited certain symptoms, such as abdominal pain, jaundice, and melena. The tumor size ranged from 2.6-9 cm. Eight of the eleven cases showed a submucosal tumor with central ulceration, which may be a characteristic form of GISTs of the ampulla of Vater. In six cases, the diagnosis of GIST was made by the study of biopsies collected endoscopically from the ulcerating lesions. Although GISTs are submucosal tumors, biopsy studies are useful for GIST of the ampulla of Vater because the tumor cells are exposed at the ulcerating lesions.
| Age (yr) | Gender | Symptoms | Size (cm) | Biopsy | Surgery | Ref. | Year |
| 83 | Female | - | 3.2 | - | PD | [11] | 2012 |
| 59 | Male | Jaundice | 7.6 | GIST | PD | [10] | 2010 |
| 55 | Female | Epigastric pain | 4.5 | - | PD | [9] | 2009 |
| 57 | Male | Melena | 2.6 | GIST | Local resection | [8] | 2007 |
| 69 | Male | Melena | 3.0 | Negative | PD | [7] | 2007 |
| 44 | Male | Weight loss | 9.0 | GIST | PD | [6] | 2007 |
| 65 | Female | Jaundice | 6.0 | GIST | Local resection | [5] | 2006 |
| 44 | Male | Jaundice | 8.0 | Mesenchymal tumor | - | [2] | 2005 |
| 68 | Female | Weight loss | 4.5 | - | PD | [4] | 2004 |
| 37 | Female | Melena | 5.5 | GIST | PD | [1] | 2004 |
| 77 | Male | Pallor | 4.0 | GIST | PD | [3] | 2001 |
The differential diagnosis may include neuroendocrine tumor, gangliocytic paraganglioma, and intra-ampullary-type carcinoma, and there are also a few case reports of leiomyoma and leiomyosarcoma. In cases of neuroendocrine tumor, gastroduodenal endoscopy typically reveals a yellowish submucosal tumor with dilated vessels and erosion on the surface. Endoscopic ultrasonography demonstrates a low-echoic mass originating from the second/third layer. The diagnostic rate of biopsy is as low as 14%[12], and some reported cases were diagnosed by endoscopic ultrasonography fine needle aspiration[13]. In cases of gangliocytic paraganglioma, gastroduodenal endoscopy reveals a submucosal tumor with erosion and ulceration on the surface, located near the ampulla of Vater. By endoscopic ultrasonography, the tumor can typically be visualized as well circumscribed, located in the submucosal layer, and involving the muscularis propria. In cases of intra-ampullary-type carcinoma, gastroduodenal endoscopy reveals an enlarged papilla, and endoscopic ultrasonography shows an irregularly shaped low-echoic mass. Given these characteristics, a diagnosis can be reached by performing gastroduodenal endoscopy and endoscopic ultrasonography.
We cite this case as GIST of the ampulla of Vater because the GIST was macroscopically located at the ampulla of Vater. By definition, the ampulla of Vater is a field that is anatomically surrounded by the sphincter of Oddi. Therefore, GISTs of the ampulla of Vater should arise from the sphincter of Oddi based on the anatomical definition. In the case presented in this report, the GIST pathologically arose from the duodenal muscularis; of the reported ten cases, none of the reports mentioned the sphincter of Oddi. It is noteworthy to consider whether GISTs arising from the sphincter of Oddi actually exist. When GISTs of the ampulla of Vater are encountered, the relationship between the GISTs and the sphincter of Oddi is important to assess.
Among the ten patients who underwent surgery, eight cases involved pancreatoduodenectomy, and two were local resections. GISTs rarely metastasize to regional lymph nodes. There is only one reported GIST case with lymph nodes metastasis, and this particular patient also had liver metastasis[2]. The gold standard for GIST treatment is surgical resection without rupture of the tumor capsule. If technically feasible, local resection may be considered. However, when the location of the lesion presents associated difficulties, a pancreatoduodenectomy should be performed for GIST of the ampulla of Vater.
In general, adjuvant therapy with a tyrosine kinase inhibitor is recommended for patients with high-risk tumors, i.e., a tumor size > 10 cm, mitotic count > 10/50 HPF, and tumor rupture. However, no clear consensus exists regarding the cutoff that should be used to select patients for adjuvant therapy. In our case, the patient underwent no adjuvant therapy because he was in the low-risk group according to the NIH consensus criteria for risk stratification of GISTs. However, GISTs located at nongastric sites are associated with less favorable outcomes than are stomach GISTs. The patient in this case report has been doing well without recurrence during the one-and-a-half years since surgery, and we will continue to monitor him with a strict follow-up schedule.
A 36-year-old, previously healthy man presented with a loss of consciousness lasting a few minutes.
Physical examination showed mild anemia of the palpebral conjunctivae.
Gastrointestinal bleeding.
The peripheral blood cell count showed anemia with a hematocrit of 24.5%; the liver and renal function tests were normal.
Gastroduodenal endoscopy showed a submucosal tumor with central ulceration at the ampulla of Vater.
Biopsies collected from the ulcerating lesion revealed a spindle-cell neoplasm and tumor cell positivity for c-kit and CD34.
The patient underwent pancreatoduodenectomy.
Gastrointestinal stromal tumor (GIST) of the ampulla of Vater is extremely rare, with only 11 cases reported in the literature.
This case report illustrates one of the rare causes of gastrointestinal bleeding; moreover, GIST of the ampulla of Vater can cause certain symptoms, such as abdominal pain, jaundice, and melena.
This article reports a rare case of a GIST of the ampulla of Vater.
P- Reviewers: Le Bian AZ, Worni M S- Editor: Wen LL L- Editor: A E- Editor: Wu HL
| 1. | Kim SH, Kim JH, Baik GH, Baek I, Hahn T, Oh SO, Kim JB, Park SH, Chang WK, Kim DJ. Malignant gastrointestinal stromal tumor of the ampulla of Vater: a case report. Korean J Gastroenterol. 2004;43:66-70. [PubMed] |
| 2. | Matsushita M, Kobayashi Y, Kobayashi H, Nagasawa M, Sato Y, Nakamura H. A case of gastrointestinal stromal tumour of the ampulla of Vater. Dig Liver Dis. 2005;37:275-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Takahashi Y, Noguchi T, Takeno S, Uchida Y, Shimoda H, Yokoyama S. Gastrointestinal stromal tumor of the duodenal ampulla: report of a case. Surg Today. 2001;31:722-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Wellmann K, Gohla G, Wenk H. [Malignant gastrointestinal stromal tumor (GIST) of the papilla vateri. A rare tumor entity]. Chirurg. 2004;75:196-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Filippou DK, Pashalidis N, Skandalakis P, Rizos S. Malignant gastrointestinal stromal tumor of the ampulla of Vater presenting with obstructive jaundice. J Postgrad Med. 2006;52:204-206. [PubMed] |
| 6. | Koçer NE, Kayaselçuk F, Calişkan K, Ulusan S. Synchronous GIST with osteoclast-like giant cells and a well-differentiated neuroendocrine tumor in Ampula Vateri: coexistence of two extremely rare entities. Pathol Res Pract. 2007;203:667-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Moss AC, Callery MP, Falchuk KR. Gastrointestinal [corrected] stromal tumor of the ampulla of vater mimicking a duodenal ulcer. Clin Gastroenterol Hepatol. 2007;5:A26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Singhal D, Kumar M, Sud R, Chaudhary A. Image of the month. Gastrointestinal Stromal Tumor of the Ampulla of Vater. Arch Surg. 2007;142:899-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Jerraya H, Bel Haj Salah R, Ben Mena K, Haouet K, Zaouche A. Stromal tumor of the ampulla of vater: report of a case and systematic review of reported cases. Tunis Med. 2009;87:556-559. [PubMed] |
| 10. | Park JE, Dong SH, Cho KH, Jang JY, Kim HJ, Kim BH, Chang YW, Chang R. [Successful resection of locally advanced gastrointestinal stromal tumor of the ampulla of Vater after treatment with imatinib]. Korean J Gastroenterol. 2010;56:39-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Brauner E, Kuten J, Ben-Ishay O, Hershkovitz D, Kluger Y. Gastrointestinal stromal tumor of the ampulla of Vater. Isr Med Assoc J. 2012;14:588-589. [PubMed] |
| 12. | Hartel M, Wente MN, Sido B, Friess H, Büchler MW. Carcinoid of the ampulla of Vater. J Gastroenterol Hepatol. 2005;20:676-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | El Hajj II, El Chafic AH, Cramer H, Al-Haddad M. Ampullary carcinoid tumors diagnosed by endoscopic ultrasound-guided fine needle aspiration in two patients with biliary and pancreatic duct obstruction. Endoscopy. 2011;43 Suppl 2 UCTN:E422-E423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |