Published online Apr 28, 2014. doi: 10.3748/wjg.v20.i16.4662
Revised: January 19, 2014
Accepted: February 17, 2014
Published online: April 28, 2014
Processing time: 157 Days and 13.3 Hours
AIM: To investigate the protective effect of glutamine (Gln) on intestinal injury and the bacterial community in rats exposed to hypobaric hypoxia environment.
METHODS: Sprague-Dawley rats were divided into control, hypobaric hypoxia (HH), and hypobaric hypoxia + Gln (5.0 g/kg BW·d) (HG) groups. On the first 3 d, all rats were placed in a normal environment. After the third day, the HH and HG groups were transferred into a hypobaric chamber at a simulated elevation of 7000 m for 5 d. The rats in the HG group were given Gln by gavage daily for 8 d. The rats in the control and HH groups were treated with the same volume of saline. The intestinal morphology, serum levels of malondialdehyde (MDA), superoxide dismutase (SOD), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), interferon-gamma (IFN-γ) and diamino oxidase (DAO) were examined. We also evaluated the expression levels of occludin, toll-like receptor 4 (TLR4), nuclear factor-κB p65 (NF-κB p65) and myeloid differentiation factor 88 (MyD88), and examined the bacterial community in caecal contents.
RESULTS: Hypobaric hypoxia induced the enlargement of the heart, liver, lung and kidney, and caused spleen atrophy. Intestinal villi damage was also observed in the HH group. Supplementation with Gln significantly alleviated hypobaric-induced damage to main organs including the intestine, increased serum SOD (1.14 ± 0.03 vs 0.88 ± 0.04, P < 0.05) and MDA (8.35 ± 1.60, P < 0.01) levels and decreased serum IL-6 (1172.13±30.49 vs 1407.05 ± 34.36, P < 0.05), TNF-α (77.46 ± 0.78 vs 123.70 ± 3.03, P < 0.001), IFN-γ (1355.42 ± 72.80 vs 1830.16 ± 42.07, P < 0.01) and DAO (629.30 ± 9.15 vs 524.10 ± 13.34, P < 0.001) levels. Moreover, Gln significantly increased occludin (0.72 ± 0.05 vs 0.09 ± 0.01, P < 0.001), TLR4 (0.15 ± 0.05 vs 0.30 ±0.09, P < 0.05), MyD88 (0.32 ± 0.08 vs 0.71 ± 0.06, P < 0.01), and NF-κB p65 (0.16 ± 0.04 vs 0.44 ± 0.03, P < 0.01) expression levels and improved the intestinal bacterial community.
CONCLUSION: Gln treatment protects from intestinal injury and regulates the gut flora imbalance in hypoxia environment. These effects may be related to the TLR4/MyD88/NF-κB signaling pathway.
Core tip: Gastrointestinal problems at high altitudes are common. Gut microbes may also play an important role in host health. Glutamine has been demonstrated to be an important source of fuel for the gut. In the study, we investigated the protective effect of glutamine on intestinal barrier damage induced by hypobaric hypoxia. The research provides a basic understanding of possible mechanism of hypobaric hypoxia-induced damage of intestinal barrier function and bacterial community imbalance. The altered bacterial communities in the intestine and the toll-like receptor 4/myeloid differentiation factor 88/nuclear factor-κB signal pathway may represent the significant therapeutic targets for the prevention/treatment of intestinal barrier dysfunction and consequent intestinal diseases.
- Citation: Xu CL, Sun R, Qiao XJ, Xu CC, Shang XY, Niu WN. Protective effect of glutamine on intestinal injury and bacterial community in rats exposed to hypobaric hypoxia environment. World J Gastroenterol 2014; 20(16): 4662-4674
- URL: https://www.wjgnet.com/1007-9327/full/v20/i16/4662.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i16.4662
High altitudes create a special type of environment because the atmospheric pressure is lower than it is at sea level. However, more than 140 million people permanently live at a high altitude (> 2500 m) in North America, Central America, South America, East Africa, and Asia. Furthermore, every year several hundred thousand people from lowland areas move to higher altitudes for work or travel. High altitude hypoxia is a challenge for people residing in or visiting high altitudes. The exposure to high altitude causes severe damage to different organs, especially the intestinal tract. The incidence of digestive system disease is reported to be higher among high-altitude residents and immigrants[1,2]. The primary function of the intestinal tract is to regulate water, electrolyte and nutrient transport. To perform these functions, the epithelium lining the intestinal tract is in close contact with the gastrointestinal lumen. Because the lumen is connected to the external environment and may have a high bacterial and antigen load, the epithelium must also prevent pathogenic agents within the gastrointestinal lumen from gaining access to internal tissues[3]. Hypoxia may induce severe primary intestinal barrier dysfunction, promote bacterial and endotoxic translocation, and cause systemic inflammatory response; it is the major factor causing high-altitude multiple organ dysfunction syndrome[4]. During studies of the complex physiological function of the intestine, we noted that the intestine is not only an important organ of digestion and nutrient absorption, but also has immunomodulatory, endocrine, and mucosal barrier functions. Intestinal mucosal barrier function is an important part of the barrier system of the body and has been studied by many researchers. It is composed of a mechanical barrier, an immune barrier, a chemical barrier, and a biological barrier. The different structures, molecular mechanisms, and biological functions of each barrier allow them to collectively defend against the invasion of foreign antigens through combined signaling pathways[5]. Although acute hypobaric hypoxia is the most common pattern, studies on this issue are limited.
Glutamine (Gln) has not traditionally been used as a nutritional supplement because it is synthesized endogenously and is considered a “non-essential amino acid”[6]. However, some studies have found that the effect of Gln is far more than that of a “non-essential amino acid”. Gln consumed as a “conditionally essential amino acid” is a special nutrient under physiological conditions and maintains normal immunological function under stress or pathological conditions. Gln plays a significant role in adjusting the cellular metabolism and cellular immune function[7]. Gln is a major source of energy for enterocytes and supports nucleotide biosynthesis. Additionally, Gln may protect epithelial cells against endotoxin/oxidant-related injury and enhance the expression of heat stress proteins following stress in gastrointestinal tract therapy[8]. Long-term treatment with Gln that was started before advanced age prevented the loss of body weight without limiting sarcopenia and had a beneficial effect on enterocytes in very old rats[9]. Currently, the protective effect of Gln on intestinal mucosal barrier function is still unknown under hypobaric hypoxia.
Gut microbes may also play an important role in host health[10]. In the absence of the gut microbiota, normal immune development and function are impaired. Understanding the influence of hypoxia on the composition of the microbial community in the intestine is crucial for regulating the microflora, and will improve gut health. Therefore, the present study was conducted to investigate the unique role of glutamine in the preservation of epithelial barrier function in the gastrointestinal tract of rats exposed to a hypobaric hypoxia environment. We observed the ultrastructure of the duodenum, jejunum, and ileum, evaluated changes in the expression of occludin in the ileum and detected several serum inflammatory mediators. In addition, we investigated the role of the toll-like receptors (TLRs)/myeloid differentiation factor 88 (MyD88)/nuclear factor-κB (NF-κB) signaling pathway in the protective effect of Gln on intestinal barrier damage induced by hypobaric hypoxia. We also analyzed the bacterial community in the intestinal contents.
The main regents used in this study were the following: L-glutamine (Xi’an Guoan, China), MDA kit (Nanjing Jiancheng, China), SOD kit (Nanjing Jiancheng, China), Total protein kit (Nanjing Jiancheng, China), 4% paraformaldehyde (Beijing Dingguo Changsheng Biotechnology Co., Ltd.), rat IL-6, TNF-α, IFN-γ, and DAO enzyme linked immunosorbent assay (ELISA) kits (RD systems, United States), NF-κB p65 and TLR4 rabbit polyclonal antibody, occludin rabbit polyclonal antibody, β-actin mouse polyclonal antibody (Santa Cruz, United States), and MyD88 rabbit polyclonal antibody (Abcam, United States).
This study was approved by the Institutional Animal Care and Use Committee of the Northwestern Polytechnical University and was conducted in accordance with the National Institutes of Health guidelines for the care and use of experimental animals. Thirty adult male Sprague-Dawley (SD) rats (200 ± 20 g) were purchased from the Experimental Animal Center of College of Medicine, Xi’an Jiaotong University.
Thirty male rats were randomly divided into three groups of ten rats each as follows: a normal control group (Control), a hypobaric hypoxia group (HH), and a hypobaric hypoxia plus Gln group (HG). For the first 3 d, all of the rats were placed in a normal environment. During this period, the rats in the HG group were given 5.0 g/kg BW/d Gln by gavage daily. The rats in the control and HH groups received intragastric administration of an equal volume of saline. The rats in HH and HG groups were transferred to a hypobaric chamber (Guizhou Fenglei Aviation Ordnance Co., Ltd, China) simulating an elevation of 7000 m for 5 d. During the hypoxia treatment, all of the rats were treated with saline or Gln as previously described. The chamber altitude was returned to sea level daily for 30 min to clean the cages, replenish the food and water and give drugs. All of the animals had free access to food and water and were weighed daily.
The rats were weighed before gavage every day, and their mental state, spontaneous activity, and eating status were monitored.
Five days after exposure to hypobaric hypoxia, the rats were anesthetized with ether, and the abdomen was opened to collect 5 mL of blood from the abdominal aorta. The blood was centrifuged at 1000 g for 10 min at 4 °C, and the serum was separated and stored in Eppendorf tubes. The general conditions of the rats and overall changes in the abdominal cavity were observed. Additionally, the heart, liver, spleen, lungs and kidney were removed from the rats and weighed. Approximately 5 cm of the duodenum, jejunum and ileum were collected into RNAase-free tubes. Caecal contents were collected and stored in freezing tubes. All of the samples were frozen by immersion in liquid nitrogen and stored at -80 °C until needed for analysis.
The rats were sacrificed after completing the hypoxia. The body weight and weights of the heart, liver, lung, kidney and spleen were determined for each animal. The organ index was calculated as percentage of body weight.
Approximately 2 cm of the duodenum, jejunum and ileum were obtained and cut open longitudinally and transversely. Then each collected intestinal segment was washed with normal saline immediately, fixed in 4% formaldehyde at 4 °C for 24 h, rinsed with phosphate buffered solution (PBS) and embedded in paraffin. The tissue was consecutively cut into 4-μm thick sections that were stained with hematoxylin and eosin (HE). The intestinal morphology was observed using a fluorescence microscope (Nikon, Japan), and the length and area of the intestinal villi were measured and calculated according to the following equation: Area = 2πrh, where r represents the radius of the villus and h is the villus height.
Approximately 0.1 mL serum was used to detect the SOD activity and measure the MDA content. The SOD activity was measured using the hydroxylamine method. The MDA content was measured by the thiobarbutiric acid colorimetric method. The kits were used according to the manufacturer’s instructions. The absorbance was measured at 550 and 532 nm, respectively, using an ultraviolet spectrophotometer (HITACHI, Japan). The activity of SOD was expressed as units per ml. The MDA content was calculated using the following formula: (nmol/mL) = [A(sample) - A(sample blank)]/[A(standard) - A(standard blank)], where A represents the absorbance value.
The levels of IL-6, TNF-α, IFN-γ, and DAO in the serum were detected using commercially available ELISA kits according to the manufacturer’s recommended protocol. A Synergy HT Multi-Detection Microplate Reader (Bio-Tek) was used to read the optical density at 450 nm. The concentrations of IL-6, TNF-α, IFN-γ, and DAO in the samples were determined using a standard curve.
Ileum mucosal tissues (100 mg) were homogenized in 1 mL lysis buffer (Sangon Biotech, China). The total protein was extracted with a Protein Extract Kit (Beyotime, China) according to the manufacturer’s instructions. The protein concentration was measured via a bicinchoninic acid assay using a BioRad protein microassay (BioRad, Hercules, CA). An aliquot containing 30 μg of protein was diluted in loading buffer (loading buffer: sample = 5:1, v/v) and heated to 98 °C for 10 min. The protein sample was separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The protein was transferred onto a 0.45 μm-pore polyvinylidene difluoride membrane (PVDF, Immuno-Blot, BioRad) at 100 V for 1 h. The membranes were blocked at room temperature for 2 h with 5% fat-free milk in PBS-T (PBS with 0.1% Tween-20). The following primary antibodies were used: NF-κB p65 (1:500), TLR4 (1:1000), MyD88 (1:500), occludin (1:500), and β-actin (1:1000). The primary antibodies were incubated at 4 °C overnight. After washing with PBS-T (0.1% BSA), the membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary anti-rabbit antibody (diluted 1:3000; Boster Co., Wuhan, China) for 2 h at room temperature. After additional washing, bound conjugates were detected with ECL SuperSignalTM West Pico substrate (Piece, Rockford, IL, United States). The proteins were visualized by exposing the blot to an X-ray film and were photographed with a digital camera. The net intensities of individual bands were measured using Quantity One (version 4.6.2). The relative expression levels of the proteins were expressed as the gray value of the target band over the gray value of β-actin in the same sample.
Genomic DNA in caecal contents was extracted using the E.N.Z.A. ® DNA Kit (Omega Bio-Tek) according to the manufacture’s protocol with a slight modification, then identified by 1% agarose gel electrophoresis. DNA purity and concentration were analyzed using the ultraviolet spectrophotometer (HITACHI, Japan). According to the specific sequence region (533R-27F) in the 16S rRNA gene that covering the V1-V3 region, the bar-coded primers 27F and 533R containing the A and B sequencing adaptors were synthesized and used to amplify this region. The forward primer (B-27F) was 5’-CCTATCCCCTGTGTGCCTTGGCAGTCTCAGAGAGTTTGATCCTGGCTCAG-3’, where the sequence of the B adaptor is shown in italics and underlined. The reverse primer (A-533R) was 5’-CCATCTCATCCCTGCGTGTCTCCGACTCAGNNNNNNNNNNTTACCGCGGCTGCTGGCAC-3’, where the sequence of the A adaptor is shown in italics and underlined and the series of Ns represent an eight-base sample specific barcode sequence. The identified DNA was subjected to polymerase chain reaction (PCR) using TranStartFastpfu DNA Polymerase (MBI. Fermentas, United States) in a 20 μL volume containing 5 mmol each of the primer, 10 ng of template DNA, 5 × FastPfu Buffer, and 1 U of FastPfu DNA Polymerase. PCR was performed in a thermocycler (Gene Amp® PCR System 9700, ABI, United States). The PCR profile included denaturation at 95 °C for 2 min, followed by 25 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s, and extension at 72 °C for 30 s, and a final extension at 72 °C for 5 min. Triplicate PCR products of the same sample were mixed, and then detected by 2% agarose gels electrophoresis containing ethidium bromide. PCR products were recycled and purified with an AxyPreDNA gel extraction kit (Axygen, China) according to the manufacturer’s instructions. The recycled PCR products were visualized on agarose gels. Furthermore, the PCR products were quantitatively determined using QuantiFluorTM-ST Fluoremeter (Promega, United States) and PicoGreen® dsDNA Quantitation Reagent (Invitrogen, Germany). Following quantitation, the amplification products from each reaction mixture were pooled in equimolar ratios based on their concentrations and were subjected to emulsion PCR (emPCR) using RocheGS FLX Titanium emPCR kits to generate amplification libraries. Amplification pyrosequencing was performed from the A-end using a 454/Roche A sequencing primer kit on a Roche Genome Sequencer GS FLX Titanium platform at Majorbio Bio-Pharm Technology Co., Ltd., Shanghai, China.
Most of the data except for the bacterial community analysis are presented as mean ± SEM. The data were analyzed by one-way analysis of variance and Student’s t-test (version 9.1, SAS, NC, United States). Differences were considered to be statistically significant at P < 0.05. The pyrosequencing data were subjected to bioinformatic analysis. Prior to analysis, the original pyrosequencing data must be filtered and optimized to obtain the valid and trimed sequences through Seqcln and Mothur (http://sourceforge.net/projects/seqclean/http://www.mothur.org/wiki/Main_Page). Then, these trimed sequences were analyzed from two aspects: operational taxonomic units (OTUs) cluster (97% similarity) and taxonomy which were mainly performed on Mothur (http://www.mothur.org) and compared with the Bacterial SILVA database (http://www.arb-silva.de/), by methods of kmer searching (http://www.mothur.org/wiki/Align.seqs) and UCHIME (http://drive5.com/uchime). Rarefaction analysis and Good’s coverage for the nine libraries were determined. Community figure was generated using R tools according to the data from document “tax.phylum.xls”. Heatmap figure was generated using Vegan-package (distance measure with Bray-Curtis; cluster analysis with complete).
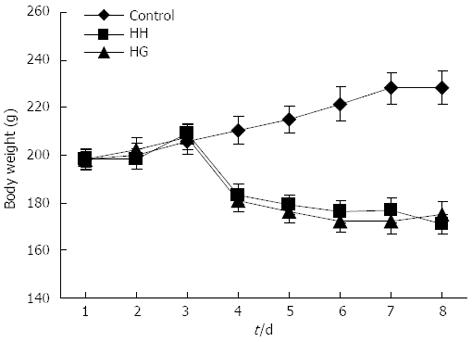
There were no animal deaths during the experiment. The rats in the control group were active and energetic and had no pathological reaction in the abdominal cavity that was visible to the naked eye. The rats in the HH and HG groups were less active and were notably more tired. Prominent swelling was observed in their intestinal canals, and the intestinal mucosa was congested. While in the hypobaric chamber, the food intake was significantly lower in the HH and HG groups than in the control group. Thus, reduced body weight was observed in all rats in the groups exposed to hypobaric hypoxia (Figure 1). On the fifth day of exposure to the hypobaric chamber, the body weights of the rats in group HG increased and were higher than those in the HH group. The eyes of the rats from the HH and HG groups were reddish brown. Table 1 showed that the heart, liver, kidney, and lung indices in the HH group were significantly increased compared to the control group (P < 0.01; P < 0.05; P < 0.05; P < 0.001, respectively). However, the spleen index in the HH group decreased compared to the control group (P < 0.05).
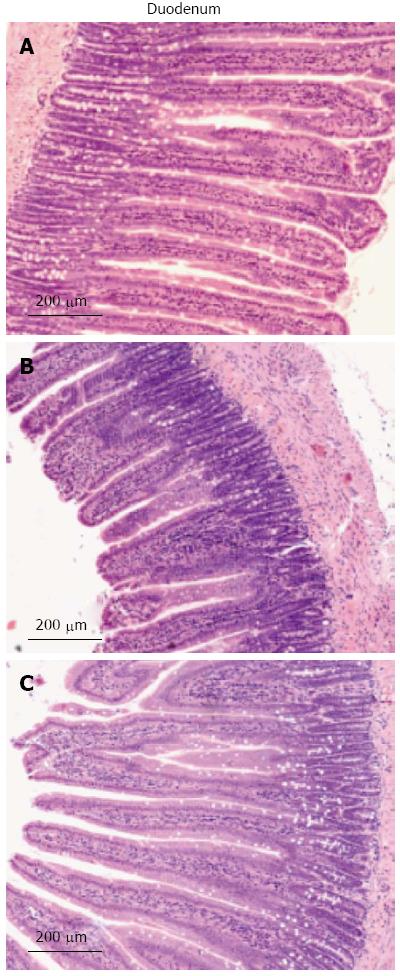
The intestinal villi with intact epithelia were dense and long and showed an ordered arrangement in the control group. Additionally, the intestinal mucosa was smooth and thick. There were no detected defects in the intestinal mucosa and villi using a light microscope (Figure 2). The intestinal villi in the HH group were sparse, short, and defective and had a scattered arrangement. In addition, the lodged and exfoliated villi became thinner than those in the control group. The tight junctions between the intestinal epithelial cells were widened. The intestinal mucosa was exfoliated and showed signs of atrophy. Compared with the HH group, the intestinal villi in the HG group were relatively intact, long, and dense and showed an orderly arrangement. A statistical analysis showed that the height of the intestinal villi (P < 0.001), the thickness of the mucosa (P < 0.001), and the villi area (P < 0.001) were significantly decreased in the HH group as compared to the control group (Table 2). Treatment with Gln alleviated the damage to the intestine morphology and structure in rats exposed to hypobaric hypoxia.
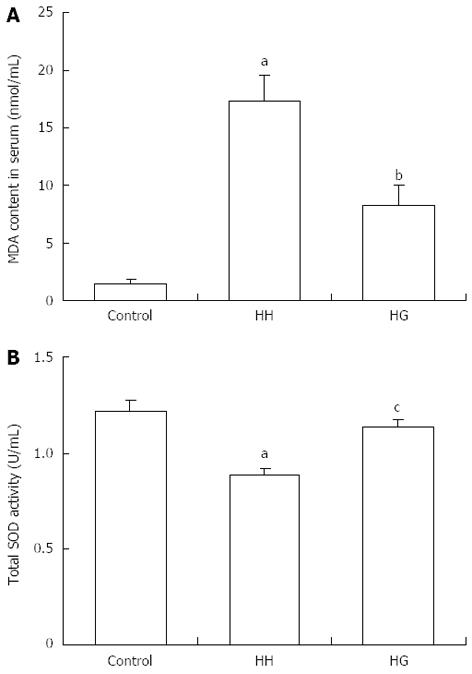
The total serum total SOD activity and MDA concentration in the HH group were significantly lower than those in the control group (P < 0.001) (Figure 3). However, supplementation with Gln significantly increased (P < 0.05) the total SOD activity and decreased (P < 0.01) the MDA concentration in serum compared to the HH group. The HH group values were significantly increased compared to the control group.
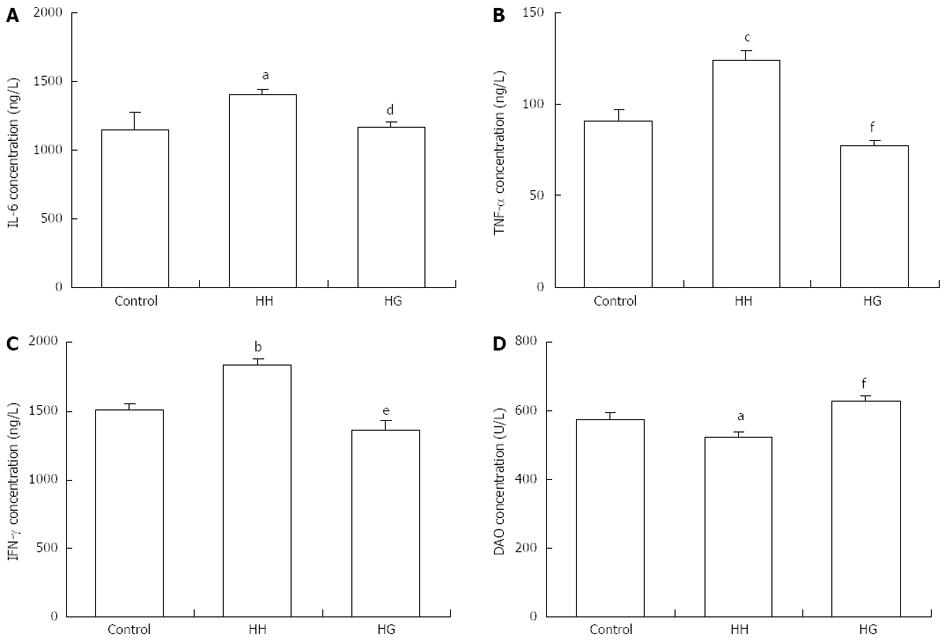
As shown in Figure 4, serum levels of IL-6 (P < 0.05), TNF-α (P < 0.001), and IFN-γ (P < 0.01) in the HH group were significantly higher than those in the control group. This result suggests the presence of hypoxia-induced inflammatory response. The levels of IL-6 (P < 0.05), TNF-α (P < 0.001), and IFN-γ (P < 0.01) in the serum of rats from the HG group were lower than those from the HH group. Moreover, serum DAO levels in the HH and HG groups were lower than those in the control group (P < 0.05). The administration of Gln significantly increased serum DAO levels compared to the HH group (P < 0.001). These results suggested that supplementation with Gln reversed hypoxia-induced increases of inflammatory mediators.
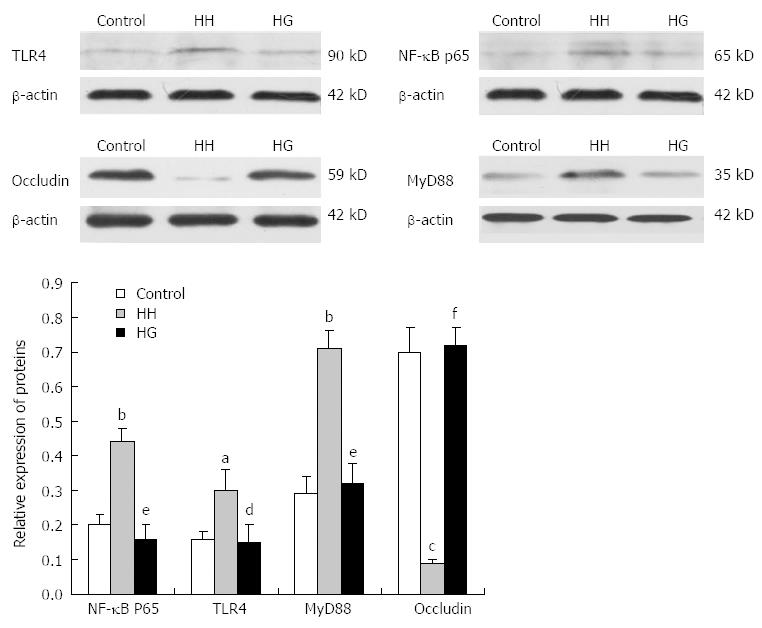
Western blotting analysis (Figure 5) showed that the expression levels of NF-κB p65 (P < 0.01), MyD88 (P < 0.01) and TLR4 (P < 0.05) in the HH group were higher than those in the control group. Supplementation with Gln significantly decreased the expression levels of NF-κB p65 (P < 0.01), MyD88 (P < 0.01), and TLR4 (P < 0.05) compared with the HH group. However, the influence of hypobaric hypoxia on occludin expression was contrary to the expression of NF-κB p65, MyD88 and TLR4. The expression level of occludin in the HH group was decreased significantly as compared to the control group. The supplementation with Gln rescued the hypoxia-induced reduction of occludin, and the HG group showed increased occludin expression.
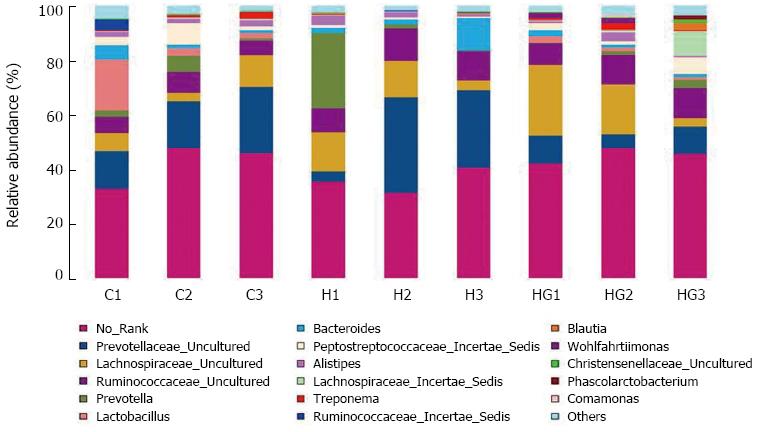
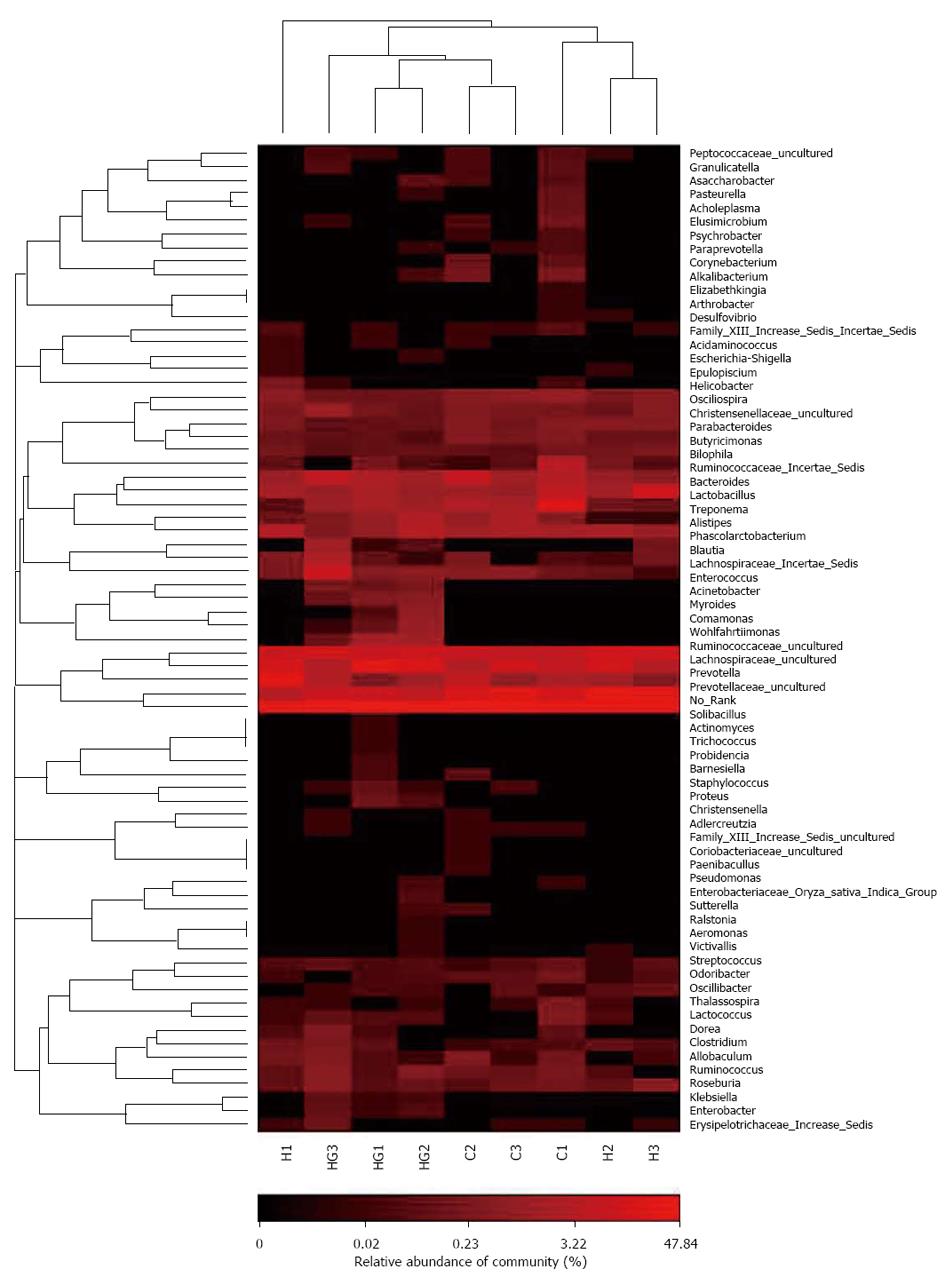
A total of 80521 valid reads and 9679 OTUs were obtained from the nine samples through 454 pyrosequencing analysis. The rarefaction curves tended to approach the saturation plateau (data not shown). Good’s coverage estimations revealed that 92%-95% of the species were obtained in all of the samples. All of the sequences were classified from phylum to genus according to the program Mothur using the default setting, and 14 different genus groups were identified from these samples. The nine libraries showed very dissimilar 16S rRNA profiles at the genus level distribution (Figure 6). The HG libraries included the maximum number of genera and included the following: No_Rank, Prevotellaceae_uncultured, Prevotella, Lactobacillus, Lachnospiraceae_uncultured, Ruminococcaceae_uncultured, Bacteroides, Peptostreptococcaceae_Incertae_Sedis, Alistipes, Lachnospiraceae_Incertae_Sedis, Treponema, Ruminococcus, and Wohlfahritiimonas. These were the most important groups and accounted for 96% of the reads. The HH libraries showed relatively simple diversity and contained the lowest number of Lactobacillus, Peptostreptococcaceae_Incertae_Sedis and Treponema, and the highest number of Prevotellaceae_uncultured and Prevotella. Compared with the other two groups, the numbers of Acinetobacter, Comamonas, Enterobacter, and Enterococcus Wohlfahrtiimonas in the HG libraries were high. A hierarchically clustered heatmap analysis based on the bacterial community profiles at the family level indicated that the HH samples clustered with the control and HG samples in order (Figure 7).
Gastrointestinal problems at high altitudes are common. Special geological and climatic environments might cause the decrease of body resistance and the increase of susceptibility to intestinal diseases observed for humans or animals exposed to high altitudes. There were currently no effective measures to prevent or treat intestinal diseases. In the current study, the body weight of rats decreased after exposure to hypobaric hypoxia at a stimulated elevation of 7000 m for 5 d. However, if we supplemented the rats with Gln daily for eight consecutive days, including three days before entry into hypobaric chamber, the body weight of the Gln-treated rats recovered on the eighth day. Moreover, hypobaric hypoxia induced the enlargement of the heart, liver, kidney, and lung, and caused spleen atrophy. However, supplementation with exogenous glutamine effectively alleviated the occurrence of the above pathological phenomenon. We know that Gln as a conditionally essential amino acid, has many roles in the human body. Gln may enhance immune function in individuals who are critically ill and immune suppressed, prevent infection in postsurgical patients, and support the integrity of the gut mucosa after intestinal damage. In the presence of critical illness and catabolic stress, the body’s glutamine consumption exceeds the normal supply. Thus, Gln becomes an “essential” amino acid[11,12].
The intestinal mucosa actively participates in host defense by engaging the mucosal immune system[3]. However, the intestinal mucosa and villi of the rats were seriously injured by the hypobaric hypoxia environment. The villi height and crypt depth of the small intestine were significantly decreased. In addition, the intestinal villi had bizarre shape changes in the form of partial loss, sloughing and vacuolization because of the hypobaric hypoxia environment. The insufficient energy synthesis caused by hypoxia decreases the frequency of cilia swing, slows peristalsis, and inhibits self-cleaning in the intestinal tract. In addition, the blood and oxygen supplies required for the normal functioning of the intestinal mucosa vary greatly under different conditions. The special anatomical structures of the intestinal microvilli were extremely sensitive to hypoxia[13,14]. Hypobaric hypoxic environments can also aggravate damage to the intestinal villi[15]. However, the intestinal mucosa and villi of the rats supplemented with Gln (5.0 g/kg•d) were normal and intact. Gln is a key factor in maintaining mucosal structure and may have special effects on the maintenance of tight junction and permeability of the intestinal mucosa. Oral glutamine may be effective in protecting the human intestinal mucosa[16], enhancing the villi height of the jejunum and ileum[17], preventing jejunal atrophy and mitigating the overall disruption[18].
High altitude is characterized by hypobaric hypoxia, which is considered an acute physiological stress leading to oxidative stress[19]. Oxidative stress describes the steady state level of oxidative damage in a cell, tissue, or organ, caused by the reactive oxygen species (ROS). ROS also triggers lipid peroxidation that is a chain reaction that provides a continuous supply of free radicals by oxidizing the polyunsaturated fatty acids in membranes and causing oxidative cell damage. MDA is formed as an end product of lipid peroxidation and acts as a main marker of endogenous lipid peroxidation[20,21]. In the current study, supplementation with Gln significantly decreased MDA levels in serum. This result indicated that Gln could protect rats from hypoxia-induced lipid peroxidation. Although a variety of mechanisms contribute to protection against ROS-mediated cell and tissue injury, intracellular AOEs, including SOD, are considered to play a major role. SOD catalyzes the conversion of superoxide radical to hydrogen peroxide. In the present study, SOD activities in the serum were substantially increased in rats treated with Gln. Therefore, antioxidant enzymes can alleviate the toxic effects of ROS and limit the effects of oxidant molecules on tissues. The antioxidant enzymes are active in the defense against oxidative cell injury because they are free radical scavengers[22].
Studies have shown that the function of the intestinal barrier may be regulated by a network of multiple cytokines including ILs, IFNs, and TNF-α[23]. An imbalance of pro-inflammatory and anti-inflammatory cytokines is another important mechanism of intestinal mucosal injury. It is well established that hypobaric hypoxia causes mucosal hyperpermeability in vivo. Subsequently, the activity of innate immune cells is increased, and this is associated with the activation of the mucosal immune system[24]. TNF-α, IL-6, and IFN-γ are important inflammatory factors that play important roles in various inflammatory reactions and are highly correlated with the severity of inflammation[25]. INF-γ is a cytokine that is critical for innate and adaptive immunity against viral and bacterial infections[26]. Increases in serum TNF-α, IL-6, and INF-γ after hypobaric hypoxia stimulation were observed in the study. However, treatment with Gln significantly decreased the levels of TNF-α, IL-6, and INF-γ. This result suggests that Gln may improve the permeability of the intestinal mucosa and protect the intestine.
DAO belongs to the class of copper-containing amine oxidases that convert primary amines to corresponding aldehydes, hydrogen peroxide, and ammonia. Human DAO may play an important role in histamine metabolism. Histamine is a potent pharmacological agent with profound biological effects[27]. The levels of serum DAO is a useful marker of intestinal mucosal integrity that indicates the function and structure of the intestine[28]. Gln treatment significantly increased serum DAO concentration. Tight junction (TJ) proteins, including occludin, claudins, and cytoskeleton proteins play a critical role in maintaining the intestinal barrier integrity. Occludin was the first transmembrane protein discovered in the tight junction. Occludin plays a crucial role in the assembly or maintenance of epithelial tight junctions[29]. The absence of occludin increases the ion permeability of TJs[30]. In the present study, hypobaric hypoxia caused a significant decrease in the expression of occludin. This result indicates that hypobaric hypoxia exposure leads to intestinal barrier dysfunction and increased intestinal permeability. However, supplementation with Gln effectively alleviated the decrease of occludin expression levels in rats living in a hypobaric hypoxia environment. Under stress conditions, such as hypobaric hypoxia, the physiological level of Gln is inadequate, and Gln can repair intestinal mucosa damage. Therefore, Gln must be supplemented. Gln is best known for its ability to serve as a source of fuel for cells, such as enterocytes, renal epithelial cells, hepatocytes, neurons, and immune cells[31]. The enteral administration of glutamine stimulates intestinal mucosal protein synthesis, protects enterocytes from apoptosis, and promotes many functional activities of immune cells[32]. Therefore, after Gln treatment lymphocytes can secrete cytokines in the hypobaric hypoxia environment. The cytokines protect intestinal immunity. The synthesis of glutathione, a major endogenous antioxidant in mammalian cells, requires glutamine as a precursor[33]. Gln in combination with N-acetyl cysteine and zinc partially restores the tight junction integrity[33,34], decreases the intestinal mucosal permeability, and maintains the intestinal integrity, similar to occludin. In short, the protective effect of Gln on intestinal mucosa barrier function may be exerted via a variety of mechanisms.
Complex intestinal microbial communities are believed to provide some benefits to their host[35] and are now the focus of many research efforts. There are currently few published reports examining the effect of hypobaric hypoxia on the microbiome. In the present study, there were significant decreases in Lactobacillus, Treponema, and Peptostreptococcaceae_Incertae_Sedis and obvious increases in Prevotellaceae_uncultured and Prevotella in the caecal contents of the HH group. These results suggest that hypoxia may influence the composition of the microbial community in the intestine. The potential pathogens and probiotics are important members of the intestinal microbiota. The numbers of Lactobacillus, Comamonas, Enterobacter, Peptostreptococcaceae_Incertae_Sedis, Acinetobacter, Enterococcus and Wohlfahrtiimonas in the HG libraries were higher than those in the HH library. The data suggest that supplementation with Gln for animals in a hypobaric hypoxia environment improved the microbial community. Changes in the composition and diversity of the bacterial community in the intestine may occur following a breach of the intestinal microfloral barrier, which results from deficiencies in the host immune defense system or damage to the intestinal mucosal barrier. Recent studies have shown that hypoxia alone can damage the function of the gastrointestinal barrier and cause flora imbalance in rats[36]. Supplementation with Gln alleviated intestinal mucosal injury and increased bacterial translocation in rats exposed to high-altitude hypoxia[4,36,37].
Toll-like receptors (TLRs) are a family of pattern-recognition receptors that play a key role in the innate immune system. As a key transmembrane protein closely related to bacterial recognition, TLR4 is thought to be involved in the first immune barrier of the gastrointestinal tract. NF-κB is the final effector molecule of the TLR4 signaling pathway. TLRs trigger a complex signaling cascade involving different adaptor proteins, kinases and transcriptional factors. Thus, various TLRs have been shown to activate both NF-κB and the mitogen-activated protein kinase pathway via MyD88. MyD88 is a common adaptor molecule that is recruited towards the Toll/IL-1 receptor domain of TLRs. NF-κB induces the transcription and translation of inflammatory cytokines and leads to the massive release of inflammatory mediators[38-40]. Locally, these molecules can lead to the apoptosis of intestinal mucosal epithelial cells and damage the tissues and organs of the intestinal tract. TLR4 promotes the proliferation of epithelial cells and inhibits intestinal bacterial translocation[41]. TLR4 might play an important role in recruiting granulocytes after intestinal damage[42]. Our result suggests that hypobaric hypoxia upregulated TLR4 expression and activated the TLR4/MyD88/NF-κB signaling pathway. Moreover, the activation of TLR4/MyD88/NF-κB signaling was consistent with changes in serum levels of TNF-α, IL-6, and IFN-γ and damage to the morphology and structure of the intestinal mucosa. Furthermore, we found that the expression of TLR4/MyD88/NF-κB signaling proteins was inversely correlated with the expression level of occludin under hypobaric hypoxic conditions. Thermal damage to the intestinal permeability increased distant organ injury that was associated with significantly reduced occludin expression and TLR4 activation, but this injury was attenuated in TLR4-deficient mice[43]. In addition, the activation of TLR4 can also alter the cellular localization of occludin[44]. This redistribution of occludin might damage barrier function. Thus, hypobaric hypoxia-induced activation of TLR4/MyD88/NF-κB may influence TJ complexes and eventually cause damage to the intestinal barrier that results in bacterial translocation. Luo et al[37] also found that TLR4 and NF-κB expression was increased in rat intestinal tissues after acute hypoxia exposure. Pyrrolidinedithiocarbamic acid treatment reversed TLR4 and NF-κB upregulation and alleviated the damage to the intestinal tract and bacterial translocation. These results suggest that the TLR4/MyD88/NF-κB signaling pathway may be related to the mechanism of damage in intestinal barrier function and changes in the bacterial community caused by a hypobaric hypoxic environment.
In summary, our results showed that a hypobaric hypoxia environment causes pathological changes in many rat organs including damage to the intestinal villi, increased expression of cytokines, and activation of the TLR4/MyD88/NF-κB signaling pathway. Gln can protect the intestinal mucosal barrier and regulate the diversity and the composition of the intestinal bacterial community. The altered bacterial communities in the intestine and TLR4/MyD88/NF-κB signal pathway may represent significant therapeutic targets for the prevention/treatment of intestinal barrier dysfunction and consequent intestinal diseases.
We are grateful to Wei Li, Chen Dong, Tiaotiao Han, Xiaobo Gao and Sheng Bao for their technical help.
High altitude hypoxia can cause severe damage to different organs including the intestine. Up to now, there are no effective measures to prevent and treat intestinal diseases. The main reason may be that the mechanism of hypobaric hypoxia-induced intestinal barrier function damage even intestinal diseases is unclear. Many studies indicate that glutamine supplementation preserves the gut barrier function and prevents permeability to toxins and pathogens from the gut lumen into mucosal tissue and circulation. However, the molecular mechanism regulating the effects of glutamine on intestinal barrier function is poorly understood, especially under hypobaric hypoxia environment.
Glutamine can become situationally essential amino acids such as gastrointestinal disease. Supplementation of glutamine can bring many benefits, including facilitating nitrogen metabolism, fueling the cells that line the intestine, supporting protein synthesis, and serving as a critical substrate for the cellular immune response. In the area of protection of gastrointestinal health with glutamine, the research hotspot is to elucidate the mechanism underlying the effect of glutamine on intestinal barrier function in physiological and pathological conditions.
Glutamine is the most abundant amino acid in the body. However, under the condition of extreme stress, the body may need more glutamine than it can make. Previous studies focused on the application effect of glutamine in food, medicine and feed. Its underlying mechanisms are still unknown. Hypobaric hypoxia is one major kind of environmental stress at high altitudes for humans, which usually causes damage to organs including the intestine and even induces intestinal diseases. Up to now, there are no effective measures to prevent and treat it. However, many studies pay more attention to the effect of hypobaric hypoxia on the nervous system and respiratory system. Little is known about the mechanism of intestine barrier dysfunction caused by hypobaric hypoxia and the protective effect of glutamine on intestine barrier dysfunction. The present study was conducted to explore the role of glutamine in the preservation of intestinal barrier function and maintaining the flora balance in rats exposed to hypobaric hypoxia environment, and investigate the role of the TLRs/MyD88/NF-κB signal pathway and tight junction protein occludin in the protective effect of Gln against intestinal barrier damage induced by hypobaric hypoxia.
The results indicate that glutamine can play a role to protect the intestinal barrier function damage and regulate the diversity and composition of intestinal bacterial community under hypobaric hypoxia environment. The altered bacterial communities in the intestine and TLR4/MyD88/NF-κB signal pathway may represent the significant therapeutic targets for the prevention/treatment of intestinal barrier dysfunction and consequent intestinal diseases.
Hypobaric hypoxia: hypobaric hypoxia that is particularly more likely to happen for humans at high altitude areas and for pilots in flight is a condition where the body is deprived of a sufficient supply of oxygen from the air to supply for body tissues whether in quantity or molecular concentration; intestinal barrier function: intestinal barrier function regulates transport and host defense mechanisms at the mucosal interface with the outside world, and the barrier consists of an intrinsic layer, including epithelial cells and tight junctions, and an extrinsic layer, which is comprised of bacteria and a coating of mucus with high concentrations of secretory IgA. Glutamine: glutamine with many functions in the body is an amino acid that is used as a nutritional supplement in the treatment of a variety of diseases.
This is a well-designed study aimed to investigate the beneficial effects of glutamine on intestinal damage in an animal model exposed to hypobaric hypoxic environment. The key findings presented here are of interest. The results are quite convincing and it will be interesting to reproduce them in humans.
P- Reviewers: Ierardi E, Ocker M, Yuan H S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Zhang DN
| 1. | Zhou B, Yang DZ, Zhou QQ. The SEM observation of small intestinal mucosa in the rabbits under simulated high altitude hypoxia. Zhonghua Xiaohua Ganzangbing Zazhi. 2009;18:751-753. |
| 2. | Recavarren-Arce S, Ramirez-Ramos A, Gilman RH, Chinga-Alayo E, Watanabe-Yamamoto J, Rodriguez-Ulloa C, Miyagui J, Passaro DJ, Eza D. Severe gastritis in the Peruvian Andes. Histopathology. 2005;46:374-379. [PubMed] |
| 3. | Shen L. Functional morphology of the gastrointestinal tract. Curr Top Microbiol Immunol. 2009;337:1-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Zhou QQ, Yang DZ, Luo YJ, Li SZ, Liu FY, Wang GS. Over-starvation aggravates intestinal injury and promotes bacterial and endotoxin translocation under high-altitude hypoxic environment. World J Gastroenterol. 2011;17:1584-1593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (5)] |
| 5. | Wu WM, Zhang FX. Research advances in plateau hypoxia and gut barrier injury. Shijie Huaren Xiaohua Zazhi. 2009;17:1432-1436. |
| 6. | Neu J, Mihatsch WA, Zegarra J, Supapannachart S, Ding ZY, Murguía-Peniche T. Intestinal mucosal defense system, Part 1. Consensus recommendations for immunonutrients. J Pediatr. 2013;162:S56-S63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Bai YY, Yu MK, Liu ZY. Immunoloregulation effect of glutamine on intestines. Zhongguo Linchuang Kangfu. 2006;10:153-155. |
| 8. | Wischmeyer PE. Clinical applications of L-glutamine: past, present, and future. Nutr Clin Pract. 2003;18:377-385. [PubMed] |
| 9. | Meynial-Denis D, Bielicki G, Beaufrère AM, Mignon M, Mirand PP, Renou JP. Glutamate and CO2 production from glutamine in incubated enterocytes of adult and very old rats. J Nutr Biochem. 2013;24:688-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 10. | Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313-323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3922] [Cited by in RCA: 3462] [Article Influence: 216.4] [Reference Citation Analysis (0)] |
| 11. | Lehmann C, Pavlovic D, Zhou J, Wuttke U, Saeger D, Spassov A, Hung O, Cerny V, Witter T, Whynot S. Intravenous free and dipeptide-bound glutamine maintains intestinal microcirculation in experimental endotoxemia. Nutrition. 2012;28:588-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Gao JS, Yang SL. Advance in causes and mechanisms of intestinal injury. Shijie Huaren Xiaohua Zazhi. 2009;17:1540-1544. |
| 14. | Kelly CJ, Colgan SP. Targeting Hypoxia to Augment Mucosal Barrier Function. J Epithel Biol Pharmacol. 2012;5:67-76. |
| 15. | Wu WM, Zhang FX, Zhang P. Influence of plateau hypoxia on the tissue injury and expression of HIF-1a and iNOS in intestinal mucosa of rats. Jiefangjun Yixue Zazhi. 2010;35:592-594. |
| 16. | Daniele B, Perrone F, Gallo C, Pignata S, De Martino S, De Vivo R, Barletta E, Tambaro R, Abbiati R, D’Agostino L. Oral glutamine in the prevention of fluorouracil induced intestinal toxicity: a double blind, placebo controlled, randomised trial. Gut. 2001;48:28-33. [PubMed] |
| 17. | Zhou X, Wu X, Yin Y, Zhang C, He L. Preventive oral supplementation with glutamine and arginine has beneficial effects on the intestinal mucosa and inflammatory cytokines in endotoxemic rats. Amino Acids. 2012;43:813-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Yi GF, Carroll JA, Allee GL, Gaines AM, Kendall DC, Usry JL, Toride Y, Izuru S. Effect of glutamine and spray-dried plasma on growth performance, small intestinal morphology, and immune responses of Escherichia coli K88+-challenged weaned pigs. J Anim Sci. 2005;83:634-643. [PubMed] |
| 19. | Bailey DM, Davies B. Acute mountain sickness; prophylactic benefits of antioxidant vitamin supplementation at high altitude. High Alt Med Biol. 2001;2:21-29. [PubMed] |
| 20. | Najeeb Q, Bhaskar N, Masood I, Wadhwa S, Kaur H, Ishaq S. Malondialdehyde (MDA) and superoxide dismutase (SOD) levels-distinguishing parameters between benign and malignant pleural effusions. Free Rad Antiox. 2012;2:8-11. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Singh M, Thomas P, Shukla D, Tulsawani R, Saxena S, Bansal A. Effect of subchronic hypobaric hypoxia on oxidative stress in rat heart. Appl Biochem Biotechnol. 2013;169:2405-2419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | El-Demerdash FM, Jebur AB, Nasr HM. Oxidative stress and biochemical perturbations induced by insecticides mixture in rat testes. J Environ Sci Health B. 2013;48:593-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427-434. [PubMed] |
| 24. | Wu QJ, Zhou YM, Wu YN, Zhang LL, Wang T. The effects of natural and modified clinoptilolite on intestinal barrier function and immune response to LPS in broiler chickens. Vet Immunol Immunopathol. 2013;153:70-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 26. | Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163-189. [PubMed] |
| 27. | Elmore BO, Bollinger JA, Dooley DM. Human kidney diamine oxidase: heterologous expression, purification, and characterization. J Biol Inorg Chem. 2002;7:565-579. [PubMed] |
| 28. | Hamada Y, Shinohara Y, Yano M, Yamamoto M, Yoshio M, Satake K, Toda A, Hirai M, Usami M. Effect of the menstrual cycle on serum diamine oxidase levels in healthy women. Clin Biochem. 2013;46:99-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Shang X, Wang P, Liu Y, Zhang Z, Xue Y. Mechanism of low-frequency ultrasound in opening blood-tumor barrier by tight junction. J Mol Neurosci. 2011;43:364-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 30. | Yu AS, McCarthy KM, Francis SA, McCormack JM, Lai J, Rogers RA, Lynch RD, Schneeberger EE. Knockdown of occludin expression leads to diverse phenotypic alterations in epithelial cells. Am J Physiol Cell Physiol. 2005;288:C1231-C1241. [PubMed] |
| 31. | Young VR, Ajami AM. Glutamine: the emperor or his clothes? J Nutr. 2001;131:2449S-259S; discussion 2449S-259S;. [PubMed] |
| 32. | Pithon-Curi TC, Schumacher RI, Freitas JJ, Lagranha C, Newsholme P, Palanch AC, Doi SQ, Curi R. Glutamine delays spontaneous apoptosis in neutrophils. Am J Physiol Cell Physiol. 2003;284:C1355-C1361. [PubMed] |
| 33. | Rao RK, Samak G. Role of Glutamine in Protection of Intestinal Epithelial Tight Junctions. J Epithel Biol Pharmacol. 2012;5:47-54. |
| 34. | Maes M, Leunis JC. Normalization of leaky gut in chronic fatigue syndrome (CFS) is accompanied by a clinical improvement: effects of age, duration of illness and the translocation of LPS from gram-negative bacteria. Neuro Endocrinol Lett. 2008;29:902-910. [PubMed] |
| 35. | Flint HJ, Bayer EA, Rincon MT, Lamed R, White BA. Polysaccharide utilization by gut bacteria: potential for new insights from genomic analysis. Nat Rev Microbiol. 2008;6:121-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1088] [Cited by in RCA: 1156] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 36. | Zhang FX, Yang WC, Deng ZY, Wu WM, Wu HP, Chen JY, Wang Y, Yang YJ. Effect of glutamine on change of intestinal microecology in rats exposed to acute high altitude. Zhongguo Weishengtai Zazhi. 2010;22:1-4. |
| 37. | Luo H, Guo P, Zhou Q. Role of TLR4/NF-κB in damage to intestinal mucosa barrier function and bacterial translocation in rats exposed to hypoxia. PLoS One. 2012;7:e46291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (1)] |
| 38. | Noreen M, Shah MA, Mall SM, Choudhary S, Hussain T, Ahmed I, Jalil SF, Raza MI. TLR4 polymorphisms and disease susceptibility. Inflamm Res. 2012;61:177-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (1)] |
| 39. | Wullaert A. Role of NF-kappaB activation in intestinal immune homeostasis. Int J Med Microbiol. 2010;300:49-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 40. | Covert MW, Leung TH, Gaston JE, Baltimore D. Achieving stability of lipopolysaccharide-induced NF-kappaB activation. Science. 2005;309:1854-1857. [PubMed] |
| 41. | Fukata M, Michelsen KS, Eri R, Thomas LS, Hu B, Lukasek K, Nast CC, Lechago J, Xu R, Naiki Y. Toll-like receptor-4 is required for intestinal response to epithelial injury and limiting bacterial translocation in a murine model of acute colitis. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1055-G1065. [PubMed] |
| 42. | Soares AL, Coelho FR, Guabiraba R, Kamal M, Vargaftig BB, Li L, Li J, Tavares-de-Lima W, Ryffel B. Tumor necrosis factor is not associated with intestinal ischemia/reperfusion-induced lung inflammation. Shock. 2010;34:306-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 43. | Peterson CY, Costantini TW, Loomis WH, Putnam JG, Wolf P, Bansal V, Eliceiri BP, Baird A, Coimbra R. Toll-like receptor-4 mediates intestinal barrier breakdown after thermal injury. Surg Infect (Larchmt). 2010;11:137-144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 44. | de La Serre CB, Ellis CL, Lee J, Hartman AL, Rutledge JC, Raybould HE. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am J Physiol Gastrointest Liver Physiol. 2010;299:G440-G448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 680] [Article Influence: 45.3] [Reference Citation Analysis (0)] |