Published online Apr 21, 2014. doi: 10.3748/wjg.v20.i15.4421
Revised: January 4, 2014
Accepted: January 14, 2014
Published online: April 21, 2014
Processing time: 219 Days and 14 Hours
AIM: To investigate the expression and prognostic value of CCL2 in gastric cancer, as well as its relationship with tumor hypoxia.
METHODS: Tumor tissues from 68 gastric cancer patients (GC) were analyzed, and the expression of CCL2 and hypoxia-inducible factor 1 alpha (HIF-1α) in tumor tissues was detected by immunohistochemistry. Statistical evaluations that were used included univariate log-rank tests of Kaplan-Meier curves and multivariate Cox regression model analysis.
RESULTS: CCL2 was highly expressed in 66.2% (45/68) of gastric cancer specimens. The distribution of CCL2 expression in tumor tissue was consistent with that of HIF-1α. Patients with high CCL2 expression in GC had a lower overall survival rate [50.6 mo (95%CI: 44.44-56.93) vs 64.6 mo (95%CI: 60.27-68.94), P = 0.013].
CONCLUSION: CCL2 expression correlates closely with HIF-1α expression in gastric cancer. CCL2 may be an independent prognostic marker for GC.
Core tip: The authors have performed immunohistochemical analysis of CCL2 and hypoxia-inducible factor 1 alpha (HIF-1α) in consecutive formalin-fixed paraffin-embedded sections of 68 gastric tumor samples taken from gastric cancer patients. The expression of the monocyte chemotactic protein-1/CCL2 is associated with the expression of HIF-1α. The research results showed a correlation between the expression of both proteins in gastric carcinoma. The authors have statistically analyzed the relationship of CCL2 expression with the clinicopathological characteristics of the patients and their survival time. The expression of CCL2 could be used as a prognostic biomarker for gastric cancer.
- Citation: Tao LL, Shi SJ, Chen LB, Huang GC. Expression of monocyte chemotactic protein-1/CCL2 in gastric cancer and its relationship with tumor hypoxia. World J Gastroenterol 2014; 20(15): 4421-4427
- URL: https://www.wjgnet.com/1007-9327/full/v20/i15/4421.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i15.4421
Gastric cancer (GC) is one of the most prevalent malignancies in the world[1], and it is the fourth most common cancer in men and the fifth in women around the world. Approximately 8% of the total diagnosed cases and 10% of annual cancer deaths are attributed to gastric cancer worldwide[2]. Presently, surgical resection remains the only curative treatment option. However, most patients have advanced cancer in stages III or IV and show lymphatic metastasis at the time of diagnosis. Currently, there is no specific biomarker that has been detected in GC for clinically diagnostic and prognostic purposes[3]. Therefore, a set of prognostic molecular biomarkers is needed for gastric cancer to improve the design and evaluation of individualized therapeutic strategies for this lethal disease.
Hypoxia is a characteristic of the tumor microenvironment that may play a critical role in tumor angiogenesis, survival response, invasion and metastasis[4]. Accumulating evidence suggests that tumor hypoxia is an independent marker of poor prognosis in patients with various types of cancer, including cervical cancer, breast cancer, head and neck cancer, soft-tissue sarcoma, cutaneous melanoma and prostatic adenocarcinoma[5]. Hypoxia can activate relevant gene expression through hypoxia-inducible factors (HIF), an important transcription factor family that includes HIF-1, HIF-2 and HIF-3. HIF is a heterodimer composed of an alpha and a beta subunit, in which the HIF-1α protein is a master regulator of the hypoxic response[6].
Hypoxic regions of solid tumors are often characterized by the large accumulation of macrophages, which contribute to tumor angiogenesis and development[7]. The trophic effect of tumor hypoxia on tumor-associated macrophages (TAMs) is clinically relevant, as a high TAM number is considered to be a negative prognostic marker in various human malignancies, including Hodgkin’s disease, glioma, cholangiocarcinoma and breast carcinoma[8]. Monocyte chemotactic protein-1 (MCP-1/CCL2), a member of the CC family of chemokines, is known to recruit monocytes and macrophages to inflammation sites and tumors[9]. The elevated expression of a number of monocyte chemoattractants, including CCL2, by both cancer and stromal cells has been shown to positively correlate with increased TAM numbers in several human tumors[10]. CCL2 has been demonstrated to regulate monocyte and macrophage infiltration and reported to be present in tumor sites, suggesting a role for this chemokine in TAM recruitment.
To better characterize the clinicopathological significance of CCL2 in gastric cancer, we hypothesized that CCL2 might be a potential prognostic biomarker for gastric cancer, and its expression would be associated with HIF-1α expression.
Formaldehyde-fixed and paraffin-embedded gastric carcinoma samples were obtained from the Pathology Department of Jinling Hospital. All of the pathological diagnoses were confirmed by two independent pathologists. None of the patients had received any preoperative treatments. Of all the patients, 73.5% were male, 26.5% were female, and the mean age was 49.86 years. All patients were followed from the date of surgery to the date of their death or the end of the study in December 2011. The data of patients who were lost to follow-up or who died of causes other than GC were censored in the survival analysis. The study was approved by the Ethics Committee of Jinling Hospital.
To evaluate the CCL2 protein expression in clinical samples that had been embedded in paraffin blocks, we stained the sections as follows. Three micrometer continuous sections were cut on slides for immunohistochemical analysis. Antigen retrieval was achieved by pressure cooking the slides with citric acid buffer at pH 6.0 for 1 min. Endogenous peroxidase activity was blocked by immersing the slides in 3% H2O2 for 10 min, and the background, nonspecific binding was reduced by incubating the slides with 5% bovine serum albumin (BSA) in PBS for 5 min. The continuous slides were incubated overnight at 4 °C with rabbit monoclonal CCL2 antibody (1:500 dilution, Santa Cruz) and mouse monoclonal HIF-1α antibody (1:100 dilution, MAB1935, R and D), respectively. Finally, the slides were washed five times in PBS 1 ×, pH 7.4, for 5 min. To reduce variability, all samples were processed at the same time in a single experiment using a single batch of antibody diluted in PBS with BSA. Slides were then washed in PBS and incubated sequentially with biotinylated goat anti-rabbit/mouse IgG at a dilution of 1:500 for 30 min at 37 °C. The reaction product was developed using diaminobenzidine tetrahydrochloride. Finally, the slides were counterstained with hematoxylin. Subsequently, the tissues were washed in distilled water for 5 min, dehydrated sequentially and mounted in resinous mountant.
The evaluation of CCL2 expression was performed independently by two experienced pathologists, who were blinded to the clinical data. The staining results for CCL2 were scored semi-quantitatively by calculating the immunostaining intensity and the distribution of the percentage of positive cells. The percentage of positive tumor cells was determined in at least 5 areas under 400 × magnification and averaged. The mean percentage was then divided into five categories: 0, < 5%; 1, 5%-25%; 2, 26%-50%; 3, 51%-75%; and 4, > 75%. The staining intensity was calculated by assigning no coloring, slightly yellow, brown yellow and tan stains to values of 0, 1, 2 and 3, respectively. Finally, we calculated the product of staining intensity and positive cell percentage: ≤ 5 was defined as low expression, and ≥ 6 as high expression.
To further study the relationship between CCL2 and HIF-1α in gastric cancer, the spatial distributions of CCL2 and HIF-1α expression were analyzed in pairs.
Differences between clinicopathological variables and the expression of CCL2 were examined by the χ2 test. Survival curves were calculated using the Kaplan-Meier method and compared by the log-rank test. Multivariate Cox regression model analysis was applied to assess the prognostic values of protein expression. The confidence level for statistical inference was 95% (P < 0.05). Statistical analysis was performed using SPSS software (Version 16.0, Chicago, IL, United States).
Patients with gastric cancer were interviewed by telephone, and additional data were collected from the medical records. The clinicopathological characteristics of the 68 patients are shown in Table 1. The ages of the patients ranged from 24 to 59 years, with a mean age of 49.82 years. According to the American Joint Committee on Cancer (AJCC, 2010) classification, there were 33 stage III/IV patients and 35 stage I/II patients. The patients were followed for a period of 1-84 mo. Two patients did not receive a full follow-up, and 37 patients died during the follow-up period.
| Clinical characteristic | CCL2 expression level | P value1 | |
| High | Low | ||
| Gender | 0.091 | ||
| Male | 36 | 14 | |
| Female | 9 | 9 | |
| Age (yr) | 0.772 | ||
| < 49.82 | 16 | 9 | |
| ≥ 49.82 | 29 | 14 | |
| Tumor diameter (cm) | 0.595 | ||
| ≤ 5 | 8 | 2 | |
| 5-10 | 8 | 5 | |
| > 10 | 29 | 16 | |
| Differentiation grade | 0.571 | ||
| Well | 2 | 2 | |
| Moderate | 16 | 10 | |
| Poor | 27 | 11 | |
| Histopathology | 0.387 | ||
| Tubular | 14 | 6 | |
| Poorly | 19 | 11 | |
| Signet-ring | 7 | 1 | |
| Mucinous | 5 | 5 | |
| Lymph node metastasis | 0.954 | ||
| Yes | 14 | 7 | |
| No | 31 | 16 | |
| AJCC stage2 | 0.268 | ||
| I/II | 21 | 14 | |
| III/IV | 24 | 9 | |
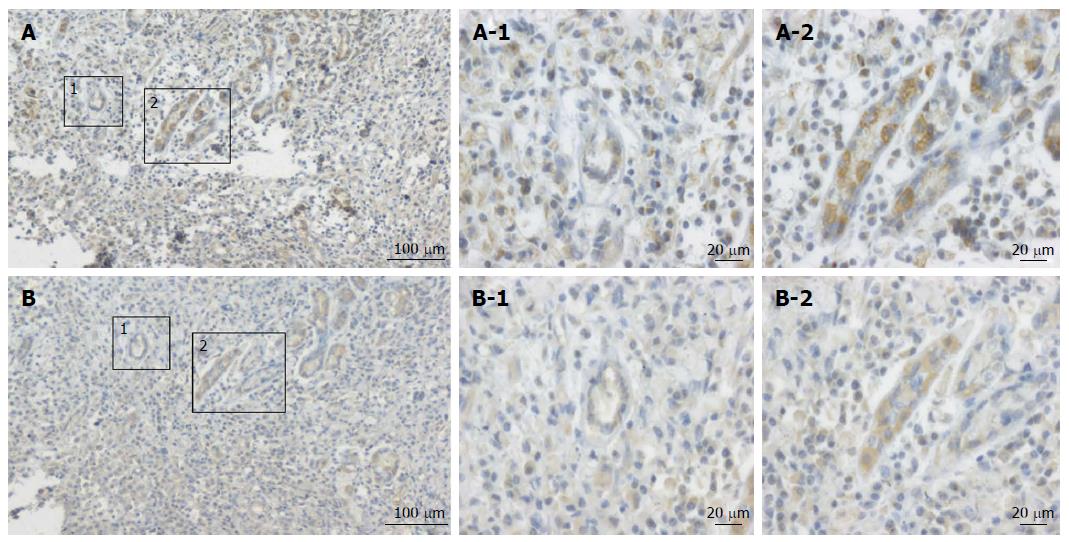
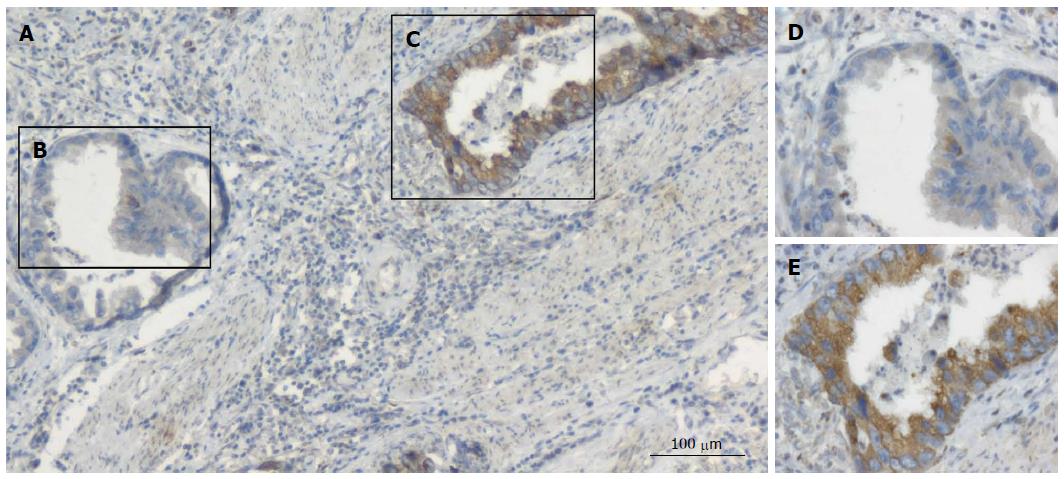
The expression levels of MCP-1 protein in GC were detected by immunostaining. Typical immunohistochemical findings of CCL2 in gastric tumor tissue are shown in Figures 1 and 2. CCL2 protein was found to be located in the cytoplasm of the malignant cells, and we repeated the experiment twice to exclude false positive results. Immunohistochemical analysis showed that, in gastric carcinoma, 45 (66.2%) of 68 tumor samples had high CCL2 expression.
To evaluate whether the expression of the inflammatory cytokine CCL2 was associated with HIF-1α expression, the expression levels of CCL2 and HIF-1α were measured in consecutive sections. The expression of HIF-1α was consistent with the spatial distribution of CCL2 in tumor cells (Figure 1). The consistency of spatial distribution of the expression of these two proteins also suggests that CCL2 expression is related to tumor hypoxia.
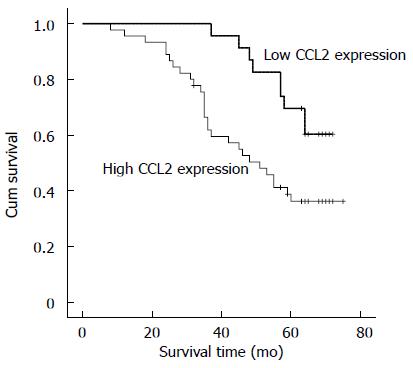
Survival curves were calculated using the Kaplan-Meier method and compared using the log-rank test. The follow-up time was censored if the patient died from another cause rather than gastric cancer or was lost during the follow-up period. Patients with high levels of CCL2 expression in gastric cancer (P < 0.005) had a statistically significant correlation with poor overall survival. (Figure 3; 50.6 mo (95%CI: 44.44-56.93) vs 64.6 mo (95%CI: 60.27-68.94), P = 0.013)). The independent effects of all significant factors were evaluated in a multivariate Cox regression model. The result demonstrated that tumor CCL2 expression level (P = 0.045, HR = 2.311, 95%CI: 1.019-5.241) and AJCC stage (P = 0.004, HR = 3.242, 95%CI: 1.467-7.163) were independent prognostic factors for gastric cancer patients, and other factors showed no statistical significance with prognosis (Table 2).
| Survival time | ||
| HR (95%CI) | P value | |
| Gender | 1.198 (0.531-2.703) | 0.664 |
| Male vs female | ||
| Tumor volume | 1.086 (0.660-1.788) | 0.744 |
| > 5 cm vs < 5 cm | ||
| Differentiation | 0.637 (0.310-1.310) | 0.221 |
| Mid + High vs Low | ||
| Lymph node | 1.109 (0.484-2.541) | 0.806 |
| Yes vs No | ||
| AJCC stage | 3.242 (1.467-7.163) | 0.004 |
| I/II vs III/IV | ||
| CCL2 expression | 2.311 (1.019-5.241) | 0.045 |
| High vs Low | ||
Advanced gastric cancer is characterized by the rapid emergence of systemic metastasis, resulting in poor prognosis due to a lack of curative treatment options. However, there is no single marker for GC. Therefore, research to identify specific markers for this malignant tumor is important and necessary. Solid tumors need new blood vessels to support their growth by providing enough nutrients and oxygen. As tumors grow, the diffusion distance from the existing vascular supply increases, which results in hypoxia[11]. Hypoxia is a common feature of the microenvironment of a diverse range of solid tumors including gastric cancer. Hypoxia plays a critical role in various cellular and physiologic events, including cell proliferation, survival, angiogenesis, immunosurveillance, metabolism, and tumor invasion and metastasis, and it is often associated with poor prognosis[4,12].
HIF-1α is one of the principal mediators of homeostasis in response to hypoxia in human tissues. Under hypoxic conditions, HIF-1α produces an active heterodimer and drives the transcription of a number of genes important for cell survival, immune reaction and chemokine production[6]. Hypoxic tumors secrete higher amounts of chemoattractants and other factors that enhance monocyte/macrophage attachment to and migration through the tumor vasculature. Recent evidence has shown that TAM may accumulate in high numbers in hypoxic areas of breast, prostate and ovarian carcinoma due to the hypoxic release of macrophage chemoattractants[13].
CCL2, also known as MCP-1, is a small cytokine and a highly potent chemokine that attracts and activates monocytes/macrophages to sites of tissue injury and inflammation, as well as to tumor sites. CCL2 acts as an immune inhibitor in tumors. CCL2 is produced by endothelial cells, fibroblasts, epithelial cells, smooth muscle cells, astrocytes, macrophages, microglial cells, and even certain tumor cells themselves[14]. Furthermore, several recent investigations have shown that CCL2 gene expression was upregulated in hypoxic regions. CCL2 expression levels were dependent on the O2 concentration and duration of the hypoxic exposure[15]. The CCL2 gene contains several binding sites for HIF in the promoter, which is believed to account for increased CCL2 expression in response to hypoxia-induced HIF stabilization[16]. However, the biological significance of CCL2 in the tumor microenvironment appears particularly complex, and the relationship between HIF-1α and CCL2 in gastric tumors has not been clarified.
In our study, we analyzed the expression of CCL2 and HIF-1α in gastric cancer samples and found high expression levels of CCL2 in primary gastric cancer by immunohistochemical analysis. Both the tumor cells and stromal cells were observed to express CCL2, and the distribution of HIF-1α expression was consistent with that of CCL2 expression in tumor sections. The consistency of the spatial distribution of the two proteins indicated that CCL2 expression was related to tumor hypoxia.
Studies have demonstrated that CCL2 is expressed in several tumors including glioma[17], melanoma[18], ovarian carcinoma[19], and uterine cervical tumors[20]. Significantly higher levels of CCL2 expression were found in the epithelial region of various tumors, including breast cancer[21]. It has also been shown that CCL2, both at the mRNA and protein levels, is expressed mainly in the epithelial regions of prostate cancer tissues[22].
Because tumor cells produce CCL2, it is considered to have an important role in the progression and invasion of cancer. This role has been confirmed in many types of cancer, such as prostate cancer[23] and breast cancer[24]. In addition, interrupting the CCR2/CCL2 interaction using a CCL2 specific antibody or CCR2 siRNA markedly reduced the recruitment of monocytes/macrophages and delayed tumor progression and metastasis[25]. This suggests that CCL2 expression within tumors may be a critical determinant of monocyte recruitment, as CCR2 is highly expressed in classical monocytes. Based on the expression patterns of CCL2 and CCR2, we hypothesize that this ligand/receptor pair is a critical determinant of the recruitment of classical monocytes. It has also been reported that CCL2 may be a candidate molecular tumor marker, which could be targeted for cancer immunotherapy[26].
Consistent with the clinicopathological significance, gastric cancer patients with high expression levels of CCL2 in tumor cells exhibited poor overall survival in our study. Thus, high expression of CCL2 was an indicator of poor clinical prognosis. Therefore, we propose that CCL2 might be a valuable predictive marker of gastric carcinoma, as it is correlated with cancer stage according to the AJCC tumor staging system. Because CCL2 is involved in many aspects of the tumor microenvironment, it may be a potential molecular target for malignant tumor therapy.
The authors wish to thank Dr. Xiaoxia Lu for her help in data collection.
Gastric carcinoma is the fourth most common cancer in men and the fifth in women around the world. Tumor specific biomarkers in gastric carcinoma may be helpful for clarifying histological heterogeneity and the underlying molecular mechanisms. A set of better molecular biomarkers for the prognosis of gastric cancer is needed to design and evaluate individualized therapeutic strategies for this malignancy.
Elevated expression of CCL2 had been showed in various cancers. However, few studies have investigated the correlation between CCL2 and gastric carcinoma. Previous studies have indicated that CCL2 gene expression is regulated in hypoxic regions, but the correlation between CCL2 expression and hypoxia-inducible factor-1α (HIF-1α) expression has not been observed. In this study, the authors demonstrated that CCL2 protein was regulated in gastric carcinoma and showed a close correlation with HIF-1α expression.
The research data showed the correlation between the expression of these two proteins in gastric carcinoma and found that CCL2 may be a prognostic marker of gastric carcinoma. Moreover, high expression of CCL2 could be an indicator of poor clinical prognosis in patients with gastric carcinoma.
The study suggested that examination of CCL2 and HIF-1α expression by immunohistochemistry analysis could be used as an effective way to identify patients at high risk of tumor progression, thus allowing the optimization of the individual treatment of patients with gastric carcinoma.
Monocyte chemotactic protein-1 (MCP-1/CCL2), a member of the CC family of chemokines, is known to recruit monocytes and macrophages to sites of inflammation and tumor. HIF is an important transcription factor family that includes HIF-1, HIF-2 and HIF-3. HIF is a heterodimer composed of an alpha and a beta subunit, in which HIF-1α determines HIF-1 activity.
The authors have performed immunohistochemical analysis of CCL2 and HIF-1 proteins in consecutive formalin-fixed paraffin-embedded sections of 68 gastric tumor samples taken from gastric cancer patients and have statistically analyzed the relationship of CCL2 expression with the clinicopathological characteristics of the patients and their survival time. This manuscript investigated the expression and prognostic value of CCL2 in gastric cancer and its relationship with tumor hypoxia. The authors found the histological distribution of CCL2 expression to be consistent with that of HIF-1α, and high CCL2 expression was associated with low survival rate.
P- Reviewers: Kim H, Kim SY, Lin CY, Sala N S- Editor: Cui XM L- Editor: Wang TQ E- Editor: Zhang DN
| 1. | de Martel C, Forman D, Plummer M. Gastric cancer: epidemiology and risk factors. Gastroenterol Clin North Am. 2013;42:219-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 277] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 2. | Guggenheim DE, Shah MA. Gastric cancer epidemiology and risk factors. J Surg Oncol. 2013;107:230-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 379] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 3. | Kumar RK, Shankar EM, Ganapathy E, Raj SS, Ebrahim AS, Farooq SM. Gastric Carcinoma: A review on epidemiology, current surgical & chemotherapeutic options. Gastric Carcinoma- New Insights into Current Management. Croatia: InTech 2013; . |
| 4. | Ruan K, Song G, Ouyang G. Role of hypoxia in the hallmarks of human cancer. J Cell Biochem. 2009;107:1053-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 355] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 5. | Vaupel P, Mayer A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev. 2007;26:225-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1540] [Cited by in RCA: 1690] [Article Influence: 93.9] [Reference Citation Analysis (0)] |
| 6. | Adams JM, Difazio LT, Rolandelli RH, Luján JJ, Haskó G, Csóka B, Selmeczy Z, Németh ZH. HIF-1: a key mediator in hypoxia. Acta Physiol Hung. 2009;96:19-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 110] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 7. | Galdiero MR, Garlanda C, Jaillon S, Marone G, Mantovani A. Tumor associated macrophages and neutrophils in tumor progression. J Cell Physiol. 2013;228:1404-1412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 322] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 8. | Riboldi E, Porta C, Morlacchi S, Viola A, Mantovani A, Sica A. Hypoxia-mediated regulation of macrophage functions in pathophysiology. Int Immunol. 2013;25:67-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 9. | Ferreira FO, Ribeiro FL, Batista AC, Leles CR, de Cássia Gonçalves Alencar R, Silva TA. Association of CCL2 with lymph node metastasis and macrophage infiltration in oral cavity and lip squamous cell carcinoma. Tumour Biol. 2008;29:114-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Zhang J, Patel L, Pienta KJ. CC chemokine ligand 2 (CCL2) promotes prostate cancer tumorigenesis and metastasis. Cytokine Growth Factor Rev. 2010;21:41-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 218] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 11. | Liao D, Johnson RS. Hypoxia: a key regulator of angiogenesis in cancer. Cancer Metastasis Rev. 2007;26:281-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 517] [Cited by in RCA: 543] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 12. | Rohwer N, Lobitz S, Daskalow K, Jöns T, Vieth M, Schlag PM, Kemmner W, Wiedenmann B, Cramer T, Höcker M. HIF-1alpha determines the metastatic potential of gastric cancer cells. Br J Cancer. 2009;100:772-781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Siveen KS, Kuttan G. Role of macrophages in tumour progression. Immunol Lett. 2009;123:97-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 275] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 14. | Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29:313-326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3016] [Cited by in RCA: 2908] [Article Influence: 181.8] [Reference Citation Analysis (0)] |
| 15. | Bosco MC, Puppo M, Pastorino S, Mi Z, Melillo G, Massazza S, Rapisarda A, Varesio L. Hypoxia selectively inhibits monocyte chemoattractant protein-1 production by macrophages. J Immunol. 2004;172:1681-1690. [PubMed] |
| 16. | Mojsilovic-Petrovic J, Callaghan D, Cui H, Dean C, Stanimirovic DB, Zhang W. Hypoxia-inducible factor-1 (HIF-1) is involved in the regulation of hypoxia-stimulated expression of monocyte chemoattractant protein-1 (MCP-1/CCL2) and MCP-5 (Ccl12) in astrocytes. J Neuroinflammation. 2007;4:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 150] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 17. | Wang H, Zhang L, Zhang IY, Chen X, Da Fonseca A, Wu S, Ren H, Badie S, Sadeghi S, Ouyang M. S100B promotes glioma growth through chemoattraction of myeloid-derived macrophages. Clin Cancer Res. 2013;19:3764-3775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 18. | Harlin H, Meng Y, Peterson AC, Zha Y, Tretiakova M, Slingluff C, McKee M, Gajewski TF. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res. 2009;69:3077-3085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 711] [Cited by in RCA: 894] [Article Influence: 55.9] [Reference Citation Analysis (0)] |
| 19. | Arnold JM, Huggard PR, Cummings M, Ramm GA, Chenevix-Trench G. Reduced expression of chemokine (C-C motif) ligand-2 (CCL2) in ovarian adenocarcinoma. Br J Cancer. 2005;92:2024-2031. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Wu HH, Lee TH, Tee YT, Chen SC, Yang SF, Lee SK, Ko JL, Wang PH. Relationships of single nucleotide polymorphisms of monocyte chemoattractant protein 1 and chemokine receptor 2 with susceptibility and clinicopathologic characteristics of neoplasia of uterine cervix in Taiwan women. Reprod Sci. 2013;20:1175-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Fujimoto H, Sangai T, Ishii G, Ikehara A, Nagashima T, Miyazaki M, Ochiai A. Stromal MCP-1 in mammary tumors induces tumor-associated macrophage infiltration and contributes to tumor progression. Int J Cancer. 2009;125:1276-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 207] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 22. | Lu Y, Cai Z, Galson DL, Xiao G, Liu Y, George DE, Melhem MF, Yao Z, Zhang J. Monocyte chemotactic protein-1 (MCP-1) acts as a paracrine and autocrine factor for prostate cancer growth and invasion. Prostate. 2006;66:1311-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 194] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 23. | Kirk PS, Koreckij T, Nguyen HM, Brown LG, Snyder LA, Vessella RL, Corey E. Inhibition of CCL2 Signaling in Combination with Docetaxel Treatment Has Profound Inhibitory Effects on Prostate Cancer Growth in Bone. Int J Mol Sci. 2013;14:10483-10496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Yoshimura T, Howard OM, Ito T, Kuwabara M, Matsukawa A, Chen K, Liu Y, Liu M, Oppenheim JJ, Wang JM. Monocyte chemoattractant protein-1/CCL2 produced by stromal cells promotes lung metastasis of 4T1 murine breast cancer cells. PLoS One. 2013;8:e58791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 25. | Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222-225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2327] [Cited by in RCA: 2221] [Article Influence: 158.6] [Reference Citation Analysis (0)] |
| 26. | Fridlender ZG, Buchlis G, Kapoor V, Cheng G, Sun J, Singhal S, Crisanti MC, Wang LC, Heitjan D, Snyder LA. CCL2 blockade augments cancer immunotherapy. Cancer Res. 2010;70:109-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 151] [Article Influence: 9.4] [Reference Citation Analysis (0)] |